Abstract
Most of the FDA-approved therapeutic monoclonal antibodies are full-size IgG molecules with a molecular weight of about 150 kDa. A major problem for such large molecules is their poor penetration into tissues (e.g., solid tumors) and poor or absent binding to regions on the surface of some molecules (e.g., on the HIV envelope glycoprotein) which are fully accessible only by molecules of smaller size. Therefore, much work especially during the last decade has been aimed at developing novel scaffolds of much smaller size and high stability. Immunoglobulin-based scaffolds including Fab (~50 kD), ScFv (~30 kD), and VH domain (termed domain antibody, dAb) (~15 kD) have been well established. Recently, a new scaffold based on human IgG1 CH2 domain (~15 kD) was also proposed (termed nanoantibody, nAb). Binders based on a CH2 scaffold could also confer some effector functions. Here, we describe the design, expression, purification, and characterization of engineered CH2 and VH domains.
Keywords: Domain antibody, VH, Nanoantibody, CH2, Library construction, Phage display, Stability, Engineered antibody domains
1. Introduction
Monoclonal antibodies (mAbs) with high affinity and specificity are now well-established therapeutics and invaluable tools for biological research. It appears that their use will continue to expand in both targets and disease indications. However, because of the fundamental problem for full-size mAbs, a large amount of work has been aimed at developing small-size binders with scaffolds based on various highly stable human and nonhuman molecules during the last decade (1–8). A promising direction is the development of binders based on the heavy or light chain variable region of an antibody; these fragments with size ranging from 11 to 15 kDa were called “domain antibodies” or “dAbs” (7, 9). A unique kind of antibodies composed only of heavy chains (designated HCAbs) (10) are naturally formed in camels, dromedaries and llamas, and their variable regions (referred to as VHH) can also recognize antigens as single domain fragments (11). Not only is the overall size of the dAbs much smaller than that of full-size antibodies but also their paratopes are concentrated over a smaller area so that the dAbs provide the capability of interacting with novel epitopes that are inaccessible to conventional antibodies or antibody fragments with paired light and heavy chain variable domains.
The structure of the antibody constant domains is similar to that of the variable domains consisting of β strands connected mostly with loops or short helices. The second domain of the α, δ, and γ heavy chain constant regions, CH2, is unique in that it exhibits very weak carbohydrate-mediated interchain protein–protein interactions in contrast to the extensive interchain interactions that occur between other domains. The expression of murine and human CH2 in bacteria which does not support glycosylation results in a monomeric protein (12, 13). It has been hypothesized that the CH2 domain (CH2 of IgG, IgA and IgD, and CH3 of IgE and IgM) could be used as a scaffold and could offer additional advantages compared to those of the dAbs because it contains binding sites or portions of binding sites conferring effector and stability functions (termed nanoantibodies, Nabs) (14). It was found previously that an isolated murine CH2 was relatively unstable at physiological temperature with a temperature of 50% unfolding (Tm) slightly higher than 37°C, while the Tm value of human CH2 was 54.1°C which was still significantly lower than that of other small scaffolds such as the 10th type III domain of human fibronectin (FN3) (12, 13, 15). The relatively low stability of human CH2 increases the probability of protein aggregation or degradation when it is engineered for binding to antigens and mediating effector functions. Therefore, further improvement of stability of the CH2 scaffold is important.
Here we describe the design, expression, purification, and characterization of engineered CH2 domains, construction of a CH2-based phage display library, a methodology to increase stability of CH2 and comparison with VH-based engineered domains. We use the term engineered antibody domains (eAds) to denote both variable and constant domains that are engineered to confer functions additional to their native ones.
2. Materials
2.1. Cloning of CH2 into Phagemid pComb3X
- Primers are designed based on sequence of human IgG1 constant domain.
Sfi I CH2 upstream: 5′- TTC GCT ACC GTG GCC CAG GCG GCC GCA CCT GAA CTC CTG GGG GGA CC −3′ Sfi I CH2 downstream: 5′- GTG ATG GTG CTG GCC GGC CTG GCC TTT GGC TTT GGA GAT GGT TTT CTC −3′
High Fidelity PCR Master (Roche, Indianapolis, IN), or other high-fidelity PCR systems may be used.
QIAquick Gel Extraction Kit (Qiagen, Valencia, CA).
Restriction enzyme Sfi I (New England Biolabs, Ipswich, MA).
Vector phagemid pComb3X (16).
Ligation kit: Rapid DNA Ligation Kit (Roche, Indianapolis, IN).
Plasmid Mini Kit (Qiagen, Valencia, CA).
2× YT medium (1 L): Tryptone, 16 g; yeast extract, 10 g and NaCl, 5 g.
Ampicillin (Sigma-Aldrich, St. Louis, MO): stock 100 mg/ml store −20°C, working at 100 μg/ml.
E. coli TG1 K12 Δ D( lac-proAB ) supE thi hsdD5/F′ traD36 proA+B lacIq lacZΔM15.
2.2. Expression and Purification of CH2 Domain
SB medium (1 L): Tryptone, 30 g; yeast extract, 20 g; MOPS, 10 g; adjust pH value to 7.0 with 1 M NaOH.
IPTG (BioGolden, MO): stock 1 M, working at 1 mM as inducer on the lacZ suppressor for HB2151 cell expression.
Buffer A: 50 mM Tris–HCl, 450 mM NaCl, pH 8.0.
Buffer B: Buffer A + 200 mM Imidazole.
Polymyxin B sulfate: 0.5 mu/ml (Sigma-Aldrich, St. Louis, MO).
Nickel column: 1 ml HiTrap Chelating HP Ni-NTA column (GE Healthcare, NJ).
FPLC (GE Healthcare, NJ).
Protein loading buffer (6×): 0.35 M Tris–HCl pH 6.8, 10.28% SDS, 0.6 M dithiothreitol (DTT), 36% glycerol (V/V), and 0.06% bromophenol blue, store at −20°C.
E. coli HB2151: K12 ara Δ(lac-proAB) thi/F’ proA+B lacIq lacZΔM15.
2.3. Primers for Library Construction
A published strategy for library construction was used here as an example (17).
Sfi I N terminus primer: 5′ ACGT GGCCCAGGCGGCC GCA CCT GAA CTC CTG 3′ Sfi I C terminus primer: 5′ ACGT GGCCGGCCT GGCC TTT GGC TTT GGA GAT GGT TTT CTC GAT G 3′ Loop BC primer Fw: 5′ AAG TTC AAC TGG TAC GTG 3′ Loop BC primer Rv: 5′CAC GTA CCA GTT GAA CTT GCC AKM AKM AKM AKM AKM AKM AKM AKM AKM AKM CAC CAC CAC GCA TGT GAC 3′ Loop FG primer Rv: 5′ GAT GGT TTT CTC GAT GGG GCC AKM AKM AKM AKM AKM AKM GTT GGA GAC CTT GCA CTT G 3′
2.4. Ligation of CH2 Fragments Containing Mutations with Phagemids
Restriction enzymes SfiI: see above.
T4 DNA Ligase, 400,000 units/ml (New England Biolabs, Ipswich, MA).
2.5. Purification, Concentration, and Desalting of Ligations
QIAquick PCR Purification Kit (Qiagen, Valencia, CA).
Centrifugal filter: Amicon Ultra-4 with a cutoff of 3,000 MW (Millipore, Billerica, MA).
2.6. Electroporations
TG1 electroporation-competent cells (Stratagene, La Jolla, CA).
Gene Pulser/MicroPulser Cuvettes (Bio-Rad, Hercules, CA).
Gene Pulser (Bio-Rad, Hercules, CA).
S.O.C. Medium: 2% tryptone, 0.5% yeast extract, 10 mM sodium chloride, 2.5 mM KCl, 10 mM MgCl2, 10 mM MgSO4, 20 mM glucose.
2.7. Preparation of CH2-Based Library
2× YT medium: see above.
Glucose (Sigma-Aldrich, St. Louis, MO): Stock 20% keep at room temperature, sterilized by filtration.
M13KO7 helper phage (Invitrogen, Carlsbad, CA).
Antibiotics: 100 mg/ml ampicillin and 50 mg/ml kanamycin.
HiSpeed Plasmid Maxi Kit.
2.8. Primers for Construction of CH2 Mutant (m01) with Increased Stability
Omp: 5′ AAG ACA GCT ATC GCG ATT GCA G 3′ gIIIF: 5′ ATC ACC GGA ACC AGA GCC ACC AC 3′ L/C primer Fw: 5′ TCA GTC TTC TGC TTC CCC CCA AAA CCC AAG GAC 3′ L/C primer Rv: 5′ TGG GGG GAA GCA GAA GAC TGA CGG TCC CCC CAG 3′ K/C primer Fw: 5′ CCC ATC GAG TGC ACC ATC TCC AAA GCC AAA GGC 3′ K/C primer Rv: 5′ GGA GAT GGT GCA CTC GAT GGG GGC TGG GAG GGC 3′
2.9. Measurement of the Antibody Domain Stability
Circular dichroism (CD): AVIV Model 202 CD Spectrometer (Aviv Biomedical, NJ).
Differential scanning calorimetry (DSC): VP-DSC MicroCalorimeter (MicroCal, MA).
Spectrofluorometry: Fluorometer Fluoromax-3 (HORIBA Jobin Yvon, NJ).
3. Methods
Cloning, expression, and purification of a CH2 domain are performed based on modification of methods used for dAbs.
3.1. RNA Isolation and cDNA Synthesis
Lymphocyte isolation and cDNA synthesis are performed according to previous protocol (16).
3.2. Cloning of CH2 into Phagemid pComb3X
-
PCR for CH2 domain amplification: Mix (for one reaction)
Reagent Volume (μl) Final concentration Sterile double-dist. water 21 PCR Master Mix (2×) 25 1× CH2 upstream primer (10 μM) 1.5 300 nM CH2 downstream primer (10 μM) 1.5 300 nM cDNA (1 μg/μl) 1 1 μg Final volume 50 Thermal cycling.Temperature Time Cycles Initial denaturation 94°C 2 min 1× Denaturation 94°C 15 s 10× Annealing 55°C 30 s Elongation 72°C 25 s Denaturation 94°C 30 min + 5 s
Cycle elongation for each succes sive cycle25× Annealing 55°C Elongation 72°C Final elongation 72°C 7 min 1× Cooling 4°C Forever - Digestion of insert DNA and vector.
Components CH2 fragment (μl) pComb3X (μl) Final concentration Insert DNA x (2 μg) – Vector pComb3x – y (10 μg) 10× NEBuffer 2 5 5 1× BSA (10 mg/ml) 0.5 0.5 100 μg/ml SfiI (20 u/μl) 4 3 ddH2O 40.5 − x 41.5 − y Final volume 50 -
Purification of digested insert and vector.
The digested products are run on agarose gel and purified according to the manual of the Kit.
-
Ligation.
Dissolve insert DNA and vector DNA in Dilution Buffer (5×) to a final volume of 5 ml in a sterile reaction vial as follows:Components Volume (μl) Final concentration Insert DNA (digest CH2 fragment) (see Note 1) 3 Vector DNA (digested pComb3X) 1 DNA dilution buffer (5×) 1 1× ddH2O – Total 5 Add 5 μl T4 DNA Ligation Buffer (2×) to each reaction vial;
Add 0.5 μl T4 DNA Ligase (5 U/μl);
Mix thoroughly;
Incubate for 5 min at 15–25°C.
Transformation of TG1 competent cells with the ligation product.
-
Plasmid extraction.
The positive clone is verified by direct sequencing and used for transformation of HB2151 for expression.
3.3. Expression and Purification of CH2
Pick up single colony from fresh transformants and grow it in 5 ml SB medium containing 100 μg/ml Amp with shaking at 250 rpm and 37°C.
After incubation for about 3 h, transfer the 5 ml culture to a 2-L flask containing 500 ml SB medium with 100 μg/ml Amp. Shake the flask at 250 rpm and 37°C.
When the OD600 reaches 0.7–1, add 500 μl 1 mM IPTG to induce protein expression with shaking at 250 rpm and 37°C overnight (see Note 2).
Harvest the cells by centrifugation at 6,000 rpm (~ 4,500×g, rotor: JLA-10.500, Beckman Coulter, CA) for 15 min at 4°C. Remove the supernatant and resuspend the pellet in 50 ml PBS (pH 7.4), and add 500 μl polymyxin B sulfate.
Rotate the tube at room temperature for 30 min.
Centrifuge the sample at 15,000 rpm (~18,000×g, rotor: JA-20, Beckman Coulter, CA) at 4°C for 45 min. The supernatant is filtered by using a 0.45-μM filter (Nalgene, NY). Then FPLC is used for protein purification.
Wash the pump A and B with corresponding buffer A and B.
Before loading the sample from the Superloop to the HiTrap Chelating HP, allow 5 ml buffer A to flow through the route.
Load the sample from the Superloop to the HiTrap Chelating HP. Collect flow through for analysis on SDS-PAGE to examine the efficiency of the HiTrap Chelating HP.
Wash the column by 15 ml buffer A.
Wash the column by 10 ml buffer B with increasing concentration from 0 to 20% (see Note 3).
Increase the concentration of buffer B to 100%.
Collect the fractions eluted by 8 ml buffer B with 0.8-ml aliquot per tube.
Stop the pump after elution. Wash the system by ddH2O including Superloop, pump, injector, etc.
Analyze the collections on SDS-PAGE.
3.4. Construction of a CH2-Based Library of Mutants
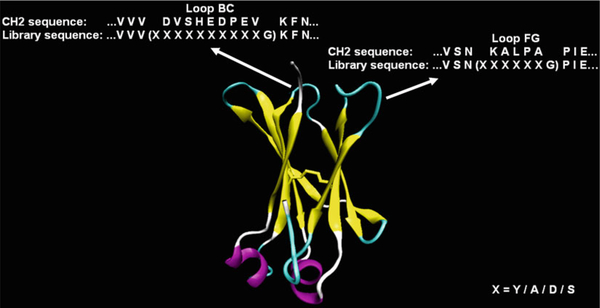
The strategies for library construction are various. Here, one published strategy is used as an example (Fig. 1) (17). The CH2 structure is represented by VMD 1.8.7 (18). Limited mutagenesis (A, S, Y, D) is introduced to loop BC (loop 1) and loop FG (loop 3). The procedure is shown (Fig. 2)
Fig. 1.
Design of a CH2-based library (17). Mutations are introduced in loop BC and loop FG with two additional amino acids.
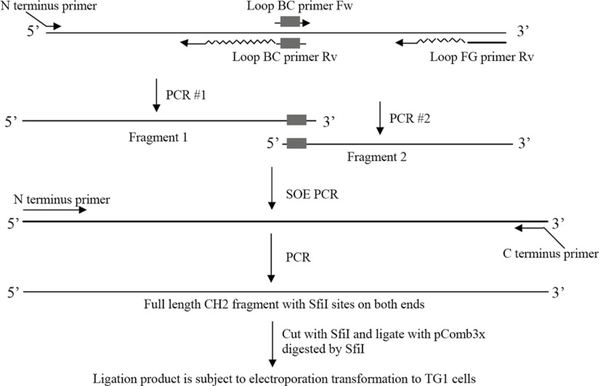
Fig. 2.
PCR strategy for amplification of CH2 fragments with mutations for library construction.
-
First round of PCR to get fragment 1 with mutations on loop BC (loop 1) and fragment 2 with mutations on loop FG (loop 3).
For amplification of fragment 1.Reagent Volume (μl) Final concentration Sterile double-dist. water 43 PCR Master Mix (2×) 50 1× N terminus primer (10 μM) 3 300 nM Loop BC primer Rv (10 μM) 3 300 nM pComb3X-CH2 (2 ng/μl) 1 0.02 ng/μl Final volume 100 For amplification of fragment 2.Reagent Volume (μl) Final concentration Sterile double-dist. water 43 PCR Master Mix (2×) 50 1× Loop BC primer Fw (10 μM) 3 300 nM Loop FG primer Rv (10 μM) 3 300 nM pComb3X-CH2 (2 ng/μl) 1 0.02 ng/μl Final volume 100 -
After purification of these two fragments, SOE (Splicing by Overlapping Extension) PCR is performed to get intact DNA fragment with mutations on both loop BC and loop FG. The volume of fragment 1 ( X μl) and fragment 2 ( y μl) is determined by the 1:1 molar ratio of fragment 1 to 2
Reagent Fragment Volume (μl) Sterile double-dist. Water – Primers and Template DNA 1 x 2 y Pipet the mixture with x + y μl High Fidelity PCR Master in a thin-walled PCR tube on ice and mix well.
Thermal cycling.Temperature Time Cycles Initial denaturation 94°C 2 min 1× Denaturation 94°C 15 s 10× Annealing 55°C 30 s Elongation 72°C 30 min Final elongation 72°C 10 min 1× Cooling 4°C Forever -
Then, PCR is performed again to amplify the intact DNA fragment.
For one reaction.Reagent Volume (μl) Final concentration Sterile double-dist. water 43 PCR Master Mix (2×) 50 1× N terminus primer (10 μM) 3 300 nM C terminus primer (10 μM) 3 300 nM SOE PCR product (see Note 4) 1 Final volume 100 Prepare about 100 μg intact DNA after purification by QIAquick Gel Extraction Kit (see Note 5) for digestion to get 30 μg digested insert DNA.
-
Digest intact DNA and phagemid vector pComb3X, and ligate them.
Digestion.Components Volume (μl) Final concentration Intact DNA x (up to 100 μg) – pComb3X – y (up to 300 μg) 10× NEBuffer 2 200 100 1× BSA (10 mg/ml) 20 10 100 μg/ml SfiI (20 u/μl) 200 90 – ddH2O 1,580 − x 800 − y Final volume 2,000 1,000 Incubate at 50°C overnight. Run the digested products on 1% agarose gels, purify the DNA with gel extraction kit (elute the DNA with ultra pure water), and quantify it (see Note 6).
Ligation (see Note 7).Components Volume (μl) Final concentration Digested insert DNA x (up to 30 μg) Digested pComb3X y (up to 100 μg) Molar ratio of mole of insert DNA/mole of vector 3:1 10× buffer for T4 DNA ligase buffer 100 1× T4 DNA ligase (400 u/μl) 100 ddH2O 800 − x − y Final volume 1,000 Incubate at 16°C for 72 h.
3.5. Concentration and Desalting of Ligated Products
The ligated products are purified by QIAquick PCR Purification Kit according to the manufacturer’s instructions.
Concentrate the purified ligated products by Microcon Ultracel YM-3 (Millipore) to 50–100 μl (see Note 8).
3.6. Electroporations and Library Preparation
Pre-warm 850 ml 2× YT medium containing 2% glucose (w/v) and 150 ml S.O.C. medium at 37°C. Chill 50 gene pulser cuvettes on ice. At the same time thaw, on ice, 2 ml of TG1 electroporation-competent cells.
Divide 2 ml of TG1 competent cells into five prechilled 1.5-ml Eppendorf tubes with 400 μl each. Add 10–20-μl ligations to each tube and pipet gently to mix. Transfer 41–42 μl mixtures to each cuvette. Gently tap the cuvette on the bench to make the mixture fill out the bottom of the cuvette.
Electroporate at 1.8 kV, 25 μF, and 200 Ω. Flush the cuvette immediately with 960 ml and then twice with 2 ml of pre-warmed S.O.C. medium and combine the 3 ml in a 2-L flask. After all electroporations are completed, about 150 ml electroporation product is obtained.
Shake at 250 rpm for 45–60 min at 37°C. Spread the electroporation product with serial dilution on 2× YT agar plates containing 2% glucose (w/v) and 100 μg/ml of ampicillin. Incubate the plates overnight at 37°C for calculation of the electroporation efficiency and size of the library.
Transfer 150 ml culture to 850 ml 2×YT medium to make 1 L culture containing 100 μg/ml ampicillin and 2% glucose. Shake for additional 2 h at 37°C.
Take 1 ml of the culture and measure the cell density by reading OD600. Calculate the total number of cells by multiplying the OD600 value by 5 × 108 (estimated number of cells in 1 ml culture when OD600 reaches 1) and the culture volume (1,000 ml in this case). Add 10 MOI (multiplicity of infection) of M13KO7 helper phage to the culture. Incubate at 37°C for 30 min, shaking for homogenization every 10 min.
Spin down the cells at 6,000 rpm (~4,500 × g, rotor: JLA-10.500, Beckman Coulter, CA) for 15 min. Resuspend in 2 L 2 × YT medium containing 100 μg/ml of ampicillin and 50 μg/ml of kanamycin. Incubate at 250 rpm overnight at 30°C.
Spin at 6,000 rpm (~4,500 × g, rotor: JLA-10.500, Beckman Coulter, CA) for 15 min at 4°C. Save the bacterial pellet for phagemid preparation using, for example, the Qiagen HiSpeed Plasmid Maxi Kit. For phage precipitation, transfer the supernatant to a clean 2L flask and add ¼ volume of 20% (w/v) PEG8000 and 2.5 M NaCl solution. Mix well and incubate on ice for at least 1 h.
Spin at 12,000 rpm (~14,000 × g, rotor: JA-14, Beckman Coulter, CA) for 20 min at 4°C. Discard the supernatant. Resuspend the phage pellet in 50 ml PBS, pH 7.4 by pipetting up and down along the side of the centrifuge bottle by using a 10-ml pipet.
Spin at 6,000 rpm (~4,500 × g, rotor: JLA-10.500, Beckman Coulter, CA) for 10 min at 4°C. Transfer the supernatant to a clean 200-ml flask and add ¼ volume of 20% (w/v) PEG8000 and 2.5 M NaCl solution. Mix well and incubate on ice for 1 h.
Spin at 12,000 rpm (~14,000 × g, rotor: JA-14, Beckman Coulter, CA) for 20 min. Discard the supernatant. Resuspend the phage pellet in 50 ml PBS, pH 7.4.
Spin at 6,000 rpm (~4,500 × g, rotor: JLA-10.500, Beckman Coulter, CA) for 10 min at 4°C. Transfer the supernatant to a clean 200-ml flask.
Measure the concentration of phage by reading OD280 (1 OD280 = 2.33 × 1012/ml). Add the same volume of sterilized glycerol and mix well. Aliquot the phage to make sure that each contains phage particles at least 100 times the total number of transformants (calculated in step 4). Store the phage at −80°C. The CH2 phage library is now ready for panning.
3.7. Construction of the CH2 Mutant m01
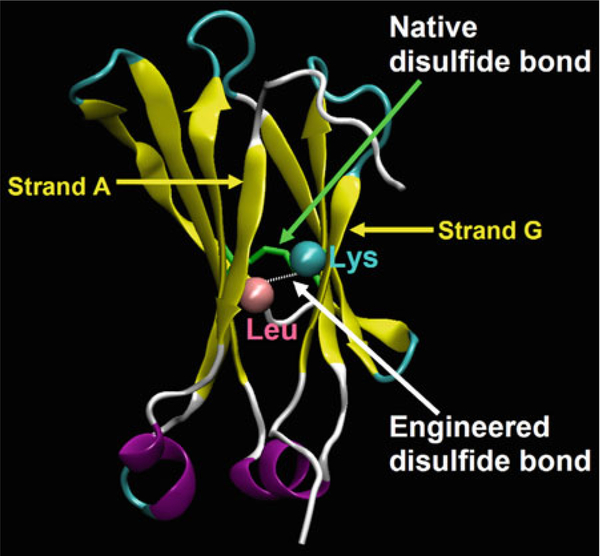
An additional disulfide bond is designed to be introduced between the N-terminal strand A and the C-terminal one G (Fig. 3) (13). The CH2 structure is represented by VMD 1.8.7 (18).
Fig. 3.
Design of m01 based on the CH2 structure (13). The distance between two Cαs forming the native disulfide bond (indicated by green arrow) is 6.53 Å. An engineered disulfide bond is introduced between Leu12 and Lys104 which were replaced by cysteines (indicated by white arrow ).
-
PCR for amplification of three fragments with mutations.
For amplification of fragment A.Reagent Volume (μl) Final concentration Sterile double-dist. water 21 PCR Master Mix (2×) 25 1× Omp (10 μM) 1.5 300 nM L/C primer Rv (10 μM) 1.5 300 nM pComb3X-CH2 (2 ng/μl) 1 0.02 ng/μl Final volume 50 For amplification of fragment B.Reagent Volume (μl) Final concentration Sterile double-dist. water 21 PCR Master Mix (2×) 25 1× L/C primer Fw (10 μM) 1.5 300 nM K/C primer Rv (10 μM) 1.5 300 nM pComb3X-CH2 (2 ng/μl) 1 0.02 ng/μl Final volume 50 For amplification of fragment C.Reagent Volume (μl) Final concentration Sterile double-dist. water 21 PCR Master Mix (2×) 25 1× K/C primer Fw (10 μM) 1.5 300 nM gIIIF (10 μM) 1.5 300 nM pComb3X-CH2 (2 ng/μl) 1 0.02ng/μl Final volume 50 -
After purification of these three fragments, SOE PCR is performed to get intact DNA fragment with replacement of L and K by two Cs. The volume of fragment A (x μl), fragment B (y μl) and fragment C (z μl) is determined by the 1:1:1 molar ratio of three fragments
Reagent Fragment Volume Sterile double-dist. water – Primers and template DNA A y B x C z Pipet the mixture with x+y+z μl High Fidelity PCR Master in a thin-walled PCR tube on ice and mix well.
Thermal cycling.Temperature Time Cycles Initial denaturation 94°C 2 min 1× Denaturation 94°C 15 s 10× Annealing 55°C 30 s Elongation 72°C 30 min Final elongation 72°C 10 min 1× Cooling 4°C forever - Amplification of SOE PCR product.
Reagent Volume (μl) Final concentration Sterile double-dist. water 43 PCR Master Mix (2×) 50 1× Omp (10 μM) 3 300 nM gIIIF (10 μM) 3 300 nM SOE PCR product 1 Final volume 100 Digestion, ligation, and transformation. See the method in Subheading 3.2
3.8. Stability Measurements of CH2, m01, and m36
CH2, m01, and m36, a domain antibody against HIV (19), are expressed and purified by the method in Subheading 3.3. The native disulfide bond in CH2 and the introduced disulfide bond are verified by using mass spectrometry.
- Circular dichroism (CD).
- Dissolve the purified proteins in PBS (see Note 9) at the final concentration of 0.49 mg/ml.
- Record the wavelength spectra at 25°C using a 0.1-cm path-length cuvette for native structure measurements.
- Measure the thermal stability at 216 nm by recording the CD signal in the temperature range of 25–90°C with heating rate 1°C/min.
- Differential scanning calorimetry (DSC).
- Concentrate the three proteins to 1.5 mg/ml (see Note 10) in PBS (pH 7.4).
- Use 1°C /min as heating rate and scan the samples from 25 to 100°C.
- Spectrofluorometry.
- Dilute all the proteins in buffer A to final concentration of 10 μg/ml in the presence of urea from 0 to 8 M.
- Record the emission spectra from 320 to 370 nm at 25°C with excitation wavelength at 280 nm.
- Correct the fluorescence spectra by the background fluorescence (buffer + denaturant).
- Use fluorescence intensity at 340 nm to evaluate the unfolding.
The stabilities of CH2, m01, and m36 are summarized in Table 1. The Tm value of m01 is higher than that of CH2 and m36. Interestingly, thermostabilitiy of an eAd is typically increased by 10°C after introduction of an additional disulfide bond (20). The strategy is similar to that used in the design of m01.
Table 1.
Comparison of stabilities of CH2, m01, and m36 by different methods
| Protein | Midpoint |
||
|---|---|---|---|
| CD | DSC | Spectrofluorometry | |
| (°C) | Urea concentration (M) | ||
| CH2 | 54.1 | 55.4 | 4.2 |
| m01 | 73.8 | 73.4 | 6.8 |
| m36 | 63.7 | 62.1 | 4.4 |
Acknowledgments
This work was supported by the Intramural AIDS Targeted Antiviral Program (IATAP), National Institutes of Health (NIH), the NIAID (NIH) Intramural Biodefense Program, and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Normally, 1–2-μg DNA fragment is more than enough for subsequent ligation and transformation.
Sufficient air exchange is very important for high yield expression. If the flask is capped too tightly, the yield will be very poor. It is an option to use flasks with vented caps.
The sample eluted by imidazole at low concentrations (0–20 mM) could be collected and analyzed on SDS-PAGE to minimize the loss of the proteins.
The amount of SOE product could be optimized for high amplification efficiency. For example, 1:1, 1:10, and 1:100 dilutions of the template (SOE product) could be tested.
It is very important to remove the trace of the buffer. Otherwise, the residual salts will decrease the efficiency of subsequent digestion, ligation, and electroporation.
In some cases the digestion of phagemid vectors may not be complete due to bad quality of DNA. To address this problem, additional treatment is needed to further purify the phagemids before digestion, or use more SfiI to digest for longer time, for example, overnight.
Before large-scale ligation can be performed, it is highly recommended to take two ligation tests. One is to assess the suitability of the vector and inserts for high-efficiency ligation and transformation. This can be accomplished through assembling small reactions either with vector only (test for vector self-ligation) or with both vector and insert, and transforming chemically competent cells like DH5α. The other is to determine the optimal ratio between insert and vector for the highest efficiency of ligation. This can be accomplished through assembling small reactions with insert and vector in different molar ratios, such as 3:1, 2:1, and 1:1, and transforming chemically competent cells.
The desalting of DNA samples is a key step to the success of electroporations. High concentration of ions in the DNA solution will result in a long and intense pulse in electroporations, which causes cell damage or rupture. We found that at least 1,000-time dilution of DNA solution was needed to generate time constants of 4.6–5.0 ms in electroporations that generally gave the highest efficiency.
The use of the buffer could be optimized to get low background noise and obtain more reliable data.
The concentration of the protein could be optimized. If the signal is low, then concentration could be increased.
References
- 1.Kolmar H, Skerra A (2008) Alternative binding proteins get mature: rivalling antibodies. FEBS J 275:2667. [DOI] [PubMed] [Google Scholar]
- 2.Skerra A (2007) Alternative non-antibody scaffolds for molecular recognition. Curr Opin Biotechnol 18:295–304 [DOI] [PubMed] [Google Scholar]
- 3.Nygren PA, Skerra A (2004) Binding proteins from alternative scaffolds. J Immunol Methods 290:3–28 [DOI] [PubMed] [Google Scholar]
- 4.Binz HK, Amstutz P, Pluckthun A (2005) Engineering novel binding proteins from non-immunoglobulin domains. Nat Biotechnol 23:1257–1268 [DOI] [PubMed] [Google Scholar]
- 5.Hey T, Fiedler E, Rudolph R, Fiedler M (2005) Artificial, non-antibody binding proteins for pharmaceutical and industrial applications. Trends Biotechnol 23:514–522 [DOI] [PubMed] [Google Scholar]
- 6.Holliger P, Hudson PJ (2005) Engineered antibody fragments and the rise of single domains. Nat Biotechnol 23:1126–1136 [DOI] [PubMed] [Google Scholar]
- 7.Holt LJ, Herring C, Jespers LS, Woolven BP, Tomlinson IM (2003) Domain antibodies: proteins for therapy. Trends Biotechnol 21:484–490 [DOI] [PubMed] [Google Scholar]
- 8.Saerens D, Ghassabeh GH, Muyldermans S (2008) Single-domain antibodies as building blocks for novel therapeutics. Curr Opin Pharmacol 8:600–608 [DOI] [PubMed] [Google Scholar]
- 9.Ward ES, Gussow D, Griffiths AD, Jones PT, Winter G (1989) Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature 341:544–546 [DOI] [PubMed] [Google Scholar]
- 10.Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N, Hamers R (1993) Naturally occurring antibodies devoid of light chains. Nature 363:446–448 [DOI] [PubMed] [Google Scholar]
- 11.Muyldermans S, Cambillau C, Wyns L (2001) Recognition of antigens by single-domain antibody fragments: the superfluous luxury of paired domains. Trends Biochem Sci 26:230–235 [DOI] [PubMed] [Google Scholar]
- 12.Feige MJ, Walter S, Buchner J (2004) Folding mechanism of the CH2 antibody domain. J Mol Biol 344:107–118 [DOI] [PubMed] [Google Scholar]
- 13.Gong R, Vu BK, Feng Y, Prieto DA, Dyba MA, Walsh JD, Prabakaran P, Veenstra TD, Tarasov SG, Ishima R, Dimitrov DS (2009) Engineered human antibody constant domains with increased stability. J Biol Chem 284: 14203–14210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimitrov DS (2009) Engineered CH2 domains (nanoantibodies). MAbs 1:26–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hackel BJ, Kapila A, Wittrup KD (2008) Picomolar affinity fibronectin domains engineered utilizing loop length diversity, recursive mutagenesis, and loop shuffling. J Mol Biol 381:1238–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Zhu Z, Xiao X, Dimitrov DS (2009) Construction of a human antibody domain (VH) library. Methods Mol Biol 525:81–99, xiii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao X, Feng Y, Vu BK, Ishima R, Dimitrov DS (2009) A large library based on a novel (CH2) scaffold: identification of HIV-1 inhibitors. Biochem Biophys Res Commun 387:387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(33–38):27–38 [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Zhu Z, Feng Y, Dimitrov DS (2008) Human domain antibodies to conserved sterically restricted regions on gp120 as exceptionally potent cross-reactive HIV-1 neutralizers. Proc Natl Acad Sci USA 105:17121–17126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagihara Y, Mine S, Uegaki K (2007) Stabilization of an immunoglobulin fold domain by an engineered disulfide bond at the buried hydrophobic region. J Biol Chem 282:36489–36495 [DOI] [PubMed] [Google Scholar]