Abstract
Adrenocortical tumors are common and incidentally discovered in up to 14% of axial imaging studies performed for other indications. Most of these tumors are nonfunctioning but may require removal because of the risk of adrenocortical carcinoma. Unfortunately, most clinical and imaging features are still not accurate enough to allow definitive diagnosis and an increasing number of patients undergo adrenalectomy to exclude a cancer diagnosis. Adrenocortical carcinoma is an aggressive malignancy with no effective therapy for patients with locally advanced and metastatic disease. Studies using new genomic approaches including mRNA, miRNA, methylation, and CGH profiling have identified dysregulated genes and pathways that may have clinical implications in improved molecular diagnosis and prognostication of adrenocortical cancer (ACC). In this review, we highlight recent advances in the molecular diagnosis of adrenocortical tumors.
Keywords: adrenal neoplasm, adrenocortical carcinoma, genomics, diagnosis, miRNA, mRNA, epigenetics, methylation, CGH
INTRODUCTION
Adrenocortical tumors are common, incidentally discovered on abdominal imaging studies, and are found in as many as 32% of cases at autopsy [1]. Most of adrenocortical tumors are nonfunctioning but may require removal because of the risk of adrenocortical carcinoma. Adrenocortical carcinoma (ACC) is a rare tumor with an incidence of 1–2 per million annually [2]. The prognosis of patients with ACC is poor with a 5-year survival rate less than 35% [3]. Unfortunately, clinical, biochemical, and imaging features in the majority of patients found to have a localized adrenocortical tumor do not reliably exclude a cancer diagnosis and an increasing number of patients are undergoing adrenalectomy [4–6]. The Weiss histologic criteria are commonly used to distinguish between benign and malignant adrenocortical tumors, with Weiss score 3 or greater indicating a malignant tumor. However, the Weiss criteria include the assessment of subjective criteria and there have been reports of cases in which patients were diagnosed with a cortical adenoma and go on to develop recurrent and or metastatic ACC.
Although the molecular pathogenesis of ACC is poorly understood, several rare monogenic disorders (Li—Fraumeni, Beckwith—Wiedemann syndromes) in which individuals develop ACC have led to the identification of common somatic genetic changes in sporadic ACC. In addition, genome-wide mRNA and miRNA expression, CGH, and methylation profiling studies in ACC have demonstrated several dysregulated genes and pathways which may be involved in adrenocortical carcinogenesis, and which may serve as diagnostic and prognostic markers [7–9] (Table I). We review our current understanding of the molecular pathogenesis of adrenocortical carcinoma and the clinical implications of the recent studies which have characterized the molecular landscape of these tumors.
TABLE I.
Summary of Molecular Changes Associated With ACC
| Genes | |
| Diagnostic | SERPING1, MRPL48, TM7SF2, DDB1, NDUFS8, PRDX5 [22] |
| IL13RA2, HTR2B, CCNB2, RARRES2, SLC16A9 [23] | |
| SF-1 [37], HTR2B and ANLN [40] | |
| IGF-2 and Ki67 [24] | |
| Prognostic | Ki67/MIB1 [41] |
| Zinc-finger transcription factor Snail [42] | |
| Estrogen receptor (ER) [43] | |
| BUB1B, PINK1 [21,38] | |
| Matrix metalloproteinase 2 [62] | |
| Glucose transporter GLUT1 [47] | |
| Large homolog 7 Drosophila (DLG7) [38] | |
| Steroidogenic factor (SF-1) [37] | |
| ERCC1 (excision repair cross-complementing) [48] | |
| MiRNA | |
| Diagnostic | miR 483–5p, miR 511, miR 503 [8,49,63] |
| Prognostic | miR-139–5p, miR-195, miR-503, miR-1202, miR-1275 [52,64] |
| Pathways | Cell cycle, retinoic acid signaling, cholesterol and lipid metabolism. toll-like receptor 4, complement system, and antigen presentation [39,40] |
| Chromosomal alterations [65] | |
| Prognosis | Gains-6q, 7q, 12q, 19p and loss-3,8, 10p, 16q, 17q, 19q |
| Regions with dysregulated gene expression | Better prognosis-16p, 5q |
| Poor prognosis-1q, 22q, 6q, 10p, 6p |
Genetic Predisposition to ACC
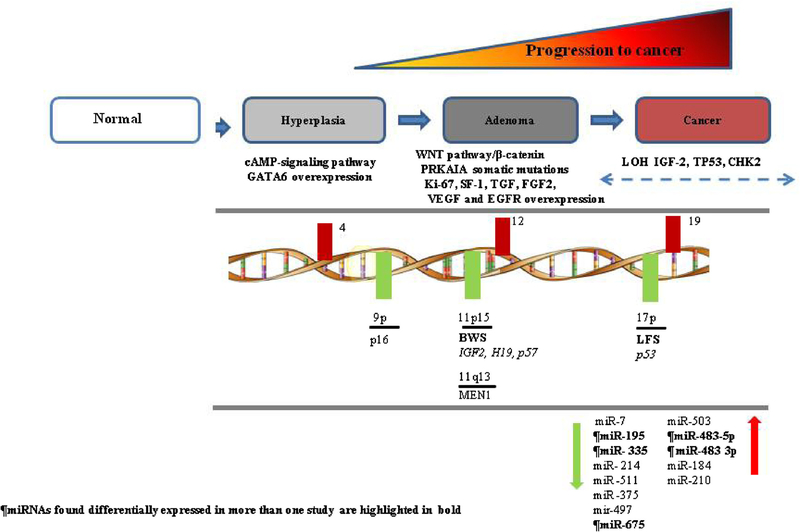
Genetic predisposition to ACC has been associated with several familial cancer syndromes; Li–Fraumeni, Beckwith–Wiedemann, Gardner, and Multiple Endocrine Neoplasia type 1 (MEN1). Furthermore, inactivating mutations in tumor suppressor genes and activating mutations in oncogenes responsible for these familial cancer syndromes have also been found to be present as somatic mutations in sporadic cases of ACC [3,10,11]. Based on these studies a working model of the molecular pathogenesis of ACC is summarized in Figure 1.
Fig. 1.
Pathogenetic model of adrenocortical tumors.
Li–Fraumeni Syndrome (TP53)
Li–Fraumeni syndrome results from a germ-line mutation in the TP53 gene (17p13.1). This mutation is inherited in an autosomal dominant manner and is present in 70% of cases [12]. ACC develops in approximately 3–4% of patients with Li–Fraumeni syndrome, usually manifesting before the age of 20 years [12]. In addition, inactivating somatic mutations in the TP53 gene have also been observed in sporadic ACC in 20–33% in exons 5–8 [12,13] and in 25% in exons 2–11 [12]. In addition, a substitution of histidine for arginine at codon 337 has been shown in the development of childhood ACC in 1 in 10 carriers of this missense mutation [12]. The outcome of the resulting mutation is the inability of the p53 protein to initiate cell growth arrest, DNA repair and apoptosis in response to severe cellular DNA damage [13]. Li–Fraumeni syndrome is also characterized by the development of soft tissue sarcomas, osteosarcomas, breast cancer, brain tumors, and leukemia at an early age. Screening for germline TP53 mutations in patients with apparently sporadic ACC is recommended, especially in pediatric cases but also in adults as 4% were recently reported to have a germline mutation [14].
Beckwith–Wiedemann Syndrome (11p15)
The majority of cases with Beckwith–Wiedemann syndrome are de novo, but in 15% of cases it is inherited [12]. In these familial cases, there is a defect in genomic imprinting (genes are expressed either from the maternal or paternal allele) of the 11p15 chromosome locus [15]. Genes affected in this region harbors the insulin-like growth factor 2 (IGF-2), cyclin-dependent kinase inhibitor 1C (CDKN1C, p57kip2), and H19 genes [12]. Patients with Beckwith–Wiedemann syndrome often have a loss of the maternal locus and a gain in the paternal locus. This results in overexpression of IGF-2 and a decrease in p57kip2 and H19 since IGF-2 is expressed on the paternal allele and the other two genes are on the maternal allele [15]. The characteristic features of Beckwith–Wiedemann syndrome are macrosomia, exomphalos, macroglossia, abdominal wall defects, ear anomalies, renal abnormalities, and cleft palate. Five percent of patients with Beckwith–Wiedemann syndrome develop ACC as well as other tumors, including nephroblastoma, hepatoblastoma, rhabdomyosarcoma, and nesideoblastosis [12].
Carney Complex
Carney complex results from inactivating mutations in the protein kinase A regulatory subunit (PRKAR1A) gene. Patients with Carney complex develop primary pigmented adrenocortical disease with hypercortisolism, abnormal pigmentation of the skin, cardiac myxomas, and other neoplasms. Somatic inactivating mutations or allelic losses of the PRKAR1A locus at 17q22–24 are also seen in sporadic cases of adrenocortical adenoma and ACCs [16].
Gardner Syndrome (5q21, APC gene, and Wnt Pathway)
Mutations in the adenomatous polyposis coli (APC) gene are known to cause hereditary colorectal cancer. Several genetic changes in 5q21 of this gene are associated with Gardner syndrome [15]. This is an autosomal dominant disorder that manifests with gastrointestinal polyps, osteomas, soft tissue tumors, epidermal cysts, desmoids tumors, and periampullary cancer. Patients with this syndrome are also at risk of developing endocrine malignancies such as the cribriform variant of papillary thyroid cancer and ACC.
Activation of the Wnt pathway, which results in the aberrant accumulation of β-catenin in the cytoplasm and nucleus, has been implicated in the pathogenesis of colorectal cancer. Given the association of ACC and Gardner syndrome, it is logical to presume that this pathway may contribute to sporadic ACC. ACCs have altered β-catenin localization as a result of activation mutations which are present in approximately one-fourth of tumors [17]. The presence of activating mutations in the beta-catenin (CTNNB1) gene is associated with worse outcome and alterations in the Wnt/β-catenin pathway may serve as prognostic markers for ACC [12,18,19].
MEN 1 Syndrome
MEN 1 is caused by inactivating mutations in the MEN1 gene located in the chromosomal region 11q13. Patients with MEN 1 develop pituitary tumors, parathyroid tumors, and pancreatic neuroendocrine tumors. In addition, patients are also at risk of developing multiple lipomas, angiomas, and adrenocortical tumors. Majority (55%) of those affected with this syndrome develop adrenocortical tumors, only a few cases of ACC have been reported [12]. Somatic inactivating mutations in MEN1 are uncommon in sporadic ACC.
Molecular Markers
Given the current diagnostic limitations of making a definitive diagnosis of ACC for localized adrenal neoplasm, genomic studies are shedding light on consistent dysregulated genes and pathways involved in the molecular pathogenesis of ACC and that could possibly be used for molecular classification and or diagnosis of adrenocortical tumors (Tables I and II).
TABLE II.
Summary of Significant Prognostic Markers in ACC
| Author | Patients (n) | Marker genes | Positive ACC samples (n) | Overall survival | Metastasis | Disease free survival | Extent of disease |
|---|---|---|---|---|---|---|---|
| Morimoto et al. 2008 [41] | 17 | Ki-67/MIB1 | 6/17 (35.2%) | NAa | NA | Increase in Ki-67 (≥7%) correlated with decreased disease free survival | NA |
| Waldmann et al. 2008 [42] | 26 | Snail | 17/26 (65.3%) | Snail positive 34 mos; Snail negative 127 mos | 5 out of 6 tumors (83.3%) with Snail positive expression | NA | 11 out of 12 (91.6%) stage III and IV were Snail positive |
| Shen et al., 2009 [44] | 17 | ER | 8/17 (47%) | 60% survival at 5 years in ER+ vs. 0% survival at 5 years in ER− | 1 out of 8 (12.5%) in ER+ patients, 5 out of 9 (55.5%) ER-patients | NA | 8/17 (47%)stage I and II, 9/17 (53%) stage III and IV |
| De Reynies et. al. 2009 [21] | 153 | DLG7, PINK1 BUB1B, PINK1 | 32/153 (20.9%) | BUB1B and PINK1 expression in ACC indicate better overall survival | NA | Combined DLG7and PINK1 expression indicate better disease free survival | NA |
| Volante et. al., 2006 [62] | 100 (50 ACC, 50 ACA) | MMP-2 | 37/50 (74%) | MMP-2+: 31 mos MMP-2−: 74.8 mos | NA | Shorter disease free interval (P = 0.05) | NA |
| Fenske et. al., 2009 [47] | 167 ACC, 15 ACA, 4 normal | GLUT1 | 55/167 (33%) | NA | NA | Reduced disease free survival (P < 0.01) | NA |
| Ronchi et. al., 2009 [48] | 163 ACC, 15 ACA, 8 normal | ERCC1 | 75/163(46%) | ERCC1+ :8 mos ERCC1-: 24 mos | NA | NA | NA |
| 2010 Sbiera et al., [37] | 162 ACC, 52 adenoma, 6 normal, | SF-1 | 158/161 (98%) | SF1+ 14 mos S− 49.8 mos | SF1+ 8.8 mos SF− 37.7 mos | ||
NA; not analyzed.
Gene Expression Analysis of Adrenocortical Tumors
There have been many genome-wide gene expression profiling studies of ACC which have identified several diagnostic molecular markers [20–29]. One of the most consistently overexpressed genes in ACC found in these studies is the insulin-like growth factor-2 (IGF-2). IGF-2 is involved in cell growth and development, and exerts its action through the IGF-1 receptor (IGF-1R). IFG-2 mRNA and protein overexpression is also seen in sporadic ACC. Furthermore, loss of heterozygosity (LOH) at 11p15, the locus of IGF-2, occurs in 67% of ACC (compared to 13% of adenomas) [12]. Regardless of the underlying genetic mechanism, expression of IGF-2 is >100-fold higher in 60–90% of ACC compared to adrenocortical adenoma and or normal adrenal cortex [15,24]. The expression level of both IGF-2 and Ki-67 is 96% sensitive and 100% specific for distinguishing benign from malignant adrenocortical tumors [24]. IGF-2 expression in pediatric ACC is similar to adrenocortical adenomas [30]. On the other hand, overexpression of IGF-IR has been shown to be significantly higher in pediatric ACC as compared to adrenocortical adenoma and was associated with presence of metastatic disease. In adult tumors, IGF-1R expression was similar in ACC and benign adrenocortical tumors [30]. In addition to IGF-2, basic fibroblast growth factor (FGF2), transforming growth factor (TGF) α, TGF-β1, and vascular endothelial growth factor (VEGF) also regulate growth and function of adrenal glands [31]. VEGF is overexpressed in ACC as compared to adrenal adenomas [32].
In most studies, the expression of MEN1 has been shown to be similar between adrenocortical adenoma and ACC [12]. Because LOH on 11q13 is common, examination of the gene expression levels at this chromosomal locus showed 25 genes that were downregulated. Validation of these genes by real time quantitative RT-PCR identified 6 genes (SERPING1, MRPL48, TM7SF2, DDB1, NDUFS8, PRDX5) with high diagnostic accuracy for distinguishing ACC from adrenocortical adenomas with an overall accuracy of 87–91% [23].
In another genome-wide expression study, 37 genes were found to be significantly differentially expressed in ACC [22] and five genes (IL13RA2, HTR2B, CCNB2, RARRES2, SLC16A9) were validated to have high diagnostic accuracy for ACC [22]. Soon and colleagues, also showed the combination of IGF-2 and MAD2L1 had high accuracy for distinguishing between benign and malignant tumors with a 100% sensitivity and 95% specificity [33]. However, the expression of MAD2L1 and CCNB1 are focal in some ACC.
Several other diagnostic markers have been studied in ACC and include SF-1 (steroidogenic factor), glucocorticoid receptor (GR), parathyroid hormone related protein (PTHrP), and osteopontin [34–37]. SF-1 was found to be overexpressed in transcriptome analysis and immunohistochemistry [38]. By immunohistochemistry analysis, SF-1 was a good diagnostic marker for ACC (sensitivity, specificity, positive predictive value, and negative predictive value for SF-1 were 99, 100, 100, and 97%, respectively) [37]. In addition, SF-1 overexpression (immunohistochemistry and RNA) was also shown to be a prognostic factor and associated with shorter overall survival and recurrence free survival in ACC in German and French cohorts. Another marker GR is a ligand-dependent nuclear transcription factor and was found to be overexpressed in ACC. Immunohistochemistry demonstrated positive staining in 94% of ACC and negative staining in 98% of adenomas (P < 0.001). This finding was validated in an independent cohort of adrenocortical tumors and 14 of 18 ACCs (78%) demonstrated positive nuclear staining whereas 32 of 33 ACAs (94%) were negative (<0.001). Lastly, the PTHrP is an oncoprotein, which has been found to influence tumor proliferation and differentiation. PTHrP/β2-microglobulin ratio was significantly higher in the ACC samples (0.008 ± 0.014) than in benign samples (0.001 ± 0.001, P < 0.006). The level of PTHrP mRNA expression were positively correlated with the extent of disease (McFarlane stage (r2 = 0.225, P < 0.0001)), Weiss score (r2 = 0.175, P < 0.004), and metastases (P < 0.05) [35].
A meta-analysis of genome-wide expression and comparative genomic hybridization (CGH) studies by Szabo et al. [39], showed several pathways to be dysregulated in ACC, cell cycle (c-MYC, CDC25B, CCNB2, CDC2, TOP2A CCNE1, CDK2, CDK7, UBC, MDM-2), retinoic acid signaling (RXRA, ALDH1A1, ALDH1A1–3) cholesterol and lipid metabolism (LXRA, NR1H3, PPARG, CD36, ABCA1, ABCG1, SREBF1, APOE), toll-like receptor 4, complement system, and antigen presentation (underexpressed SERPING1 and MHCII). In another combined analysis, similar (cell cycle, retinoic acid signaling, complement system, and antigen presentation) pathways were found to be dysregulated in ACC [38,40]. Using gene set enrichment analysis of data from published studies [39] and their own data (gene expression and CGH on 11 tumor samples), Zsippai et al. [40] show 46 out of 101 chromosome aberrations correlate with significant gene expression alterations. Furthermore, they found that overexpression of aniline (ANLN) and underexpression of serotonin receptor 2B (HTR2B) are novel biomarkers for malignancy [40].
In addition to evaluation of diagnostic markers, there have been studies which have evaluated prognostic markers for ACC (Table II). Ki67 (also referred to MIB1) has been shown to be a prognostic marker for several malignancies. The Ki67 labeling index in 17 ACC was recently analyzed to determine its prognostic value [41]. A Ki67 index of ≥7% was significantly associated with lower disease-free survival in patients with a Weiss score of ≤6 [41]. The zinc-finger transcription factor Snail, which regulates epithelial-to-mesenchymal transition, was detected in 65% (17/26) of ACC [42]. No Snail expression was identified in normal adrenocortical tissue. The expression of Snail was associated with more aggressive disease (6 of 14 stage I or II positive for Snail as compared to 11 of 12 stage III–IV ACC, P = 0.01). Survival was also associated with the expression of Snail in ACC samples with a median survival of 127 months in patients with Snail-negative tumors as compared to 34 months in patients with Snail-positive tumors. Ki67 index was directly associated with the amount of Snail expression [42]. The estrogen receptor (ER), particularly ERβ is mainly expressed in the zona glomerulosa and fasciculata. In ACC, downregulation of ERβ and upregulation of ERα is associated with patient outcome, as has been reported in patients with breast cancer [43]. In a study of 17 patients with ACC, nearly half the tumor samples expressed ER [44]. The 5-year survival rate for those with ER-positive tumors was significantly better than for patients with ER-negative tumors (60% vs. 0%) [44]. In another study, analysis of a combination of BUB1B and PINK1 expression in ACC tumor samples was also associated with overall survival [21]. The higher expression level of DLG7 and reduced expression of PTEN-induced putative kinase 1 (PINK1) has also been associated with lower disease-free survival in ACC [21,38]. Interestingly, another group has validated DLGAP5 -PINK1 and BUB1-PINK1 expression in combination as predictor of outcome in adult and pediatric ACCs from Brazilian cohort. In adult ACCs, they found a Δct cutoff of ≤3.2 and ≤3.14 accurately predicted disease-free survival (AUC = 0.92) and overall survival (AUC = 0.90) [45]. However, in pediatric ACCs, these molecular predictors were not associated with disease-free or overall survival. Overexpression of MMP2 and GLUT1 has also been associated with worse overall survival in patients with ACC [46,47]. ERCC1 (excision repair cross-complementing) is a DNA repair enzyme and is overexpressed in ACC. Ronchi et al. [48] have reported that higher ERCC1 expression level is associated with a poor prognosis in patients who received platinum-based chemotherapy for ACC. Lastly, a recent study demonstrated two clusters of prognosis in ACC with genes involved in transcription and cell cycle in the poor-outcome group and the good outcome group was enriched for genes regulating cell metabolism and intracellular transport [38]. These and other studies suggest that several molecular markers may be useful for prognostication in patients with ACC but larger cohort studies will be necessary to determine their clinical application for guiding patient follow up and the use of adjuvant therapy in the future.
MicroRNA Profiling in Adrenocortical Tumors
MicroRNAs are small noncoding RNAs involved in post-transcriptional regulation of gene expression, which have been associated with tumorigenesis in various malignancies. MicroRNA expression in ACC has been performed in six studies (five in adult and one in pediatric ACC) [7,8,49–51]. Underexpression of miR-195 and overexpression of miR-483–5p in ACC have been observed in two studies with a relatively large set of tumor samples analyzed [7,8]. Underexpression of miR-195 was also associated with worse overall survival [52] and miR-483–5p had a high diagnostic accuracy for distinguishing benign from malignant adrenocortical tumors. Interestingly, miR-483–5p maps to intron 2 of the IGF-2 gene and IGF-2 is co-expressed with this microRNA. The combination of these two microRNAs has high diagnostic accuracy for distinguishing benign from malignant adrenocortical tumors and may be linked to IGF-2 overexpression [7]. In another study, expression difference in miR-503 and miR-511 were reported to have high accuracy for differentiating between benign and malignant adrenocortical tumors (100% sensitivity, 93% specificity [52]). Doghman et al. [50] analyzed pediatric adrenocortical tumors and found 26 significantly differentially expressed microRNAs. A recent study by Schimtz et al. [51] analyzed microRNA expression in formalin fixed paraffin tissue samples and validated the results in an independent cohort of 15 primary ACC. Using miR-675 and 335 expression cut off of >6 and >8.8, predicted a malignant tumor in 60% of ACC. Lastly, miR-139–5p has been reported to be overexpressed in ACC and is associated with poor outcome [53]. Specifically, miR-139–5p was upregulated in recurrent ACCs, suggesting that this miRNA may be a marker of recurrent ACCs [52].
Comparative Genomic Hybridization (CGH)
CGH has been utilized to study chromosomal aberrations in ACC. In general, ACC is associated with significant chromosomal losses and gains as compared to benign tumors [12]. These chromosomal changes have also been associated with ACC tumor size [54]. Recent studies have also shown that specific genetic aberrations in ACC tumor samples are associated with prognosis in patients with ACC [55]. Specifically, gains in chromosomes 6q, 7q, 12q, and 19p, and losses in chromosomes 3, 8, 10p, 16q, 17q, and 19q, have been associated with a significantly worse survival, independent of tumor size, tumor weight and grade, and functional status of the tumor [54]. Increased chromosomal alterations have been consistently observed in ACC [55]. A diagnostic model was developed by combining DNA copy number analysis at six loci (5q22, 7p12.1, 11p13, 13q31.1, 16q22.1, and 22q12.1). This model distinguished carcinomas from adenomas in an independent validation cohort of 79 tumors with a sensitivity of 100% and specificity of 83%. Interestingly, the altered loci found in this study includes well-known oncogenes and tumor suppressors gene such as fibroblast growth factor receptor 4 (FGFR4) at 5q35; cyclin-dependent kinase 2 (CDK2) and cyclin-dependent kinase 4 (CDK4) at 12q13; GINS complex subunit 2 (Psf2 homolog) (GINS2) at 16q24; TPX2, microtubule-associated, homolog (TPX2); cyclin E1 (CCNE1) at 19q13; ubiquitin-conjugating enzyme E2C (UBE2C) and v-myb myeloblastosis viral oncogene homolog (avian)-like 2 (MYBL2) at 20q11, melanocortin receptor 1 (MC1R) at 16q24, and suppression of tumorigenicity 13 (ST13) at 22q12. Based on tumor DNA from 21 tumors two prognostic groups were identified based on chromosomal alterations and one group had a worse survival which was validated in an independent cohort of 25 tumors samples (P < 0.05) [55].
DNA Methylation Profiling of Adrenocortical Tumors
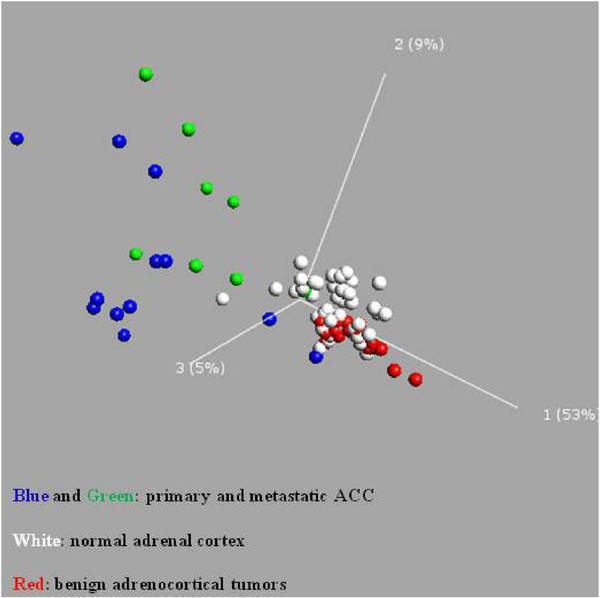
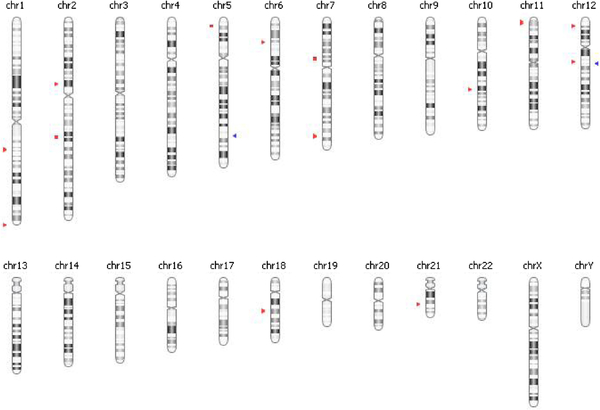
Epigenetic changes have been implicated in the development of cancer and such changes have been found to have diagnostic, prognostic, and therapeutic implications [56]. Epigenetics refers to changes in gene expression that are not due to changes in the DNA sequence [57]. The most well-established epigenetic change is DNA methylation of cytosines, by DNA methyl transferase enzymes. Cytosines associated with guanines are called CpG dinucleotides, and those found in CpG rich regions are called CpG islands, the majority of which are in the 5′ regulatory (promoter) regions of genes [58–60]. DNA methylation has been implicated in affecting a number of different cellular processes including apoptosis, the cell cycle, DNA damage repair, growth factor response, signal transduction, and tumor architecture, all of which can contribute to the initiation and progression of cancer [61]. We recently performed genome-wide DNA methylation profiling of adrenocortical tumors and normal adrenal cortex [66]. From this analysis, we have found that ACC samples were globally hypomethylated and the methylation patterns were distinctly different in normal, benign, and primary malignant and metastatic ACC tissue samples (Fig. 2). CpG island methylator phenotype (CIMP) has been proposed as a key mechanism for cancer development and progression. In our comparison, we also found CIMP in ACC samples as compared to benign tumor samples (Fig. 3).
Fig. 2.
Principal component analysis of genome-wide methylation in normal adrenal cortex, and benign and malignant adrenocortical tumors.
Fig. 3.
Chromosomal location of CpG island methylator phenotype in primary ACC as compared to benign adrenocortical tumors. Sites in red: Hypermethylated and blue: Hypomethylated.
SUMMARY
Advances in genomic technologies have provided some insight into the pathogenesis of ACC, and molecular markers for the diagnosis and prognosis of ACC. There are, however, considerable differences in the candidate molecular markers identified among these studies of a rare malignancy suggesting that ACC may have a heterogeneous genetic basis beyond just methodological differences among these studies and relatively small sample numbers analyzed. Future studies encompassing a large set of tumor samples with integrated pangenomic analysis of the same tumor are needed to result in molecular markers which could be clinically applied and possibly define the genetic basis of ACC and therapeutic targets.
Footnotes
Conflicts of interest: None.
REFERENCES
- 1.Kloos RT, Gross MD, Francis IR, et al. : Incidentally discovered adrenal masses. Endocr Rev 1995;16:460–484. [DOI] [PubMed] [Google Scholar]
- 2.Kuruba R, Gallagher SF: Current management of adrenal tumors. Curr Opin Oncol 2008;20:34–46. [DOI] [PubMed] [Google Scholar]
- 3.Libe R, Bertherat J: Molecular genetics of adrenocortical tumours, from familial to sporadic diseases. Eur J Endocrinol 2005;153:477–487. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher SF, Wahi M, Haines KL, et al. : Trends in adrenalectomy rates, indications, and physician volume: A statewide analysis of 1816 adrenalectomies. Surgery 2007;142:1011–1021 discussion 1011–21. [DOI] [PubMed] [Google Scholar]
- 5.Murphy MM, Witkowski ER, Ng SC, et al. : Trends in adrenalectomy: A recent national review. Surg Endosc 2010;24:2518–2526. [DOI] [PubMed] [Google Scholar]
- 6.Saunders BD, Wainess RM, Dimick JB, et al. : Trends in utilization of adrenalectomy in the United States: Have indications changed? World J Surg 2004;28:1169–1175. [DOI] [PubMed] [Google Scholar]
- 7.Patterson EE, Holloway AK, Weng J, et al. : MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer 2011;117:1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soon PS, Tacon LJ, Gill AJ, et al. : miR-195 and miR-483–5p identified as predictors of poor prognosis in adrenocortical cancer. Clin Cancer Res 2009;15:7684–7692. [DOI] [PubMed] [Google Scholar]
- 9.Baehner FL, Lee M, Demeure MJ, et al. : Genomic signatures of cancer: Basis for individualized risk assessment, selective staging and therapy. J Surg Oncol 2011;103:563–573. [DOI] [PubMed] [Google Scholar]
- 10.Igaz P, Wiener Z, Szabó P, et al. : Functional genomics approaches for the study of sporadic adrenal tumor pathogenesis: Clinical implications. J Steroid Biochem Mol Biol 2006;101: 87–96. [DOI] [PubMed] [Google Scholar]
- 11.Bertherat J, Bertagna X: Pathogenesis of adrenocortical cancer. Best Pract Res Clin Endocrinol Metab 2009;23:261–271. [DOI] [PubMed] [Google Scholar]
- 12.Soon PS, McDonald KL, Robinson BG, et al. : Molecular markers and the pathogenesis of adrenocortical cancer. Oncologist 2008;13:548–561. [DOI] [PubMed] [Google Scholar]
- 13.Herbet M, Feige JJ, Thomas M: Insights into the role of genetic alterations in adrenocortical tumorigenesis. Mol Cell Endocrinol 2009;300:169–174. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann LJ, Heinze B, Fassnacht M, et al. : TP53 germline mutations in adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab 2012;97:E476–E485. [DOI] [PubMed] [Google Scholar]
- 15.Barlaskar FM, Hammer GD: The molecular genetics of adrenocortical carcinoma. Rev Endocr Metab Disord 2007;8:343–348. [DOI] [PubMed] [Google Scholar]
- 16.Bertherat J, Groussin L, Sandrini F, et al. : Molecular and functional analysis of PRKAR1A and its locus (17q22–24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res 2003; 63:5308–5319. [PubMed] [Google Scholar]
- 17.Berthon A, Martinez A, Bertherat J, et al. : Wnt/beta-catenin signalling in adrenal physiology and tumour development. Mol Cell Endocrinol 2012;351:87–95. [DOI] [PubMed] [Google Scholar]
- 18.Ragazzon B, Libé R, Gaujoux S, et al. : Transcriptome analysis reveals that p53 and {beta}-catenin alterations occur in a group of aggressive adrenocortical cancers. Cancer Res 2010;70:8276–8281. [DOI] [PubMed] [Google Scholar]
- 19.Tissier F, Cavard C, Groussin L, et al. : Mutations of beta-catenin in adrenocortical tumors: Activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res 2005;65:7622–7627. [DOI] [PubMed] [Google Scholar]
- 20.Slater EP, Diehl SM, Langer P, et al. : Analysis by cDNA microarrays of gene expression patterns of human adrenocortical tumors. Eur J Endocrinol 2006;154:587–598. [DOI] [PubMed] [Google Scholar]
- 21.de Reynies A, Assié G, Rickman DS, et al. : Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol 2009;27:1108–1115. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Ranvier GG, Weng J, Yeh RF, et al. : Identification of biomarkers of adrenocortical carcinoma using genomewide gene expression profiling. Arch Surg 2008;143:841–846 discussion 846. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Ranvier GG, Weng J, Yeh RF, et al. : Candidate diagnostic markers and tumor suppressor genes for adrenocortical carcinoma by expression profile of genes on chromosome 11q13. World J Surg 2008;32:873–881. [DOI] [PubMed] [Google Scholar]
- 24.Soon P, Gill AJ, Benn DE, et al. : Microarray gene expression and immunohistochemistry analyses of adrenocortical tumours identify IGF2 and Ki-67 as useful in differentiating carcinomas from adenomas. Endocr Relat Cancer 2009;16:573–583. [DOI] [PubMed] [Google Scholar]
- 25.de Fraipont F, El Atifi M, Cherradi N, et al. : Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic Acid microarrays identifies several candidate genes as markers of malignancy. J Clin Endocrinol Metab 2005; 90:1819–1829. [DOI] [PubMed] [Google Scholar]
- 26.Velazquez-Fernandez D, Laurell C, Geli J, et al. : Expression profiling of adrenocortical neoplasms suggests a molecular signature of malignancy. Surgery 2005;138:1087–1094. [DOI] [PubMed] [Google Scholar]
- 27.West AN, Neale GA, Pounds S, et al. : Gene expression profiling of childhood adrenocortical tumors. Cancer Res 2007;67:600–608. [DOI] [PubMed] [Google Scholar]
- 28.Giordano TJ, Thomas DG, Kuick R, et al. : Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am J Pathol 2003;162:521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giordano TJ, Kuick R, Else T, et al. : Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res 2009;15:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almeida MQ, Fragoso MCBV, Lotfi CFP, et al. : Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. J Clin Endocrinol Metab 2008;93: 3524–3531. [DOI] [PubMed] [Google Scholar]
- 31.Fassnacht M, Libé R, Kroiss M, et al. : Adrenocortical carcinoma: A clinician’s update. Nat Rev Endocrinol 2011;7:323–335. [DOI] [PubMed] [Google Scholar]
- 32.Kolomecki K, Stepien H, Bartos M, et al. : Usefulness of VEGF, MMP-2, MMP-3 and TIMP-2 serum level evaluation in patients with adrenal tumours. Endocr Regul 2001;35:9–16. [PubMed] [Google Scholar]
- 33.Soon PS, Gill AJ, Benn DE, et al. : Microarray gene expression and immunohistochemistry analyses of adrenocortical tumors identify IGF2 and Ki-67 as useful in differentiating carcinomas from adenomas. Endocr Relat Cancer 2009;16:573–583. [DOI] [PubMed] [Google Scholar]
- 34.Tacon LJ, Soon PS, Gill AJ, et al. : The glucocorticoid receptor is overexpressed in malignant adrenocortical tumors. J Clin Endocrinol Metab 2009;94:4591–4599. [DOI] [PubMed] [Google Scholar]
- 35.Rizk-Rabin M, Assie G, Rene-Corail F, et al. : Differential expression of parathyroid hormone-related protein in adrenocortical tumors: Autocrine/paracrine effects on the growth and signaling pathways in H295R cells. Cancer Epidemiol Biomarkers Prev 2008;17:2275–2285. [DOI] [PubMed] [Google Scholar]
- 36.Weismann D, Briese J, Niemann J, et al. : Osteopontin stimulates invasion of NCI-h295 cells but is not associated with survival in adrenocortical carcinoma. J Pathol 2009;218:232–240. [DOI] [PubMed] [Google Scholar]
- 37.Sbiera S, Schmull S, Assie G, et al. : High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors. J Clin Endocrinol Metab 2010;95:E161–E171. [DOI] [PubMed] [Google Scholar]
- 38.Ragazzon B, Assie G, Bertherat J: Transcriptome analysis of adrenocortical cancers: From molecular classification to the identification of new treatments. Endocr Relat Cancer 2011;18: R15–R27. [DOI] [PubMed] [Google Scholar]
- 39.Szabo PM, Tamási V, Molnár V, et al. : Meta-analysis of adrenocortical tumour genomics data: Novel pathogenic pathways revealed. Oncogene 2010;29:3163–3172. [DOI] [PubMed] [Google Scholar]
- 40.Zsippai A, Rita Szabó D, Szabó PM, et al. : mRNA and micro-RNA expression patterns in adrenocortical cancer. Am J Cancer Res 2011;1:618–628. [PMC free article] [PubMed] [Google Scholar]
- 41.Morimoto R, Satoh F, Murakami O, et al. : Immunohistochemistry of a proliferation marker Ki67/MIB1 in adrenocortical carcinomas: Ki67/MIB1 labeling index is a predictor for recurrence of adrenocortical carcinomas. Endocr J 2008;55:49–55. [DOI] [PubMed] [Google Scholar]
- 42.Waldmann J, Feldmann G, Slater EP, et al. : Expression of the zinc-finger transcription factor Snail in adrenocortical carcinoma is associated with decreased survival. Br J Cancer 2008;99: 1900–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barzon L, Masi G, Pacenti M, et al. : Expression of aromatase and estrogen receptors in human adrenocortical tumors. Virchows Arch 2008;452:181–191. [DOI] [PubMed] [Google Scholar]
- 44.Shen XC, Gu CX, Qiu YQ, et al. : Estrogen receptor expression in adrenocortical carcinoma. J Zhejiang Univ Sci B 2009;10: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fragoso MC, Almeida MQ, Mazzuco TL, et al. : Combined expression of BUB1B, DLGAP5, and PINK1 as predictors of poor outcome in adrenocortical tumors: Validation in a Brazilian cohort of adult and pediatric patients. Eur J Endocrinol 2012; 166:61–67. [DOI] [PubMed] [Google Scholar]
- 46.Volante M, Sperone P, Bollito E, et al. : Matrix metalloproteinase type 2 expression in malignant adrenocortical tumors: Diagnostic and prognostic significance in a series of 50 adrenocortical carcinomas. Mod Pathol 2006;19:1563–1569. [DOI] [PubMed] [Google Scholar]
- 47.Fenske W, Völker HU, Adam P, et al. : Glucose transporter GLUT1 expression is an stage-independent predictor of clinical outcome in adrenocortical carcinoma. Endocr Relat Cancer 2009;16:919–928. [DOI] [PubMed] [Google Scholar]
- 48.Ronchi CL, Sbiera S, Kraus L, et al. : Expression of excision repair cross complementing group 1 and prognosis in adrenocortical carcinoma patients treated with platinum-based chemotherapy. Endocr Relat Cancer 2009;16:907–918. [DOI] [PubMed] [Google Scholar]
- 49.Tombol Z, Szabó PM, Molnár V, et al. : Integrative molecular bioinformatics study of human adrenocortical tumors: Micro-RNA, tissue-specific target prediction, and pathway analysis. Endocr Relat Cancer 2009;16:895–906. [DOI] [PubMed] [Google Scholar]
- 50.Doghman M, El Wakil A, Cardinaud B, et al. : Regulation of insulin-like growth factor-mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors. Cancer Res 2010;70:4666–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmitz KJ, Helwig J, Sheu SY, et al. : Differential expression of microRNA-675, microRNA-139–3p and microRNA-335 in benign and malignant adrenocortical tumours. J Clin Pathol 2011;64:529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh P, Soon PS, Feige JJ, et al. : Dysregulation of microRNAs in adrenocortical tumors. Mol Cell Endocrinol 2012;351:118–128. [DOI] [PubMed] [Google Scholar]
- 53.Cherradi N, Chabre O, Feige JJ: Role of miRNA in ACC. Session: Molecular Pathogenesis of ACC-new insights from array studies. In: International Adrenal Cancer Symposium Feb 18–19, Wurzburg, Germany (abstract). 2011. [Google Scholar]
- 54.Stephan EA, Chung TH, Grant CS, et al. : Adrenocortical carcinoma survival rates correlated to genomic copy number variants. Mol Cancer Ther 2008;7:425–431. [DOI] [PubMed] [Google Scholar]
- 55.Barreau O, de Reynies A, Wilmot-Roussel H, et al. : Clinical and pathophysiological implications of chromosomal alterations in adrenocortical tumors: An integrated genomic approach. J Clin Endocrinol Metab 2012;97:E301–E311. [DOI] [PubMed] [Google Scholar]
- 56.Bielinska M, Parviainen H, Kiiveri S, et al. : Review paper: Origin and molecular pathology of adrenocortical neoplasms. Vet Pathol 2009;46:194–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egger G, Liang G, Aparicio A, et al. : Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004;429: 457–463. [DOI] [PubMed] [Google Scholar]
- 58.Bock C, Paulsen M, Tierling S, et al. : CpG island methylation in human lymphocytes is highly correlated with DNA sequence, repeats, and predicted DNA structure. PLoS Genet 2006;2:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esteller M: Epigenetics in cancer. N Engl J Med 2008;358: 1148–1159. [DOI] [PubMed] [Google Scholar]
- 60.Jones PA, Baylin SB: The epigenomics of cancer. Cell 2007; 128:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnstone RW: Histone-deacetylase inhibitors: Novel drugs for the treatment of cancer. Nat Rev Drug Discov 2002;1:287–299. [DOI] [PubMed] [Google Scholar]
- 62.Volante M, Buttigliero C, Greco E, et al. : Pathological and molecular features of adrenocortical carcinoma: An update. J Clin Pathol 2008;61:787–793. [DOI] [PubMed] [Google Scholar]
- 63.Patterson EE, Holloway AK, Weng J, et al. : MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer 2010;117:1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ozata DM, Caramuta S, Velázquez-Fernández D, et al. : The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocr Relat Cancer 2011;18:643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bussey KJ, Demeure MJ: Genomic and expression profiling of adrenocortical carcinoma: Application to diagnosis, prognosis and treatment. Future Oncol 2009;5:641–655. [DOI] [PubMed] [Google Scholar]
- 66.Rechache NS, Wang Y, Stevenson HS, Killian JK, Edelman DC, Merino M, Zhang L, Nilubol N, Stratakis CA, Meltzer PS, Kebebew E. DNA methylation profiling identifies global methylation differences and markers of adrenocortical tumors. J Clin Endocrinol Metab 2012. [Epub ahead of print] PMID: 22472567. [DOI] [PMC free article] [PubMed] [Google Scholar]