Abstract
Recent successes in the application of epigenetic drugs for the treatment of myelodys-plastic syndrome have raised questions on the safety of long-term administration of DNA methylation inhibitors. We treated preweaned cancer prone ApcMin/+ (Min) mice continuously with the DNA methylation inhibitor zebularine in their drinking water to determine the effects of the drug on normal mouse development as well as cancer prevention. Zebularine caused a tissue-specific reduction in DNA methylation at B1 short interspersed nucleotide elements in the small and large intestines of female Min mice but not in other organs examined after chronic oral treatment. No significant difference in the average weights of mice was observed during the treatment. In addition, analysis of global gene expression of colonic epithelial cells from the females indicated that only 3% to 6% of the genes were affected in their expression. We did not detect toxicity and abnormalities from the histopathologic analysis of liver and intestinal tissues. Lastly, we tested whether prevention of tumorigenesis can be achieved with chronic oral administration of zebularine in Min mice. The average number of polyps in Min females decreased from 58 to 1, whereas the average polyp number remained unaffected in Min males possibly due to differential activity of aldehyde oxidase. Taken together, our results show for the first time that long-term oral administration of zebularine causes a gender-specific abrogation of intestinal tumors while causing a tissue-specific DNA demethylation. Importantly, prolonged treatment of mice with epigenetic drugs resulted in only minor developmental and histologic changes.
It is widely accepted that the development of cancer is a multistep process, each step of which occurs as a result of a specific genetic event (1). Moreover, recent advances in epigenetics have led us to believe that aberrant DNA methylation and histone modification patterns play an important role in tumorigenesis (2–4). Epigenetic changes have been noted in normal tissues, preinvasive lesions, and high-risk tissues, potentially serving as targets of chemoprevention (5–8). Therefore, epigenetic intervention using pharmacologic inhibitors to completely abrogate or delay the process of carcinogenesis may be feasible. In fact, several studies have shown that the modulation of histone modifications and/or DNA methylation prevents tumorigenesis (9–11).
Zebularine, a novel inhibitor of DNA methylation, has been shown to have anticancer properties in vivo and in vitro (12–15). Unlike 5-azacytidine and 5-aza-2′-deoxycytidine, which are chemically labile, zebularine is stable, making it possible to deliver the drug orally (14). However, chronic use of demethylating agents is of concern because genome-wide hypomethylation has been associated with chromosomal instability and cancer in mice (16, 17). Whether hypomethylation of DNA causes cancer in humans has yet to be confirmed (18). Previous published studies have not addressed the long-term toxicity of zebularine and have mainly dealt with the anticancer properties of the drug. Therefore, it is important to explore the effects of zebularine in the entire animal following chronic administration of the methylation inhibitor. The effect of chronic DNA methylation inhibition in mice should suggest whether there is a potential for safe long-term therapy in man.
In the present study, we explored the chemopreventive properties of zebularine in a murine intestinal cancer model and extended our studies on the toxicity of the compound. We first tested whether chronically administered zebularine could prevent or delay tumor progression in ApcMin/+ (Min) mice. Min mice have a nonsense mutation in the adenomatous polyposis coli (Apc) gene, which leads to the development of multiple adenomas in the intestines along with other phenotypes such as anemia, splenomegaly, and impaired development of proliferative tissues (19–21). We found that oral administration of zebularine resulted in decreased tumorigenicity. Remarkably, zebularine displayed a gender-specific antitumor activity in Min mice, possibly due to increased levels of aldehyde oxidase, which metabolizes the drug to uridine more readily in males than in females (22).
We further analyzed the effects of chronic zebularine treatment in the normal tissues using Min mice. The level of DNA methylation in all organs was unaffected with the exception of gastrointestinal tract of the females. Analysis of global gene expression levels in colonic epithelial cells showed that whereas the methylation level decreased by 50% in the colon, the expression of only ~5% of genes was affected. Finally, examination of the mice showed that there was no adverse effect on the growth rate and the structural integrity of intestinal and hepatic tissues of these mice in both gender groups. Our work is the first demonstration of intestinal tumor abrogation in mouse by an oral epigenetic drug with an extensive analysis of side effects on normal tissues at the same time. Zebularine proves to be a demethylating agent that causes low toxicity in mice when administered for a prolonged time.
Materials and Methods
Animal care and drug treatment
C57/BL/6 female and C57BL/6 ApcMin/+ male mice were purchased from The Jackson Laboratory and were maintained in the facilities at Zilkha Neurogenetic Institute (Los Angeles, CA). The wild-type C57BL/6 female mice were crossed with C57BL/6 ApcMin/+ male mice. Min mice were given drinking water containing 3% sucrose and 0.2 mg/mL zebularine ad libitum starting at day 7 post-birth until they were 120 ± 3 days old, at which point the mice were sacrificed. The weights of mice were monitored by weighing each mouse weekly on a Mettler balance. All animal work on the C57BL/6 mice was done in accordance with University of Southern California Institutional Animal Care and Use Committee guidelines.
Preparation of colonic epithelial cells and polyp analysis
The entire gastrointestinal tract of Min mice was removed (duode num to rectum) immediately after sacrifice and washed with PBS. The intestinal tract was cut longitudinally and rinsed with PBS. HBSS (Ca2+, Mg2+-free, 30 mmol/L EDTA, pH 8.0) was used to wash 1-cm squares lacking tumor from small and large intestines. The mucosa was incubated in HBSS with EDTAfor 20 min at 37°C and placed on a shaker at maximum speed at room temperature for 15 min. The solution was centrifuged at 1,000 rpm for 2 min, supernatant was removed, and the epithelial cell pellet was stored at −80°C for extraction of nucleic acids at a later time point. The remaining intestine was fixed with 70% ethanol and was subjected to polyp analysis under a dissecting microscope. All adenomas present along the entire length of the intestine were counted.
Nucleic acid isolation
DNAwas prepared from frozen tissue samples and intestinal epithelial cells using previously described methods (23). Total RNA was collected from intestinal epithelial cells with the mirVana miRNAIsolation Kit (Ambion) according to the manufacturer’s protocol.
Microarray analysis
The microarray analysis was done at the Microarray Core Facility at Childrens Hospital Los Angeles (Los Angeles, CA). Equal amounts of purified RNA from colonic epithelial cells of the females were pooled together and submitted for cRNA preparation and array hybridization. An Affymetrix GeneChip Mouse Gene 430 2.0 Array was used for hybridization on GeneChip Scanner 3000 (Affymetrix, Inc.). Data analysis was done by the Microarray Core Facility using S-score and dChip algorithms (24, 25).
Quantitative real-time reverse transcription-PCR analysis
Total RNA (5 μg) was reverse transcribed with Moloney murine leukemia virus reverse transcriptase (Invitrogen) and random primers (Invitrogen). The reverse transcription was carried out in a total volume of 50 μL as previously described (26). The quantitation of mRNA levels was carried out with the real-time fluorescence detection method as previously described (12, 27). All samples were normalized to the reference gene, Gapdh. The primer and probe sequences for Ireg1 are as follows: forward, 5′-AGGAGTCATTGCTGCTAGAATCG-3′; probe, 5′ 6-FAM-ACACAGTTGCTGCAAGAAAATGTAATTGAATCTGAABHQ-1 3′; and reverse, 5′-TGCACACCATTGATAATGCCTC-3′. The conditions for real-time reverse transcription-PCR are 94°C for 9 min followed by 45 cycles at 94°C for 15 s and 60°C for 1 min.
Quantitation of DNA methylation by Ms-SNuPE
Genomic DNA (4 μg) was treated with sodium bisulfite as previously described (14). The PCR primers used were 5′-TTAGTGGGGTATTTTTATTTGTATGG-3′ (forward) and 5′-AAATATCCTAAAAATACAAACTACAC-3′ (reverse). An initial denaturation at 94°C for 3 min was followed by 94°C for 45 s, 56°C for 45 s, and 72°C for 45 s for 40 cycles. Primers used for Ms-SNuPE analysis were 5′-GTTAATGAGGAATTTTAATTTTG-3′, 5′-TTTTATGTATAGTTAGGATAG-3′, and 5′-TATTTTTGAGTTTGTTTTTTTTGTAA-3′. Conditions for primer extension were 94°C for 1 min, 50°C for 30 s, and 72°C for 20 s.
Quantitation of DNA methylation by pyrosequencing
Bisulfite-converted DNA was used for pyrosequencing analysis as previously described (18). Briefly, the PCR product of each gene was used for individual sequencing reaction. Streptavidin-Sepharose beads (Amersham Biosciences) and Vacuum Prep Tool (Biotage AB) were used to purify the single-stranded biotinylated PCR product according to the manufacturer’s recommendation. The primers used for the PCR were 5′-GTGTAGTTTTGGTTGTTTTG-3′ (forward) and 5′-Biotin-ACTTAACTACTAACAACTCTAACTAA-3′ (reverse). The appropriate sequencing primer was annealed to the purified PCR product and used for a pyrosequencing reaction using the PSQ 96HS system (Biotage). The sequencing primer used was 5′-AGTGTTGGGATTAAAGG-3′. Raw data were analyzed with the allele quantitation algorithm using the provided software.
Preparation of histologic sections
Tissues were fixed in buffered formalin and processed for histologic analyses using standard protocols. The larger macroscopically visible polyps were embedded in separate paraffin blocks. The rest of the gastrointestinal tract was embedded in its entirety in at least two paraffin blocks.
Results
Chronic zebularine treatment causes diminished tumor multiplicity in mice
To determine whether chronic administration of zebularine prevents the formation of intestinal tumors, we treated ApcMin/+ (Min) mice with zebularine dissolved in the drinking water. After zebularine treatment, the formation of intestinal polyps in female Min mice was prevented significantly (P < 0.0001) whereas the polyp count remained unaffected in the males (P = 0.98; Fig. 1). Only 3 of 11 females had developed polyps detectable with a dissecting microscope. Microscopic examination of the intestinal polyps in the small intestine of male and female Min mice after H&E staining of tumor sections showed that the polyps found in the males and untreated females were large compared with those found in the treated females (Fig. 2). Therefore, zebularine, when administered orally, had effectively prevented or delayed the formation intestinal polyps in a gender-specific manner in Min mice.
Fig. 1.
Effects of chronic zebularine administration on the formation of intestinal tumors in mice. Min mice were given 0.2 mg/mL zebularine dissolved in 3% sucrose water daily for 113 ± 3 d. Mice were sacrificed at day 120. The gastrointestinal (GI) tract was removed, cut longitudinally, and washed with PBS. The entire gastrointestinal tract was analyzed for the presence of intestinal tumor under a dissecting microscope. P = 0.98 for males and P < 0.0001 for females (t test).
Fig. 2.
H&E staining of intestinal polyps after chronic zebularine treatment of Min mice. Min mice were given 0.2 mg/mL zebularine dissolved in 3% sucrose water daily for 113 ± 3 d. Mice were sacrificed at day 120. The gastrointestinal tract was removed, cut longitudinally, and washed in PBS. H&E-stained sections were observed for the presence of small polyps undetectable under a dissecting microscope. Untreated and treated males and untreated females had similarly sized polyps, but the treated females had smaller polyps at lower frequency. A, untreated male; B and C, treated male; D, untreated female; E and F, treated female.
Gender- and tissue-specific demethylation by zebularine in Min mice
We assessed the effect of zebularine treatment in normal tissues by measuring the change in DNA methylation in various organs of the Min mice. Methylation analysis of brain, heart, lungs, liver, kidneys, epidermal skin cells, spleen, stomach, and small and large intestines was conducted by pyrosequencing of the B1 repetitive element. B1 is a short interspersed nucleotide element found ubiquitously in the genome and therefore serves as a good marker for genome-wide DNA methylation changes (18). Very little change in DNA methylation in the male mice was detected in all of the tissues examined after zebularine treatment (Fig. 3A). However, a significant decrease in B1 methylation was observed in the small intestines (P = 0.018) and the colon (P < 0.0001; Fig. 3B). Methylation analysis of DNAfrom individual female mice showed that an appreciable level of demethylation was seen in mice that did not develop intestinal polyps, and the methylation level in the intestines of females that developed polyps was comparable to that of the treated male mice (Supplementary Fig. S1). These results imply that DNA methylation plays a causal role in the formation of polyps in these mice. Methylation analysis of small and large intestines of the Min mice at the Igf2 gene revealed that a statistically significant demethylation was seen only in the large intestine. Furthermore, the methylation at Igf2 decreased by 25% in the colon, whereas the global methylation had decreased by 40%, indicating that the monoallelic gene Igf2 was less sensitive to the chronic zebularine administration than the rest of the genome (Supplementary Fig. S2). Taken together, demethylation was found in the small intestine and colon but not in other organs, suggesting that the activity of zebularine may be more specific than was presumed. Additionally, female Min mice were consistently found to respond to zebularine treatment whereas the males remained insensitive to the drug.
Fig. 3.
Gender- and tissue-specific demethylation by zebularine in Min mice. Min mice were given 0.2 mg/mL zebularine dissolved in 3% sucrose water daily for 113 ± 3 d. Mice were sacrificed after treatments were completed. DNA was purified from various tissues and subjected to methylation analysis by pyrosequencing at the B1 repetitive element. The methylation of treated males (A) was not affected in all tissues tested by prolonged zebularine treatment whereas the DNA methylation in gastrointestinal tracts of treated females (B) was affected. White columns, methylation level of untreated mice; black columns, zebularine-treated group. Columns, mean percent methylation; bars, SD. t test for large intestine, P = 0.6 for males and P < 0.0001 for females; for small intestine, P = 0.26 for males and P = 0.018 for females.
Effects of chronic zebularine administration in Min mice
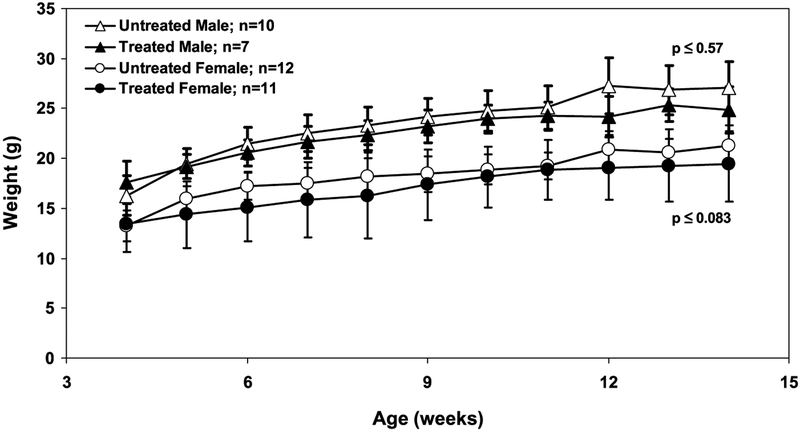
Three of 21 (14%) Min mice became moribund between weeks 5 and 7 of the treatment at the effective zebularine dose of 0.2 mg/mL, regardless of the gender; however, the majority of the mice survived the entire treatment period, displayed normal behavior, and remained apparently healthy. During the course of the treatment, the weight of each mouse was measured weekly. Whereas zebularine-treated male and female mice did not show a statistically significant retardation in growth compared with the control mice, they weighed slightly less than the control group (Fig. 4). Treatment with 0.5 mg/mL zebularine caused 100% lethality, whereas 0.1 mg/mL treatment resulted in 100% survival, indicating that this transgenic mouse strain may be extremely sensitive to dose changes.
Fig. 4.
Effects of chronic administration of zebularine on the weights of male and female Min mice. Min mice were given 0.2 mg/mL zebularine dissolved in 3% sucrose water daily for 113 ± 3 d. Mice were weighed weekly during the treatment beginning at week 4 from birth. The average weights of untreated and treated males and females were calculated for each group. No statistical significance was achieved between the weights of the untreated and treated mice, regardless of gender. Open triangles, untreated males; closed triangles, treated males; open circles, untreated females; closed circles, treated females.
Splenomegaly, a phenotype commonly observed in the Min mice, was observed in untreated male and female Min mice and treated males at the time of euthanasia (21). However, a significant decrease in the weight and size of spleen was noted in the treated female group (P = 0.0004; Supplementary Fig. S3A). Splenomegaly may occur in response to hyperfunction, increased hematopoiesis, hemolytic anemia, myeloproliferative disorders, or viral infection. It has been reported that Min mice suffer from anemia as a result of intestinal bleeding (19). Remarkably, a significant increase in the RBC count was observed in the females after zebularine treatment (P = 0.017), suggesting that the females responded to zebularine favorably and lost the phenotypes characteristic of untreated Min mice (Supplementary Fig. S3B). Taken together, these results suggest that all animals under treatment experienced a slight decrease in their weight, but the females were relieved of some of the Min phenotype as a result of chronic administration of zebularine.
Microarray analysis of colonic epithelial cells in female Min mice
To assess the effect of chronic zebularine treatment on the global gene expression profile, we carried out an Affymetrix microarray analysis using RNAextracted from normal colonic epithelial cells not containing polyps from female mice. Because the greatest demethylation was observed in the colon of the females, we expected to see the greatest changes in gene expression in this tissue. Microarray results were analyzed using the following algorithms to ensure that biases on the analytic methods were minimized: dChip, which is a model-based analysis that estimates gene expression indexes (25), and S-score, which calculates the relative change in probe pair intensities directly (24). Using the dChip method, we found that 1,713 (3.8%) probe sets of a total of 45,101 probe sets were up-regulated ≥2-fold and 1,066 (2.4%) probe sets were down-regulated ≥2-fold. According to the S-score algorithm, the number of probe sets that yielded a significant signal was 1,392 (P = 0.01). This is notable, given that 50% of genes are associated with CpG islands in their promoters (28). We further analyzed our data using the S-score algorithm to select a set of significant probe sets (P = 10−5) and analyzed it using the L2L database that allows the comparison of published mammalian microarray studies with our data and provides an insight into the biological processes that are affected by chronic zebularine treatment (29).
From this analysis, we found 10 up-regulated and 5 down-regulated genes that were involved in peptide cross-linking, digestion, acute-phase response, regulation of cell adhesion, arginine metabolism, urea cycle intermediate metabolism, aldehyde metabolism, and iron ion homeostasis (Table 1A and B). One gene of interest is Slc40a1 or Ireg1, a gene that plays a critical role in intestinal iron absorption and cellular iron release (30). Real-time reverse transcription-PCR confirmed that the expression of Ireg1 was indeed higher in the untreated female Min mice than in the treated females although it did not reach statistical significance (Supplementary Fig. S4). Notably, Ireg1 is known to be up-regulated in the intestine of mice with hemolytic anemia, which is also known to be associated with splenomegaly (31). These results show that zebularine treatment was associated with the disappearance of splenomegaly and anemia and provide a direct link between a physiologic observation and a molecular analysis.
Table 1.
Genes up-regulated or down-regulated in mouse colonic epithelial cells after zebularine treatment
| Biological process | Gene symbol | Probe ID | Description |
|---|---|---|---|
| (A) Genes up-regulated in colonic epithelial cells after chronic zebularine treatment of the Min mice | |||
| Peptide cross-linking | Tgm2 | 1455900_X_AT | transglutaminase 2 |
| 1433428_X_AT | |||
| 1437277_X_AT | |||
| Digestion | Ppy | 1420440_AT | pancreatic polypeptide |
| Akr1b10 | 1448894_AT | aldo-keto reductase family 1, member B10 (aldose reductase) | |
| Fabp2 | 1418438_AT | fatty acid binding protein 2, intestinal | |
| Acute-phase response | Reg3a | 1448290_AT | regenerating islet-derived 3α |
| 1416297_S_AT | |||
| Reg3g | 1448872_AT | regenerating islet-derived 3 γ | |
| Regulation of cell adhesion | Tgm2 | 1455900_X_AT | transglutaminase 2 |
| 1433428_X_AT | |||
| 1437277_X_AT | |||
| Arginine metabolism | Ddah1 | 1429298_AT | dimethylarginine dimethylaminohydrolase 1 |
| Otc | 1420525_A_AT | ornithine carbamoyltransferase | |
| Urea cycle intermediate metabolism | Ddah1 | 1429298_AT | dimethylarginine dimethylaminohydrolase 1 |
| Otc | 1420525_A_AT | ornithine carbamoyltransferase | |
| Aldehyde metabolism | Aldh1a1 | 1416468_AT | aldehyde dehydrogenase 1 family, member A1 |
| Akr1b10 | 1448894_AT | aldo-keto reductase family 1, member B10 (aldose reductase) | |
| (B) Genes down-regulated in colonic epithelial cells after chronic zebularine treatment of the Min mice | |||
| Peptide cross-linking | Tgm3 | 1440150_AT | transglutaminase 3 |
| Digestion | Slc15a1 | 1419343_AT | solute carrier family 15 (oligopeptide transporter), member 1 |
| Ctse | 1418989_AT | cathepsin E | |
| Regulation of cell adhesion | Lama3 | 1427512_A_AT | laminin, α3 |
| Iron ion homeostasis | Slc40a1 | 1417061_AT | solute carrier family 40 (iron-regulated transporter), member 1 |
| 1447227_AT | |||
NOTE: Min mice were given 0.2 mg/mL zebularine dissolved in 3% sucrose-water daily for 113 ± 3 d. Mice were sacrificed on day 120. RNA from colonic epithelial cells was subjected to microarray analysis and analyzed using S-score algorithm (P < 10−5). An L2L array was used to obtain biological processes that were significantly affected (29).
Taken together, our data showed that the expression of only a small percentage of genes in colonic epithelial cells was affected after zebularine administration, in contrast to the dramatic DNA methylation changes in the same tissue. In addition, the change in expression of at least one gene may have occurred as a result of loss of the Min phenotype and not zebularine toxicity. This suggests that chronic methylation inhibition may not dramatically disturb the global gene expression patterns while causing a considerable decrease in DNA methylation levels and may be less detrimental to normal tissues than anticipated.
Histology of Min mice after chronic zebularine treatment
We next investigated whether the chronic administration of a pharmacologic inhibitor would generate inflammation or tissue damage. Because zebularine is mainly metabolized by aldehyde oxidase in the liver and causes the greatest demethylation in the intestines, we examined the histopathology of these two organs. H&E-stained liver and intestines showed that the architecture of liver tissue was intact, and no obvious histologic changes were noted in the livers of males and females after chronic zebularine treatment (Fig. 5A–D). Similarly, no gross abnormalities were observed in the small intestines of these animals (Fig. 5E–H). In addition, the absence of acute or chronic inflammation and the lack of disruption of the tissue architecture integrity provide evidence that zebularine was minimally toxic to these organs during prolonged administration. Afew localized fibroses were found in the large intestine in 2 of 11 treated females. Because these fibroses were not a general feature of the entire intestine, it is unclear whether they were induced by zebularine. This suggests that zebularine had effectively delayed the progression of carcinogenesis and reduced the size and number of the intestinal polyps while causing very little damage to the development and maintenance of these organs.
Fig. 5.
H&E staining of liver and small intestine after chronic zebularine treatment of Min mice. Min mice were given 0.2 mg/mL zebularine dissolved in 3% sucrose water daily for 113 ± 3 d. Mice were sacrificed at day 120. The liver was washed in PBS several times. The gastrointestinal tract was removed, cut longitudinally, and washed in PBS. H&E-stained tissue sections were assessed for histologic abnormalities. Liver: untreated male (A), treated male (B), untreated female (C), and treated female (D); small intestine: untreated male (E), treated male (F), untreated female (G), and treated female (H).
Discussion
Our study shows that chronic oral zebularine treatment inhibited the formation of intestinal adenomas in Min mice in a gender-specific manner. The examination of the Min mice disclosed that zebularine has prevented tumorigenesis at the earliest stage. At the same time, zebularine was shown to be quite selective in terms of inhibiting DNA methylation and caused no observable toxicity in the majority of the mice that underwent chronic treatment. These results provide evidence that may help to broaden the application of DNA methylation inhibitors clinically.
One intriguing aspect of our study was the gender-specific response of the Min mice to the drug. In plasma, zebularine is rapidly converted into uridine, uracil, and dihydrouracil by the liver enzyme aldehyde oxidase (29, 32, 33). Aldehyde oxidase is responsible for the metabolism of many antiviral and anticancer agents (34, 35). Interestingly, aldehyde oxidase activity is influenced by the levels of testosterone and growth hormones, leading to gender, species, and strain differences in the enzyme activity (36–39). A recent publication has shown that zebularine metabolism is also influenced by the differential aldehyde oxidase activity, and the enzyme activity of male mice ranked the highest among the animals tested whereas the female mice had virtually no aldehyde oxidase activity (22). This may explain the gender-specific effect of zebularine seen in the Min mice. Administration of an inhibitor of aldehyde oxidase in combination with zebularine to male mice might increase the efficacy of the drug. Several aldehyde oxidase inhibitors such as raloxifene are used clinically (40, 41).
We showed for the first time that zebularine causes a tissue-specific demethylation in the female Min mice. Previously, DNA methylation levels had been analyzed using normal tissues from the tumor-bearing organ, but we went further to study the methylation levels of various other organs. Because the inhibition of DNA methyltransferases by zebularine occurs after DNA incorporation (42), it is possible that demethylation occurred in the most actively dividing cells and/or tissues, which in this case was the colon and the small intestine. The life spans of colonic and small intestinal epithelial cells are 4 to 8 and 3 to 4 days, respectively (43, 44), which is considerably shorter than the turnover of skin epidermal cells (45 days; ref. 45). These three types of tissues were analyzed for methylation, and a considerable amount of demethylation was detected in large and small intestines whereas no decrease in methylation was seen in skin epidermal cells, indicating that the turnover of these cells may be responsible for the tissue-specific demethylation. It would be of interest to determine whether zebularine activity is dependent on the rate of cell division in vivo as it is in vitro (13).
The third facet of our study dealt with toxicity resulting from the chronic administration of a pharmacologic agent and the feasibility of prolonged use of a DNA methylation inhibitor in patients and high-risk individuals for cancer chemoprevention. The greatest decrease in DNA methylation by zebularine was observed in the colon; however, the expression of 95% of genes in the same tissue was not disrupted. Notably, whereas global methylation, as measured at the B1 repetitive sequence, decreased by 40%, the methylation level of an imprinted gene, Igf2, decreased by 25%, suggesting that the monoallelically methylated regions may be less sensitive to zebularine treatment than the rest of the genome. This is a significant finding that strengthens the notion that zebularine is relatively nontoxic in mice, and which may allay the concerns that the use of demethylating agents is detrimental to the operation of normal biological processes (16, 17).
Indeed, it is encouraging that some of the genes with changes in their expression profile (i.e., Ireg1) contributed to the restoration of a more normal phenotype in these mice. H&E staining showed that after almost 4 months of zebularine treatment, no histologic damage was found in the liver and intestines of the Min mice. In fact, zebularine has been shown to be minimally toxic when administered i.p. to tumor-bearing mice for up to 1 year (15). Our study is consistent with previous findings that zebularine has low toxicity in mouse models of cancer; however, caution would be essential in using this drug in patients because primates are very sensitive to the parenteral administration of zebularine (46).
Zebularine is the first orally administered DNA methylation inhibitor that has activity against a variety of cancer models in mice. We have shown that zebularine effectively prevents intestinal tumors in mice at the earliest stages when administered chronically. The gender- and tissue-specific activity of zebularine suggests that zebularine affects and uses very specific metabolic pathways. Notably, zebularine is an orally administered compound that was given chronically with no significant toxicity. The demonstration of a positive outcome with chronic oral administration of a methylation inhibitor is encouraging in that alternative orally bioavailable compounds with this mechanism of action may prove highly beneficial for chronic oral dosing either as preventive or adjuvant therapies.
Supplementary Material
Acknowledgments
We thank Drs. G. Liang and G. Egger for discussion and critical reading of the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest
P.A. Jones, P.W. Laird, and A.S. Yang: Ownership Interest TherEpi Corporation. The other authors disclosed no potential conflicts of interest.
Supplementary data for this article are available at Cancer Prevention Research Online (http://cancerprevres.aacrjournals.org/).
References
- 1.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759–67. [DOI] [PubMed] [Google Scholar]
- 2.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev 2002;16:6–21. [DOI] [PubMed] [Google Scholar]
- 3.Fraga MF, Ballestar E, Villar-Garea A, et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 2005;37:391–400. [DOI] [PubMed] [Google Scholar]
- 4.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet 2002;3: 415–28. [DOI] [PubMed] [Google Scholar]
- 5.Crawford YG, Gauthier ML, Joubel A, et al. Histologically normal human mammary epithelia with silenced p16(INK4a) overexpress COX-2, promoting a premalignant program. Cancer Cell 2004;5: 263–73. [DOI] [PubMed] [Google Scholar]
- 6.Holm TM, Jackson-Grusby L, Brambrink T, Yamada Y, Rideout WM III, Jaenisch R. Global loss of imprinting leads to widespread tumorigenesis in adult mice. Cancer Cell 2005;8:275–85. [DOI] [PubMed] [Google Scholar]
- 7.Holst CR, Nuovo GJ, Esteller M, et al. Methylation of p16(INK4a) promoters occurs in vivo in histologically normal human mammary epithelia. Cancer Res 2003;63:1596–601. [PubMed] [Google Scholar]
- 8.Kopelovich L, Crowell JA, Fay JR. The epigenome as a target for cancer chemoprevention. J Natl Cancer Inst 2003;95:1747–57. [DOI] [PubMed] [Google Scholar]
- 9.Belinsky SA, Klinge DM, Stidley CA, et al. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Res 2003;63: 7089–93. [PubMed] [Google Scholar]
- 10.Eads CA, Nickel AE, Laird PW. Complete genetic suppression of polyp formation and reduction of CpG-island hypermethylation in Apc(Min/+) Dnmt1-hypomorphic mice. Cancer Res 2002;62: 1296–9. [PubMed] [Google Scholar]
- 11.Laird PW, Jackson-Grusby L, Fazeli A, et al. Suppression of intestinal neoplasia by DNA hypomethylation. Cell 1995;81:197–205. [DOI] [PubMed] [Google Scholar]
- 12.Cheng JC, Weisenberger DJ, Gonzales FA, et al. Continuous zebularine treatment effectively sustains demethylation in human bladder cancer cells. Mol Cell Biol 2004;24:1270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng JC, Yoo CB, Weisenberger DJ, et al. Preferential response of cancer cells to zebularine. Cancer Cell 2004;6:151–8. [DOI] [PubMed] [Google Scholar]
- 14.Cheng JC, Matsen CB, Gonzales FA, et al. Inhibition of DNA methylation and reactivation of silenced genes by zebularine. J Natl Cancer Inst 2003;95:399–409. [DOI] [PubMed] [Google Scholar]
- 15.Herranz M, Martin-Caballero J, Fraga MF, et al. The novel DNA methylation inhibitor zebularine is effective against the development of murine T-cell lymphoma. Blood 2005;107:1174–7. [DOI] [PubMed] [Google Scholar]
- 16.Eden A, Gaudet F, Waghmare A, Jaenisch R. Chromosomal instability and tumors promoted by DNA hypomethylation. Science 2003;300:455. [DOI] [PubMed] [Google Scholar]
- 17.Gaudet F, Hodgson JG, Eden A, et al. Induction of tumors in mice by genomic hypomethylation. Science 2003;300:489–92. [DOI] [PubMed] [Google Scholar]
- 18.Yang AS, Estecio MR, Doshi K, Kondo Y, Tajara EH, Issa JP. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res 2004;32:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 1990;247: 322–4. [DOI] [PubMed] [Google Scholar]
- 20.Su LK, Kinzler KW, Vogelstein B, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 1992;256:668–70. [DOI] [PubMed] [Google Scholar]
- 21.You S, Ohmori M, Pena MM, et al. Developmental abnormalities in multiple proliferative tissues of Apc(Min/+) mice. Int J Exp Pathol 2006; 87:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klecker RW, Cysyk RL, Collins JM. Zebularine metabolism by aldehyde oxidase in hepatic cytosol from humans, monkeys, dogs, rats, and mice: influence of sex and inhibitors. Bioorg Med Chem 2006; 14:62–6. [DOI] [PubMed] [Google Scholar]
- 23.Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res 1991; 19:4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy RE, Archer KJ, Miles MF. Empirical validation of the S-Score algorithm in the analysis of gene expression data. BMC Bioinformatics 2006; 7:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Wong WH. Model-based analysis of oligo-nucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A 2001;98:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez-Zulueta M, Bender CM, Yang AS, et al. Methylation of the 5′ CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res 1995;55:4531–5. [PubMed] [Google Scholar]
- 27.Eads CA, Danenberg KD, Kawakami K, Saltz LB, Danenberg PV, Laird PW. CpG island hypermethylation in human colorectal tumors is not associated with DNA methyltransferase overexpression. Cancer Res 1999;59:2302–6. [PubMed] [Google Scholar]
- 28.Takai D, Jones PA. Comprehensive analysis of CpG islands in human chromosomes 21 and 22. Proc Natl Acad Sci U S A 2002;99:3740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman JC, Weiner AM. L2L: a simple tool for discovering the hidden significance in microarray expression data. Genome Biol 2005;6:R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 2005;1:191–200. [DOI] [PubMed] [Google Scholar]
- 31.Latunde-Dada GO, Vulpe CD, Anderson GJ, Simpson RJ, McKie AT. Tissue-specific changes in iron metabolism genes in mice following phenylhydrazine-induced haemolysis. Biochim Biophys Acta 2004;1690:169–76. [DOI] [PubMed] [Google Scholar]
- 32.Beumer JH, Joseph E, Egorin MJ, Covey JM, Eiseman JL. Quantitative determination of zebularine (NSC 309132), a DNA methyltransferase inhibitor, and three metabolites in murine plasma by high-performance liquid chromatography coupled with on-line radioactivity detection. J Chromatogr B Analyt Technol Biomed Life Sci 2006;831: 147–55. [DOI] [PubMed] [Google Scholar]
- 33.Beumer JH, Joseph E, Egorin MJ, et al. A mass balance and disposition study of the DNA methyltransferase inhibitor zebularine (NSC 309132) and three of its metabolites in mice. Clin Cancer Res 2006;12:5826–33. [DOI] [PubMed] [Google Scholar]
- 34.Garattini E, Mendel R, Romao MJ, Wright R, Terao M. Mammalian molybdo-flavoenzymes, an expanding family of proteins: structure, genetics, regulation, function and pathophysiology. Biochem J 2003;372:15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitamura S, Sugihara K, Nakatani K, et al. Variation of hepatic methotrexate 7-hydroxylase activity in animals and humans. IUBMB Life 1999; 48:607–11. [DOI] [PubMed] [Google Scholar]
- 36.Al-Salmy HS. Inter-strain variability in aldehyde oxidase activity in the mouse. Comp Biochem Physiol C Toxicol Pharmacol 2002;132:341–7. [DOI] [PubMed] [Google Scholar]
- 37.Kurosaki M, Demontis S, Barzago MM, Garattini E, Terao M. Molecular cloning of the cDNA coding for mouse aldehyde oxidase: tissue distribution and regulation in vivo by testosterone. Biochem J 1999; 341:71–80. [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshihara S, Tatsumi K. Involvement of growth hormone as a regulating factor in sex differences of mouse hepatic aldehyde oxidase. Biochem Pharmacol 1997;53:1099–105. [DOI] [PubMed] [Google Scholar]
- 39.Yoshihara S, Tatsumi K. Purification and characterization of hepatic aldehyde oxidase in male and female mice. Arch Biochem Biophys 1997;338:29–34. [DOI] [PubMed] [Google Scholar]
- 40.Obach RS. Potent inhibition of human liver aldehyde oxidase by raloxifene. Drug Metab Dispos 2004;32:89–97. [DOI] [PubMed] [Google Scholar]
- 41.Obach RS, Huynh P, Allen MC, Beedham C. Human liver aldehyde oxidase: inhibition by 239 drugs. J Clin Pharmacol 2004;44:7–19. [DOI] [PubMed] [Google Scholar]
- 42.Zhou L, Cheng X, Connolly BA, Dickman MJ, Hurd PJ, Hornby DP. Zebularine: a novel DNA methylation inhibitor that forms a covalent complex with DNA methyltransferases. J Mol Biol 2002;321: 591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Croft DN, Levitan R. DNA loss, cell loss and epithelial. Proc R Soc Med 1970;63 Suppl:15–6. [PMC free article] [PubMed] [Google Scholar]
- 44.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992;119:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergstresser PR, Taylor JR. Epidermal “turnover time”—a new examination. Br J Dermatol 1977;96: 503–9. [DOI] [PubMed] [Google Scholar]
- 46.Jonhson W, Harder J, Naylor J, et al. A pharmaco-kinetic/pharmacodynamic approach to evaluating the safety of zebularine in non-human primates. 2006: 97th AACR Annual Meeting Washington DC. p.1311. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.