Abstract
Transcranial direct current stimulation (tDCS) is a low intensity neuromodulation technique shown to elicit therapeutic effects in a number of neuropsychological conditions. Independent randomized sham-controlled trials and meta- and mega-analyses demonstrate that tDCS targeted to the left dorsolateral prefrontal cortex can produce a clinically meaningful response in patients with major depressive disorder (MDD), but effects are small to moderate in size. However, the heterogeneous presentation, and the neurobiology underlying particular features of depression suggest clinical outcomes might benefit from empirically informed patient selection. In this review, we summarize the status of tDCS research in MDD with focus on the clinical, biological, and intrinsic and extrinsic factors shown to enhance or predict antidepressant response. We also discuss research strategies for optimizing tDCS to improve patient-specific clinical outcomes. TDCS appears suited for both bipolar and unipolar depression, but is less effective in treatment resistant depression. TDCS may also better target core aspects of depressed mood over vegetative symptoms, while pretreatment patient characteristics might inform subsequent response. Peripheral blood markers of gene and immune system function have not yet proven useful as predictors or correlates of tDCS response. Though further research is needed, several lines of evidence suggest that tDCS administered in combination with pharmacological and cognitive behavioral interventions can improve outcomes. Tailoring stimulation to the functional and structural anatomy and/or connectivity of individual patients can maximize physiological response in targeted networks, which in turn could translate to therapeutic benefits.
Keywords: Transcranial electrical stimulation (TES), major depression, antidepressant treatment, neuromodulation, direct current
1. Introduction
Depression presents a major burden to global health, affecting more than 300 million people [1]. The impact of depression is compounded by the limited success of first-line antidepressant therapies where only a third of patients with major depressive disorder (MDD) are expected to remit after initial treatment [2]. Research that could promote the translation of more effective, accessible and individually-tailored antidepressant therapies into clinical practice is thus of major importance for reducing the economic and personal toll of this common and debilitating disorder. Transcranial direct current stimulation (tDCS) is a neuromodulation technique that uses low amplitude direct current (usually ≤2 mA) to enhance or suppress cortical excitability [3]. TDCS has shown therapeutic potential in a range of neuropsychiatric disorders, including MDD [4].
Conventional administration of tDCS involves a 2-electrode montage applied to the scalp to deliver constant current. In animal studies [5] with replication in humans [6], the positively charged electrode (i.e. anode) has been shown to increase cortical excitability locally, while an inhibitory effect is observed with the negatively charged electrode (cathode). Excitatory and inhibitory effects occur by shifting membrane potentials towards depolarization and hyperpolarization, respectively, and the biophysical effects of tDCS are shown to endure after stimulation. A preponderance of existing data supports that tDCS neuromodulation has probable antidepressant effects [7 8]. Though still experimental, due to its simple setup, portability, safety and low cost, tDCS could thus present a valuable noninvasive and relatively accessible treatment option for MDD. However, the antidepressant mechanisms of tDCS, identifying which individuals are most likely to benefit from treatment, and the dosing parameters optimal for achieving individualized therapeutic response, remain critical barriers to translation. In this review, we appraise recent evidence regarding the efficacy and selection of tDCS for treating individuals with major depression. We also discuss gaps in knowledge regarding the potential systems-level antidepressant mechanisms of tDCS, and research strategies to optimize and tailor tDCS to enhance its therapeutic effects.
2. The efficacy of tDCS for the treatment of depression
A growing amount of data from open-label, or randomized clinical trials (RCTs) suggests that tDCS can elicit antidepressant effects in patients with MDD [8-11]. The majority of prior depression trials have targeted the dorsolateral prefrontal cortex (DLPFC) for modulation by tDCS due to 1) its role in the top-down regulation of mood and of subcortical regions involved in emotional responses, 2) observed hypo- metabolism or activation and lateralized effects in depression imaging studies [12 13], and 3) its proximity to the scalp for external stimulation. To enhance activity (or reduce hypoactivity), the excitatory electrode (anode) has been used to target the left DLPFC (F3, international 10–20 EEG system) in the majority of studies, while the return electrode (cathode) has been placed over the right DLPFC or a neutral region [8-11]. Though results from individual studies are less consistent, likely influenced by the inclusion of small and heterogeneous samples, antidepressant effects have been reported in the majority of investigations. The estimation of effect sizes has been made possible via meta- and/or mega analyses [8 11], which differ in that former aggregates previously reported results while the latter includes reexamination of raw data. Specifically, the most recently published meta-analyses including 7 RCTs and n=259 patients [11], reported effects for active versus sham tDCS considered small-to-medium (pooled odds ratio (OR) for response characterized as ≥50% improvement in depressive symptoms: 1.63, 95% CI 1.26–2.12; and for remission of symptoms: 2.5, 95% CI 1.26–2.49) and low publication bias. Antidepressant effects were also significant and viewed as clinically meaningful when examining clinical outcomes as a continuous variable (Hedges’ g = 0.37; 95% CI 0.04–0.7). A subsequently published mega-analysis [8] re-analyzing raw data from n=289 participants from 6 RCTs found more robust clinical effects (OR for response: 2.44, 95% CI 1.38–4.32, and remission: 2.38, 95% CI 1.22–4.64, and depression improvement β = 0.347, 95% CI 0.12–0.57). Further, when examining the number necessary to treat (NNT) (i.e., the number of patients necessary to treat to prevent one undesirable outcome) compared to other depression treatment studies, response and remission were found as likely for tDCS as for commonly prescribed antidepressants and neuromodulation with repetitive transcranial magnetic stimulation (rTMS).
In a recently completed non-inferiority study, and the largest single trial to date including n=245 patients divided into three treatment arms (tDCS plus oral placebo, sham tDCS plus escitalopram, or sham tDCS plus oral placebo), both tDCS and drug therapy were found to be significantly more effective than placebo. However, in this trial (the ELECT-tDCS study), the non-inferiority margin for tDCS was not met, such that even though treatment efficacy was not shown to differ significantly across treatment modalities, pharmacotherapy was judged superior [14]. Further, another relatively large and recently completed multisite tDCS RCT including N=130 patients showed that clinical improvement, though significant, was similar in patients receiving both active and sham tDCS [15]. Although placebo effects may account for these unexpected findings, results have also brought to question whether the transient stimulation designed to mimic scalp sensations during sham also elicits biophysical effects. Taken together, the available evidence supports that left DLPFC tDCS can ameliorate depression symptoms, although there is not currently clear evidence to support that tDCS has a clinical advantage over standard treatments. However, as discussed below, it is possible that tDCS might be more effective for particular forms of depressive illness and that further optimization of treatment parameters tailored to individual patients may substantially improve efficacy.
3. Clinical factors associated with tDCS response in depression
Depression is a heterogeneous disorder, where a DSM diagnosis allows for 200+ possible symptom combinations. Different constellations of symptoms and their severity, the presence of comorbid disorders, demographics, and psychiatric and treatment histories are amongst some of the factors that could impact individual antidepressant response [16-18]. Patient selection may thus similarly affect tDCS antidepressant outcomes. To date, independent studies have shown that tDCS is clinically beneficial in mild-to-moderate, severe as well as refractory depression [8 11]. However, combined analyses of individual patient data [8], found inverse relationships between response or remission rates and the level of treatment resistance. Therefore, as for other antidepressant treatments including including ECT and rTMS [19-21], individuals who have failed previous antidepressant trails also appear less likely to respond to tDCS. Notably, although some individual studies have examined more specific features of illness with regard to tDCS outcomes (e.g., number and duration of past depressive episodes, age of onset, duration of illness), small samples have limited interpretation of these potential moderators. The effects of tDCS for patients with different diagnostic classifiers or psychiatric comorbidies are also less studied. However, tDCS has been shown to reduce depressive symptoms in individuals with both unipolar and bipolar depression [22]. Further, in megaanalyses, bipolar depression was also shown to be a predictor of tDCS clinical improvement. Other predictors of response included severe depression, being female and melancholic depression [8].
At least two studies have explored relationships between tDCS response and clinical dimensions of depression in independent depression samples using factors derived from the Hamilton (HDRS) or Montgomery–Åsberg depression (MADRS) rating scales. One study using data from a previously published RCT (n=64) [23] used a three factor model of MADRS items, including (i) dysphoria (reported sadness, pessimistic thoughts, and suicidal thoughts), (ii) retardation (apparent sadness, concentration difficulties, lassitude, and inability to feel) and (iii) vegetative symptoms (inner tension, reduced sleep and reduced appetite) to examine differential effects of tDCS on these features [24]. Results showed significant improvements in all three MADRS factors following a 3-week course of tDCS. However, only the dysphoria and retardation factors differentiated the active and sham groups; similar findings were observed after a follow-up open-label phase of tDCS treatment. Focusing instead on addressing how pretreatment features of depressive illness might relate to subsequent tDCS response, another study used a published HDRS six factor structure, including (i) anxiety/ somatization, (ii) body weight, (iii) cognitive disturbances, (iv) circadian fluctuations, (v) retardation, and (vi) sleep disturbances, to examine predictors of tDCS response [25]. Including n=171 participants with unipolar and bipolar depression from 3 independent open-label trails, results from this study showed significant relationships between baseline cognitive disturbances, retardation and anxiety/somatization factors with tDCS response and no moderating effects of age, sex or type of depression diagnosis. Though requiring replication, these results provide initial evidence to support that tDCS may preferentially target core features of depressive illness over vegatative sympotoms, and that patients with particular clinical profiles might benefit more from treatment. Notwithstanding, to better understand how features of depression in individual patients might impact tDCS outcomes, much larger and well-characterized samples are clearly needed. Data-driven approaches might be particularly informative for identifying clinical predictors and correlates of response to allow for more tailored treatment strategies.
4. Biological factors associated with tDCS response in depression
Using blood-oxygen-level dependent (BOLD) and arterial spin labeling (ASL) perfusion imaging in animals, anodal tDCS is shown to lead to increased activation [26] and CBF [27] in specific brain areas, supporting that physiological effects could lead to downstream neuroplastic processes. BOLD fMRI has been applied in human tDCS studies and the results showed that tDCS elicits long-lasting, polarity dependent changes in BOLD signal and network connectivity [28-31]. Similarly, ASL perfusion MRI has also shown polarity dependent changes in regional cerebral blood [31 32].
Neurotrophic factors, the proteins (including brain derived neurotrophic factor (BDNF)) supporting neuroplasticity, are shown as altered in MDD, and are related to antidepressant effects [33 34]. In line with the neurotrophic hypothesis of depression [33], animal tDCS induces long-lasting synaptic potentiation and BDNF-dependent synaptic plasticity [35], suggesting a possible mechanism of clinical response. To establish whether changes in BDNF and other neurotrophic factors might associate with tDCS or sertraline treatment in patients with MDD, plasma-levels of BDNF and neurotrophins 3 (NT-3) and 4 (NT-4), nerve growth factor (NGF) and glial cell line derived neurotrophic factor (GDNF) were measured patients with MDD (n=73) before and after randomization to active/sham tDCS or sertraline/placebo as part of the SELECT-tDCS RCT [36 37]. No changes in BDNF or other neurotrophic factors were shown to predict or relate to treatment response for either treatment modality [36 37]. However, a separate RCT investigating the non-inferiority of tDCS to escitalopram (ELECT-TDCS study, n=236, [14]), found that baseline NGF plasma levels predicted depression improvement for tDCS versus escitalopram.
Disruptions in immune system function are repeatedly implicated in pathophysiology of MDD [38], interact with neurotrophic factors [39], and are shown to be affected by neuromodulation therapies [40]. However, in the ELECT and SELECT tDCS studies [14 41], cytokines were not shown associate with clinical response though they decreased over time across treatment groups (the ELECT trial measured interleukins (IL) IL-1 ß, IL-6, IL-8, IL-10, IL-12p70, IL-18, IL-33, tumor necrosis factor-alpha (TNF-alpha), and its soluble receptors sTNFr1 and sTNFr2, and the SELECT trail measured IL-2, IL-4, IL-6, IL-10, IL-17a, TNF-alpha, IFN-γ). Though early changes in neurotrophic processes and immune response (i.e. changes occurring shortly after the initiation of treatment) might also predict or signal subsequent changes in clinical outcome, trajectories of change in these biomarkers have not been investigated in relation to tDCS treatment in MDD. With the exception of NGF, viable leads are mostly lacking at this time. However, peripheral blood biomarkers of neuroplasticity and inflammation might still prove relevant for determining predictors and correlates of tDCS response with further research in larger samples.
5. Dosing parameters and tDCS response in depression
The tDCS “dose” required to induce optimal neurobiological effects may depend on electrode montage, size, or other parameters (current intensity, polarity, duration), as well as the context of stimulation. Different RCTs of tDCS in MDD have manipulated dose by varying current intensity (0.5 to 2.5 mA), duration (20-30 minutes) and the number (usually 5-15) and frequency of tDCS sessions (once or twice daily, or on alternate days). In a prior meta-analysis of studies using different parameters, tDCS charge per electrode surface area (C/cm2), and total summed charge were not shown to significantly influence clinical outcomes, though a trend suggested higher current charges may elicit larger antidepressant effects [11 23]. However, in megaanalysis of individual patient data, both greater tDCS charge and longer session duration were found to statistically improve antidepressant response, suggesting future trials might be optimized accordingly.
Though almost all modern RCTs of MDD have targeted the left DLPFC for excitatory stimulation, cathode position, which also affects the path of electric current through the brain, has varied across studies. However, clinical outcomes do not appear to differ for montages using the right DLPFC (F4, 10-20 EEG location) or the right supraorbital area for cathode placement, which have been used in the majority of MDD studies. While F3 anodal tDCS may better engage dorsal forebrain-limbic systems involved in mood regulation, bitemporal tDCS may more effectively engage ventro-limbic circuits [42 43] involved in emotion reactivity. Notably, computational modelling has shown that anodal F3 stimulation with extra-cephalic right upper arm cathode placement is more effective for inducing changes in current flow in deeper ventro-limbic structures [42 43], though no RCTs have compared this montage to other montages typically used in MDD. Since dysregulated activity in dorsal prefrontal-limbic circuits and in subcortical hippocampal-amygdala-striatal-thalamic circuits are linked with depression pathophysiology and repeatedly observed in structural and functional imaging studies of depression [44 45], it appears likely that modulation of both systems by tDCS may work to elicit a therapeutic response. Further, patient-specific clinical characteristics and/or symptom profiles may ultimately dictate if a given tDCS montage may be more effective for a particular patient. Finally, studies using stimulation targets informed by individual functional and structural anatomy, and that address links between regional current density in relation to changes in clinical response might be of important value for better optimizing and tailoring tDCS treatment in MDD.
6. Intrinsic and extrinsic moderators of tDCS response in depression
Since low intensity stimulation shifts the balance of neural excitation and inhibition [46], other brain network dynamics can influence whether or not downstream changes in brain activity occur with tDCS [47]. Indeed, animal studies show that weak, but simultaneous polarization of a large number of neurons are amplified in an already active neural network [48]. TDCS neuromodulation is thus partially dependent on the concurrent activity of particular neural circuits, where changes are more likely to occur in activated over inactive networks [49]. These biophysical interactions also explain why tDCS effects on behavior are mostly observed when cognitive or behavioral probes are used together with stimulation [50 51]. However, in MDD, tDCS is suggested to affect pathological network activity selectively without a behavioral probe [49] and is usually applied without simultaneous manipulation of behavioral state. Nonetheless, the therapeutic effects of tDCS might still be strengthened by intrinsic or extrinsic potentiation of neural function. For example, pharmacological therapies perturb intrinsic state by modulating neurotransmission and antidepressant effects might be greater with combination drug therapy. Data from several individual trials as well as from mega-analysis support this hypothesis where the initiation or augmentation of antidepressant drug treatment with tDCS is shown to significantly improve clinical efficacy [41 52].
Cognitive behavioral interventions, which can elicit response rates similar to antidepressant medications [53], present a means for extrinsic modulation of depression-related brain circuits. For DLPFC tDCS, cognitive behavioral interventions are suggested to modulate top-down neural processing to regulate emotional states by engaging underactive prefrontal circuits to regulate overactive subcortical limbic circuits [54]. Notably, initial evidence supports that F3 anodal tDCS combined with cognitive behavioral therapy can produce both acute and lasting antidepressant effects [55]. Further, an open-label study combining tDCS with cognitive emotional training (CET), was shown to produce significant clinical improvements in medication-resistant major depression (41% achieved response criterion), as well as improvements in self ratings of psychological symptoms, ruminations and quality of life [56]. Though not a controlled trial, these findings thus suggest that tDCS used in combination with psychological interventions may improve efficacy, even in refractory depression. Prior studies have also attempted to prime DLPFC neural circuitry in MDD using neurocognitive training coupled with tDCS. In a prior RCT, patients receiving cognitive control/working memory training combined with active or sham tDCS (n=37) showed significant antidepressant effects, though between treatment groups differences were not observed [57]. However, older, higher-performing individuals showed particular benefits of combination therapy. A subsequent RCT using another variant of cognitive control training with concurrent tDCS showed antidepressant effects across all conditions at the end of the 5-session treatment series and sustained improvements in depressive symptoms in patients that received active cognitive training plus tDCS at 3-week follow-up [58]. Preliminary evidence thus suggests that pharmacotherapy and cognitive training used to augment tDCS may enhance or prolong antidepressant outcomes.
7. Individual optimization to improve tDCS treatment in depression
The extent to which tDCS can affect neural properties relies on the penetration of current and characteristics of the skull, CSF distribution and cortical morphology. Identical stimulation parameters may thus induce different physiological, behavioral, and therapeutic effects across individuals [9]. For example, even for a well-defined stimulation area, accuracy of electrode placement may vary based on individual anatomy. The diffusivity of current flow can also influence neural targeting, and is greater for larger scalp electrodes as used in most prior depression RCTs. Strategies to mitigate (or leverage) these factors could maximize tDCS therapeutic response in individual patients. Though it is not yet clear whether more focal or diffuse stimulation might improve clinical outcomes in MDD, electrode configurations are now available that allow for high-definition (HD) tDCS [59]. HD-tDCS consists of 4 small “return” disk electrodes arranged around a center electrode, which determines the direction of unifocal modulation without requiring a regionally separated anode and cathode [60]. On direct comparison, HD-tDCS produces changes in brain excitability that exceed the magnitude and duration of standard tDCS [61]. However, no published RCTs have yet compared the therapeutic efficacy of conventional versus HD-tDCS in MDD. Also, as relevant to the spatial focality of stimulation, prior tDCS trials of MDD have performed mostly the same left DLPFC electrode localization procedures without attempting to standardize electrode placement based on skull size and brain morphology, which can impact neural engagement [62]. For example, prior studies have shown that MRI-guided neuronavigation reduces inter-subject variability for DLPFC targeting for rTMS [63]. Further, targeting rTMS to DLPFC using structural anatomy [64] as well as functional coordinates extracted from meta-analysis of functional neuroimaging studies resulted in greater anti-depressive effects in MDD patients compared to standard localization methods (i.e., 5 cm or 10-20 method) [65]. The use of MRI-guided neuronavigation to target the DLPFC may similarly reduce inter-individual variability to optimize tDCS.
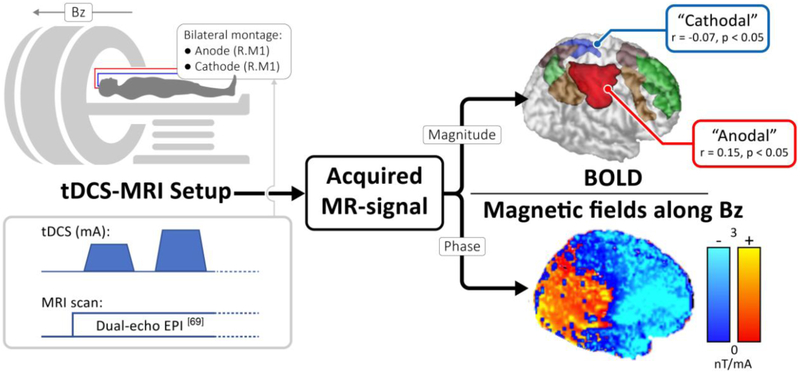
Computational modeling of tDCS electric field current direction and intensity suggest the perturbation of distinct neural regions for different tDCS montages [66]. To understand the relationships between tDCS dose parameters and electric-field distributions based on individual anatomy (e.g., scalp and skull thickness, CSF and gray and white matter, topography of the cortex as well as white matter anisotropic conductivities), modeling of electric field distributions in individual patients using structural MRI can improve neural targeting. Recently, our group has developed MRI-based electric current mapping techniques to measure tDCS induced electric currents in the human brain in vivo by tracking corresponding changes in magnetic fields or phase maps [67-69]. Thus, unlike head models, or other imaging modalities that provide surrogate markers of tDCS stimulation, it is possible to visualize the magnetic field induced by tDCS current with high spatial resolution in individual subjects. In line with results from modeling experiments [70], simultaneous tDCS-MRI shows that tDCS induces cortical electrical field changes under the electrodes as well as in brain regions connected to the stimulation site [Figure 1.]. Realtime MRI mapping of tDCS neural engagement in individual subject can ensure modulation of targeted networks to maximize possible antidepressant effects.
Figure 1. Simultaneous mapping of tDCS electric currents and functional changes in a sample subject.
Concurrent tDCS-MRI data can be acquired as shown, with the phase and magnitude of the MR signal encoding the current induced magnetic field (along Bz) and BOLD-contrast respectively. While the former is linearly proportional to tDCS electric currents along an orthogonal direction (Ampere’s Law), the latter is an established marker for tracking brain activity. An ICA analysis on the BOLD-data identified brain networks including the default mode network (green) and the executive networks (brown). The analysis also identified two regions underneath the anode and cathode electrodes (labelled "anodal" and "cathodal"), which were found to correlate significantly with the applied tDCS current.
8. Conclusion
A growing body of evidence suggests that tDCS can elicit antidepressant effects. Though tDCS-related clinical response obtained using conventional F3 anodal tDCS has not been demonstrated as superior to standard first-line antidepressants in terms of overall efficacy, benefits include its minimal side effects and low risk for adverse events, low cost and accessibility. However, cumulating evidence suggests that tDCS can be further optimized to enhance its therapeutic effects. Optimization can occur by manipulating dose (including electrode size, configuration and charge), using combination pharmacological or cognitive therapies designed to sensitize brain circuits linked with disease pathophysiology, and refining spatial targeting (electrode placement) and stimulation based on individual structural and functional anatomy and connectivity. Understanding of how electric fields penetrate the brain to engage and modulate particular neural circuits will also advance efforts to tailor treatment, which can be achieved by computational modelling and novel in-vivo mapping of tDCS magnetic fields and current density. Though it is not yet clear whether particular patient characteristics can inform which individuals most likely to benefit from tDCS therapy, recent research suggests that tDCS elicits antidepressant effects in both unipolar and bipolar depression, but is less suited for treatment resistant patients. Research also suggests tDCS may better target dimensions of depressive illness, and that patients with particular pre-treatment symptom profiles will have a greater response. At present, measures of gene and immune system function have shown fewer links with tDCS-related clinical response, but few studies have addressed this question. Future research, including imaging guided tDCS, more comprehensive clinical and cognitive phenotyping and data-driven approaches to identify more salient predictors of response are still needed.
Highlights:
TDCS of the left dorsolateral prefrontal cortex can reduce depressive symptoms
TDCS may be less suited for treatment-resistant depression
Combining tDCS with pharmaco- or psychotherapies may enhance therapeutic outcomes
Optimizing tDCS parameters to individual patients can improve physiological response
Acknowledgements:
Funding: This work was supported by the National Institutes of Health, including Grant Nos. MH110526 (to KLN and DJW), and K24 MH102743 (to KLN).
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.WHO. World Health Organization, Depression. (http://www.who.int/mental_health/management/depression/definition/en/)
- 2.Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv 2009;60(11):1439–45 doi: 10.1176/appi.ps.60.11.1439 . [DOI] [PubMed] [Google Scholar]
- 3.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000;527 Pt 3:633–9 doi: PHY_1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moffa AH, Brunoni AR, Nikolin S, Loo CK. Transcranial Direct Current Stimulation in Psychiatric Disorders: A Comprehensive Review. The Psychiatric Clinics of North America 2018;41(3):447–63 doi: 10.1016/j.psc.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Bindman LJ, Lippold OC, Redfearn JW. The Action of Brief Polarizing Currents on the Cerebral Cortex of the Rat (1) during Current Flow and (2) in the Production of Long-Lasting after-Effects. J Physiol 1964;172:369–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001;57(10):1899–901 [DOI] [PubMed] [Google Scholar]
- 7.Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clinical Neurophysiology 2014;125(11):2150–206 doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, Blumberger DM, et al. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. The British Journal of Psychiatry 2016;208(6):522–31 doi: bjp.bp.115.164715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Journal of Psychiatric Research 2013;47(1):1–7 doi: 10.1016/j.jpsychires.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Kalu UG, Sexton CE, Loo CK, Ebmeier KP. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychological Medicine 2012;42(9):1791–800 doi: 10.1017/S0033291711003059. [DOI] [PubMed] [Google Scholar]
- 11.Shiozawa P, Fregni F, Bensenor IM, Lotufo PA, Berlim MT, Daskalakis JZ, et al. Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. The International Journal of Neuropsychopharmacology 2014;17(9):1443–52 doi: 10.1017/S1461145714000418. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Human Brain Mapping 2008;29(6):683–95 doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, et al. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biological Psychiatry 2008;63(4):369–76 doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 14.Brunoni AR, Moffa AH, Sampaio-Junior B, Borrione L, Moreno ML, Fernandes RA, et al. Trial of Electrical Direct-Current Therapy versus Escitalopram for Depression. N Engl J Med 2017;376(26):2523–33 doi: 10.1056/NEJMoa1612999. [DOI] [PubMed] [Google Scholar]
- 15.Loo CK, Husain MM, McDonald WM, Aaronson S, O'Reardon JP, Alonzo A, et al. International randomized-controlled trial of transcranial Direct Current Stimulation in depression. Brain Stimulation 2018;11(1):125–33 doi: 10.1016/j.brs.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Fregni F, Marcolin MA, Myczkowski M, Amiaz R, Hasey G, Rumi DO, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. The International Journal of Neuropsychopharmacology 2006;9(6):641–54 doi: 10.1017/S1461145705006280. [DOI] [PubMed] [Google Scholar]
- 17.Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychological Medicine 2012;42(5):967–80 doi: 10.1017/S0033291711001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chekroud AM, Gueorguieva R, Krumholz HM, Trivedi MH, Krystal JH, McCarthy G. Reevaluating the Efficacy and Predictability of Antidepressant Treatments: A Symptom Clustering Approach. JAMA Psychiatry 2017;74(4):370–78 doi: 10.1001/jamapsychiatry.2017.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Archives of General Psychiatry 2000;57(5):425–34 [DOI] [PubMed] [Google Scholar]
- 20.Lisanby SH, Husain MM, Rosenquist PB, Maixner D, Gutierrez R, Krystal A, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology 2009;34(2):522–34 doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]
- 21.Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med 2006;354(12):1243–52 doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 22.Donde C, Neufeld NH, Geoffroy PA. The Impact of Transcranial Direct Current Stimulation (tDCS) on Bipolar Depression, Mania, and Euthymia: a Systematic Review of Preliminary Data. Psychiatr Q 2018;89(4):855–67 doi: 10.1007/s11126-018-9584-5. [DOI] [PubMed] [Google Scholar]
- 23.Loo CK, Alonzo A, Martin D, Mitchell PB, Galvez V, Sachdev P. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. The British Journal of Psychiatry 2012;200(1):52–9 doi: 10.1192/bjp.bp.111.097634. [DOI] [PubMed] [Google Scholar]
- 24.Alonzo A, Chan G, Martin D, Mitchell PB, Loo C. Transcranial direct current stimulation (tDCS) for depression: analysis of response using a three-factor structure of the Montgomery-Asberg depression rating scale. Journal of Affective Disorders 2013;150(1):91–5 doi: 10.1016/j.jad.2013.02.027 [DOI] [PubMed] [Google Scholar]
- 25.Brunoni AR, Junior RF, Kemp AH, Lotufo PA, Bensenor IM, Fregni F. Differential improvement in depressive symptoms for tDCS alone and combined with pharmacotherapy: an exploratory analysis from the Sertraline vs. Electrical Current Therapy for Treating Depression Clinical Study. The International Journal of Neuropsychopharmacology 2014;17(1):53–61 doi: 10.1017/S1461145713001065. [DOI] [PubMed] [Google Scholar]
- 26.Takano Y, Yokawa T, Masuda A, Niimi J, Tanaka S, Hironaka N. A rat model for measuring the effectiveness of transcranial direct current stimulation using fMRI. Neuroscience Letters 2011;491(1):40–3 doi: 10.1016/j.neulet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Wachter D, Wrede A, Schulz-Schaeffer W, Taghizadeh-Waghefi A, Nitsche MA, Kutschenko A, et al. Transcranial direct current stimulation induces polarity-specific changes of cortical blood perfusion in the rat. Experimental Neurology 2011;227(2):322–7 doi: 10.1016/j.expneurol.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Polania R, Paulus W, Nitsche MA. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Human Brain Mapping 2012;33(10):2499–508 doi: 10.1002/hbm.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saiote C, Turi Z, Paulus W, Antal A. Combining functional magnetic resonance imaging with transcranial electrical stimulation. Front Hum Neurosci 2013;7:435 doi: 10.3389/fnhum.2013.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polania R, Paulus W, Antal A, Nitsche MA. Introducing graph theory to track for neuroplastic alterations in the resting human brain: a transcranial direct current stimulation study. Neuroimage 2011;54(3):2287–96 doi: 10.1016/j.neuroimage.2010.09.085. [DOI] [PubMed] [Google Scholar]
- 31.Kwon YH, Jang SH. The enhanced cortical activation induced by transcranial direct current stimulation during hand movements. Neuroscience Letters 2011;492(2):105–8 doi: 10.1016/j.neulet.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 32.Stagg CJ, Lin RL, Mezue M, Segerdahl A, Kong Y, Xie J, et al. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. The Journal of Neuroscience 2013;33(28):11425–31 doi: 10.1523/JNEUROSCI.3887-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanson ND, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: A critical reappraisal. Neuropsychopharmacology 2011;36(13):2589–602 doi: 10.1038/npp.2011.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biological Psychiatry 2000;47(12):1043–9 doi: S0006322300002286 [pii]. [DOI] [PubMed] [Google Scholar]
- 35.Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 2010;66(2):198–204 doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunoni AR, Machado-Vieira R, Zarate CA Jr., Vieira EL, Vanderhasselt MA, Nitsche MA, et al. BDNF plasma levels after antidepressant treatment with sertraline and transcranial direct current stimulation: results from a factorial, randomized, sham-controlled trial. European Neuropsychopharmacology 2014;24(7):1144–51 doi: 10.1016/j.euroneuro.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunoni AR, Machado-Vieira R, Zarate CA Jr., Vieira EL, Valiengo L, Bensenor IM, et al. Assessment of non-BDNF neurotrophins and GDNF levels after depression treatment with sertraline and transcranial direct current stimulation in a factorial, randomized, sham-controlled trial (SELECT-TDCS): an exploratory analysis. Progress in Neuro-psychopharmacology & Biological Psychiatry 2015;56:91–6 doi: 10.1016/j.pnpbp.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry 2009;65(9):732–41 doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabakman R, Lecht S, Sephanova S, Arien-Zakay H, Lazarovici P. Interactions between the cells of the immune and nervous system: neurotrophins as neuroprotection mediators in CNS injury. Prog Brain Res 2004;146:387–401 [DOI] [PubMed] [Google Scholar]
- 40.van Buel EM, Patas K, Peters M, Bosker FJ, Eisel UL, Klein HC. Immune and neurotrophin stimulation by electroconvulsive therapy: is some inflammation needed after all? Translational Psychiatry 2015;5:e609 doi: 10.1038/tp.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunoni AR, Valiengo L, Baccaro A, Zanao TA, de Oliveira JF, Goulart A, et al. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA psychiatry 2013;70(4):383–91 doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- 42.Bai S, Dokos S, Ho KA, Loo C. A computational modelling study of transcranial direct current stimulation montages used in depression. Neuroimage 2014;87:332–44 doi: 10.1016/j.neuroimage.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 43.Ho KA, Bai S, Martin D, Alonzo A, Dokos S, Loo CK. Clinical Pilot Study and Computational Modeling of Bitemporal Transcranial Direct Current Stimulation, and Safety of Repeated Courses of Treatment, in Major Depression. J ECT 2015;31(4):226–33 doi: 10.1097/YCT.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 44.MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Molecular Psychiatry 2011;16(3):252–64 doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 45.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature 2008;455(7215):894–902 doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reato D, Rahman A, Bikson M, Parra LC. Effects of weak transcranial alternating current stimulation on brain activity-a review of known mechanisms from animal studies. Front Hum Neurosci 2013;7:687 doi: 10.3389/fnhum.2013.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tehovnik EJ. Electrical stimulation of neural tissue to evoke behavioral responses. Journal of Neuroscience Methods 1996;65(1):1–17 [DOI] [PubMed] [Google Scholar]
- 48.Reato D, Rahman A, Bikson M, Parra LC. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. The Journal of Neuroscience 2010;30(45):15067–79 doi: 10.1523/JNEUROSCI.2059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bikson M, Name A, Rahman A. Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Front Hum Neurosci 2013;7:688 doi: 10.3389/fnhum.2013.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boggio PS, Bermpohl F, Vergara AO, Muniz AL, Nahas FH, Leme PB, et al. Go-no-go task performance improvement after anodal transcranial DC stimulation of the left dorsolateral prefrontal cortex in major depression. Journal of Affective Disorders 2007;101(1-3):91–8 doi: 10.1016/j.jad.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 51.Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences of the United States of America 2009;106(5):1590–5 doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valiengo L, Bensenor IM, Goulart AC, de Oliveira JF, Zanao TA, Boggio PS, et al. The sertraline versus electrical current therapy for treating depression clinical study (select-TDCS): results of the crossover and follow-up phases. Depression and Anxiety 2013;30(7):646–53 doi: 10.1002/da.22079. [DOI] [PubMed] [Google Scholar]
- 53.DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nature reviews. Neuroscience 2008;9(10):788–96 doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lener MS, Iosifescu DV. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: a review of the literature. Annals of the New York Academy of Sciences 2015;1344:50–65 doi: 10.1111/nyas.12759. [DOI] [PubMed] [Google Scholar]
- 55.D'Urso G, Mantovani A, Micillo M, Priori A, Muscettola G. Transcranial direct current stimulation and cognitive-behavioral therapy: evidence of a synergistic effect in treatment-resistant depression. Brain Stimulation 2013;6(3):465–7 doi: 10.1016/j.brs.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Martin DM, Teng JZ, Lo TY, Alonzo A, Goh T, Iacoviello BM, et al. Clinical pilot study of transcranial direct current stimulation combined with Cognitive Emotional Training for medication resistant depression. Journal of Affective Disorders 2018;232:89–95 doi: 10.1016/j.jad.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 57.Brunoni AR, Boggio PS, De Raedt R, Bensenor IM, Lotufo PA, Namur V, et al. Cognitive control therapy and transcranial direct current stimulation for depression: a randomized, double-blinded, controlled trial. Journal of Affective Disorders 2014;162:43–9 doi: 10.1016/j.jad.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 58.Segrave RA, Arnold S, Hoy K, Fitzgerald PB. Concurrent cognitive control training augments the antidepressant efficacy of tDCS: a pilot study. Brain Stimulation 2014;7(2):325–31 doi: 10.1016/j.brs.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 59.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimulation 2009;2(4):201–7, 07 e1 doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villamar MF, Wivatvongvana P, Patumanond J, Bikson M, Truong DQ, Datta A, et al. Focal modulation of the primary motor cortex in fibromyalgia using 4×1-ring high-definition transcranial direct current stimulation (HD-tDCS): immediate and delayed analgesic effects of cathodal and anodal stimulation. The journal of Pain 2013;14(4):371–83 doi: 10.1016/j.jpain.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Kuo HI, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, et al. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimulation 2013;6(4):644–8 doi: 10.1016/j.brs.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 62.Seibt O, Brunoni AR, Huang Y, Bikson M. The Pursuit of DLPFC: Non-neuronavigated Methods to Target the Left Dorsolateral Pre-frontal Cortex With Symmetric Bicephalic Transcranial Direct Current Stimulation (tDCS). Brain Stimulation 2015;8(3):590–602 doi: S1935-861X(15)00858-X [pii] 10.1016/j.brs.2015.01.401. [DOI] [PubMed] [Google Scholar]
- 63.Rusjan PM, Barr MS, Farzan F, Arenovich T, Maller JJ, Fitzgerald PB, et al. Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Human Brain Mapping 2010;31(11):1643–52 doi: 10.1002/hbm.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herbsman T, Avery D, Ramsey D, Holtzheimer P, Wadjik C, Hardaway F, et al. More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biological Psychiatry 2009;66(5):509–15 doi: 10.1016/j.biopsych.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 65.Fitzgerald PB, Hoy K, McQueen S, Maller JJ, Herring S, Segrave R, et al. A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology 2009;34(5):1255–62 doi: 10.1038/npp.2008.233. [DOI] [PubMed] [Google Scholar]
- 66.Csifcsak G, Boayue NM, Puonti O, Thielscher A, Mittner M. Effects of transcranial direct current stimulation for treating depression: A modeling study. Journal of Affective Disorders 2018;234:164–73 doi: 10.1016/j.jad.2018.02.077. [DOI] [PubMed] [Google Scholar]
- 67.Jog M, Smith RX, Jann K, Dunn W, Wu A, Wang DJ. In-vivo Evidence of transcranial Direct Current Stimulation (tDCS) induced Magnetic Field Changes in Human Brain using MRI. Proc. Inter. Soc. Magn. Reson. Med 2015;24:515 [Google Scholar]
- 68.Jog MV, Smith RX, Jann K, Dunn W, Lafon B, Truong D, et al. In-vivo Imaging of Magnetic Fields Induced by Transcranial Direct Current Stimulation (tDCS) in Human Brain using MRI. Sci Rep 2016;6:34385 doi: 10.1038/srep34385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jog MS, Yan L, Jann K, Wang DJJ. Proceedings #16. Evaluation of a novel MRI technique for mapping in-vivo currents and hemodynamic changes during tDCS. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation 2017;10(4):e64–e66 doi: 10.1016/j.brs.2017.04.109. [DOI] [Google Scholar]
- 70.Sadleir RJ, Vannorsdall TD, Schretlen DJ, Gordon B. Transcranial direct current stimulation (tDCS) in a realistic head model. Neuroimage 2010;51(4):1310–8 doi: 10.1016/j.neuroimage.2010.03.052. [DOI] [PubMed] [Google Scholar]