Graphical Abstract
A cloud-based wearable IoT aldehyde sensor system for asthma research and management
1. Introduction
Asthma is one of the most common chronic diseases in children, affecting approximately 6.1 million children under 18 in the US alone (8.3% of the pediatric population) [1]. It is estimated to be the leading cause of missed school days and the third-ranking cause of hospitalization for children [2]. The annual cost of treating pediatric asthma is $3.2 billion dollars [3]. Triggers of pediatric asthma exacerbations such as air pollution, tobacco smoke, allergies, and airway infections are well known. However, how the interaction between environmental exposure and the patient’s biological response determines susceptibility and timing of such events is less clear. One significant barrier to causal understanding is the lack of objective measures of exposure metrics correlated with patient physiological responses and activities.
Air pollution is known to be associated with asthma exacerbations [4–6]. For example, formaldehyde, a common air pollutant known to irritate airways, is found in tobacco smoke, furniture, paintings, synthetic carpets, and other everyday sources [7–13]. Previous studies have suggested a significant positive association between formaldehyde exposure and asthma exacerbations [14–22]. However, currently no sensing system is available to researchers which can continuously monitor an individual patient’s exposure to formaldehyde and correlate it with the patient’s symptoms and physiological responses. Formaldehyde sensors based on metal oxide technology and colorimetric assays have been developed before [23–25], but not in wearable formats suitable for real-life monitoring. In addition, to make large-scale epidemiological studies feasible, it’s likely a cloud-based IoT-like system is required to handle large-scale sensor deployment, management, data storage, and analytics [26–34].
To address this unmet need, we have developed a wearable IoT aldehyde sensor integrated with a cloud-based informatics system. The wearable IoT aldehyde sensor is based on electrochemical fuel cell technology and can measure formaldehyde in air at levels as low as 30ppb, below the OSHA lower limit of 0.75ppm (8 hour time-weighted average) [35,36]. The final device resembles a wrist watch with dimensions of 36mm × 42mm × 11mm and a weight of 35g. In use, the device can continuously operate over 7 days without recharging. The built-in Bluetooth Low Energy (BLE) capability allows it to wirelessly upload data to an Android smartphone and display data in real time. The smartphone also functions as a gateway to an Amazon cloud-based informatics system which handles sensor data storage, management and analytics. Asthma researchers and physicians can visualize and analyze the sensor data through a web browser.
2. Material and Methods
2.1. System Overview
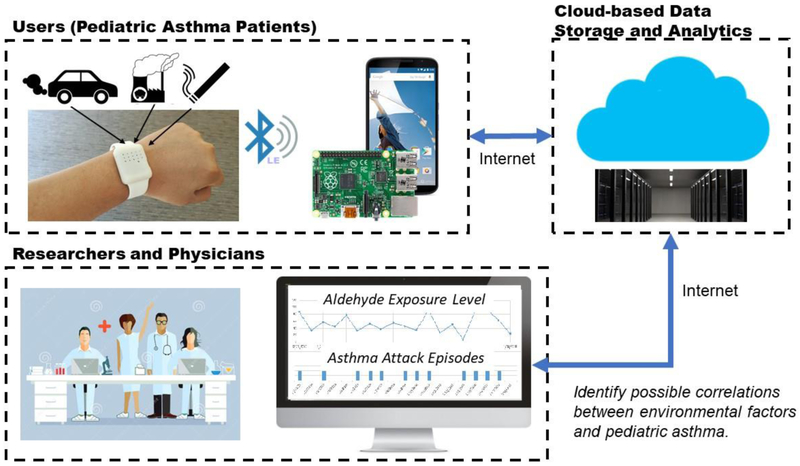
The architecture of the system is shown in Figure 1, which consists of three major components: (1) a wearable sensor on the wrist, (2) a BLE-capable gateway with internet connectivity (such as a smartphone or a computer), and (3) a cloud-based data analytics system.
Figure 1.
Architecture of the cloud-based wearable IoT aldehyde sensor system for asthma research and management.
The wearable device utilizes an electrochemical fuel cell for aldehyde sensing and a digital CMOS sensor for temperature and humidity monitoring. Low-power, miniaturized electronics is employed to build a self-contained data acquisition and transmission module, including an analog front-end (AFE), a BLE-capable wireless microcontroller, flash memory, power management circuitry and a lithium battery.
The wearable sensor was designed to resemble a wrist watch. When powered on, the device will continuously measure ambient environmental conditions at a sampling interval of 1 dataset per second. Measurement data is averaged every minute and stored in an on-board flash memory. Both real-time and historical data can be accessed by a BLE-capable gateway using custom software communication protocol.
An Android-based application was developed on a Google Nexus 5X smartphone serving as a gateway. Once a BLE connection is established between the application and the wearable sensing device, historical and real-time measurement data is retrieved, displayed, and stored. The app also uploads the data to a cloud-based informatics system through Internet for centralized data storage, visualization and analysis.
This cloud-based data analytics system is implemented on Amazon Web Services (AWS) with a simple dashboard configured to demonstrate the basic analytics capabilities. Users can access this cloud-based analytics system via a secure web interface. Time-stamped measurement data can also be downloaded to a local computer for quantitative analysis using data analysis software.
2.2. Wearable Sensor Specifications
The U.S. Environmental Protection Agency (EPA) estimates that the average in-home formaldehyde exposure levels range from 0.10 to 3.8 parts per million (ppm), while outdoor levels in U.S. urban area are in the range of 11 to 20 parts per billion (ppb) [37]. The Occupational Safety and Health Administration (OSHA) requires work place exposures to formaldehyde to be less than 0.75ppm as an eight-hour time-weighted average (TWA) [35]. Studies also suggest that the time-weighted average of indoor formaldehyde exposure levels should be limited to 0.1 parts-per-million (ppm) [38]. Based on these guidelines, the target detection range for aldehyde was set to be 30 ppb to 10 ppm.
Certain weather conditions are also significant asthma triggers, including high relative humidity (RH) and freezing temperatures [39,40]. Moreover, it is important to calibrate the fuel cell sensor output against temperature and humidity levels, as the sensor response can be affected by these factors. Therefore, a calibrated digital temperature and RH sensor was included.
A sturdy package is necessary for a wearable sensor to be used in real-world environments. Also, the wearable sensor should be as small and light as possible, especially for pediatric users. For end-user convenience, it is also desirable to make such devices rechargeable with a battery life longer than 24 hours. For these reasons, all internal components were packaged into the size of a wrist watch (36 × 42 × 11mm). A USB 2.0 micro-B port was provided for charging with standard USB chargers (5.5V).
Bluetooth Low-Energy (BLE) is a low-power wireless data transmission technology widely adopted by smartphones and IoT devices. It is currently estimated that billions of devices are BLE-ready across the globe [41]. Given a smartphone app, end users will be able to view sensor data in real-time and contribute to research by sharing their data. Moreover, readily available commercial microcontrollers with BLE functions have been developed for emerging IoT devices. Therefore, a low-power BLE-enabled, small-footprint microcontroller was selected to build our wearable sensor platform.
2.3. Fuel Cell Aldehyde Sensor
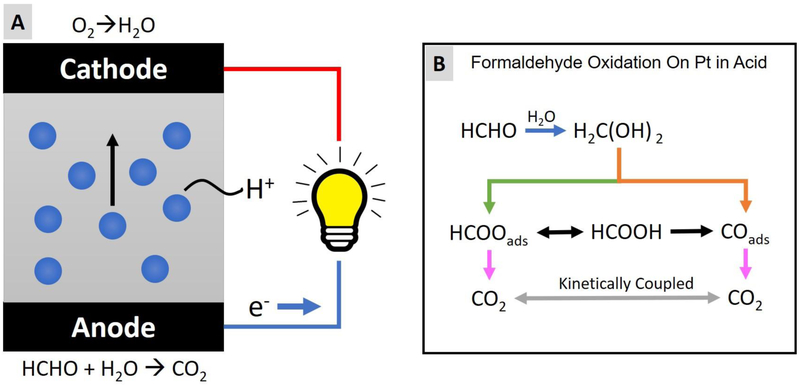
A fuel cell aldehyde sensor is a two-electrode amperometric electrochemical sensor, which converts chemical energy into electrical currents. The energy conversion is accomplished by a sandwich wafer structure (Figure 2.A): two outer layers (anode and cathode) of a fuel cell sensor are coated with powdered Platinum (Pt) catalyst materials and an intermediate aqueous acidic electrolyte layer is constructed for protons to pass through [42]. Aldehyde is oxidized at the anode (Figure 2.C) while oxygen is reduced at the cathode (Figure 2.B). The generated protons travel through the intermediate layer (a dilute aqueous acid electrolyte soaked into a porous plastic separator) to reach the cathode side and react with oxygen. If an electric load is connected between the anode and cathode, the generated electrons will flow through the external circuit to neutralize the system and complete the reaction. Consequently, an electrical current is generated.
Figure 2.
Working principle of a formaldehyde fuel cell sensor. (A) Illustration of the structure and working principle of a formaldehyde fuel cell sensor. (B) The reaction scheme of formaldehyde oxidation on platinum in acid.
The electrode reactions in a formaldehyde/Pt fuel-cell can be expressed as follows [43]:
Previous studies revealed that the electro-oxidation of formaldehyde on a platinum electrode in an acid medium have multiple pathways with various intermediate steps (Figure 2.B) [44–47]. As a result, the energy conversion efficiency is likely smaller than the formula shown. According to the manufacturer, the fuel cell sensor used in this work (Dart Sensors 2-FE5) on average yields two electrons for every formaldehyde molecule consumed. One possible hypothesis is that the reaction is dominated by the methylene glycol molecule which is formed when formaldehyde dissolves in water. Each methylene glycol molecule contains two —OH groups each releasing a hydrogen atom to the catalyst to react, while the rest portion of the molecule (H2CO2—) is oxidized non-electrochemically.
Besides various aldehyde gases, this fuel cell sensor also responds to other types of interfering gases. These undesirable responses to some of the common interfering substances have been tested by the manufacturer and listed in Table 1.
Table 1.
The cross-sensitivities of the fuel cell sensor to common interfering gases (provided by the manufacturer).
| Substance | Cross-Sensitivity (%) |
|---|---|
| CO | 1 |
| H2 | 0.1 |
| SO2 | 12 |
| Cl2 | −3 |
| NH3 | 0 |
| CO2 | 0 |
| Ethanol | 45 |
| Phenol | 7 |
| Water vapor | 0* |
Step changes in %RH produce short term transient response.
This wearable sensor operates in the diffusion regime, thus no sampling actuator (e.g. a pump) is required. Aldehyde gas present in the ambient environment diffuses into the fuel cell through an opening (2mm in diameter) on the sensor package. A porous hydrophobic membrane filter is placed above the opening to prevent liquid from getting into the sensor.
2.4. Temperature and Humidity Sensor
A fully calibrated digital temperature and relative humidity sensor (HTU21D) from TE Measurement Specialties is employed in the wearable sensor to continuously monitor the temperature and relative humidity levels in the ambient environment. Temperature and relative humidity are measured by internal transducers with accuracies of ±0.3°C and ±2%, respectively. The output signals are digitized by a built-in ADC and stored in the internal registers of the chip.
2.5. Electronic System Design
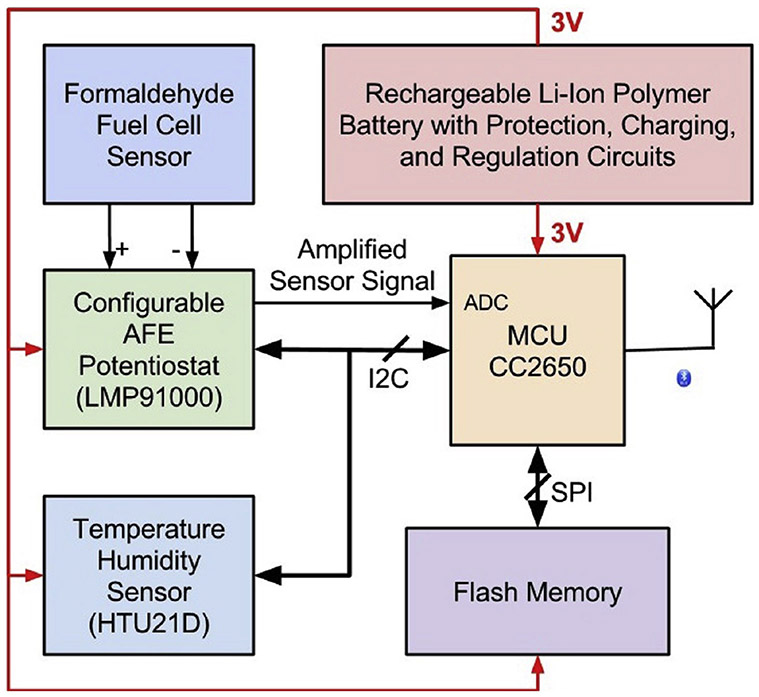
Figure 3 shows the complete block diagram of the electronic system. The core of the electronics system is an ultra-low power BLE-compatible microcontroller (Texas Instruments, CC2650F128RSM) with a footprint of 4 × 4 mm, which is programmed to control and monitor all peripheral devices in the system.
Figure 3.
Block diagram of the design of the electronic system.
A low-power configurable AFE potentiostat chip (LMP91000), designed to support various 2-lead and 3-lead electrochemical sensors, was employed to interface between the formaldehyde sensor and the built-in ADC in the microcontroller. The electrical current from the formaldehyde fuel cell sensor is amplified by the internal TIA with programmable gains and subsequently quantized by the ADC. This potentiostat chip can be easily configured to adapt to other electrochemical sensors in the future.
A low-power 1-megabyte flash memory device (MX25R8035F) was added to the system for data storage. At a sampling interval of 1 dataset/min, this flash memory can store over 100 days of data, which is sufficient for current applications. In the case the memory is full, the program automatically wraps to the beginning of the flash memory.
All electronic components were powered through a 3-volt voltage regulator (TPS78230) powered by a rechargeable lithium-ion polymer battery (DTP4015245) with built-in protection circuitry. Moreover, a lithium-ion polymer battery charging controller (MCP73831) was connected between the USB charging port and the lithium battery for safe charging.
2.6. Firmware Design
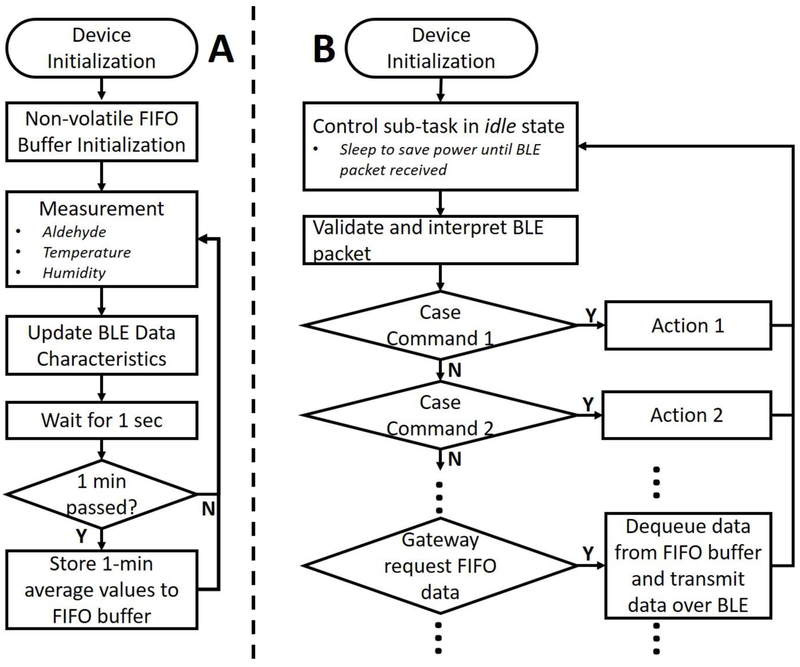
Besides the transducing mechanisms, two essential functions are required for a practical wearable sensing device: (1) sensor data acquisition and storage and (2) real-time and historical measurement data retrieval. The firmware was developed on TI BLE-Stack v2.2.x SDK with TI’s real-time operating system (RTOS). In the data acquisition task (Figure 4.A), measurement data was taken every second and stored in the on-chip random-access memory (RAM). Both digital (temperature and relative humidity) and analog (aldehyde fuel cell) sensors were used in this wearable device. Every second, the temperature and humidity sensor measurements were acquired, while the output current of the aldehyde fuel cell was amplified by a transimpedance amplifier and digitized with the built-in 12-bit ADC on the microcontroller.
Figure 4.
Simplified flowchart of the firmware design. (A) Flowchart for sensor data acquisition and storage. (B) Flowchart of the BLE communication and control.
The external flash memory was managed as a non-volatile FIFO buffer, where measurement data can be stored. To reduce memory usage, 1-minute consecutive measurement data was averaged before it was saved in the FIFO buffer. Additionally, local and UTC timestamps can be transmitted to the wearable sensor and stored in the FIFO buffer from a gateway device for synchronization. Historical data stored in the FIFO buffer can be retrieved by a gateway device using custom BLE commands (Figure 4.B).
2.7. Bluetooth-Low Energy Communication Protocols
BLE devices communicate using the Generic Attributes (GATT) Profile protocol, which is based on the Attribute protocol (ATT). Sensor information and measurement data are divided and encapsulated in logical entities called characteristics, and multiple characteristics are grouped into a service to serve a specific purpose.
A BLE service was designed and implemented for data transmission and device configuration. It consisted of five characteristics: one for transmitting general-purpose data (TX), one for receiving general-purpose data (RX), and the other three (DATA) contain sensor measurement data. TX and RX characteristics were configured to be 20-bytes in length, the maximum value allowed in BLE. A custom packet-based communication protocol was designed for sensor configuration and historical data retrieval. Data characteristics were defined to store measurement data (temperature, relative humidity, and aldehyde level) in single-precision floating-point format (4-bytes) and updated every second. In future developments, the number of data characteristics can be increased, and the sampling frequency can be reconfigured based on the requirements to support other sensing modalities.
2.8. Aldehyde Sensor Calibration
To evaluate the responsivity and baseline at different temperatures, a calibration experiment setup (Figure 5) was designed and built to test the fully assembled prototype device.
Figure 5.
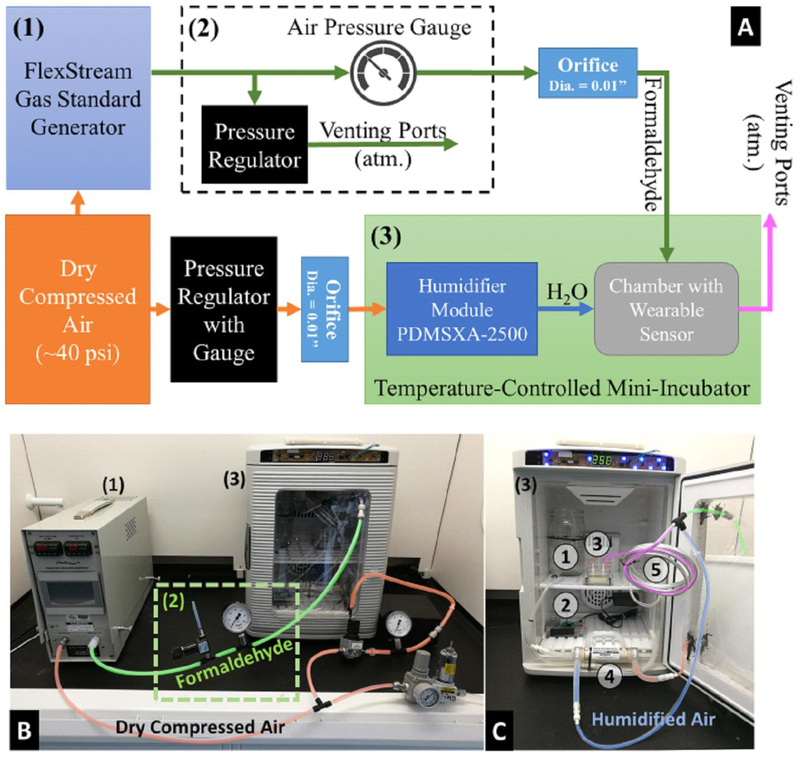
Calibration Setup. (A) Block diagram of calibration setup. (B) Overview of the calibration setup in a fume hood. (C) Internal view of the temperature-controlled mini-incubator. ① Pure water to humidify the dry compressed air. ② Raspberry Pi 3 Model B for data acquisition. ③ Test chamber with wearable sensor inside. ④ PermSelect® Silicone membrane module (PDMSXA-2500). ⑤ Long aluminum tubing for gas mixing and equalizing the temperature of the gases generated by the gas standard generator.
A certified gas standard generator (Kin-Tek FlexStream™ Base Module) was utilized to generate a formaldehyde-air mixture of know concentrations using a permeation tube method. The output formaldehyde level was determined from the output flow rate of gases derived from a compressed air source (~40 psi). To limit the rate of gas flow supplied to the test chamber, the output port of the machine was connected to a pressure relieving regulator, an air-pressure gauge and a 0.01-inch orifice (Figure 5.2).
In addition, the formaldehyde fuel cell sensor requires a relative humidity range of 15–95%, while no humidity is allowed in the gas standard generator. Based on this, a custom humidifier was built using a PermSelect® Silicone membrane module (PDMSXA-2500). Air flow was derived from the same compressed air, regulated by a pressure regulator and 0.01-inch orifice, and fed into the humidifier module.
Both the formaldehyde-air mixture and humidified air were fed to one tube for mixing and directed into a test chamber holding the wearable sensor. The test chamber and the humidifier module were placed in a modified temperature-controlled mini-incubator (Benchmark Scientific, H2200-HC, Figure 5.3) for temperature control.
2.9. Sensor Validation and Reproducibility Tests
Calibration parameters were stored in the on-chip non-volatile flash memory, converting the sensor measurement from ADC readings (arbitrary unit, a.u.) to corresponding ambient aldehyde concentration (parts-per-billion, ppb). Later, two more experiments were conducted to validate the sensor performance; one was performed with standard gas generated at five different concentrations to validate the accuracy of the calibrated sensor measurement, and the other was carried out with standard gas generated at a certain level (206 ppb) for multiple times to examine the reproducibility of the sensor output.
3. Results
3.1. Electronics System
A standard FR-4 four-layer printed circuit board (PCB) was designed and fabricated with a footprint of 16 × 28 × 1.6 mm (excluding the formaldehyde fuel cell sensor and lithium battery). Once the PCB was received, electronic components were manually populated and tested in the lab.
Firmware was developed using a prototype device built with evaluation modules and breadboards. The firmware image was then loaded into a freshly fabricated sensor. Finally, power consumption was benchmarked using a benchtop power supply (Agilent E3631A) and a digital multimeter (Keithley 2000), typically below 400uA at 4V. Other functions (e.g. BLE communication, data acquisition, data retrieval, etc.) were tested with the help of a Linux-based personal computer and dedicated testing programs implemented in Python 3 using the bluepy library.
3.2. Fuel Cell Sensor Package
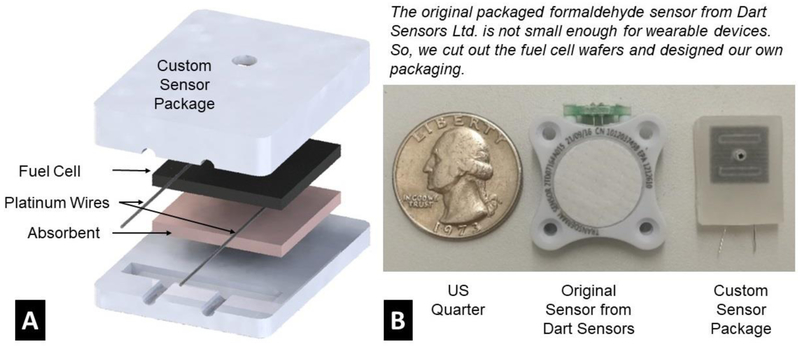
The commercial formaldehyde fuel cell sensor (Dart Sensors Ltd.) comes with a robust package, whose footprint (24 × 27 × 6 mm) is too big to fit into a smartwatch form with other electronic components. However, the core of the fuel cell sensor is two wafers with dimensions of 11 × 11 × 1 mm. These smaller wafers allow re-packaging of the sensor to meet the size requirements.
Custom sensor packages were fabricated with polypropylene (PP) in two pieces (Figure 6). Both pieces were milled out of a polypropylene sheet of 1/8-inch thickness using a computer-controlled Benchtop CNC machine (Roland, MDX-40a). A slot of 11.3 × 11.3 × 2.5 mm was milled in the top piece to hold the fuel cell sensors, and a ventilation port of 2 mm in diameter was opened in the center of the slot, permitting ambient air to diffuse to the surface of the sensor. The bottom piece was a rigid polypropylene sheet that matched the outline of the top piece, keeping the fuel cell wafers in place. After assembly, the top and bottom pieces were welded together around the edges using a soldering iron heated to 350°C.
Figure 6.
Fuel Cell Sensor Package. (A) 3D illustration of custom fuel cell packaging. (B) Comparing the footprints of a custom packaged sensor, an original sensor, and a US quarter.
Two platinum wires were embedded in the enclosure to contact the anode and cathode of the fuel cell. The other ends of the wires were later soldered onto an assembled and tested PCB to form electrical connectivity for data acquisition.
3.3. Wearable Device Package and Assembly
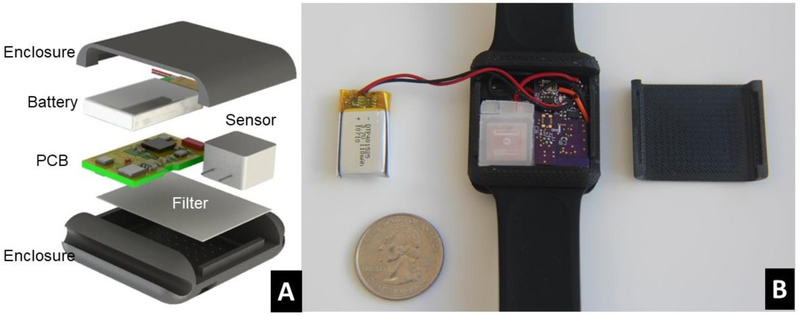
A smart watch enclosure (Figure 7.A) with an array of small venting ports (1mm diameter) on the top surface was designed in SolidWorks and prototyped using a high-resolution 3D printer. A porous hydrophobic filter made of polypropylene was attached to the inner surface of the enclosure with adhesives, preventing water from getting into the device and reducing the influence of convection air flows on the fuel cell sensors. The electronic components (PCB, battery, fuel cell sensor) were then assembled into the enclosure. The back cover of the enclosure was then fixed and glued onto the top cover to form good sealing.
Figure 7.
Wearable Sensor Enclosure Design and Assembly. (A) 3D illustration of the design of our wearable sensor. (B) An assembled prototype wearable sensor.
A groove was designed on each side of the enclosure, compatible with a wide range of quick release silicone-based bands for smart watches. At the final assembly stage, a pair of smart watch bands were glued onto the wearable device.
Prototype wearable sensors (Figure 7.B) were fabricated and tested in a lab environment. The average power consumption measured was approximately 900 μW (3V * 300 μA). The total weight of the device is about 15g (excluding watch bands).
3.4. Sensor Calibration and Validation
After a prototype wearable sensor was assembled and tested, the device was calibrated using the experiment setup described in Figure 5. Measurement data (temperature, relative humidity, and aldehyde level) was continuously collected by an external single-board Linux-based computer (Raspberry Pi 3 Model B) via BLE wireless communication sampling at a frequency of 1 Hz. The transferred data was then time-stamped and stored in the mini-computer in CSV format. After every experiment, the acquired measurement data was downloaded to a PC and analyzed with MATLAB.
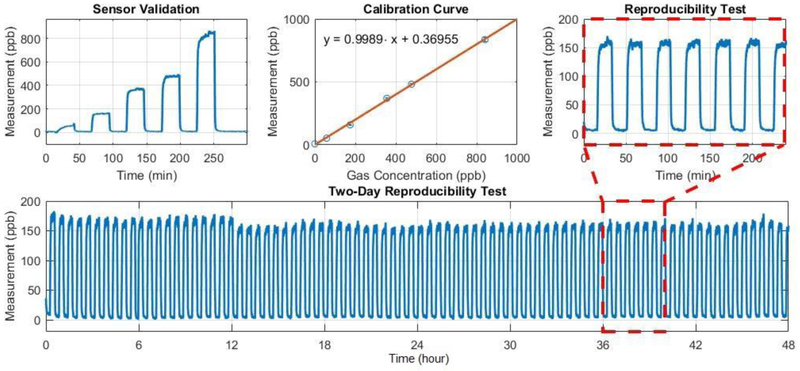
After updating the calibration parameters, sensor validation and reproducibility, experiments were conducted to verify the performance of the calibrated wearable sensor. Figure 8 shows a sample result from a calibrated prototype device. The sensor measurement data was consistent with the gas concentration generated by the calibration setup. An extended reproducibility test was conducted for two days. The overall standard deviations of the measurement results were 8.2 ppb (gas on) and 6.2 ppb (gas off), respectively. This should be sufficient for our current application.
Figure 8.
Sample measurement data from a calibrated wearable sensor prototype.
3.5. Smartphone App – User-Interface and Gateway to Cloud
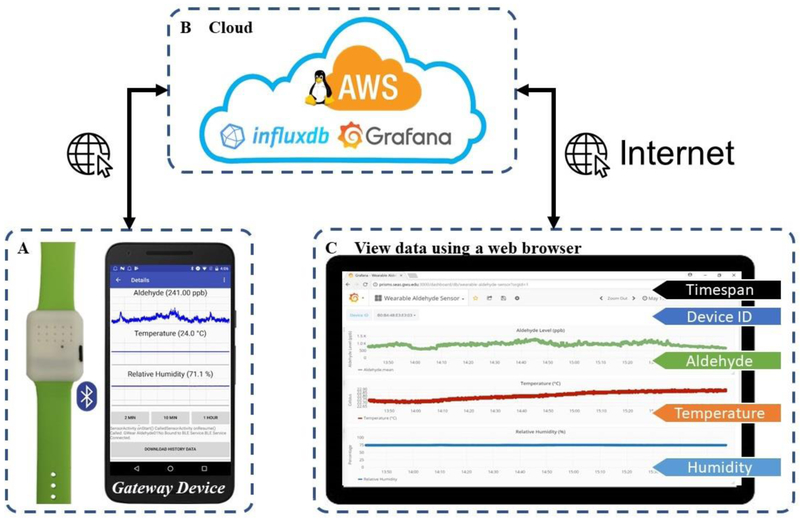
An Android-based smartphone application (Figure 9.A) was developed to demonstrate a proof-of-concept gateway device. This application has four major functions: (1) discover and list nearby BLE-enabled devices, (2) display and plot incoming BLE data packets in real-time when connected to a wearable device, (3) retrieve historical data from the wearable device, and (4) synchronize recorded data with a cloud-based database system for data analytics.
Figure 9.
Demonstration of the wearable IoT sensor system. (A) a prototype wearable sensor and a Google Nexus 5X smartphone with the proof-of-concept Android app and internet connectivity, (B) a cloud-based informatics system for centralized data management deployed on an Amazon Web Services (AWS) EC2 Server, (C) a Google Chrome web browser displaying the uploaded data.
Once a wearable device is connected to the smartphone app, real-time measurement data (temperature, relative humidity, and formaldehyde level) will be acquired by the smartphone via BLE characteristics at a frequency of 1 Hz. A button is provided for the user to retrieve historical data stored on the wearable sensor. The downloaded data will be time-stamped, stored in the memory of the smartphone. Three time series graphs, based on an open-source graph plotting library (GraphView), were provided on the graphical user interface (GUI) of the app to display the measurement data.
When internet is available, the stored measurement data will be uploaded to the cloud database system (InfluxDB, details in Section 3.6) using Influxdb-java library [48]. This client library utilizes the InfluxDB HTTP API to communicate with the database server, in which information is organized in JSON format and transmitted to the server over the internet. The app uses the IP address of the database server, username, and password for connection and authentication. During operation, a measurement from one transducer will be inserted as an individual database entry, with its timestamp and other necessary information (details in Section 3.6). The data entries are temporarily stored in an internal buffer in the RAM of the smartphone and flushed to the server as a batch every 10 seconds to reduce communication overhead.
3.6. Cloud-based Informatics System
A cloud-based informatics system (Figure 9.B) was implemented using Amazon Web Services (AWS). An Ubuntu Server 16.04 image was installed in an Elastic Compute Cloud (EC2) t2.micro instance with an 8GB Elastic Block Storage (EBS) general purpose SSD volume (gp2) for general data storage. This virtual server was managed remotely with secure shell (SSH). Two open-source software packages, InfluxDB and Grafana, were deployed to store sensor measurement data and provide necessary analytics functions.
InfluxDB is an open-source database designed for managing time series data for applications like IoT, DevOps, and data analytics, etc. [49]. Administrator username and password for InfluxDB were configured during installation, and a time-series database for development and demonstration is created afterwards. Every entry in the database represents a measurement from one transducer at a specific time, which consists of five essential fields: (1) time-stamp, (2) MAC address of the BLE wearable device, (3) name of the transducer, (4) measurement value, and (5) unit of measurement. The database entries are stored on the EBS volume, allowing the storage space to be scaled as the amount of data increases.
A web-based data visualization and management system was implemented with Grafana. It is an open-source software for web-based time series data analytics and monitoring [50,51], which supports various data sources (including InfluxDB) and has a built-in web server with configurable dashboards for data visualization. A dashboard (Figure 9.C) was configured to display uploaded measurement data, identified by wearable device ID (BLE MAC address) and time span, in three time plots. This dashboard can refresh at a frequency of 5 seconds to display uploaded data timely.
To track the cloud system status and performance, various metrics of the EC2 instance are monitored using Amazon CloudWatch. Nine metrics involving CPU usage, networking, and storage are monitored: CPUUtilization, CPUCreditUsage, NetworkIn, NetworkOut, StatusCheckFailed_Instance, StatusCheckFailed System, DiskWriteBytes, and DiskReadBytes. By monitoring StatusCheckFailed, Amazon CloudWatch can automatically restart the EC2 instance to minimize system down-time when it crashes.
EC2 instances can be connected through either its public DNS, public IP, or private IP. When the EC2 instance is relaunched or restarted, these addresses are changed, which can cause problems with reconnecting the informatics system. To address this issue, the EC2 instance is mapped to an Elastic IP address [52], a static public IP address that is automatically mapped to the instance’s IP. This ensures that the IP of the instance is always known, including when the instance’s IP is changed due to an instance failure. The subdomain prisms.seas.gwu.edu is set to point to the Elastic IP address.
Our sensors are also compatible with the informatics systems being developed in the NIH PRISMS project [51].
3.7. Tobacco Smoke Sensing
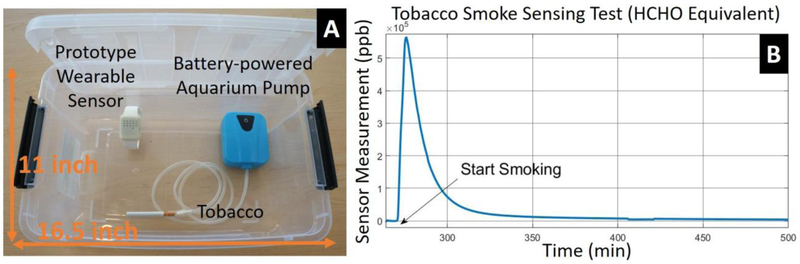
One potential application of the wearable aldehyde sensor is environmental tobacco smoke detection and quantification due to the aldehyde and methanol content presented in tobacco smoke [53]. An experimental setup was built (Figure 10.A) to quantify the sensor response to tobacco smoke and study its potential as a tobacco smoke sensor. A 12-Quart plastic storage box with a cover was used as the test chamber. A prototype wearable sensor was placed inside the test chamber with a cigarette and a battery-powered aquarium pump. Tobacco smoke was then generated by burning the cigarette. A constant air flow was provided by the pump to pass through the cigarette to facilitate the burning process. The test chamber was placed in a chemical fume hood and a 1/4-inch diameter hole was drilled on the cover of the chamber for ventilation.
Figure 10.
Tobacco smoke sensing test in a plastic box with cover. (A) Experiment setup. A prototype wearable device is placed inside a plastic box (16.49"L × 10.98"W × 6.53"H) with a cigarette continuously driven by air flow generated by a battery-powered aquarium pump. A venting port (1/4-inch diameter) was drilled on the plastic cover for slow ventilation. This setup was placed in a fume hood during experiment. (B) A sample result from tobacco smoke sensing test retrieved from the cloud.
Figure 10.B shows typical measurement data from one such tobacco smoke sensing test. The cigarette was consumed in 1 minute after the experiment began, and the sensor output reached its peak value in about 5 minutes, followed by an exponential decay as tobacco smoke diffused out of the chamber.
The sensor reading reached around 600 ppm at its peak, which is equivalent to 200 ppb in a 140 square-feet room (assuming 2.5 meters tall). In real-world settings, ventilation and air-conditioning may significantly lower this concentration. However, one previous study showed that formaldehyde concentration can reach 250ppb in a poorly ventilated room after 10-15 cigarette smoke [9], which is in the detectable range of our sensor.
4. Discussion
Specificity has been one of the major challenges for low-power miniature gas sensors [54]. The formaldehyde fuel cell sensor used in this work has measurable responses to various aldehydes and other interference substances, such as alcohol and SO2. Its specificity is mainly determined by the catalysts coated on the surfaces of the fuel cell. Other types of miniature gas sensors, such as metal oxide sensors, also suffer specificity issues [55]. Although various techniques have been developed to increase the specificity and selectivity of gas sensors [54–57], it is still one of the biggest challenges for miniature gas sensors. One possible method to improve specificity is to incorporate multiple sensing modalities working under different sensing principles to increase the dimensionality of the measurement data and apply signal processing techniques (e.g. principle component analysis) to distinguish and quantify various gas contents [58,59].
Although our custom packaged fuel cell sensor was calibrated with a standard gas generator and the results were consistent across various experiments, the wearable sensors exhibited baseline fluctuations during real-world on-wrist tests. Four possible causes may contribute to these fluctuations: (1) the fluctuation of the temperature gradient across the fuel cell sensor thickness [60], (2) movement-induced air convection, (3) motion artifacts, and (4) electromagnetic interference (EMI). The first one appeared to have the most significant effect in our field tests. This thermal gradient variation and potential EMI can be solved by encapsulating the fuel cell sensor in a grounded metallic enclosure. The thermal gradient effect can also be compensated in software by measuring it with two temperature sensors. Sources (2) and (3) can be reduced by smaller pore size membrane filters and more secure mechanical fit designs, respectively. Based on our extensive tests, we found that it is critical to construct the fuel cell sensor package with a proper thermal design, minimizing the temperature gradient between the two sensor electrodes. For instance, wrapping the sensor with a thick layer of thermal insulation material can significantly reduce the fluctuations in on-body deployment tests.
The fuel cell sensors have transient responses to fast changes in ambient relative humidity. Our experiment results showed that it generally took 1-5 min for the sensor to recover. According to the manufacturer, this is likely caused by thermal effects, where excessive water molecules dissolved into the acid-containing proton exchange membrane and generated heat. These transient responses can be removed in post-processing, based on relative humidity data. Further studies are required to validate if such transient responses can be corrected with the relative humidity measurements.
We also realized that some of the chemical residue on the circuit board introduced during fabrication can evaporate and interact with the fuel cell sensors, generating a baseline shift. Also, the releasing rate depends on temperature, which makes the baseline shift change significantly with temperature. We have reduced this baseline shift by soaking the PCB in ethanol (99%) for 1 hour, thoroughly brushing it under running pure water, and drying it using compressed air. In future designs, it would be preferable to have the sensor air pathway isolated from the circuit board to minimize the residue effects.
Despite these challenges, fuel cell sensors have many advantages as a sensing modality for wearable gas monitors. For example, the sensing element used in this work has a small footprint (11 × 11 × 2 mm) and does not require a power supply for operation. The lower limit of detection (LLOD) can reach 15-30 ppb in a well-controlled (lab) environment. The performance of each individual sensor is consistent over an extended period (months). Further, the platinum-based fuel cell sensor only responds to substances that can be reduced or oxidized at a platinum electrode, which intrinsically provides certain specificity [61]. In our field tests, the major source of interference observed was due to various types of alcohols.
When combined with other wearable environmental sensors [62–65] and physiological sensors such as a wheezing detection patch or a spirometer, this wearable IoT sensor platform can provide objective exposure data correlated with asthma symptoms and physiological responses. It can also be used in a citizen-science environmental monitoring system to map air pollution at much higher spatial and temporal resolutions than currently available. Other applications include new house and vehicle inspections and furniture selections which may benefit from on-site formaldehyde sensing.
The service life of the fuel cell sensor is 3 years as listed in its datasheet. We have fabricated and tested several sensors for over a year and they still function today. Regarding the long-term stability and sensor responsivity, it is a general practice to recalibrate a sensor every 3 to 6 months. We did not notice degradation in sensor responsivity within 1 month of normal usage.
Our current design has limitations in tobacco smoke sensing and quantification, since it is very unlikely to characterize a source that generate thousands of gases with only one sensor. Moreover, aldehydes and methanol only account for a small portion of the chemical composition of tobacco smoke [53]. Future studies could incorporate other sensing modalities and use signal processing techniques such as principle component analysis and machine learning-based clustering algorithms to determine the potential to increase both sensitivity and specificity of tobacco smoke sensing. Other sensing modalities to be explored could include metal oxide sensors, particulate matter sensors, voltammetry techniques, temporal profiles of sensor responses, and gas-specific absorbing layers.
5. Conclusion
In this paper, we describe a personalized wearable IoT sensor platform for monitoring ambient aldehyde level. A self-contained wearable aldehyde sensing device with the size comparable to a smart watch was designed and built using commercial aldehyde fuel cell sensors and state-of-the-art low-power electronics. The average power consumption measured was 900μW, enabling the device to last over a week with a fully charged 100mAh lithium battery. The prototype devices were calibrated in the laboratory with a permeation tube based standard gas generator. The lower limit of detection (LLOD) was 30ppb and upper detection limit was larger than 10ppm. A dedicated Android App was developed on a Google Nexus 5X smartphone, serving as a gateway to wirelessly retrieve sensor data and upload it to a cloud-based informatics system implemented on AWS for storage, visualization and analysis. Further engineering improvements are necessary to minimize the fluctuations of the fuel cell sensor due to temperature gradient changes in on-body deployments.
We envision this wearable environmental IoT sensor platform will be used by physicians and researchers to continuously monitor the aldehyde exposure level of asthma patients over time. The aldehyde exposure levels can be correlated with asthma attack events and various symptoms to discover any possible relationship. In the future, this system can potentially be applied for personalized asthma management if a causal relation is identified between aldehyde exposure and asthma exacerbations for a given individual.
Highlights.
A self-contained wearable sensor was designed, prototyped and tested to continuously monitor temperature, relative humidity, and aldehyde level.
The lower limit of detection (LLoD) of aldehyde level is smaller than 30ppb in lab environment.
A smartphone app was developed to retrieve sensor data and upload it to a cloud-based analytics system.
Further efforts are needed to minimize the fluctuation of the fuel cell sensors in on-body deployments.
Acknowledgements
Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number U01EB021986. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Dr. Walter King, Dr. Katherine Sward, Dr. Neal Patwari, and Philip Lundrigan for helpful discussions.
Biography
Baichen Li completed his Master's degree in Biomedical Engineering at The George Washington University (GWU) in 2013. He worked in a medical device startup as an electrical engineer before he returned to pursue a Ph.D. degree in 2015 at GWU. Currently, he is a doctoral student in the Nanophotonics and Microfluidics Lab at GWU, conducting research on wearable sensors, wireless sensor network, point-of-care diagnostic devices, and microfluidics.
Quan Dong is a Ph.D. candidate at Biomedical Engineering Department, George Washington University. He started his Ph.D. study in 2013. The subject of his research is development of wearable medical devices, ambulatory monitoring devices, and microfluidics devices.
Scott Downen worked in industry for a medical device design consulting company as a design engineer before returning to complete his Master's in Biomedical Engineering at The George Washington University in 2017. His research focused on using machine vision to determine human emotional state using machine learning methodologies. He is currently working on his Ph.D. at The George Washington University with a focus in wearable electronic sensors, wireless sensor networks, and microfluidics.
Nam Tran is pursuing a BS degree in Biomedical Engineering at The George Washington University. His research contribution currently focuses on integrating biomedical sensors into a cloud architecture.
James Hunter Jackson is a Clinical Research Coordinator at Children's National Medical Center, providing comprehensive education of asthma pathophysiology and medication administration in an outpatient clinical setting. He earned M.S. in Biochemistry & Molecular Biology at Georgetown University in 2015.
Dinesh Pillai, MD is a board-certified pediatrician and pediatric pulmonologist in the Division of Pulmonary Medicine at Children’s National Health System (CNHS), an Associate Professor in the Departments of Pediatrics and Genomics & Precision Medicine at George Washington University (Washington, DC) and an investigator in the Center for Genetic Medicine Research (CNHS). His basic science and translational research interest centers around airway epithelium related mechanisms involved in disease development and response to treatment for asthma. Dr. Pillai also serves as site Co-Investigator for the multicenter Inner-City Asthma Consortium. Clinically, he has developed a severe asthma clinic focused on treating children with refractory asthma. Overall, Dr. Pillai’s work has led to the development of a comprehensive Severe Asthma Program at Children’s National with a systems biology approach to treating and evaluating disease mechanisms in severe asthma.
Mona Zaghloul earned PhD and MASc in Electrical Engineering and MMath in Applied Analysis and Computer Science from University of Waterloo, Canada. She is a National Academy of Inventor (NAI) Fellow and a IEEE life Fellow. She published more than 100 journal papers, 300 conferences papers, 3 books and 5 book chapters. She is the Director of the Institute of MEMS and VLSI Technology, at the George Washington University, Washington DC. Dr. Zaghloul has received numerous awards including the IEEE Circuits and Systems Jubilee Golden Medal for outstanding contribution to the IEEE Circuits and Systems Society, May 2000; she was the IEEE Sensors Council President (2009-2010). She received an Honorary Doctor Degree from University Waterloo in 2007 in recognition of academic career in the international electrical engineering community, presented to her at the celebration of the University’s 50th anniversary.
Zhenyu Li is an Associate Professor of Biomedical Engineering at The George Washington University. He received the Ph.D. degree in Electrical Engineering in 2008 from the California Institute of Technology. His current research focuses on the development of biosensors and medical devices using micro and nanotechnology, namely microfluidics, MEMS, Optofluidics and flexible electronics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN, Vital Signs: Asthma in Children — United States, 2001–2016, MMWR. Morb. Mortal. Wkly. Rep 67 (2018) 149–155. doi: 10.15585/mmwr.mm6705e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].The Centers for Disease Control and Prevention, Asthma-related Missed School Days among Children aged 5–17 Years., 2015. https://www.cdc.gov/asthma/asthma_stats/AstStatChild_Missed_School_Days.pdf (accessed May 29, 2018).
- [3].Weiss KB, Sullivan SD, Lyttle CS, Trends in the cost of illness for asthma in the United States, 1985–1994, J. Allergy Clin. Immunol 106 (2000) 493–499. doi: 10.1067/mai.2000.109426. [DOI] [PubMed] [Google Scholar]
- [4].Patel MM, Miller RL, Air pollution and childhood asthma: recent advances and future directions., Curr. Opin. Pediatr 21 (2009) 235–42. doi: 10.1097/MOP.0B013E3283267726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Diette GB, McCormack MC, Hansel NN, Breysse PN, Matsui EC, Environmental issues in managing asthma., Respir. Care 53 (2008) 602–15; discussion 616–7. [PMC free article] [PubMed] [Google Scholar]
- [6].Gilmour MI, Jaakkola MS, London SJ, Nel AE, Rogers CA, How exposure to environmental tobacco smoke, outdoor air pollutants, and increased pollen burdens influences the incidence of asthma., Environ. Health Perspect 114 (2006) 627–33. doi: 10.1289/EHP.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Salthammer T, Mentese S, Marutzky R, Formaldehyde in the Indoor Environment, Chem. Rev 110 (2010) 2536–2572. doi: 10.1021/cr800399g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lofroth G, Burton RM, Forehand L, Hammond SK, Seila RL, Zweidinger RB, Lewtas J ’, Characterization of Environmental Tobacco Smoke, Environ. Sci. Techno 23 (1989) 610–614. doi: 10.1021/es00063a015. [DOI] [Google Scholar]
- [9].Schaller KH, Triebig G, Beyer B, Formaldehyde determination in tobacco smoke--studies under experimental and actual conditions., Zentralblatt Fur Hyg. Und Umweltmedizin Int. J. Hyg. Environ. Med 189 (1989) 103–10. [PubMed] [Google Scholar]
- [10].Godish T, Formaldehyde exposures from tobacco smoke: a review., Am. J. Public Health 79 (1989) 1044–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baker RR, The generation of formaldehyde in cigarettes—Overview and recent experiments, Food Chem. Toxicol 44 (2006) 1799–1822. doi: 10.1016/j.fct.2006.05.017. [DOI] [PubMed] [Google Scholar]
- [12].Ogunwale MA, Li M, Ramakrishnam Raju MV, Chen Y, Nantz MH, Conklin DJ, Fu X-A, Aldehyde Detection in Electronic Cigarette Aerosols, ACS Omega. 2 (2017) 1207–1214. doi: 10.1021/acsomega.6b00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Quackenboss JJ, Lebowitz MD, Michaud JP, Bronnimann D, Formaldehyde exposure and acute health effects study, Environ. Int 15 (1989) 169–176. doi: 10.1016/0160-4120(89)90023-8. [DOI] [Google Scholar]
- [14].Wieslander G, Norbäck D, Björnsson E, Janson C, Boman G, Asthma and the indoor environment: the significance of emission of formaldehyde and volatile organic compounds from newly painted indoor surfaces, Int. Arch. Occup. Environ. Health 69 (1996) 115–124. doi: 10.1007/s004200050125. [DOI] [PubMed] [Google Scholar]
- [15].Annesi-Maesano I, Hulin M, Lavaud F, Raherison C, Kopferschmitt C, de Blay F, Charpin DA, Denis C, Poor air quality in classrooms related to asthma and rhinitis in primary schoolchildren of the French 6 Cities Study., Thorax. 67 (2012) 682–8. doi: 10.1136/thoraxjnl-2011-200391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McGwin G, Lienert J, Kennedy JI, Kennedy JI Jr., Formaldehyde exposure and asthma in children: a systematic review., Environ. Health Perspect 118 (2010) 313–7. doi: 10.1289/ehp.0901143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li Y-F, Gilliland FD, Peters JM, Effects of Maternal Smoking during Pregnancy and Environmental Tobacco Smoke on Asthma and Wheezing in Children, Am J Respir Crit Care Med Internet. 163 (2001) 429–436. doi: 10.1164/ajrccm.163.2.2006009. [DOI] [PubMed] [Google Scholar]
- [18].Larsson ML, Frisk M, Hallström J, Kiviloog J, Lundbäck B, Environmental Tobacco Smoke Exposure During Childhood Is Associated With Increased Prevalence of Asthma in Adults, Chest. 120 (2001) 711–717. doi: 10.1378/chest.120.3.711. [DOI] [PubMed] [Google Scholar]
- [19].Chilmonczyk BA, Salmun LM, Megathlin KN, Neveux LM, Palomaki GE, Knight GJ, Pulkkinen AJ, Haddow JE, Association between Exposure to Environmental Tobacco Smoke and Exacerbations of Asthma in Children, N. Engl. J. Med 328 (1993) 1665–1669. doi: 10.1056/NEJM199306103282303. [DOI] [PubMed] [Google Scholar]
- [20].Pandey SK, Kim K-H, A review of environmental tobacco smoke and its determination in air, TrAC Trends Anal. Chem 29 (2010) 804–819. doi: 10.1016/J.TRAC.2010.04.014. [DOI] [Google Scholar]
- [21].Nordman H, Keskinen H, Tuppurainen M, Formaldehyde asthma--rare or overlooked?, J. Allergy Clin. Immunol 75 (1985) 91–9. doi: 10.1016/0091-6749(85)90018-1. [DOI] [PubMed] [Google Scholar]
- [22].Hendrick DJ, Rando RJ, Lane DJ, Morris MJ, Formaldehyde asthma: challenge exposure levels and fate after five years., J. Occup. Med 24 (1982) 893–7. [PubMed] [Google Scholar]
- [23].Dirksen JA, Duval K, Ring TA, NiO thin-film formaldehyde gas sensor, Sensors Actuators B Chem. 80 (2001) 106–115. doi: 10.1016/S0925-4005(01)00898-X. [DOI] [Google Scholar]
- [24].Chung P-R, Tzeng C-T, Ke M-T, Lee C-Y, Formaldehyde Gas Sensors: A Review, Sensors. 13 (2013) 4468–4484. doi: 10.3390/s130404468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Qin X, Wang R, Tsow F, Forzani E, Xian X, Tao N, A Colorimetric Chemical Sensing Platform for Real-Time Monitoring of Indoor Formaldehyde, IEEE Sens. J 15 (2015) 1545–1551. doi: 10.1109/JSEN.2014.2364142. [DOI] [Google Scholar]
- [26].Riazul Islam SM, Kwak Daehan, Humaun Kabir M, Hossain M, Kwak Kyung-Sup, The Internet of Things for Health Care: A Comprehensive Survey, IEEE Access. 3 (2015) 678–708. doi: 10.1109/ACCESS.2015.2437951. [DOI] [Google Scholar]
- [27].Dimitrov DV, Medical Internet of Things and Big Data in Healthcare., Healthc. Inform. Res 22 (2016) 156–63. doi: 10.4258/hir.2016.22.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Monowar Hossain S, Hnat T, Saleheen N, Jahan Nasrin N, Noor J, Ho B-J, Condie T, Srivastava M, Kumar S, Jahan Nas-rin N, mCerebrum: A Mobile Sensing Software Platform for Development and Validation of Digital Biomarkers and Interventions, in: SenSys ’17 Proc. 15th ACM Conf. Embed. Netw. Sens. Syst Artic. No. 7, 2017. doi: 10.1145/3131672.3131694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Saleheen N, Ali AA, Hossain SM, Sarker H, Chatterjee S, Marlin B, Ertin E, Al’Absi M, Kumar S, puffMarker: A Multi-Sensor Approach for Pinpointing the Timing of First Lapse in Smoking Cessation., in: Proc. ACM Int. Conf. Ubiquitous Comput . UbiComp, NIH Public Access, 2015:pp.999–1010. [PMC free article] [PubMed] [Google Scholar]
- [30].Ko J, Lu C, Srivastava MB, Stankovic JA, Terzis A, Welsh M, Wireless Sensor Networks for Healthcare, Proc. IEEE 98 (2010) 1947–1960. doi: 10.1109/JPROC.2010.2065210. [DOI] [Google Scholar]
- [31].Chan Y-FY, Wang P, Rogers L, Tignor N, Zweig M, Hershman SG, Genes N, Scott ER, Krock E, Badgeley M, Edgar R, Violante S, Wright R, Powell CA, Dudley JT, Schadt EE, The Asthma Mobile Health Study, a large-scale clinical observational study using ResearchKit, Nat. Biotechnol 35 (2017) 354–362. doi: 10.1038/nbt.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lara OD, Labrador MA, A Survey on Human Activity Recognition using Wearable Sensors, IEEE Commun. Surv. Tutorials 15 (2013) 1192–1209. doi: 10.1109/SURV.2012.110112.00192. [DOI] [Google Scholar]
- [33].Patel S, Park H, Bonato P, Chan L, Rodgers M, A review of wearable sensors and systems with application in rehabilitation, J. Neuroeng. Rehabil 9 (2012) 21. doi: 10.1186/1743-0003-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wilhelm E, Siby S, Zhou Y, Ashok XJS, Jayasuriya M, Foong S, Kee J, Wood KL, Tippenhauer NO, Wearable Environmental Sensors and Infrastructure for Mobile Large-Scale Urban Deployment, IEEE Sens. J 16 (2016) 8111–8123. doi: 10.1109/JSEN.2016.2603158. [DOI] [Google Scholar]
- [35].Occupational Safety and Health Administration, Formaldehyde, OSHA Factsheet. (2011). https://www.osha.gov/OshDoc/data_General_Facts/formaldehyde-factsheet.pdf (accessed June 1, 2018). [Google Scholar]
- [36].Paustenbach D, Alarie Y, Kulle T, Schachter N, Smith R, Swenberg J, Witschi H, Horowitz SB, A recommended occupational exposure limit for formaldehyde based on irritation., J. Toxicol. Environ. Health 50 (1997) 217–63. [PubMed] [Google Scholar]
- [37].United States Environmental Protection Agency, Formaldehyde, 2000. https://www.epa.gov/sites/production/files/2016-09/documents/formaldehyde.pdf (accessed May 29, 2018).
- [38].Golden R, Identifying an indoor air exposure limit for formaldehyde considering both irritation and cancer hazards., Crit. Rev. Toxicol 41 (2011) 672–721. doi: 10.3109/10408444.2011.573467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dosman JA, Hodgson WC, Cockcroft DW, Effect of Cold Air on the Bronchial Response to Inhaled Histamine in Patients with Asthma, Am. Rev. Respir. Dis 144 (1991) 45–50. doi: 10.1164/ajrccm/144.1.45. [DOI] [PubMed] [Google Scholar]
- [40].Strauss RH, McFadden ER, Ingram RH, Deal EC, Jaeger JJ, Influence of heat and humidity on the airway obstruction induced by exercise in asthma., J. Clin. Invest 61 (1978) 433–40. doi: 10.1172/JCI108954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gomez C, Oller J, Paradells J, Overview and Evaluation of Bluetooth Low Energy: An Emerging Low-Power Wireless Technology, Sensors. 12 (2012) 11734–11753. doi: 10.3390/s120911734. [DOI] [Google Scholar]
- [42].King Walter John, Electrochemical sensors, US20090194417A1, 2009. https://patents.google.com/patent/US20090194417 (accessed June 8, 2018).
- [43].Sun W, Sun G, Qin B, Xin Q, A fuel-cell-type sensor for detection of formaldehyde in aqueous solution, Sensors Actuators B Chem. 128 (2007) 193–198. doi: 10.1016/J.SNB.2007.06.002. [DOI] [Google Scholar]
- [44].Guthrie JP, Carbonyl Addition Reactions: Factors Affecting the Hy drate-Hemiacetal and Hemiacetal-Acetal Equilibrium Constants, Can. J. Chem 53 (1975) 898–906. [Google Scholar]
- [45].Samjeské G, Miki A, Osawa M, Electrocatalytic oxidation of formaldehyde on platinum under galvanostatic and potential sweep conditions studied by time-resolved surface-enhanced infrared spectroscopy, J. Phys. Chem. C 111 (2007) 15074–15083. doi: 10.1021/jp0743020. [DOI] [Google Scholar]
- [46].Yeager E, Electrocatalysts for O2 reduction, Electrochim. Acta 29 (1984) 1527–1537. doi: 10.1016/0013-4686(84)85006-9. [DOI] [Google Scholar]
- [47].Pletcher D, Sotiropoulos S, A study of cathodic oxygen reduction at platinum using microelectrodes, J. Electroanal. Chem 356 (1993) 109–119. doi: 10.1016/0022-0728(93)80514-I. [DOI] [Google Scholar]
- [48].Influxdata, Java client for InfluxDB, (n.d.). https://github.com/influxdata/influxdb-java (accessed June 4, 2018).
- [49].Influxdata, Influxdb, (2018). https://github.com/influxdata/influxdb (accessed June 4, 2018).
- [50].Grafana Labs, Grafana – The open platform for analytics and monitoring, (2018). https://grafana.com/ (accessed June 3, 2018). [Google Scholar]
- [51].Lundrigan P, Min K, Patwari N, Kasera S, Kelly K, Moore J, Meyer M, Collingwood SC, Nkoy F, Stone B, Sward K, EpiFi: An In-Home Sensor Network Architecture for Epidemiological Studies, (2017). https://arxiv.org/abs/1709.02233 (accessed June 3, 2018). [Google Scholar]
- [52].Amazon, Amazon EC2 Instance IP Addressing - Amazon Elastic Compute Cloud, (n.d.). https://docs.aws.amazon.com/AWSEC2/latest/UserGuide/using-instance-addressing.html (accessed June 4, 2018).
- [53].Hoffmann D, Hoffmann I, El-Bayoumy K, The less harmful cigarette: A controversial issue. A tribute to Ernst L. Wynder, Chem. Res. Toxicol 14 (2001) 767–790. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- [54].Liu X, Cheng S, Liu H, Hu S, Zhang D, Ning H, A Survey on Gas Sensing Technology, Sensors. 12 (2012) 9635–9665. doi: 10.3390/s120709635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Moseley PT, Progress in the development of semiconducting metal oxide gas sensors: a review, Meas. Sci. Technol 28 (2017) 082001. doi: 10.1088/1361-6501/aa7443. [DOI] [Google Scholar]
- [56].Fine GF, Cavanagh LM, Afonja A, Binions R, Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring, Sensors. 10 (2010) 5469–5502. doi: 10.3390/s100605469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Berna A, Metal Oxide Sensors for Electronic Noses and Their Application to Food Analysis, Sensors. 10 (2010) 3882–3910. doi: 10.3390/s100403882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wilson A, Baietto M, Applications and Advances in Electronic-Nose Technologies, Sensors. 9 (2009) 5099–5148. doi: 10.3390/s90705099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Arshak K, Moore E, Lyons GM, Harris J, Clifford S, A review of gas sensors employed in electronic nose applications, Sens. Rev 24 (2004) 181–198. 10.1108/02602280410525977 (accessed June 6, 2018). [DOI] [Google Scholar]
- [60].Lee C-Y, Fan W-Y, Hsieh W-J, In-situ Monitoring of Internal Local Temperature and Voltage of Proton Exchange Membrane Fuel Cells, Sensors. 10 (2010) 6395–6405. doi: 10.3390/s100706395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jones TP, Harris GJ, Breakspere RJ, Thomas A, Specificity Using Electrochemical Fuel Cell Sensors In Screening And Evidential Breath Testing, in: Proc. Ninth Int. Conf. Alcohol, Drugs, Traffic Safety, San Juan, Puerto Rico, 1983: pp. 721–729. http://www.icadtsinternational.com/files/documents/1983_066.pdf (accessed June 4, 2018). [Google Scholar]
- [62].Deng Y, Chen C, Xian X, Tsow F, Verma G, McConnell R, Fruin S, Tao N, Forzani E, A Novel Wireless Wearable Volatile Organic Compound (VOC) Monitoring Device with Disposable Sensors, Sensors. 16 (2016) 2060. doi: 10.3390/s16122060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tsow F, Forzani E, Rai A, Wang R, Tsui R, Mastroianni S, Knobbe C, Gandolfi AJ, Tao NJ, A Wearable and Wireless Sensor System for Real-Time Monitoring of Toxic Environmental Volatile Organic Compounds, IEEE Sens. J 9 (2009) 1734–1740. doi: 10.1109/JSEN.2009.2030747. [DOI] [Google Scholar]
- [64].Fan D, Gong J, Ghaemmaghami B, Zhang A, Lach J, Peden DB, Characterizing and Calibrating Low-Cost Wearable Ozone Sensors in Dynamic Environments, in: 2017 IEEE/ACM Int. Conf. Connect. Heal. Appl. Syst. Eng. Technol., IEEE, 2017: pp. 300–301. doi: 10.1109/CHASE.2017.112. [DOI] [Google Scholar]
- [65].Fletcher RR, Oreskovic NM, Robinson AI, Design and clinical feasibility of personal wearable monitor for measurement of activity and environmental exposure., Conf. Proc. … Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf 2014 (2014) 874–7. doi: 10.1109/EMBC.2014.6943730. [DOI] [PMC free article] [PubMed] [Google Scholar]