Abstract
Background
Curcumin, a controversial “panacea,” has been broadly studied. Its bioactivities including antioxidant, anti-inflammatory, and especially antineoplastic activities have been documented. However, due to its extensive bioactivities, some scientists hold a skeptical point of view toward curcumin and described curcumin as a “deceiver” to chemists. The objective of this study was to explore curcumin's another possibility as a potential supplementary leading compound to cancer treatments.
Methods
Literature searches were conducted using electronic databases. Search terms such as “curcumin,” “curcumin analogues,” and so on were used. The literatures were collected and summarized. In this article, reported targets of curcumin are reviewed. The limitations of a curcumin as a therapeutic anticancer product including low bioavailability and poor targeting are mentioned. Furthermore, modified curcumin analogues and antitumor mechanisms are listed and discussed in the aspects of cell death and tumor microenvironment including angiogenesis, tissue hypoxia status, and energy metabolism.
Results
Several possible modification strategies were presented by analyzing the relationships between the antitumor activity of curcumin analogues and their structural characteristics, including the introduction of hydrophilic group, shortening of redundant hydrocarbon chain, the introduction of extra chemical group, and so on.
Conclusions
From our perspective, after structural modification curcumin could be more effective complementary product for cancer therapies by the enhancement of targeting abilities and the improvement of bioavailability.
Keywords: antitumor agent, cancer treatment, curcumin, curcumin analogues, curcumin modification
1. Introduction
Curcumin, a hydrophobic polyphenol spontaneously undergoing a keto–enol tautomerism, is isolated from the rhizome of Curcuma longa L (Fig. 1).[3] Multiple molecular targets of curcumin bring a vast pharmacological effects including antioxidant, anti-inflammatory, antimicrobial, anticarcinogenic, thrombi-suppressive, hepatoprotective, cardiovascular-protective, Alzheimer-easing, hypoglycemic, and antiarthritic activities.[1,2] Recently, it aroused a great controversy among pharmacologists and phytochemists. On the one hand, curcumin shows multiple pharmacological effects and high-dose safety feature that warrant further investigations. On the other hand, curcumin exhibits a low bioavailability.[4–6] Moreover, vast targets inevitably bring vast unnecessary side effects. Many scientists tend to believe curcumin has been releasing a “deceptive false signal” in drug-screening tests, and they also pointed out that chemicals may hit in drug screenings but unlikely to yield a drug, and curcumin seems to be one of those “chemical frauds.”[66] However, a most recent review argues that curcumin should not be dismissed and curcumin's feature of multiple molecular targets is associated with modulation instead of direct inhibition. There are many animal studies and clinical trials that show the therapeutic benefits of curcumin that should not be neglected.[67] In our opinion, it is unlikely that curcumin, as a multitarget chemical, hits a specific target without affecting others. Therefore, in this article, we summarized promising modification strategies characterized by cancer-related structural features to direct structural reforms that we hope could narrow target range to strengthen more specific effects. Modified curcumin analogues could be more effective complementary products to cancer therapies.
Figure 1.

Chemical structure of curcumin. The α,β-unsaturated β-diketone moiety undergoes keto–enol tautomerism and forms a hydrogen bond containing 6-membered ring.
1.1. Search methods for identification of studies
The following electronic databases were searched: PubMed, Google Scholar, and China National Knowledge Infrastructure. The search strategy to be used in Google Scholar is shown in Table 1. The search strategies to be used in other databases are similar.
Table 1.
Search strategy applied in Google Scholar database.

1.2. Is curcumin still worthy of further exploration?
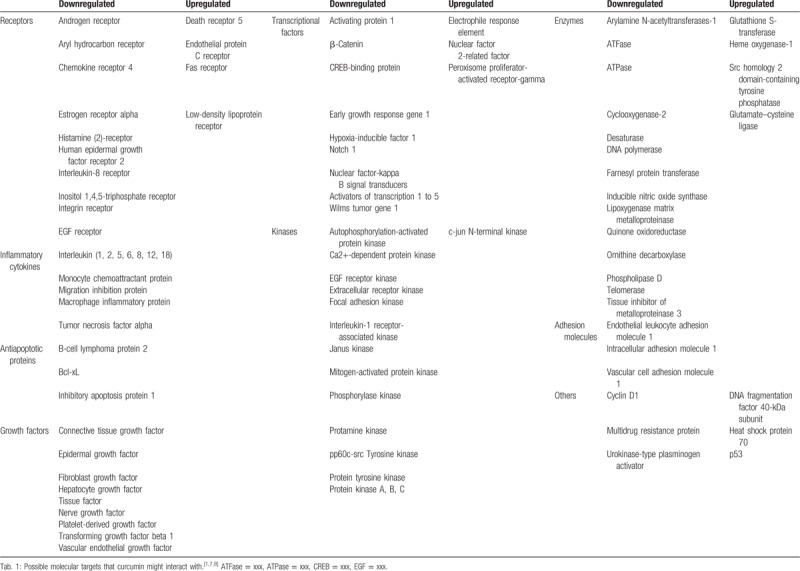
Pharmacological study of curcumin has never been stopped ever since it was isolated from turmeric. Researchers were surprised to find that curcumin performed remarkably well in antioxidant, anti-inflammatory, and even in anticancer experiments. Curcumin has proven to be effective and has also shown a multitarget nature in mechanism studies. The vast targets of curcumin make it an ideal basis for drug design. In this study, we have summarized the possible molecular targets of curcumin (Table 2).
Table 2.
Targets of curcumin.
Curcumin exhibits good antitumor activities. In a hen model, intake of daily dietary curcumin notably reduced the prevalence of spontaneous ovarian cancer by inhibiting NF-κB/signal transducer and activator of transcription 3 (STAT3) signaling pathways and by inducing nuclear factor erythroid 2/heme oxygenase 1 antioxidant pathway.[68] In human colon cancer 116 cells and cancer tissues, curcumin regulated the proliferation and apoptosis via miR-491/PEG10 pathway.[69] In addition, as an adjuvant for antitumoral DNA-damaging drugs, curcumin induced γ-H2AX foci and decreased the expression of Rad51 to sensitize lymphoma cells to DNA-damaging agents.[70] Also, curcumin resensitized gemcitabine-resistant pancreatic ductal adenocarcinoma cells to chemotherapy by attenuating the expression of Enhancer Of Zeste Homolog 2 Subunit of Polycomb Repressive Complex 2, a recently identified drug resistance regulating key player and its related lncRNA PVT1. In addition, curcumin-targeted cancer stem cells (CSCs) down-regulated self-renewal driving genes.[71] Activation of Wnt/β-catenin is critical in epithelial–mesenchymal transition and the acquisition of CSCs properties. Curcumin has been reported to reverse tobacco smoke-induced activation of Wnt/β-catenin.[72] Combined with other compounds or curcumin alone showed suppressive activities in cervical cancer cells by activating p53 and caspase-3.[73] By inhibiting JAK/STAT3 signaling pathway, curcumin attenuated the normal endothelial cells transition to tumor endothelial cells to prevent tumor-microenvironment-induced angiogenesis.[74] Numerous experimental evidences and ongoing experiment have been proving that curcumin is a promising chemopreventive natural product and worth further explorations.
1.3. Stumbling blocks between curcumin and an effective antitumor product
Curcumin exhibits an excellent antitumor activity, but there is almost no curcumin-related product clinically applied as an anticancer drug or chemopreventive medication.[75] Poor bioavailability is the leading cause that limits the benefits of curcumin. Curcumin undergoes a spontaneous keto–enol tautomerism that makes it unstable at physiological pH. In addition, curcumin is a hydrophobic polyphenol almost insoluble in water. Slow uptake and rapid metabolism features of curcumin also limit its efficacy.[76] Pharmacokinetic studies revealed that oral bioavailability of curcumin was only 1%.[77]
Besides its low bioavailability issue, broad bioactivity profile of curcumin drew skepticism. A review explained its broad bioactivity profile as pan-assay interference compounds. According to the authors, multiple bioactivities of curcumin is an assay-interfered readout rather than compound–target interactions, for example, fluorescence interference, covalent labeling of proteins, metal chelation, redox reactivity, aggregation, and so on.[78] This explanation may be plausible for experiments in vitro, but it is not able to explain large experimental and clinical trail data in vivo.[13] We suppose curcumin, as a secondary metabolites , is structurally weak-matched with a common domain, which appears as a wide target inhibition or activation. To develop a medication, one must have a specific target, and multitarget behaviors would inevitably bring wide side effects that explained multiple activities of curcumin. When it comes to a specific disease, for example, cancer, a lot of literature documented antitumor activities of curcumin; it is unlikely that a single molecule worked on so many targets without any adverse side effects. However, it is interesting that experiments in vivo proved curcumin to be safe even at a very high dose.[5,79] Possible explanations may be that either the absorption of curcumin is low or biological activities of curcumin are definite but moderate.
1.4. Current solutions for curcumin
Poor bioavailability limits the further benefits of curcumin; a lot of researchers gave their solutions. It prevented curcumin from glucuronidation to less potent metabolite curcumin glucuronides using an adjuvant piperine that is a known inhibitor of glucuronidation.[80,81] Nanoparticle technology has been applied in curcumin delivery system. With a very small particle size, highly hydrophobic curcumin is dispersed in water to the utmost extent, which helps curcumin to get into target cells.[82] In addition, liposome, micelles, phospholipid complexes delivery systems of curcumin have been developed to achieve a better bioavailability.[4,83–85]
The improvement in pharmaceutics or delivery systems of curcumin increases bioavailability indeed, but it will not change the chemical structure; curcumin still remains the “panacea” attribute. By narrowing target range to strengthen more specific effects, structural modifications of curcumin seem to be the strategy to enhance the activity as a specific anticancer product.
To strengthen pharmaceutical activities of curcumin, researchers synthesized many curcumin analogues. In a study, most of the synthesized curcumin analogues showed an increasing antioxidant activity to low-density lipoprotein (LDL) peroxidation with the increase of phenolic group number.[9] 3′-Methoxyl in curcumin was deemed to play a role in anti-inflammatory effects; analogues that contain cyclohexanone performed superiorly, and deletion of the β-diketone moiety increased its biological stability defect that was caused by the keto–enol tautomerism.[10] Curcumin was also found to be favorable in the treatment of Alzheimer disease. The study of structure–activity relationships revealed that it is the 2 aromatic rings that could bind with amyloid beta protein (Aβ) and inhibit Aβ aggregation but not the flexibility of the bridge between 2 aromatic rings.[11]
In the respect of antitumor activity, modification on curcumin also has been extensively investigated. For centuries, we have been struggling with cancers, although a lot of progresses in the mechanism of tumor genesis and development have been made and there have been plenty of methods coping with different types of cancer for instance radiotherapy, chemotherapy, and other anticancer medications. However, there are many other problems, which include drug toxicities, chemoresistance, radioresistance, cancer recurrence, and so on. In previous studies, curcumin presented a great effectiveness in vitro, but it has hardly met expectations of the clinical demands.[78] But as a basis for antitumor drug designs, there are 3-group sections in curcumin that have been structurally modified to yield more effective compounds, for instance, the aromatic rings, the β-diketone moiety, and the 2 flanking double bonds. As Mosley et al in their article pointed out, mostly, the successful anticancer compounds based on curcuminoid structures retained the conjugated α,β-unsaturated ketone moieties as a Michael receptor. They observed, as cytotoxins, compounds with more than 1 conjugated carbonyl were more active than those with a single conjugated carbonyl, which indicates that a conjugated carbonyl might be the functional group against cancer cells and cancer cells are more susceptible to multiple chemical insults.[14]
1.5. Curcumin analogues targeting cancer cells
Traditional anticancer compounds are targeting cancer cells directly. Curcumin has been documented to have direct cancer cell inhibiting effects in many cell models. Some of its analogues, therefore, have been designed to enhance the cytotoxicity directly toward cancer cells. The cytotoxicity of curcumin is mostly presumed to be related to the conjugated carbonyl, but the conjugation in curcumin tends to be unstable because of the keto–enol tautomerism. Therefore, the design strategy prefers to retain the conjugated carbonyl while avoiding the tautomerism by the deletion of the β-diketone moiety. A successful example DM-1 (Fig. 2, 15), which is a curcuminoid by a β-diketone moiety deletion based on curcumin skeleton, showed a superior toxicity to melanoma cells.[86] A series of mono-carbonyl curcumin analogues were designed and synthesized to increase stability of curcumin, among which CA15 (Fig. 2, 16) exhibited a stronger selective toxicity toward laryngeal cancer cells compared to curcumin. Similar compounds A (Fig. 2, 17) and I (Fig. 2, 18) also showed a significant antitumor activities both in triple-negative breast cancer cells in vitro and in triple-negative breast cancer and leukemia xenografts in vivo.[87,88] However with the alteration of α,β-unsaturated ketones, saturated curcumin or curcumin analogues, for example tetrahydrocurcumin (Fig. 2, 7), were proved to be much less potent, which further proved the importance of conjugated carbonyl in antitumor activity.[16,24]
Figure 2.
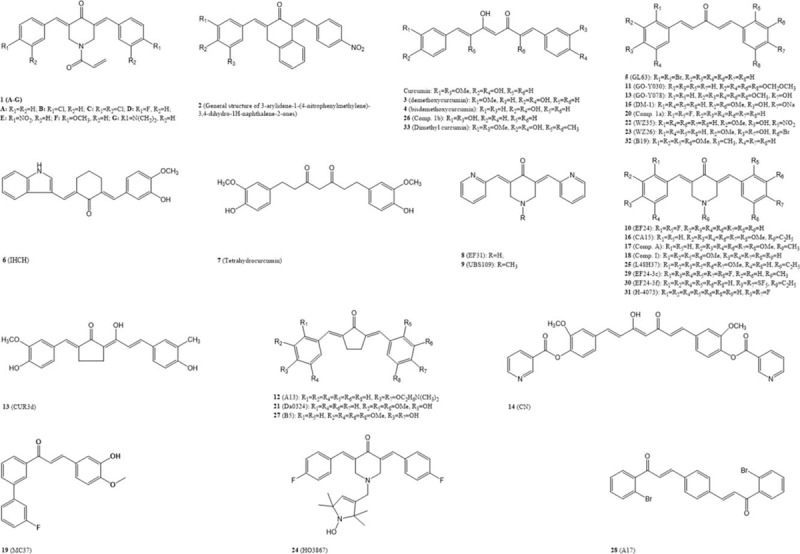
Potential antineoplastic curcumin analogues. Structures of curcumin and its analogues presented in this review have been reported to have the potential antineoplastic activity.
Based on mono-carbonyl curcumin, a designed series of piperidone curcuminoids also showed a promising outcomes in tumor inhibition.[92] Compared to curcumin, N-acryloyl series (Fig. 2, 1) were found to be more cytotoxic toward murine P388 and L1210 leukemic cells and human Molt 4/C8 and CEM neoplasms.[15] In another article, a series of α,β-unsaturated monoketone curcumin analogue based on piperidone showed remarkable cytotoxicity to leukemia cell lines.[16]
Above all, further modifications of curcumin are basically based on mono-carbonyl curcumin or piperidone curcumin with the introduction of methyl groups, hydroxy groups, fluorine, methoxy groups, hybridization, and so. Some of the examples are listed as MC37 (Fig. 2, 19),[89] 2,2′-fluorine mono-carbonyl curcumin (Fig. 2, 20),[90] and Da0324 (Fig. 2, 21).[91]
1.5.1. Curcumin analogues targeting CSCs
The CSC hypothesis accounts for the functional heterogeneity and hierarchical organization of cancerous cells. CSCs are responsible for the tumor comprising diverse cells and exhibiting a stem cell-like feature. In most cases, CSCs are drug resistant and have the abilities of self-renewal and cancer initiation to maintain the tumor existence that majorly is the reason of cancer recurrence.[98] Recent studies on curcuminoids have presented several activities by which oncogenesis could be distinctly suppressed. It has been pointed out that 3 major signaling pathways, the Wnt/β-catenin, sonic hedgehog, and Notch l pathways, may be involved in curcumin blocking CSCs self-renewal through direct or indirect influences. CSCs resistance to chemotherapy and radiotherapy is considered the main reason for cancer recurrence. A strategy using a combination of CSCs-targeted curcuminoids and conventional anticancer drug therapies has been proven to be potential to attenuate tumor resistance and recurrence.[21,26] In colorectal CSCs (ALDH+/CD133+ subpopulation of colorectal cancer cells), difluorinated curcumin (CDF) has been shown to downregulate the expression of miR-21 that would be present in high level in many cancer cells, further restore the expression of PTEN.[27,28] Recent research also revealed its role in pancreatic cancer in which CDF accumulates 3-folds more than curcumin.[23,30] GO-Y030 (Fig. 2, 11) and a water-soluble curcuminoid A13 (Fig. 2, 12) selectively inhibit the phosphorylation of STAT3, and then downregulate the expression of STAT3-mediated genes involved in cancer cell proliferation and survival, such as cyclin D1, Bcl-2, Bcl-XL, and putative STAT3 downstream targets, Notch 1 and Notch 3, that are essential for self-renewal and angiogenesis of stem cell.[20,25] By increasing the expression of cleaved PARP and cleaved casepase-3, GO-Y030 also drives CSCs to apoptosis and inhibits retinoblastoma protein phosphorylation that causes cell cycle arrest at G1 to affect CSCs cell cycle.[19,31]
1.5.2. Curcumin analogues triggering apoptosis
According to cell death morphologies, cell death includes “apoptosis,” “necrosis,” and “mitotic catastrophe.”[99] Present experimental data of curcumin and its analogues on antitumor activity mainly regard activating the apoptosis through ROS-mediated endoplasmic reticulum (ER) stress and mitochondrion-dependant pathways as the key to initiate cell death program. Acute ER stress in cancerous cells triggers the induction of a family of ER stress protein that promotes survival and growth of cancer cells, in which acute ER stress plays an overall protective role in its resistance to chemotherapy and radiation therapy. However, prolonged or severe ER stress impairs the protective mechanisms and triggers the cell death program.[32,33] In intrinsic apoptosis, intracellular stress including DNA damage, cytosolic Ca2+ overload, and ER stress triggers pro-death or pro-survival signals to a mitochondrion-centered control mechanism. When pro-death signals prevail, mitochondrial outer membrane permeabilization occurs, which leads to a dissipation of mitochondrial transmembrane potential and arrest of mitochondrial ATP synthesis. The uncoupled respiration chains lead to reactive oxygen species (ROS) overproduction and cytochrome C released into the cytosol t induces cell apoptosis.[99] Some studies show that curcumin triggers the accumulation of cytosolic Ca2+ by inhibiting the Ca2+-ATPase pump further increases the ER stress that induces the prolonged ER stress and activation of specific cell apoptosis by cleavage and activation of caspase and p23 cleavage, and downregulation of the antiapoptotic Mcl-1 protein.[34,35] Some successful curcumin analogues, WZ35 (Fig. 2, 22), WZ26 (Fig. 2, 23), HO3867 (Fig. 2, 24), L48H37 (Fig. 2, 25), 2,2′-F mono-carbonyl curcumin (Fig. 2, 20), 3,3′-OH curcumin (Fig. 2, 26), and B5 (Fig. 2, 27), have been documented to induce ROS production and accumulation that activate ROS-dependent ER stress signaling pathways or mitochondrial apoptosis pathways and finally lead to cell apoptosis.[90,33,100,101,102,103,104,105,107,108] In addition, HO3867 and L48h37 are inhibitors of STAT3 that is closely related to tumor progression and activates gene expression of Bcl-2, considered as an important antiapoptotic protein.[33,104] GL63 (Fig. 2, 5), WZ25, and A17 (Fig. 2, 28) can induce the expression of C/EBP homologous protein that is a direct effector of ER stress.[36,101,109] B5 and IHCH (Fig. 2, 6) activate autophagy through the Akt and AMPK signaling pathways.[37,108] By analyzing structures of this effective anti-apoptosis curcumin analogues, we noted that most of them are based on mono-carbonyl curcumin or piperidone curcumin, and nearly half of them are with halogen elements introduction, for example, fluorine and bromine.
1.6. Curcumin analogues influencing the tumor microenvironment
Tumor microenvironment is the cellular environment in which the tumor exists, including tumor itself, surrounding blood vessels, immune cells, fibroblasts, and so on.[107] In the process of tumor progression and metastasis, tumor microenvironment plays an essential role.[108] In this article, we discussed the roles of curcumin analogues from the aspects of tumor microenvironment including angiogenesis, hypoxia status, and lipid metabolism.
1.6.1. Curcumin analogues inhibiting angiogenesis
Angiogenesis known as the formation of new blood vessels plays a critical role in cancer progression. It channels the tumor metastasis and also facilitates the delivery of oxygen and nutrition and clearance of wastes to satisfy the rapid metabolisms during tumor proliferation. Therefore, for cancer treatments, inhibiting angiogenesis is another key to preventing tumors from growing and metastasizing. Angiogenesis inhibitors have been widely used in cancer treatment.[22] As direct angiogenesis inhibitors, natural curcumin and its analogues demethoxycurcumin (Fig. 2, 3) and bisdemethoxycurcumin (Fig. 2, 4), which occur naturally and are isolated from turmeric, were proved to be active in inhibiting vascular endothelial growth factor (VEGF) and basic fibroblast growth factor-induced neovascularization, and the downregulation of VEGF and fibroblast growth factor (FGF) is mainly mediated by its inhibitory effect on NF-κB transcription.[23,94] Structural modifications of curcumin on antiangiogenesis aim to enhance the downregulative effect of VEGF or FGF. Some other curcumin analogues such as EF24 (Fig. 2, 10) and its fluoro and pentafluorothio analogues EF-24-3c (Fig. 2, 29) and EF-24-4f (Fig. 2, 30) had a stronger antiangiogenesis effect, some, for example, 3c even showed disruptive effect on blood vessel.[18] Similar curcumin analogues include UBS109 (Fig. 2, 9), EF31 (Fig. 2, 8),[38] H-4073 (Fig. 2, 31),[109] and B19 (Fig. 2, 32).[97] Other than VEGFs, many components including growth factors and signal transducers also participated in tumor angiogenesis. A curcumin analogue GO-Y078 (Fig. 2, 13) showed angiogenesis-inhibiting effect not through the suppression of VEGF signaling but by actin disorganization.[95] A unusual modification strategy dimethyl curcumin (Fig. 2, 33) by introducing methyl groups at C2 and C6 positions enhanced the antiangiogenesis activity of curcumin. Dimethyl curcumin remained β-diketone but introduced 2 methyl groups at C2 and C6 positions to resist metabolism in vivo.[96]
1.6.2. Curcumin analogues acting under hypoxia status
In most cases, solid tumors are under hypoxia, because tumor angiogenesis results in abnormal and dysfunctional blood vessels that can hardly match with the rapid growth with a high oxygen consuming.[58] Tissue hypoxia increases the cellular ROS and leads to ER stress.[59] Therefore, tumor hypoxia enhances the risk of resistance to treatments.[60] Also, hypoxia-inducible factors (HIFs) are overexpressed in cancer cells under hypoxia status, and recent research has proven that HIFs can induce relevant gene expression that contributes to self-renewal and pluripotency of CSCs, such as Oct4 and Notch l.[44,45] When tumor under hypoxia status, CUR3d (Fig. 2, 13) and a series of curcumin analogues can downregulate HIF-1α.[39] Investigations on curcumin analogues EF31 and UBS109 for their anti-pancreatic cancer activity reveal that through downregulation of HIF-1α, heat shock proteins 90, cyclooxygenase 2, and VEGF, they also can drastically impair angiogenesis in pancreatic cancer.[38,93]
1.6.3. Curcumin analogues regulating lipid metabolism
Lipid metabolism, an important energy metabolism pathway in organisms, plays an even more critical role in tumor's energy metabolism pathways. Because of the rapid energy-consuming rate and poor oxygen-supplying channel, tumor tissues are usually under hypoxia. Therefore, glycolysis becomes the main energy supply pathway in cancer cells. However, glycolysis still cannot be sufficient for energetic and biosynthetic requirements of tumors.[47] Given the irregular alteration in both glucose and glutamine metabolisms, cancer cells are in need of fatty acids as extra energy sources that can be obtained by synthesis or uptake from exogenous environment. Increased dependence on lipid oxidation as their primary energy source has been observed in many tumor types.[48,61,62] Growing evidence have confirmed that changes in lipid metabolism are closely related to the occurrence and development of tumors.[59] Most of the enzymes required for fatty acid and cholesterol synthesis are regulated by sterol regulatory element-binding proteins (SREBPs), a transcription factor of the helix-loop-helix leucine zipper family.[49] Overexpressed SREBP1 and SREBP2 have been found in many tumor cells.[50] Intermediated by SREBP, mutant tumor protein p53 associates with sterol gene promoters. Hyperactivated genes break tissue architecture and finally lead to the tumorigenesis in breast cancer.[51] In glioblastoma, the expression of LDL receptor is essential for the survival of glioblastoma.[53] Another study illustrates that mutant epidermal growth factor receptor induces the activation of SREBP-1.[52] SREBP1-regulated fatty acid synthase is involved in the production of lipid-signaling molecules and responsible for the multidrug resistance (MDR) during cancer treatments.[54,55] According to all these evidence, lipid metabolic pathway could be a new perspective for cancer treatment and SREBPs could be the promising targets. Our research group has been seeking to understand and exploit the role of curcumin and curcumin analogues in cholesterol metabolism. In previous studies, we found curcumin inhibited ox-LDL-induced cholesterol accumulation in cultured vascular smooth muscle cells (VSMCs) by increasing the caveolin-1 expression and inhibiting nuclear translocation of SREBP-1.[63] Curcumin also induced cell cycle arrest at G1/S phase and inhibited chol: MβCD-induced VSMCs proliferation via the suppression of overactivated extracellular signal-regulated kinase signaling.[64] On the other hand, we also found curcumin could block the activation of Notch 1.[109] In addition, a curcumin analogue curcumin nicotinate, known as CN (Fig. 2, 14), newly designed by our group has proven to significantly downregulate the expression of SREBP1 and to decrease lipid accumulation in THP-1 cells through the restoration of autophagy flux.[56,57,59] Because curcumin and its analogues can regulate lipid synthesis and accumulation, we have been exploring whether curcumin and curcumin analogues play important roles in antitumor activity through the regulation of lipid metabolism. In our preliminary antitumor activity screening tests, CN indeed exhibited an excellent antitumor activity (unpublished data).
2. Discussion
2.1. Modification strategy of curcumin
Curcumin is a safe natural compound and has vast molecular targets, which makes it an ideal leading compound. However, to our knowledge, with a wide target range, one will not be effective enough to work as a therapeutic medicine without any side effects. As an antitumor molecule, curcumin is unlikely to be sufficient. Therefore, it seems inevitable to narrow down the targeting spectrum of curcumin meanwhile to enhance targeting efficacy by structural modifications.
So far, the modification strategies of curcumin focus on improving the bioavailability. Curcumin is almost insoluble in water; therefore, the introduction of hydrophilic group or shortening of redundant hydrocarbon chain might be able to increase its water solubility. In addition, the spontaneous keto–enol tautomerism of curcumin makes it unstable in the process of metabolism in vivo, for which mono-carbonyl curcumin or piperidone curcumin might be more suitable for structural modification as a starting point (Fig. 3). Further modification could be the introduction of extra chemical group, for example, halogen elements; hydroxy, nitro, and methoxy groups to increase biocompatibility and bioavailability; cyclohexanone (Fig. 2, 2) to the chain between 2 aromatic rings to increase potency against MDR in tumor[17]; and heterocycle to increase the solubility and cytotoxicity.[40] Recent studies have also mentioned the prospect of some asymmetric curcumin analogues.[41,42]
Figure 3.
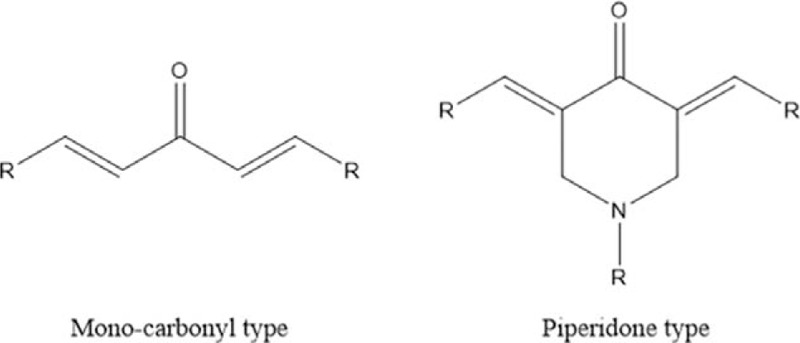
Promising structural modification types. Curcumin related mono-carbonyl type and piperidone type are promising structure modification starting points.
Modified curcumin might still not be the end. Together with the nanoparticle technology, liposome, micelles, and phospholipid complexes delivery systems, the bioavailability of curcumin analogues will be further elevated. Also, from the aforementioned successful examples, we see the potentials of curcumin analogues to work as a synergistic drug or a sensitizer of cancer chemotherapies and radiotherapies for the reasons that with different structural modification strategies, curcumin analogues can not only target cancer cells but also target tumor microenvironment (Fig. 4), for example, arresting cell cycle at G1 phase that might play a synergistic effect with radiotherapies,[43] triggering apoptosis by ROS-mediated signaling pathways, inhibiting angiogenesis by downregulation of NF-κB-mediated VEGF or FGF level, decreasing tissue hypoxia-induced HIFs overexpression, and regulating tumor lipid metabolism.
Figure 4.
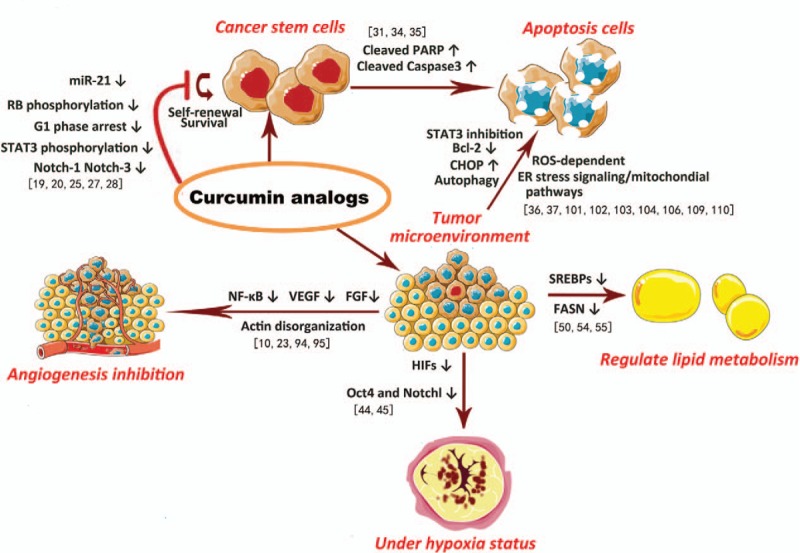
How curcumin analogues interrupt resistance and recurrence of cancer cells. In the aspects of cell death and tumor microenvironment including angiogenesis, tissue hypoxia status, and energy metabolism, modified curcumin analogues interrupt resistance and recurrence of cancer.
In summary, curcumin, as a promising compound isolated from traditional Asian dietary and medicinal plant turmeric, has drawn a massive attention. Extensive research has revealed its possible potentials for various therapeutic purposes, among which the antitumor activity has recently become a research hotspot. In cancer treatments, lots of thorny problems occur to us. Curcumin has deemed to be a multi-target agent that has its unique benefits to tumor inhibition, but its problem of low bioavailability has always been a stumbling block that have be overcome. From our perspective, narrowing target range to strengthen more specific effects, modified curcumin analogues could be more effective. The structural modifications in our review proved to be a feasible way to improve its solubility and efficacy. Mainstream cancer therapies combined with curcumin analogues as complementary agents would be a promising therapeutic strategy, yet more curcumin analogue types and the combination remain to be further explored.
2.2. Uncited reference
Acknowledgments
We thank all the colleagues who have helped to revise and to correct mistakes and inaccuracies in this article.
Author contributions
Conceptualization: Duan-Fang Liao.
Formal analysis: Qing-Zi Yan.
Investigation: Li-Mei Lin.
Methodology: Zhe Shi.
Software: Ya-Ling Zeng.
Supervision: Bo-Hou Xia.
Supervision: Duan-Fang Liao.
Validation: Qin-Hui Tuo.
Writing – original draft: De-Biao Xiang, Kai-Qiang Zhang, Ya-Ling Zeng, Qing-Zi Yan.
Writing – review and editing: Zhe Shi, Qin-Hui Tuo, Li-Mei Lin, Bo-Hou Xia, Ping Wu, Duan-Fang Liao.
Footnotes
Abbreviations: CDF = difluorinated curcumin, CN = curcumin nicotinate, CSCs = cancer stem cells, ER = endoplasmic reticulum, FGF = fibroblast growth factor, HIF-1α = hypoxia inducible factor-1α, LDL = low-density lipoprotein, MDR = multidrug resistance, ROS = reactive oxygen species, SREBPs = sterol regulatory element-binding proteins, STAT3 = signal transducer and activator of transcription 3, VEGF = vascular endothelial growth factor, VSMCs = vascular smooth muscle cells.
How to cite this article: Xiang DB, Zhang KQ, Zeng YL, Yan QZ, Shi Z, Tuo QH, Lin LM, Xia BH, Wu P, Liao DF. Curcumin: from a controversial “panacea” to effective antineoplastic products. Medicine. 2020;99:2(e18467).
DBX and KQZ contributed equally to this work.
This study was financially supported by the Construct Program of the Pharmaceutical Science Key Discipline in Hunan Province, the National Natural Science Foundation of China (81673722, 81600291, and 81773736), Natural Science Foundation of Hunan Province (2019JJ50443), and Major Projects of Hunan provincial development (S2015S501P010).
This study does not require ethic approval, because we only analyzed data from published studies.
The work has not been published previously, and is not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript for publication.
The authors report no conflicts of interest
References
- [1].Nabavi SM, Russo GL, Tedesco I, et al. Curcumin and melanoma: from chemistry to medicine. Nutr Cancer 2018. 1–2. [DOI] [PubMed] [Google Scholar]
- [2].Ahmad SS, Khan H, Danish Rizvi SM, et al. Computational study of natural compounds for the clearance of amyloid-beta: a potential therapeutic management strategy for Alzheimer's disease. Molecules 2019;24:E3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kunwar A, Simon E, Singh U, et al. Interaction of a curcumin analogue dimethoxycurcumin with DNA. Chem Biol Drug Des 2011;77:281–7. [DOI] [PubMed] [Google Scholar]
- [4].Yadav YC, Pattnaik S, Swain K. Curcumin loaded mesoporous silica nanoparticles: assessment of bioavailability and cardioprotective effect. Drug Dev Ind Pharm 2019;45:1889–95. [DOI] [PubMed] [Google Scholar]
- [5].Kharat M, McClements DJ. Recent advances in colloidal delivery systems for nutraceuticals: a case study-delivery by design of curcumin. J Colloid Interf Sci 2019;557:506–18. [DOI] [PubMed] [Google Scholar]
- [6].Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat 2014;46:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Aggarwal BB, Bhatt ID, Ichikawa H. Ravindran PN, Babu KN, Sivaraman K, et al. Curcumin–biological and medicinal properties. Turmeric: The Genus Curcuma. Boca Raton, FL: CRC Press; 2007. xxx. [Google Scholar]
- [8].Qadir MI, Naqvi S, Muhammad SA. Curcumin: a polyphenol with molecular targets for cancer control. Asian Pac J Cancer Prev 2016;17:2735–9. [PubMed] [Google Scholar]
- [9].Panahi Y, Ahmadi Y, Teymouri M, et al. Curcumin as a potential candidate for treating hyperlipidemia: a review of cellular and metabolic mechanisms. J Cell Physiol 2018;233:141–52. [DOI] [PubMed] [Google Scholar]
- [10].Liang G, Yang S, Zhou H, et al. Synthesis, crystal structure and anti-inflammatory properties of curcumin analogues. Eur J Med Chem 2009;44:915–9. [DOI] [PubMed] [Google Scholar]
- [11].Zhai P, Xia CL, Tan JH, et al. Syntheses and evaluation of asymmetric curcumin analogues as potential multifunctional agents for the treatment of Alzheimer's disease. Curr Alzheimer Res 2015;12:403–14. [DOI] [PubMed] [Google Scholar]
- [12].Haroyan A, Mukuchyan V, Mkrtchyan N, et al. Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: a comparative, randomized, double-blind, placebo-controlled study. BMC Complement Altern Med 2018;18:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Allegra A, Innao V, Russo S, et al. Anticancer activity of curcumin and its analogues: preclinical and clinical studies. Cancer Invest 2017;35:1–22. [DOI] [PubMed] [Google Scholar]
- [14].Kálai T, Kuppusamy ML, Balog M, et al. Synthesis of N-substituted 3,5-bis (arylidene)-4-piperidones with high antitumor and antioxidant activity. J Med Chem 2011;54:5414–21. [DOI] [PubMed] [Google Scholar]
- [15].Jha A, Mohapatra P, AAlHarbi S, et al. Curcumin: not so spicy after all. Mini Rev Med Chem 2017;17:1425–34. [DOI] [PubMed] [Google Scholar]
- [16].Dimmock JR, Das U, Gul HI, et al. 3-Arylidene-1-(4-nitrophenylmethylene)-3,4-dihydro-1H-naphthalen-2-ones and related compounds displaying selective toxicity and reversal of multidrug resistance in neoplastic cells. Bioorg Med Chem Lett 2005;15:1633–6. [DOI] [PubMed] [Google Scholar]
- [17].Schmitt F, Gold M, Begemann G, et al. Fluoro and pentafluorothio analogs of the antitumoral curcuminoid EF24 with superior antiangiogenic and vascular-disruptive effects. Bioorg Med Chem 2017;25:4894–903. [DOI] [PubMed] [Google Scholar]
- [18].Kudo C, Yamakoshi H, Sato A, et al. Novel curcumin analogs, GO-Y030 and GO-Y078, are multi-targeted agents with enhanced abilities for multiple myeloma. Anticancer Res 2011;31:3719–26. [PubMed] [Google Scholar]
- [19].Lin L, Liu Y, Li H, et al. Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Brit J Cancer 2011;105:212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ramasamy TS, Ayob AZ, Myint HH, et al. Targeting colorectal cancer stem cells using curcumin and curcumin analogues: insights into the mechanism of the therapeutic efficacy. Cancer Cell Int 2015;15:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang Z, Dabrosin C, Yin X, et al. In broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol 2015;35:S224–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pei H, Yang Y, Cui L, et al. Bisdemethoxycurcumin inhibits ovarian cancer via reducing oxidative stress mediated MMPs expressions. Sci Rep 2016;6:28773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Momtazi AA, Sahebkar A. Difluorinated curcumin: a promising curcumin analogue with improved anti-tumor activity and pharmacokinetic profile. Curr Pharm Des 2016;22:4386–97. [DOI] [PubMed] [Google Scholar]
- [24].Weng Q, Ren L, Guo L, et al. Curcumin analogue, A13, exhibits anti-leukemia effect via inhibiting STAT3. Tumor Biol 2016;37:9959–66. [DOI] [PubMed] [Google Scholar]
- [25].Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105–11. [DOI] [PubMed] [Google Scholar]
- [26].Roy S, Yu Y, Padhye SB, et al. Difluorinated-curcumin (CDF) restores PTEN expression in colon cancer cells by down-regulating miR-21. PLoS One 2013;8:e68543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ali S, Ahmad A, Aboukameel A, et al. Increased Ras GTPase activity is regulated by miRNAs that can be attenuated by CDF treatment in pancreatic cancer cells. Cancer Lett 2012;319:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [28].Dandawate PR, Vyas A, Ahmad A, et al. Inclusion complex of novel curcumin analogue CDF and β-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm Res 2012;29:1775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang J, Xu K, Liu P, et al. Inhibition of Rb phosphorylation leads to mTORC2-mediated activation of Akt. Mol Cell 2016;62:929–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Anania VG, Yu K, Gnad F, et al. Uncovering a dual regulatory role for caspases during endoplasmic reticulum stress-induced cell death. Mol Cell Proteomics 2016;15:2293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ryoo HD. Long and short (timeframe) of endoplasmic reticulum stress-induced cell death. FEBS J 2016;283:3718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Moore TW, Zhu S, Randolph R, et al. Liver S9 fraction-derived metabolites of curcumin analogue UBS109. ACS Med Chem Lett 2014;5:288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ye H, Wei X, Wang Z, et al. A novel double carbonyl analog of curcumin induces the apoptosis of human lung cancer H460 cells via the activation of the endoplasmic reticulum stress signaling pathway. Oncol Rep 2016;36:1640–8. [DOI] [PubMed] [Google Scholar]
- [34].Pan Y, Xiao J, Liang G, et al. A new curcumin analogue exhibits enhanced antitumor activity in nasopharyngeal carcinoma. Oncol Rep 2013;30:239–45. [DOI] [PubMed] [Google Scholar]
- [35].Zhou GZ, Xu SL, Sun GC, et al. Novel curcumin analogue IHCH exhibits potent anti-proliferative effects by inducing autophagy in A549 lung cancer cells. Mol Med Rep 2014;10:441–6. [DOI] [PubMed] [Google Scholar]
- [36].Nagaraju GP, Zhu S, Ko JE, et al. Antiangiogenic effects of a novel synthetic curcumin analogue in pancreatic cancer. Cancer Lett 2015;357:557–65. [DOI] [PubMed] [Google Scholar]
- [37].Bhullar KS, Jha A, Rupasinghe HP, et al. Novel carbocyclic curcumin analog CUR3d modulates genes involved in multiple apoptosis pathways in human hepatocellular carcinoma cells. Chem Biol Interact 2015;242:107–22. [DOI] [PubMed] [Google Scholar]
- [38].Gwavava P. Heterocyclic Cyclohexanone Curcumin Analogues Inhibit Growth of Ovarian Cancer Cells, In-Vitro [dissertationl]. Otago, New Zealand: University of Otago; 2015. [Google Scholar]
- [39].Li Q, Chen J, Luo S, et al. Synthesis and assessment of the antioxidant and antitumor properties of asymmetric curcumin analogues. Eur J Med Chem 2015;93:461–9. [DOI] [PubMed] [Google Scholar]
- [40].Kapelle IBD, Irawadi TT, Rusli MS, et al. Synthesis of asymmetric curcumin analogues from cullilawanoil using conventional and microwave method. Procedia Chem 2015;16:480–8. [Google Scholar]
- [41].Kriegs M, Kasten-Pisula U, Riepen B, et al. Radiosensitization of HNSCC cells by EGFR inhibition depends on the induction of cell cycle arrests. Oncotarget 2016;7:45122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schito L, Semenza GL. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer 2016;2:758–70. [DOI] [PubMed] [Google Scholar]
- [43].Zhang C, Zhi WI, Lu H, et al. Hypoxia-inducible factors regulate pluripotency factor expression by ZNF217-and ALKBH5-mediated modulation of RNA methylation in breast cancer cells. Oncotarget 2016;7:64527–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bae MK, Kim SH, Jeong JW, et al. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep 2006;15:1557–62. [PubMed] [Google Scholar]
- [45].Warburg O. On the origin of cancer. Science 1956;123:309–14. [DOI] [PubMed] [Google Scholar]
- [46].Ackerman D, Simon MC. Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends Cell Biol 2014;24:472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jeon TI, Osborne TF. SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol Metab 2012;23:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 2007;7:763–77. [DOI] [PubMed] [Google Scholar]
- [49].Freed-Pastor WA, Mizuno H, Zhao X, et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 2012;148:244–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Guo D, Prins RM, Dang J, et al. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci Signal 2009;2:ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Deliang G, Felicia R, Mary Y, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov 2011;1:442–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Vazquez-Martin A, Colomer R, Brunet J, et al. Pharmacological blockade of fatty acid synthase (FASN) reverses acquired autoresistance to trastuzumab (Herceptin™) by transcriptionally inhibiting ‘HER2 super-expression’ occurring in high-dose trastuzumab-conditioned SKBR3/Tzb100 breast cancer cells. Inter J Oncol 2007;31:769–76. [PubMed] [Google Scholar]
- [53].Cheng CS, Wang Z, Chen J. Targeting FASN in breast cancer and the discovery of promising inhibitors from natural products derived from traditional Chinese medicine. Evid Based Complement Alternat Med 2014;2014:232946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gong YZ, Yao HL, Sun SW, et al. SREBP-1/caveolin-1 mediate the anti-atherosclerotic effect of curcumin nicotinate in apolipoprotein E-deficient mice. Chin J Arterioscler 2014;19:975–97. [Google Scholar]
- [55].Gu HF, Li HZ, Tang YL, et al. Nicotinate-curcumin impedes foam cell formation from THP-1 cells through restoring autophagy flux. PLoS One 2016;11:e0154820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci 2012;125:5591–6. [DOI] [PubMed] [Google Scholar]
- [57].Zeeshan HM, Lee GH, Kim HR, et al. Endoplasmic reticulum stress and associated ROS. Int J Mol Sci 2016;17:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Vaupel P, Thews O, Mayer A, et al. Oxygenation status of gynecologic tumors: what is the optimal hemoglobin level? Strahlenther Onkol 2002;178:727–31. [DOI] [PubMed] [Google Scholar]
- [59].Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011;11:85–95. [DOI] [PubMed] [Google Scholar]
- [60].Kuemmerle NB, Rysman E, Lombardo PS, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther 2011;10:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yuan HY, Kuang SY, Zheng X, et al. Curcumin inhibits cellular cholesterol accumulation by regulating SREBP-1/caveolin-1 signaling pathway in vascular smooth muscle cells. Acta Pharmacol Sin 2008;29:555–63. [DOI] [PubMed] [Google Scholar]
- [62].Qin L, Yang YB, Tuo QH, et al. Effects and underlying mechanisms of curcumin on the proliferation of vascular smooth muscle cells induced by Chol:MβCD. Biochem Biophys Res Commun 2009;379:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Baker M. Deceptive curcumin offers cautionary tale for chemists. Nature 2017;541:144–5. [DOI] [PubMed] [Google Scholar]
- [64].Heger M. Drug screening: don’t discount all curcumin trial data. Nature 2017;543:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sahin K, Orhan C, Tuzcu M, et al. Chemopreventive and antitumor efficacy of curcumin in a spontaneously developing hen ovarian cancer model. Cancer Prev Res (Phila) 2018;11:59–67. [DOI] [PubMed] [Google Scholar]
- [66].Li B, Shi C, Li B, et al. The effects of curcumin on HCT-116 cells proliferation and apoptosis via the miR-491/PEG10 pathway. J Cell Biochem 2018;119:3091–8. [DOI] [PubMed] [Google Scholar]
- [67].Zhao Q, Guan J, Qin Y, et al. Curcumin sensitizes lymphoma cells to DNA damage agents through regulating Rad51-dependent homologous recombination. Biomed Pharmacother 2018;97:115–9. [DOI] [PubMed] [Google Scholar]
- [68].Yoshida K, Toden S, Ravindranathan P, et al. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis 2017;38:1036–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Liang Z, Lu L, Mao J, et al. Curcumin reversed chronic tobacco smoke exposure induced urocystic EMT and acquisition of cancer stem cells properties via Wnt/β-catenin. Cell Death Dis 2017;8:e3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mukherjee S, Debata PR, Hussaini R, et al. Unique synergistic formulation of curcumin, epicatechin gallate and resveratrol, tricurin, suppresses HPV E6, eliminates HPV+ cancer cells, and inhibits tumor progression. Oncotarget 2017;8:60904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jin G, Yang Y, Liu K, et al. Combination curcumin and (–)-epigallocatechin-3-gallate inhibits colorectal carcinoma microenvironment-induced angiogenesis by JAK/STAT3/IL-8 pathway. Oncogenesis 2017;6:e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Siviero A, Gallo E, Maggini V, et al. Curcumin, a golden spice with a low bioavailability. J Herb Med 2015;5:57–70. [Google Scholar]
- [73].Liu W, Zhai Y, Heng X, et al. Oral bioavailability of curcumin: problems and advancements. J Drug Target 2016;24:694–702. [DOI] [PubMed] [Google Scholar]
- [74].Zhongfa L, Chiu M, Wang J, et al. Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer Chemother Pharmacol 2012;69:679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Nelson KM, Dahlin JL, Bisson J, et al. The essential medicinal chemistry of curcumin: miniperspective. J Med Chem 2017;60:1620–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Singh PK, Kotia V, Ghosh D, et al. Curcumin modulates α-synuclein aggregation and toxicity. ACS Chem Neurosci 2012;4:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sehgal A, Kumar M, Jain M, et al. Piperine as an adjuvant increases the efficacy of curcumin in mitigating benzo(a)pyrene toxicity. Hum Exp Toxicol 2012;31:473–82. [DOI] [PubMed] [Google Scholar]
- [78].Sehgal A, Kumar M, Jain M, et al. Modulatory effects of curcumin in conjunction with piperine on benzo(a)pyrene-mediated DNA adducts and biotransformation enzymes. Nutr Cancer 2013;65:885–90. [DOI] [PubMed] [Google Scholar]
- [79].Lotfi-Attari J, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Co-delivery of curcumin and chrysin by polymeric nanoparticles inhibit synergistically growth and hTERT gene expression in human colorectal cancer cells. Nutr Cancer 2017;69:1290–9. [DOI] [PubMed] [Google Scholar]
- [80].Duse L, Pinnapireddy SR, Strehlow B, et al. Low level LED photodynamic therapy using curcumin loaded tetraether liposomes. Eur J Pharm Biopharm 2018;126:126–241. [DOI] [PubMed] [Google Scholar]
- [81].Woraphatphadung T, Sajomsang W, Rojanarata T, et al. Development of chitosan-based pH-sensitive polymeric micelles containing curcumin for colon-targeted drug delivery. AAPS PharmSciTech 2018;19:991–1000. [DOI] [PubMed] [Google Scholar]
- [82].Chávez-Zamudio R, Ochoa-Flores AA, Soto-Rodríguez I, et al. Preparation, characterization and bioavailability by oral administration of O/W curcumin nanoemulsions stabilized with lysophosphatidylcholine. Food Funct 2017;8:3346–54. [DOI] [PubMed] [Google Scholar]
- [83].Oliveira ÉA, Lima DS, Cardozo LE, et al. Toxicogenomic and bioinformatics platforms to identify key molecular mechanisms of a curcumin-analogue DM-1 toxicity in melanoma cells. Pharmacol Res 2017;125:178–87. [DOI] [PubMed] [Google Scholar]
- [84].Chen J, Zhang L, Shu Y, et al. Curcumin analogue CA15 exhibits anticancer effects on HEp-2 cells via targeting NF-κB. Biomed Res Int 2017;2017:4751260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pignanelli C, Ma D, Noel M, et al. Selective targeting of cancer cells by oxidative vulnerabilities with novel curcumin analogs. Sci Rep 2017;7:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Liang B, Liu Z, Cao Y, et al. Mc37, a new mono-carbonyl curcumin analog, induces G2/M cell cycle arrest and mitochondria-mediated apoptosis in human colorectal cancer cells. Eur J Pharmacol 2017;796:139–48. [DOI] [PubMed] [Google Scholar]
- [87].Liu GY, Zhai Q, Chen JZ, et al. 2,2′-Fluorine mono-carbonyl curcumin induce reactive oxygen species-mediated apoptosis in human lung cancer NCI-H460 cells. Eur J Pharmacol 2016;786:161–8. [DOI] [PubMed] [Google Scholar]
- [88].Jin R, Xia Y, Chen Q, et al. Da0324, an inhibitor of nuclear factor-κB activation, demonstrates selective antitumor activity on human gastric cancer cells. Drug Des Devel Ther 2016;10:979–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Anthwal A, Singh K, Rawat MS, et al. Synthesis of 4-piperidone based curcuminoids with anti-inflammatory and anti-proliferation potential in human cancer cell lines. Anticancer Agents Med Chem 2016;16:841–51. [DOI] [PubMed] [Google Scholar]
- [90].Rajitha B, Nagaraju GP, Shaib WL, et al. Novel synthetic curcumin analogs as potent antiangiogenic agents in colorectal cancer. Mol Carcinogen 2017;56:288–99. [DOI] [PubMed] [Google Scholar]
- [91].Saberi-Karimian M, Katsiki N, Caraglia M, et al. Vascular endothelial growth factor: an important molecular target of curcumin. Crit Rev Food Sci Nutr 2019;59:299–312. [DOI] [PubMed] [Google Scholar]
- [92].Sugiyama S, Yoshino Y, Kuriyama S, et al. A curcumin analog, GO-Y078, effectively inhibits angiogenesis through actin disorganization. Anticancer Agents Med Chem 2016;16:633–47. [DOI] [PubMed] [Google Scholar]
- [93].Koo HJ, Shin S, Choi JY, et al. Introduction of methyl groups at C2 and C6 positions enhances the antiangiogenesis activity of curcumin. Sci Rep 2015;5:14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sun L, Liu J, Lin SS, et al. Potent anti-angiogenicactivity of B19–a mono-carbonyl analogue of curcumin. Chin J Nat Med 2014;12:8–14. [DOI] [PubMed] [Google Scholar]
- [95].Suresh R, Ali S, Ahmad A, et al. The role of cancer stem cells in recurrent and drug-resistant lung cancer. Adv Exp Med Biol 2016;890:57–74. [DOI] [PubMed] [Google Scholar]
- [96].Galluzzi L, Vitale I, Abrams JM, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 2012;19:107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Chen M, Zhou B, Zhong P, et al. Increased intracellular reactive oxygen species mediates the anti-cancer effects of WZ35 via activating mitochondrial apoptosis pathway in prostate cancer cells. Prostate 2017;77:489–504. [DOI] [PubMed] [Google Scholar]
- [98].Zhang X, Chen M, Zou P, et al. Curcumin analog WZ35 induced cell death via ROS-dependent ER stress and G2/M cell cycle arrest in human prostate cancer cells. BMC Cancer 2015;15:866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zou P, Zhang J, Xia Y, et al. ROS generation mediates the anti-cancer effects of WZ35 via activating JNK and ER stress apoptotic pathways in gastric cancer. Oncotarget 2015;6:5860–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Zou P, Xia Y, Chen T, et al. Selective killing of gastric cancer cells by a small molecule targeting ROS-mediated ER stress activation. Mol Carcinogen 2016;55:1073–86. [DOI] [PubMed] [Google Scholar]
- [101].Bixel K, Saini U, Kumar Bid H, et al. Targeting STAT3 by HO3867 induces apoptosis in ovarian clear cell carcinoma. Int J Cancer 2017;141:1856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hu Y, Zhao C, Zheng H, et al. A novel STAT3 inhibitor HO-3867 induces cell apoptosis by reactive oxygen species-dependent endoplasmic reticulum stress in human pancreatic cancer cells. Anticancer Drugs 2017;28:392–400. [DOI] [PubMed] [Google Scholar]
- [103].Feng C, Xia Y, Zou P, et al. Curcumin analog L48H37 induces apoptosis through ROS-mediated endoplasmic reticulum stress and STAT3 pathways in human lung cancer cells. Mol Carcinogen 2017;56:1765–77. [DOI] [PubMed] [Google Scholar]
- [104].Liu GY, Sun YZ, Zhou N, et al. 3,3′-OH curcumin causes apoptosis in HepG2 cells through ROS-mediated pathway. Eur J Med Chem 2016;112:157–63. [DOI] [PubMed] [Google Scholar]
- [105].Shao FY, Du ZY, Ma DL, et al. B5, a thioredoxin reductase inhibitor, induces apoptosis in human cervical cancer cells by suppressing the thioredoxin system, disrupting mitochondrion-dependent pathways and triggering autophagy. Oncotarget 2015;6:30939–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Spill F, Reynolds DS, Kamm RD, et al. Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotech 2016;40:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Chaffer L, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559–64. [DOI] [PubMed] [Google Scholar]
- [108].Kumar B, Yadav A, Hideg K, et al. A novel curcumin analog (H-4073) enhances the therapeutic efficacy of cisplatin treatment in head and neck cancer. PLoS One 2014;9:e93208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sun SW, Tong WJ, Guo ZF, et al. Curcumin enhances vascular contractility via induction of myocardin in mouse smooth muscle cells. Acta Pharmacol Sin 2017;38:1329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]


