Abstract
The aims of this study were to evaluate the prevalence of metabolic syndrome (MetS) and explore the association between sleep duration and MetS. This study enrolled 8 272 adults aged 18 years and older from 6 urban and 8 rural areas during 2013 to 2014in Henan China. Participants were interviewed about demographic characteristics, lifestyle factors and medical history, and physical measurements were performed. The relationships between sleep duration and MetS were evaluated and plotted by Restricted Cubic Spline Regression. The mean age was 51.5 years (SD 14.2) and 4 916 (59.4%) were female. The crude prevalence of MetS was 30.3% and the age-standardized rate was 23.6%. Men were more likely to have MetS than women (P = .01). MetS was positively associated with age, education, smoking, drinking, BMI and sleep duration, and seemed irrelevant to occupation and sedentary behavior. In terms of individual component of MetS, high blood pressure was the most prevalent component for both men and women, while the lowest prevalent was high triglycerides in men and for women was low high-density lipoprotein cholesterol (HDL-C). There was a U-shaped relationship between sleep duration and MetS and its components. Sleep duration <6 hours or >9 hours were associated with higher risk of MetS (OR from 1.10 to 2.15). The MetS was prevalent, and more than half of total adult population was suffering from high blood pressure. Sleep duration may be a determinant of metabolic health. Both short (<6 hours) and long sleep duration (>9 hours) was linked to an increased risk of MetS.
Keywords: cross-sectional study, metabolic syndrome, sleep duration
1. Introduction
Metabolic syndrome is a cluster of risk factors that include abdominal obesity, elevated blood pressure, elevated glucose, elevated triglycerides, and reduced HDL cholesterol, which is linked to an increased risk of developing cardiovascular disease and diabetes mellitus.[1] MetS has been one of the major public-health challenges worldwide, and its estimated prevalence is about one quarter of the world population.[2] National Health and Nutrition Examination Survey data reported overall prevalence of the metabolic syndrome in the United States was 33% from 2003 to 2012 and predicted nearly 35% of adults and 50% of aged 60 years or older had MetS.[3] Data from 9 European population-based studies suggested 41% of the men and 38% of the women had MetS.[4] A nationally representative study in China showed the overall prevalence in the year 2010 was 33.9%.[5] The metabolic syndrome has a multifactorial causation. Sleep has important function in homeostasis maintenance of internal environment, which can regulate physiological, hormonal and psychological processes.[6–8] These hormonal changes may contribute to a range of adverse health outcomes, including hypertension, diabetes, cancer, depression, and mortality.[9–13] Previous epidemiologic studies have shown sleep duration had increased risk of MetS.[14–16] However, these results were inconsistent, which had been reflected in a recent meta-analysis.[17] The potential reasons may be due to ethics disparity, different geographic distribution, socio-demographic factors, as well as the diagnostic criteria.[5,18] In the study, our aim was to investigate the prevalence of MetS and the relationship between sleep duration and MetS based on cross-sectional survey conducted in 2013 to 2014 in the central part of China.
2. Methods
2.1. Study population
Data for this analysis was derived from China Chronic Disease and Risk Factors Surveillance Study. The detailed information of surveillance survey had been published previously in detail.[19–21] The survey took place between June 2013 and April 2014.
In the first stage, 14 (6 urban and 8 rural) regions were selected from a total of 159 counties referring to the proportion of urban and rural counties. In the second stage, 4 urban subdistricts or rural townships were involved at each counties county according to the probability proportional to the size of their population. In the third stage, 3 communities or villages were selected at each subdistrict or townships township with a probability proportional to the size of their population. At last, the household composition information was obtained from the government household registration system, and a sample of at least 50 households was randomly drawn. A Kish selection table was used to randomly select only 1 person aged 18 years or older from each household. Only persons who had been living at their current residence for more than half a year were eligible to participate. These households who could not be accessed during 3 attempts to visit on 3 days were replaced by another household with a similar family structure in the same village or residential area. The overall response rate was 98.8% (the cooperation rate was 92.6% and the replacement rate was 6.2%). The study protocol was approved by the ethical review committee of Centre of Disease Control and Prevention. All participants gave written informed consent for participating in this survey.
2.2. Data collection
Trained interviewers performed a door-to-door and face-to-face interview, using a comprehensive questionnaire including demographic characteristics, lifestyle factors including smoking, alcohol use, physical activities, and medical history. Physical measurements were recorded using standard methods. Individuals were asked to be fasting for 10 to 12 hours before blood extraction.
All participants were assessed at a central survey site in residential areas. Blood pressure was measured 3 times at 1-minute interval with an automated electronic device (OMRON HEM-1300, Omron, Japan), after participants had rested in the seated position for at least 5 minutes. The mean of these readings was used in all analyses. Standing height and weight were measured to the nearest 0.1 cm or 0.1 kg without heavy clothing. Body mass index (BMI) was calculated as weight in kilograms divided by standing height squared in meters (kg/m2). Waist circumference was measured in the horizontal plane midway between lowest rib and the iliac crest. Fast blood samples were also collected for all participants and the OGTT was performed to drink a solution containing 75 g of glucose for only non-diabetic individuals. Serum samples were centrifuged to store within 2 hours and shipped at -80°C to the central laboratory. Plasma glucose was measured by using glucose oxidase method or hexokinase method under a stringent quality control method. Serum lipid profile was tested by using an automated clinical chemistry analyzer (ROCHE DIAGNOSITICS, USA). All study laboratories completed a standardization and certification program.
2.3. Definition of metabolic syndrome and covariate
The MetS was defined according to Chinese guideline for type 2 diabetes.[22,23] MetS was diagnosed when 3 or more of the following criteria were met:
-
1.
abdominal obesity: waist circumference ≥ 90 cm for men and ≥ 85 cm for women;
-
2.
high blood glucose: fasting plasma glucose level ≥6.1 mmol/L or 2-hour plasma glucose (2hPG) level ≥7.8 mmol/L or previously diagnosed type 2 diabetes;
-
3.
high blood pressure: blood pressure ≥130/85 mm Hg or drug treatment for hypertension;
-
4.
high triglycerides: fasting triglycerides level ≥1.70 mmol/L;
-
5.
low HDL-C: HDL-C level <1.04 mmol/L.
Following questions were surveyed by collecting the information on sleep status: “On average, how many hours and minutes do you sleep per day?” Sedentary behaviors were extracted from: “How many hours and minutes do you usually sit, lean, or lie except sleep per day?” Based on the status of self-reported, 3 categories of smoking status were defined: never-smoker (participants who reported not smoking), current smoker (participant who reported ever smoking at least 1 cigarette or who had stopped smoking <6 months), and former smoker (participants who had stopped >6 months). Based on the frequency of alcohol drinking during the past year, participants were classified into 4 main drinking categories: never-drinker (participants who reported not drinking), low risk drinker (participants who reported drinking <41 g/day in men or <21 g/day in women), hazardous drinker (participants who reported drinking 41 to 60 g/day in men or 21 to 40 g/day in women) and harmful drinker (participant who reported drinking ≥61 g/day in men or ≥41 g/day in women).[24] Based on the guidelines for prevention and control of overweight and obesity in Chinese adults, individuals were categorized into 4 groups: underweight, normal weight, overweight and obesity, and the corresponding cut off points were 18.5, 24 and 28 kg/m2.[25]
2.4. Statistical analysis
We excluded 112 participants with missing information on sex, marital status, education, occupation and metabolic data, and those with extreme values of sleep duration (Fig. 1). Finally, total participants 8 272 participants were included in our analyses. Demographic and metabolic features were showed based on gender using percentages for categorical variables and means ± SD for continuous variables. Prevalence of MetS and its components were calculated for the overall population and different subgroups, such as age groups, gender, BMI groups, sedentary behaviors and sleep duration. Sleep duration was classified as <6, 6–9, and >9 hours. Sedentary time behavior was divided into <3, 3–6, and > 6 hours. The relationships between sleep duration and MetS were evaluated by Restricted Cubic Spline Regression, adjusting for age, sex, education, occupation, smoking category, alcohol intake, physical activities, and BMI. The number of dots was chosen to 3 according to criteria to balance best fit and overfitting. MetS rate standardization was done using Henan province population in 2010. All the statistical analyses were performed with SAS version 9.2 (SAS Institute Inc). Figures used Stata 12.0 version (StataCorp, College Station, TX, USA).
Figure 1.
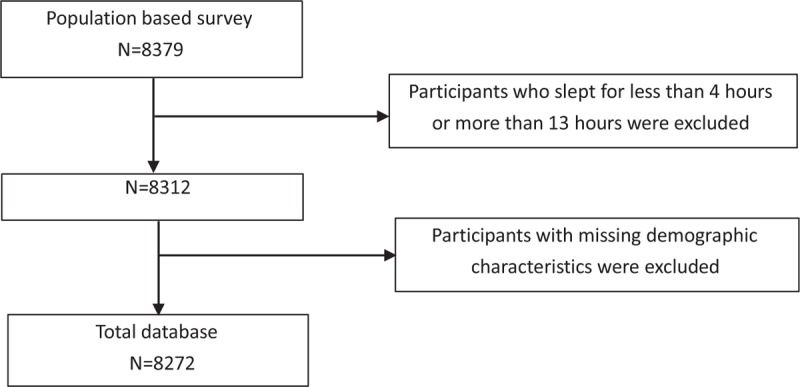
Flow diagram.
3. Results
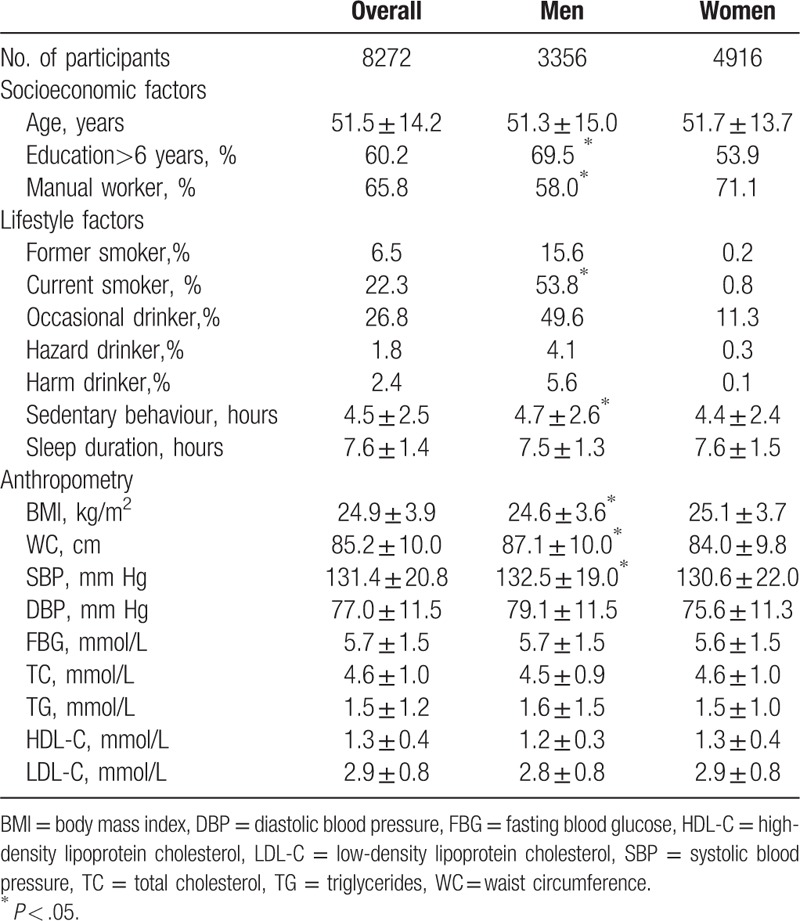
Totally 8272 participates were included in the analyses. The mean age was 51.5 years (SD 14.2) and 4 916 (59.4%) was female. Overall, 60.2% participates had been educated for more than 6 years, and 65.8% participates were manual workers. Among male participants, 53.8% were current smokers and 5.6% were harm drinkers, whereas the corresponding were 0.8% and 0.1% in women. Men had higher waist circumference and systolic blood pressure than women, nevertheless a lower BMI level. More detailed characteristics were shown in Table 1.
Table 1.
Baseline characteristics of the study population by gender.
The estimated prevalence of MetS was 30.3% (95% CI, 29.3 to 31.3), and the age-standardized rate was 23.6%. Men are more likely to have MetS than women (P = .01).
MetS was positively associated with age, education, smoking, drinking, BMI and sleep duration, and seem irrelevant with occupation and sedentary behavior (Table 2).
Table 2.
Covariate analysis of metabolic syndrome and its components.

In terms of individual component of MetS, high blood pressure was the most prevalent component for both men and women, while the lowest prevalent was high triglycerides in men and for women was low HDL-C. Compared to participants with less than 60 years old, the prevalence of components were higher in those aged 60 or over, except for low HDL-C. More than 50% of participants were suffering from high blood pressure, and one quarter was suffering from high triglycerides and low HDL cholesterol. Abdominal obesity was more common in women than in men (39.9% vs 46.5%), whereas high blood pressure, high triglycerides, and low HDL-C were more common in males than that in females (55.7% vs 50.2%, 29.0% vs 26.0%, 32.3% vs 21.6%). BMI was correlated with MetS components, about 2 times in obesity adult than that in normal and underweight group. Participants with lower sedentary behaviors had higher prevalence of high blood glucose and Low HDL-C, whereas those with short sleep duration had greater risks (Table 2).
In order to explore the relationship between sleep duration and MetS, we used restricted cubic splines regression to calculate and visualize the relationship (Fig. 2). There was a U-shaped relationship between sleep duration and MetS, and sleep duration <6 hours or >9 hours was associated with the high risk of MetS (OR from 1.10 to 2.15). In term of individual components of MetS, there was a similar trend in participants that sleep more than 9 hours. However, there was no association between participants with sleep duration <6 hours and abdominal obesity, blood pressure, triglycerides, and HDL-C.
Figure 2.

Association of sleep duration and metabolic syndrome and its components by using method of restricted cubic splines, adjusted by age, sex, education, occupation, smoking category, alcohol intake, physical activities, and BMI.
4. Discussion
Although the metabolic syndrome definition of American heart association (ADA), US national Cholesterol Education Programme Adult Treatment Panel III (ATP III), and International Diabetes Federation (IDF) were used worldwide, the Chinese Diabetes Society criteria was adopted in our analysis in view of ethnic difference. Our study provided the latest estimate of MetS prevalence among subjects aged 18 and older in the Henan province. The age-standardized prevalence of MetS was 23.6%, which indicated about 30 million adults suffered from metabolic syndrome. More than half of total adult population suffered from high blood pressure, and two fifths of participants had abdominal obesity while about one third of participants had others components. High blood pressure, high triglycerides, and low HDL-C were more prevalent in men than in women.
Asia-Pacific region was facing a significant epidemic of MetS and nearly 1/5th of the adult population or more were affected by MetS in most countries.[26] A meta-analysis with a total population of 226,653 subjects suggested a high prevalence of MetS in the range of 13.2% to 46.3% from 2005 to 2015 in 35 cross-sectional studies in mainland China.[27] Much earlier, the InterASIA study, a cross-sectional survey conducted in 2000 to 2001, revealed an age-standardized prevalence of MetS is 9.8% in men and 17.8% in women.[18] The China CDC demonstrated the prevalence of MetS was 33.9% among adults aged 18 and older in mainland China, based on the 2010 China Noncommunicable Disease Surveillance.[5] National Health and Nutrition Examination Survey reported metabolic syndrome prevalence increased from 25.3% to 34.2% during 1988 to 2012, and more than a third of all US adults met the definition and criteria for MetS in 2012.[28] The prevalence of the MetS is increasing rapidly over the past decades throughout the world and MetS is much prevalent in US than that in central China. The potential reasons may be as follows: aging population, rapid urbanization, nutrition transition, and increasingly obesity or sedentary lifestyles or ethnicity. Several surveys demonstrated that MetS is associated with an increased incidence and mortality of cardiovascular disease[29] and also associated with all-cause mortality or cancer mortality.[30] Consequently, population-wide policies should be implemented for individual-level screening and treatment.
On the other hand, the association of sleep duration and MetS also varied in different surveys. A U-shaped association between sleep duration and MetS was found in our study. A recent meta-analysis of cross-sectional studies explored a dose-response relationship between short sleep duration and MetS. A 1.5 odds for suffering MetS was found in those who slept less than 5 hours. Oppositely, long sleep was not associated with metabolic disorder.[17] A cohort study of 162,121 adults investigated short sleep duration increased the risk for MetS by 9% in healthy adults and long sleep decreased the MetS after 787,983 person-years of follow-up.[31] A summary of twelve cross-sectional studies and 3 cohort studies showed that short and long sleep durations were risky behaviors for increasing the risk of metabolic syndrome.[16]
Causal mechanisms relating sleep duration and MetS included circadian regulation of energy metabolism and hormone secretion.[6,7,32] Sleep rhythms disruption has been related to impaired metabolism and seems to influence the pathogenesis of metabolic diseases. Changes in the timing and sleep duration could also disrupt circadian rhythmicity and autonomic balance, disturbing the diurnal cardiac output rhythm, and increasing blood pressure variability.[33,34] Sleep restriction played a key role in the regulation of glucose and was associated with glucose clearance reduction and glucose production elevation, increasing glucose production, and incident diabetes.[35,36] The relationship between sleep duration and obesity, type 2 diabetes, hypertension may be mediated through changes in dietary intake. Short sleepers may have irregular eating behavior and have higher total energy intake and total fat intake.[37] Low-grade inflammation was also activated during short sleep.[38] Mechanisms underlying the association between long sleep duration and metabolic health were unclear, and future studies are required to explain the role of comorbidities and confounding factors. It may be explained by comorbidity and confounding factors.
There are several limitations in this study. Firstly, it was a cross-sectional survey, which had restricted ability to address the causal relationship. This result should be consolidated in prospective cohort study. Secondly, we used self-reported sleep duration in our study, and this may be result in information bias. Thirdly, this study only contain 1 province, and there is uncertainty for extrapolation. At last, we did not take into account insomnia symptom. Insomnia symptoms are the most common sleep complaint and highly prevalent in individuals with short sleep duration. However, previous study showed the association between short sleep duration and metabolic syndrome were similar among participants with and without insomnia symptoms.[31]
5. Conclusion
The MetS was prevalent in central China, more than half of total adult population was suffering from high blood pressure, and two fifths of participants had abdominal obesity. This study demonstrates that sleep duration may be a determinant of metabolic health. Both short (<6 hours) and long sleep duration (>9 hours) were linked to an increased risk of MetS.
Acknowledgments
We thank the participants and the regional CDC staff for conducting the survey.
Author contributions
Data curation: Li Gao, Minjie Qi, Shixian Feng.
Funding acquisition: Gang Zhou.
Investigation: Lei Fan, Zilong Hao.
Methodology: Lei Fan, Zilong Hao.
Resources: Li Gao, Minjie Qi, Shixian Feng.
Supervision: Gang Zhou.
Validation: Zilong Hao.
Writing – original draft: Lei Fan.
Lei Fan orcid: 0000-0003-0789-9226.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, DBP = diastolic blood pressure, FBG = fasting blood glucose, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, MetS = metabolic syndrome, OR = odds ratio, SBP = systolic blood pressure, TC = total cholesterol, TG = triglycerides, WC = waist circumference.
How to cite this article: Fan L, Hao Z, Gao L, Qi M, Feng S, Zhou G. Non-linear relationship between sleep duration and metabolic syndrome: A population-based study. Medicine. 2020;99:2(e18753).
LF and ZH contributed equally to this study.
We declare no competing interests.
This work was supported by programme grants from Science and Technology Department of Henan Province (182102311132), National Major Public Health Services and National Key Research and Development Project (2018YFC1311702).
We declare no competing interests.
References
- [1].Grundy SM. Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol 2005;25:2243–4. [DOI] [PubMed] [Google Scholar]
- [2].Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep 2018;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aguilar M, Bhuket T, Torres S, et al. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015;313:1973–4. [DOI] [PubMed] [Google Scholar]
- [4].Gao W. Does the constellation of risk factors with and without abdominal adiposity associate with different cardiovascular mortality risk? Int J Obes (Lond) 2008;32:757–62. [DOI] [PubMed] [Google Scholar]
- [5].Lu J, Wang L, Li M, et al. Metabolic syndrome among adults in China: The 2010 China Noncommunicable Disease Surveillance. J Clin Endocrinol Metab 2017;102:507–15. [DOI] [PubMed] [Google Scholar]
- [6].Potter GD, Skene DJ, Arendt J, et al. Circadian rhythm and sleep disruption: causes, metabolic consequences, and countermeasures. Endocr Rev 2016;37:584–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol 2012;349:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Selvi Y, Kandeger A, Boysan M, et al. The effects of individual biological rhythm differences on sleep quality, daytime sleepiness, and dissociative experiences. Psychiatry Res 2017;256:243–8. [DOI] [PubMed] [Google Scholar]
- [9].Guo X, Zheng L, Wang J, et al. Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta-analysis. Sleep Med 2013;14:324–32. [DOI] [PubMed] [Google Scholar]
- [10].Wang J, Kwok MK, Au YS, et al. Sleep duration and risk of diabetes: Observational and Mendelian randomization studies. Prev Med 2019;119:24–30. [DOI] [PubMed] [Google Scholar]
- [11].Shen J, Chrisman M, Wu X, et al. Sleep duration and risk of cancer in the Mexican American Mano-a-Mano Cohort. Sleep Health 2019;5:78–83. [DOI] [PubMed] [Google Scholar]
- [12].Ouyang P, Sun W. Depression and sleep duration: findings from middle-aged and elderly people in China. Public Health 2019;166:148–54. [DOI] [PubMed] [Google Scholar]
- [13].Cai H, Shu XO, Xiang YB, et al. Sleep duration and mortality: a prospective study of 113 138 middle-aged and elderly Chinese men and women. Sleep 2015;38:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jike M, Itani O, Watanabe N, et al. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Med Rev 2018;39:25–36. [DOI] [PubMed] [Google Scholar]
- [15].Xi B, He D, Zhang M, et al. Short sleep duration predicts risk of metabolic syndrome: a systematic review and meta-analysis. Sleep Med Rev 2014;18:293–7. [DOI] [PubMed] [Google Scholar]
- [16].Ju SY, Choi WS. Sleep duration and metabolic syndrome in adult populations: a meta-analysis of observational studies. Nutr Diabetes 2013;3:e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Iftikhar IH, Donley MA, Mindel J, et al. Sleep duration and metabolic syndrome. an updated dose-risk metaanalysis. Ann Am Thorac Soc 2015;12:1364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gu D, Reynolds K, Wu X, et al. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet 2005;365:1398–405. [DOI] [PubMed] [Google Scholar]
- [19].Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017;317:2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang LM, Zhang M, Li YC, et al. Scheme of the Chinese chronic non-communicable disease and risk factor surveillance. Zhonghua Yu Fang Yi Xue Za Zhi 2018;52:191–4. [DOI] [PubMed] [Google Scholar]
- [21].Zhao ZP, Wang LM, Li YC, et al. Provincial representativeness assessment of China Non-communicable and Chronic Disease Risk Factor Surveillance System in 2013. Zhonghua Yu Fang Yi Xue Za Zhi 2018;52:165–9. [DOI] [PubMed] [Google Scholar]
- [22].Chinese DS. China guideline for type 2 diabetes (2013). Chin J Frontiers of Med Sci (Electronic Version) 2015;7:26–89. [Google Scholar]
- [23].Chinese DS. Metabolic Syndrome Cooperative Study Group: the suggestion about metabolic syndrome from Chinese diabetes society. Chin J Diabetes Mellitus 2004;12:156–61. [Google Scholar]
- [24].World Health Organization. International Guide for Mnitoring Acohol Consumption and Rlated Harm. 2000;Geneva: WHO, 54. [Google Scholar]
- [25].Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci 2004;17 Suppl:1–36. [PubMed] [Google Scholar]
- [26].Ranasinghe P, Mathangasinghe Y, Jayawardena R, et al. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: a systematic review. BMC Public Health 2017;17:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li R, Li W, Lun Z, et al. Prevalence of metabolic syndrome in Mainland China: a meta-analysis of published studies. BMC Public Health 2016;16:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the united states, national health and nutrition examination survey, 1988–2012. Prev Chronic Dis 2017;14:E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Papakonstantinou E, Lambadiari V, Dimitriadis G, et al. Metabolic syndrome and cardiometabolic risk factors. Curr vasc pharmacol 2013;11:858. [DOI] [PubMed] [Google Scholar]
- [30].Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002;288:2709–16. [DOI] [PubMed] [Google Scholar]
- [31].Deng HB, Tam T, Zee BC, et al. Short sleep duration increases metabolic impact in healthy adults: a population-based cohort study. Sleep 2017;40:1–0. [DOI] [PubMed] [Google Scholar]
- [32].Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metab 2018;84:11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gangwisch JE. A review of evidence for the link between sleep duration and hypertension. Am J Hypertens 2014;27:1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang Y, Mei H, Jiang YR, et al. Relationship between duration of sleep and hypertension in adults: a meta-analysis. J Clin Sleep Med 2015;11:1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999;354:1435–9. [DOI] [PubMed] [Google Scholar]
- [36].Shan Z, Ma H, Xie M, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 2015;38:529–37. [DOI] [PubMed] [Google Scholar]
- [37].Dashti HS, Scheer FA, Jacques PF, et al. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv Nutr 2015;6:648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol 2007;5:93–102. [DOI] [PubMed] [Google Scholar]


