Abstract
Detection of the chronic kidney disease (CKD) progression can begin early intervention to improve the prognosis of severe non-alcoholic fatty liver disease (NAFLD). This bi-directional cross-sectional study evaluates the roles of fatty acid-binding protein (FABP) and retinol binding protein (RBP4), which are produced from inflamed liver, adipose tissue and immune cells, for the prediction of CKD progression in severe NAFLD. Ninety severe NAFLD patients with hypertension and proteinuria (NAFLD+HTN+) were enrolled and divided into CKD (n = 39) and non-CKD groups (n = 51). Among 39 NAFLD+HTN+ patients, 18 cases were categorized as CKD progression group. In comparison with CKD stable group (n = 21), the positive correlation between fold change values of hepatic fibrotic score (KPa), urinary FABP4 or urinary RBP4 versus severity of albuminuria were noted among CKD progression group. On multivariate analysis, high body mass index (BMI, >25 kg/m2), high hepatic fibrosis score (>9.5 KPa), high urinary level of vascular cell adhesion molecule-1 (VCAM-1, >2239 μg/g cr), high urinary level of FABP4 (>115 ng/g cr) and high urinary level of RBP4 (>33.5 mg/g cr) are 5 independent predictors for progressive CKD during 24 months of follow-up. Synergetic effect was noted among these 5 risk factors for the prediction of CKD progression in NAFLD+HTN+ patients. The in vitro experiments revealed that both FABP4 and RBP4 directly enhanced albumin-induced ER stress and apoptosis of human renal tubular epithelial cell line HK-2 cells and human podocytes cell lines. Through clinical and experimental approaches, this study revealed new 5 synergetic predictors including high BMI, hepatic fibrosis score, urinary level of VCAM-1, urinary level of FABP4 and RBP4, for the CKD progression in severe NAFLD patients with hypertension and proteinuria.
Keywords: albuminuria, chronic kidney disease, fatty acid-binding protein, non-alcoholic fatty liver disease, retinol binding protein
1. Introduction
Chronic kidney disease (CKD) is associated with high morbidity, mortality, and medical costs.[1] Patients with moderate and severe non-alcoholic fatty liver disease (NAFLD) are associated with risk of hypertension.[2] Systemic hypertension causes intraglomerular hypertension that leads to glomerular hypertrophy, glomerulosclerosis, and loss of kidney function. High prevalence of CKD and hypertension had been reported among NAFLD patients.[2,3]
C-reactive protein (CRP) expressed on endothelium can induce the expression of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1).[4] Elevated CRP, ICAM-1, and VCAM-1 levels are strong independent predictors of hypertension risk.[4,5] Active secretion of cytokines by the fatty liver can interfere with blood pressure homeostasis. Inflammatory markers such as CRP, ICAM-1, and VCAM-1 are produced from the liver in response to stimulation of interleukin-6 and tumor necrosis factor-α.[4,5] The presence of severe NAFLD and hypertension are independently associated with CKD progression.[6,7]
Actually, the severe NAFLD is the most rapidly growing indication for simultaneous liver kidney transplantation with poor renal outcomes.[8] A recent 6.5 years follow-up study reported that the decline in eGFR was greater in patients with higher NAFLD fibrosis score, with proteinuria, and with hypertension than those without aforementioned risk factors.[6–9]
Estimation of GFR by Cr-based equations lacks precision and accuracy due to non-renal determinants-such as non-renal removal, renal secretion, and variations in muscle mass-affecting serum Cr level. So, it is emergent to discovery surrogate liver-kidney pathogenic markers to early detection of CKD progression in cases with severe NAFLD.
Fatty acid binding protein 1/4 (FABP 1/4 from hepatocyte and adipocyte) and retinol-binding protein 4 (RBP4, from both hepatocyte and adipocyte) are highly expressed in liver and kidney of NAFLD patients.[10–15] High level of FABP and RBP predict the risk of hypertension, hepatic steatosis, inflammation, fibrosis, and renal dysfunction in NAFLD patients.[10–15] Silencing of FABP1 ameliorates hepatic steatosis and inflammation in NAFLD mice.[12] High circulating FABP4 level, which is an adipocyte and macrophages-derived bioactive molecule, had been reported in cases with NAFLD and hypertension.[13] Meanwhile, high circulating levels of FABP4 predicts severe inflammation and fibrosis in NAFLD patients.[14]
Urinary FABP had been reported as useful biomarkers for detection, monitoring, and prediction of the deterioration of renal dysfunction in case with and without diabetes.[15,16] Urinary levels of FABP1 are significantly higher in those with AKI than in hospitalized control patients without AKI as well as predict the need for acute renal replacement therapy.[16]
Persistent albuminuria has been associated with systemic inflammation and CKD progression.[17] High FABP4 plasma concentrations are associated with high plasma creatinine and low GFR in diabetes patients even in the absence of microalbuminuria or clinically relevant alterations of creatinine.[18] uFABP4 level is independently correlated with level of albuminuria and predicts yearly decline of eGFR.[18,19]
Adipocytes and hepatocytes-produced RBP4 is significantly increased in patients with severe NAFLD. [10,11,20] Additionally, urinary RBP is associated to high systolic blood pressure and CKD progression in cases with CKD.[20,21] Recent study reported that high systolic blood pressure, diastolic blood pressure, CRP, and serum RBP4 levels were independently associated with high serum FABP4 levels in NAFLD patients.
Taken together, this study aims to evaluate biomarkers including urinary adhesion molecule, FABP1, FABP4, and RBP4, for prediction the CKD progression in severe NFLAD patients with hypertension and proteinuria. Moreover, the correlation between these potential biomarkers with severity of albuminuria is analyzed in these patients. Apoptosis is one important pathway to induce the CKD progression.[22] Accumulation of albumin in kidney leads to accumulate mis-folded proteins within endoplasmic reticulum (ER) and induction of ER stress. Prolonged ER stress induced protein that promoting apoptosis.[23] Additionally, the direct effects of newly screened biomarkers on the albumin-induced kidney injury will evaluate on the in vitro experiments.
2. Methods
2.1. Study population
From October 2014 to March 2019, 330 consecutive adult patients (aged between 18 and 80 years), with abnormal liver function [>1.5X of ALT], abnormal renal function [serum creatinine >1.2 mg/dl in men, 0.9 mg/dl in women], abdominal ultrasound diagnosed severe fatty liver and hypertension (HTN) within past 3 months were screened. Finally, 120 severe NAFLD cases with HTN and proteinuria (PU, urine protein >2+ on urinalysis) were found (Fig. 1). Severe NAFLD was defined by abdominal ultrasound as well as the FibroScan) M probe with the cut-off values of controlled attenuation parameter (CAP) >325 dB/m in cases with abnormal liver function test.[24] HTN is defined as systolic blood pressure ≥140 mm Hg and diastolic blood pressure ≥90 mm Hg [4 measurements during 2 visits or current treatment of HTN with antihypertensive drugs. Diabetes was defined as a fasting blood sugar > 126 mg/dl or with medical record of history of diabetes After excluding of the 30 cases with any exclusion criteria listed below. Eventually, all 90 severe NAFLD+HTN+ cases with abnormal renal function were included for collection of clinical history of smoking habits, diabetes, CVD [acute myocardial infarction (AMI), stroke, peripheral arterial disease] registration, physical examination, average arterial pressure, and average body mass index (BMI) within 3 months after enrollment (Fig. 2).
Figure 1.
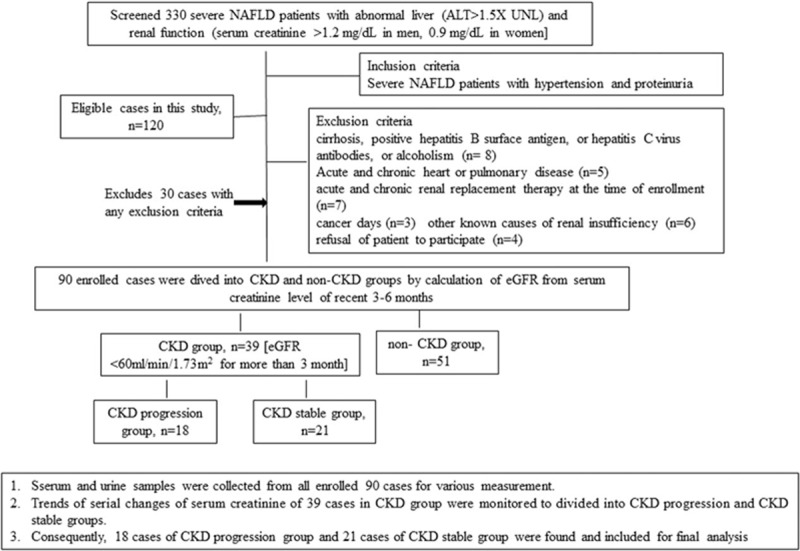
Flow chart for the process of screening and enrollment of severe NAFLD patients with hypertension (HTN) and proteinuria in this study. Three hundred thirty severe NAFLD patients with abnormal liver and renal function were screened and 120 patients met the inclusion criteria of having HTN and proteinuria. After exclusion of 30 patients having exclusion criteria, ninety severe NAFLD patients with HTN and proteinuria were enrolled, categorized, and follow-up.
Figure 2.
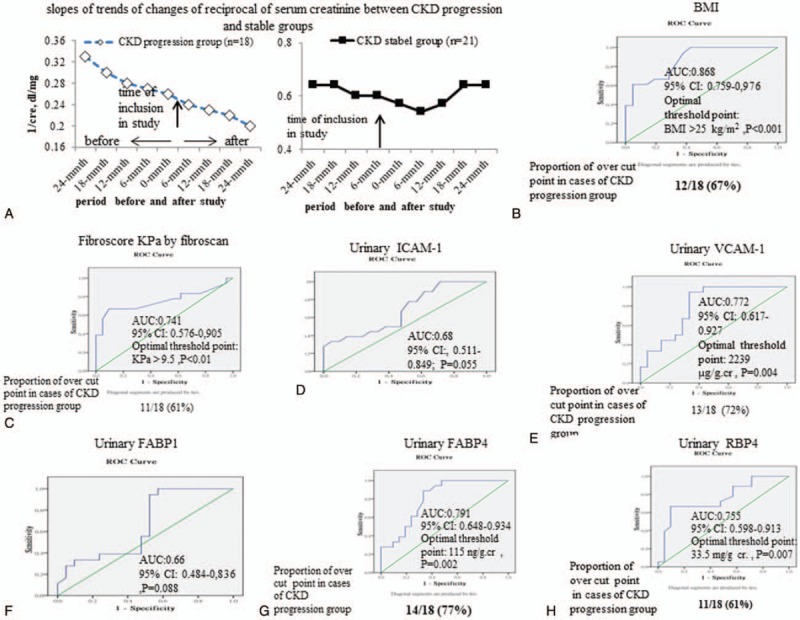
Biomarkers for the prediction of the CKD progression among severe NAFLD patients with HTN and proteinuria. (A) the average changing trends of mean values reciprocal of serum creatinine from 24 months before cases enrollment to 24 months after cases enrollment in CKD progression and CKD stable groups; Operating characteristic (ROC) curve and area under the ROC curve of (B) body mass index (BMI), (C) fibrotic score (KPa), (D-H) urinary level of sVCAM-1, sICAM-1, FABP1, FABP4and RBP, to predict the CKD progression or CKD stable. The rate of decline of renal function was evaluated by the slope of reciprocal serum creatinine (SRSC) every 6 months within 24 months before and after included in this study. The ability of urinary FABP1, FABP4, and RBP4 to predict progressive CKD was assessed using an ROC curve and the area under the curve (AUC) with 95% confidence intervals (CI) statistic. Optimal cutoffs were determined using the Youden index criterion for diagnosing CKD among severe NAFLD cases with hypertension.
Exclusion criteria were history of cancer, history of liver cirrhosis, positive hepatitis B surface antigen, or hepatitis C virus antibodies, alcohol intake ≥30 g/day in men or ≥20 g/day in women, missing information on alcohol intake, acute and chronic severe heart disease and pulmonary disease, other known causes of renal insufficiency such as infection, gastrointestinal bleeding, malnutrition, changing of body weight more than 5%, nephrotoxic agents, glomerulonephritis or urinary obstruction or receiving acute/chronic renal replacement therapy within 3 months before enrollment, follow-up of less than 24 months, immunological disorder and any other dermatological problems,[25] or a refusal to participate in the study.
2.2. Grouping of the severe NAFLD+HTN+ cases with renal dysfunctions
Further, 90 cases were divided into CKD (n = 39) and non-CKD (n = 51) groups. CKD was defined as estimated glomerular filtration rate (eGFR) <60 ml/minute/1.73 m2 or urine protein >2+ on urinalysis at the baseline examination.[6,9,11] For retrospective and prospective analysis of eGFR, recent stable serum creatinine levels either as an outpatient or a previous admission value within 24 months prior to and after enrollment were included. Patients gave written informed consent to participate in the study which was approved by the Clinical Investigation and Ethics Committee of our hospital (IRB number: 201509004AC, approved on 21/Sep/2015 and 2017–12-029BC).
2.3. Clinical, serologic and urinary laboratory assessments for NAFLD+HTN+ cases with CKD
In addition to clinical history of smoking habits, diabetes, CVD [acute myocardial infarction, stroke, registration, physical examination, average blood pressure, and average body mass index (BMI) were collected. Then, blood samples of 39 NAFLD+HTN+ cases were used to measure levels of CBC and biochemistry data, C-reactive protein (CRP), soluble intercellular adhesion molecule-1 (sICAM-1), and soluble vascular cell adhesion molecule-1 (sVCAM-1), fatty acid binding protein 1 (FABP1), FABP4, and retinol binding protein 4 (RBP4). These biomarkers were measured by commercial available ELISA kits (R&D systems, Minineapolis, MN; Lifespan, Biosciences; Thermo Fisher Scientific; Abcam, Cambridge, GB). First morning midstream specimens of spot urine (MSU) were collected in all NAFLD+HTN+ cases. All MSU specimens were centrifuged extensively to eradicate contamination with any urothelial or microbiological cell material. Supernatant was used for subsequent analysis. Samples were stored at −80°C until further processing for measurement of urinary albumin, fatty acid binding protein 1 (FABP1), FABP4, and retinol binding protein 4 (RBP4) level. Then, albuminuria was used as early indicator for renal injury as a mean value obtained from 3 MSU and expressed as the albumin (mg) to creatinine (mg) ratio (ACR).
2.4. Monitoring of renal function in NAFLD+HTN+ cases with CKD
The rate of decline of renal function was evaluated by the slope of reciprocal serum creatinine (SRSC) every 6 months within 24 months before and after included in this study (Figure 2A). Then, 39 enrolled cases with SKD were further sub-classified into CKD stable and CKD progression groups according to the changes of SRSC. CKD stable cases was defined as a slope of the regression line of less than −0.001 (dl mg−1 month−1) and the CKD progression cases were those with a slope of the regression line of more than −0.001 (dl mg−1 month−1). We compared the 2 groups with regards to clinical and laboratory parameters as the start of follow-up.
2.5. In vitro effects of FABP4 and RBP4 on albumin- induced HK cells/podocytes apoptosis
Significantly, incubation with incremental concentrations of bovine serum albumin (BSA, 25, 50, 75, 100 μg/ml) induced the significant apoptosis of HK-2 cells/podocytes which measured by MTT assay. A preliminary dose-finding experiment revealed that, among different concentrations (25, 50, 75, 100 μg/ml) of BSA, maximal apoptotic effects of BSA was noted at 100 μg/ml of BSA. After 2 hours of BSA (100 μg/ml) pretreatment, BSA-pretreated cells were incubated with increasing concentrations of human recombinant FABP4 (25, 50, 75, 100 μg/ml), or human recombinant RBP4 (25, 50, 75, 100 μg/ml) for 48 hours for dose response comparisons. Using MTS assay, cell viability test was assessed by the ability of metabolically active cells to reduce the tetrazolium salt to formazan compounds using MTS reagent (Promega, Madison, WI, USA). The absorbance of the samples was measured using a microplate reader at a 450 nm wavelength after 3 hours of incubation with MTS solution (0.19 mg/ml). The results are representative of 3 independent experiments. The percentage of cell viability of each treated group was compared to untreated group (Fig. 3).
Figure 3.
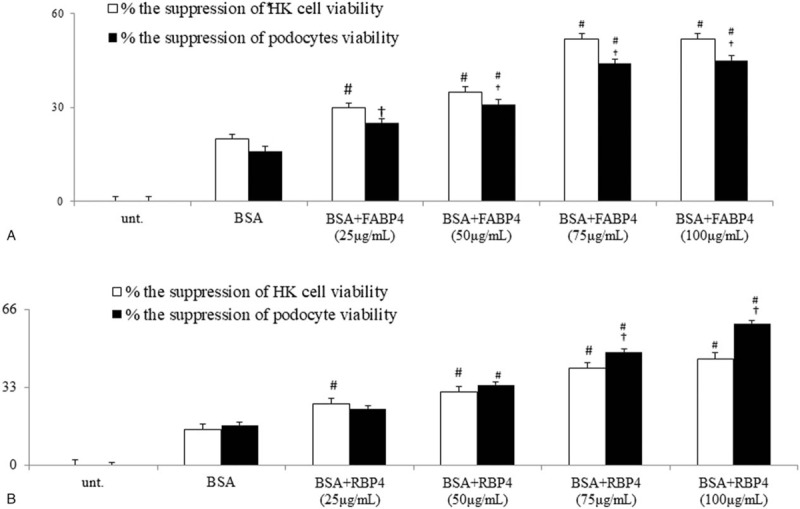
Direct in vitro effects of human recombinant FABP4 (hrFABP4) and hrRBP4 on the BSA (bovine serum albumin)-induced apoptosis and corresponding signals on cultured human proximal tubule epithelial cell line HK-2 cell and human podocyte cell line. (A-B). hrFABP4 and hrRBP4 significant dose-dependently increase the BSA-suppressed viability (MTT assay) of HK-2/podocytes; #P < .05 vs BSA group;†P < 005 vs HK-2 group.
2.6. Measurements of apoptotic activity on albumin-induced HK cells/podocytes apoptosis
Cell viability was determined using an MTT assay. Briefly, the HK cells/podocytes cells were seeded at a density of 5 × 103 cells per well into 96-well plates and, at the end of the aforementioned treatments, the cells were incubated with MTT solution (1 mg/ml final concentration stock solution in PBS per well) at 37°C for 4 hours. The medium was then removed, and the formazan crystals were dissolved with 150 μl DMSO. The absorbance at 570 nm was determined using a microplate reader (Spectra Fluo, Tecan, Sunrise, Austria). The experiments were repeated in triplicate and data were expressed as the percentages of suppression cell viability compared to the control. Cells were plated in 8-chamber glass slides. Cells were fixed in 4% paraformaldehyde and then analyzed using an Apop Tag in situ apoptosis detection kit (Chemicon, CA, USA). In terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-stained slides (×200), percentage of TUNEL-positive cells undergoing DNA fragmentation-related apoptosis were calculated by averaging 6 randomly selected areas of each slides. Alexa Fluor 488 annexin V/Dead Cell Apoptosis Kit (Invitrogen) was used to detect annexin V and PI double positive cells for evaluating caspase activation-related membrane translocation of phosphatidylserine in late apoptosis. For immunofluorescence (IF) staining, treated cells as aforementioned were fixed in paraformaldehyde followed by permeabilization with 0.025% digitonin in PBS. After washing the cells were subsequently incubated at RT with active cleaved caspase-3 (casp-3) antibodies, FITC-conjugated secondary antibody. After washing with PBS, optical section data for % of caspase-3 (+) area on each slide were evaluated.
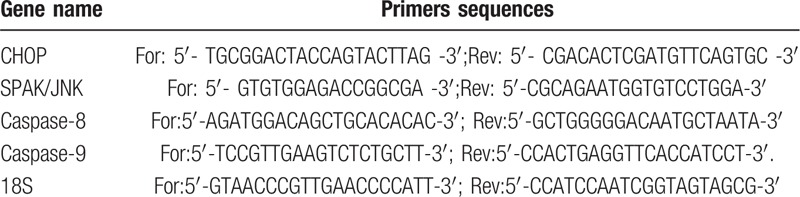
Using the same cells of albumin cytotoxicity experiments, Cell lysates of above mentioned cells were used for extraction of proteins [CHOP, phospho-MAPKp38, Bcl-2] and mRNAs [CHOP, SAPK/JNK, caspase-8, caspase-9] for ER stress and apoptosis-related markers (Table 1). These markers are the downstream signals of TNFα-related activation of apoptosis.
Table 1.
Primer of gene used for quantitative realtime PCR analysis.
2.7. Statistical analysis
Statistical analysis was performed using SPSS version 14 for Windows (SPSS Inc., Chicago, IL). Data are reported as mean (standard deviation, SD) or frequencies (%).
Differences for numerical variables were evaluated using the two-tailed independent Student t test or one-way analysis of variance. Comparisons of categorical variables between different groups were performed using the Pearson χ2 test or Fisher exact test.
Then, the ability of urinary FABP1, FABP4, and RBP4 to predict progressive CKD was assessed using an ROC curve and the area under the curve (AUC) with 95% confidence intervals (CI) statistic. Optimal cutoffs were determined using the Youden index criterion for diagnosing CKD among severe NAFLD cases with hypertension. The AUCs closer to 1 reflect more substantial differences between progressive CKD in severe NAFLD+HTN+ patients with and without high levels of urinary biomarkers.
Univariate and multivariate analyses were performed to test independent CKD risk factors predicting CKD by performing ANOVA, linear regression and binary logistic regressions, where applicable. Statistical significance was defined when “P < .05” in a “two-tailed” test with a 95% confidence interval. To evaluate the contributing effects of on presence of CKD, significant univariate risk factors were selected to enter in the multivariate regression analysis with an incrementally forward stepwise approach. The presence of high levels (higher than the cut-off value) of multivariate risk factors that predict progressive CKD, were coded “1” in the analysis, whereas the low levels of these values were coded “0”. When adding these CKD risk factors together in a multiple risk factors scale, severe NAFLD patients with HTN were further divided into different groups according to the number (0–2 and 3–4) of CKD risk factors. In logistic regression, the group with the greatest case number was defined as reference group.
3. Results
3.1. General information
Notably, 90 severe NAFLD cases with hypertension and proteinuria (NAFLD+HTN+) were noted among 330 severe NAFLD patients with abnormal liver and renal function (Fig. 1). Then, 39 (43%) CKD cases were found among the 90 enrolled severe NAFLD cases with hypertension and proteinuria (NAFLD+HTN+).
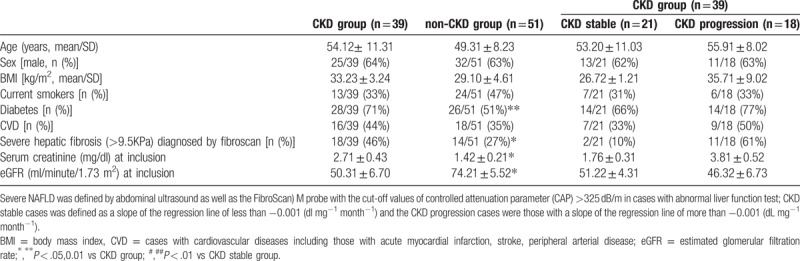
It is noteworthy that higher percentage of diabetes, much severe hepatic fibrosis, higher serum creatinine level and lower eGFR were noted among CKD group compared to non-CKD group (Table 2). Nonetheless, mean age, gender distribution, BMI, percentage of current smokers, CVD, and anti-HTN user at inclusion were not different between 2 groups.
Table 2.
Basal demographic data between different groups of severe NAFLD+HTN+ patient.
3.2. CKD progression group have much severe hepatic fibrosis and high levels of urine VCAM-1, FABP4, and RBP4 at inclusion
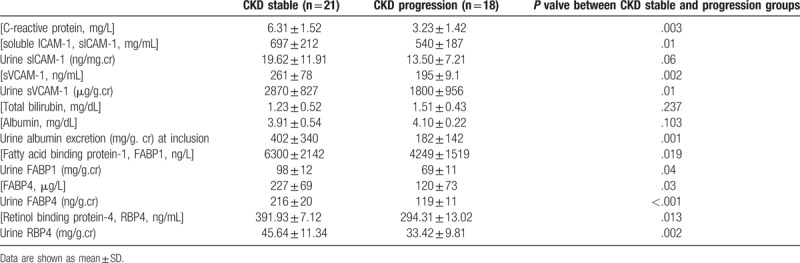
In comparison with CKD stable group, higher urine albumin excretion, higher BMI, higher percentage of CVD, higher serum creatinine level, lower eGFR, higher serum CRP, serum sICAM-1, serum sVCAM-1, serum, and urinary levels of FABP1, FABP4, and RBP4 were observed in CKD progression group (Tables 2 and 3). Nonetheless, the percentage of diabetes, serum total bilirubin level and serum albumin level were not different between the CKD progression and CKD stable groups.
Table 3.
Basal demographic data of severe NAFLD+HTN+ patients in CKD progression and CKD stable groups.
3.3. Prediction of the progression of CKD in severe NAFLD+HTN+ cases
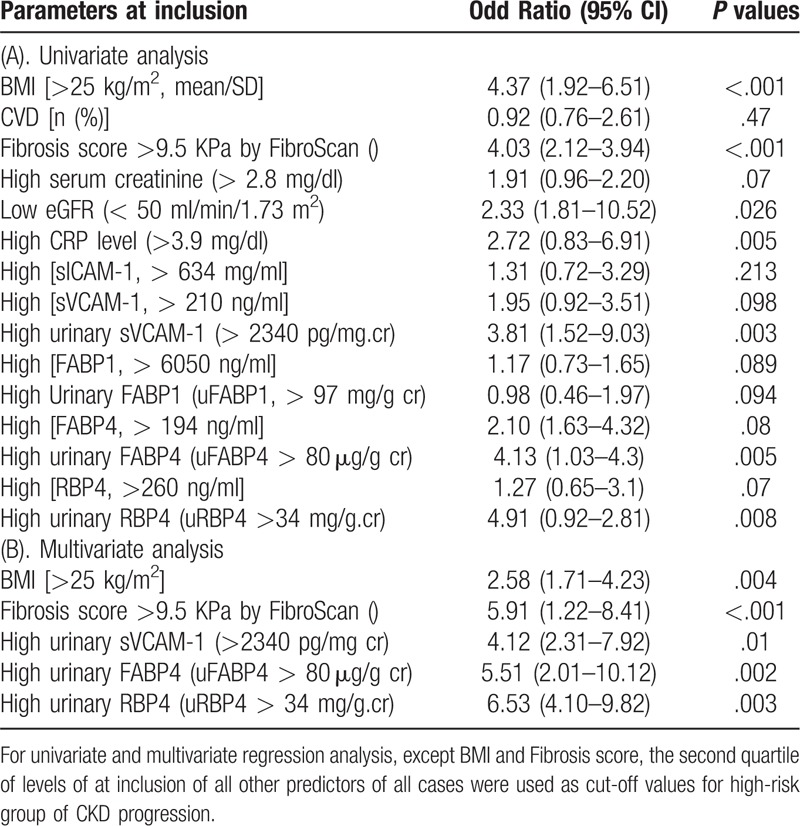
Figure 2A displayed that greater trend of decline in reciprocal serum creatinine was observed in CKD progression group than those in CKD stable groups. Then, ROC analysis was performed to find the predictability of candidate parameters for the progression of CKD (Fig. 2C-I). In ROC analysis, only 5 parameters including high BMI, severe hepatic fibrosis, high level of urinary sVCAM-1, high level of urinary FABP4, and high level of urinary RBP4 reach significance.
Meanwhile, in univariate analysis, significant predictors for the progression of CKD were high BMI, high fibrosis score, low eGFR, high serum CRP level, and high urinary levels of sVCAM-1, uFABP4, uRBP4 (Table 4). Further, in multivariate analysis, only high BMI, high fibrosis score, high level of urinary sVCAM-1, high level of urinary FABP4, and high level of urinary RBP4 are independent predictors for the progression of CKD across 24 months before and after inclusion (Table 4).
Table 4.
Regression analysis of predictive factors for the CKD progression in patients (n = 39) with severe NAFLD+HTN+.
Among these 5 significant risk predictors of CKD progression, the increasing trend of odd ratio (3.2 or 4) for cases having more than any 3 or 4 to 5 risk factors as compared to reference group (with OR = 1 for those with 0–2 risk factors) was found (Fig. 4A). These results indicated the synergetic effects of these 5 risk factors for the prediction of CKD progression in severe NAFLD patients with hypertension and proteinuria.
3.4. Correlation between the fold change value of 5 candidate predictors and albuminuria in CKD progression group
Significant positive correlation between fold change values of albuminuria and fold change values of hepatic fibrotic score (KPa), urinary FABP4 level, and urinary RBP4 level were found in cases with CKD progression (Fig. 4B-F). Fold changes were calculated by divided the value of each risk factor in CKD progression group by the mean value of CKD stable group.
Figure 4.
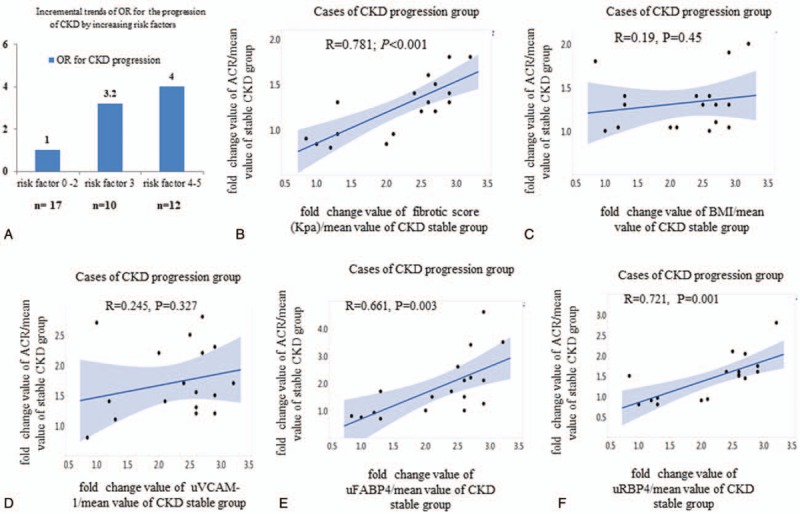
Synergetic effects of significant risk biomarkers for prediction of the CKD progression in severe NAFLD patients with HTN and proteinuria. (A) the relative risks of 0–2, 3, 4-5 risk biomarkers for prediction of CKD progression or CKD stable; (B) correlation between average fold change values of albuminuria and average fold changes of fibrotic score (KPa); (C) correlation between average fold change values of albuminuria and BMI; (D) correlation between average fold change values of albuminuria and uVCAM-1; (E) correlation between average fold change values of albuminuria and uFABP4; (F) correlation between average fold change values of albuminuria and uRBP4. Fold changes were calculated by divided the value of each risk factor in CKD progression group by the mean value of CKD stable group.
3.5. In vitro effects of FABP4 and RBP4 on BSA-induced apoptosis of HK-2 cells/podocytes.
Figure 3A-B revealed that both hrFABP4 and hrRBP4 dose-dependently enhanced the BSA-suppressed viability of HK-2 cells and podocytes. Figure 5A,C,E,F,I,J revealed similar trends of the enhancement of BSA-induced increases in percentages of caspase+, Tunel+, Annexin-V+PI+ HK-2 cells, and podocytes by concomitant incubation of hrFABP4 and hrRBP4. Correspondingly, the co-incubation of hrFABP4 and hrRBP4 have similar trends of increase in the expressions of ER stress and apoptosis markers including CHOP, SPAK/JNK, p38-MAPK, Bcl-2, caspase-3,-8,-9 proteins, and mRNAs in cell lysates of BSA-pretreated HK-2 cells and podocytes (Fig. 5B,D,G,H).
Figure 5.
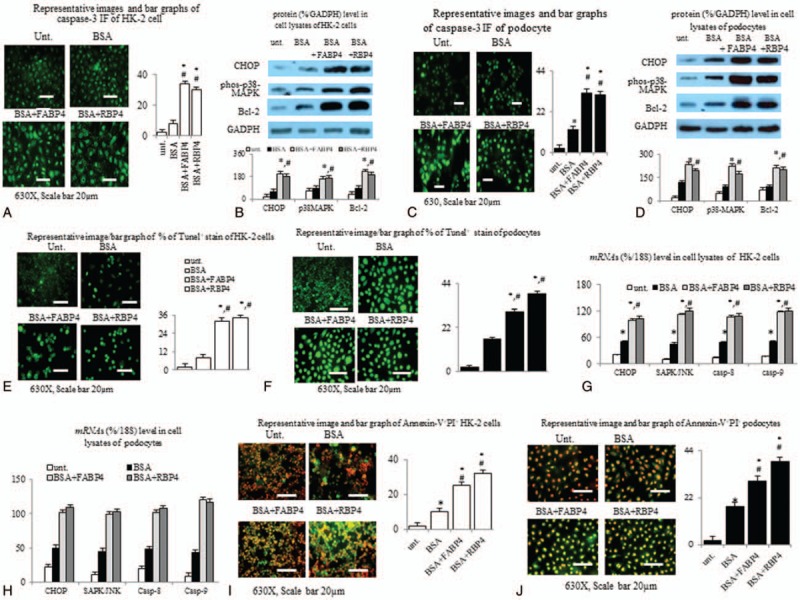
Direct in vitro effects of human recombinant FABP4 (hrFABP4) and hrRBP4 on the BSA (bovine serum albumin)-induced apoptosis and corresponding signals on cultured human proximal tubule epithelial cell line HK-2 cell and human podocyte cell line. Bar graphs and IF images of the percentage of (A,C) caspase-3 (+),(E,F)TUNEL(+) [early apoptosis] and (I,J) Annexin-V+PI+ [late apoptosis] of BSA-pretreated HK-2/podocytes that concomitantly incubation with hrFABP4 (75 μg/ml) and hrRBP4 (100 μg/ml); (B,D) Proteins and (G,H) mRNA levels of ER stress and apoptosis markers in cell lysates of BSA-pretreated HK-2 and podocytes. ∗P < .05 vs untreated (unt.) group; #P < .05 vs BSA group. The results are expressed as representative of 3 independent experiments. The percentage of cell viability of each treated group compared to untreated group were calculated.
4. Discussion
NAFLD, hypertension and proteinuria are common associated diseases with increasing long-term risk of CKD progression.[2,3,6,9,17] Detection of CKD at earlier stages of diseases offers the opportunity to initiate therapies known to attenuate CKD progression.[26,27] Treating individuals with early CKD has the potential to delay ESKD, especially among young and middle-aged individuals.[26,27] Our study investigate the clinical significance of urinary biomarkers by measurement with ambulatory spot urine samples because these samples were easy to obtain in the outpatient clinic and contamination of such samples is less than that in 24-hour urine collections.
Elevation of both serum and urinary VCAM-1 levels had been reported in patients with impaired renal function and NAFLD.[28] Recent study reported that serum VCAM-1 level predicts severe hepatic fibrosis in NAFLD patients.[29] In this study, both the severity of hepatic fibrosis and high urinary VCAM-1 are independent predictors for progressive CKD in severe NAFLD patients with hypertension and proteinuria. Persistent albuminuria has been associated with systemic inflammation and CKD progression.[17] So, it is reasonable to observe that both severe hepatic fibrosis and high urine VCAM-1 levels were significantly positive correlated with severity of albuminuria in severe NAFLD patients with hypertension and CKD.
In morbid obese NASH patients, the association between advanced fibrosis and decreases in eGFR, suggesting a common inflammatory link between liver and renal lesion.[3,30] Notably, the average BMI at inclusion in CKD progression group are significantly higher than that in CKD stable group. Further, the univariate and multivariate analysis indicated that BMI > 25 mg/cm2, cut-off value for overweight, is the independent predictor for CKD progression. Obesity has been reported as an independent risk factor of development and progression of CKD.[31] In obese patients with altered renal function, weight loss can improve proteinuria, albuminuria, and normalizes GFR.[32] Obesity is identified as a low-grade chronic inflammation state and weight control is a critical factor to reduce systemic inflammation.[33] In term of therapeutic intervention, weight control through pharmacological, surgical, lifestyle, and diet modification might reduce the CKD progression. Further prospective large-scale cohort studies are required to confirm this hypothesis.
Increased serum and urinary levels of FABP4 and RBP4 had been reported in cases with severe NALFD.[10,14,21,34] Obese individuals had significantly higher FABP4, RBP4, and VCAM-1 expressions compared with non-obese individuals.[20,35,36] FABP4, RBP4, and VCAM-1 are surrogate markers for systemic inflammation in patients with obesity, hypertension, CKD, and NAFLD.[9,11,14,21,22,36,37] Lifestyle modification to control body weight significantly reversed the raised FABP4, RBP4, and VCAM-1 levels in obese children and adolescents.[35,38] From previous and our studies, weight control to decrease inflamed adipose tissue may slow the rate of CKD progression in overweight NAFLD patients with hypertension and proteinuria. Nonetheless, detrimental effects of weight reduction had been reported in cases with CKD.[32,33] Thus, adequate estimation and monitoring of the fat mass by functional image during the process of weight control is crucial for avoid negative impacts on renal function of obese cases with CKD.
Notably, only around 60% of enrolled cases received anti-hypertension drugs at inclusion. It had been reported that adequate control of blood pressure can slow the progression of CKD. So, in addition to weight and diet control, optimal using of anti-hypertension agents is crucial to stable renal function in severe NAFLD patients with hypertension and CKD.
Induction of ER stress is the main mechanism for albumin induced the apoptosis of renal proximal tubular cell.[39] Podocyte injury is closely related to the progression of chronic kidney disease (CKD). Albumin overload in podocytes, specialized epithelial cells in the Bowman space, results in increased expression an ER stress marker and apoptosis.[40] So, it is reasonable to observe the albumin-induced apoptosis of HK-2 cells and podocytes in our in vitro experiments are associated with the up-regulation of corresponding ER stress and apoptotic cascades. FABP4 had been reported to induce apoptosis through stimulation of ER stress in human renal epithelial cells.[41] RBP4 also induced apoptosis in real tubular cells through activation of phosphorylation of JNK1 and p38 signal.[42] In addition caspases (-3,-8,-9) expression, ER stress-related DNA breakage in early apoptosis was investigated using an in situ TUNEL assay to detect the free ends of DNA after breakage in this study. For apoptosis, apoptotic cells have externalized membrane phosphatidylserine; therefore, annexin V, a calcium-dependent phospholipid-binding protein that binds to phosphatidylserine with high affinity. Overall, this study confirmed that the enhancement of albumin-induced and ER stress-mediated apoptosis by FABP4/RBP4 in human HK-2 and podocytes cell lines. These results indicated the potential roles of FABP4 and RBP4 as target for therapeutic intervention for CKD progression in severe NAFLD patients with hypertension and proteinuria.
In conclusion, this study reports high levels of liver and adipose tissue-derived inflammatory markers including urinary VCAM-1, urinary FABP4, and urinary RBP4 in severe NAFLD patients with hypertension and proteinuria. Nonetheless, large-scale study with larger patient's cohorts is required to continue before pharmacological development and clinical implication.
Acknowledgments
We especially thank Peng Chi-Yi, Ching-Han Huang, Lin Yu Chen, Ya-Wei Liu and Yi-Tsau Huang for their excellent technical supports.
Author contributions
Conceptualization: Yu-Lien Tsai, Chih-Wei Liu, Yi-Hsiang Huang, Han-Chieh Lin.
Investigation: Yu-Lien Tsai, Shiang-Fen Huang, Tzu-Hao Li, Ming-Chih Hou.
Writing – original draft: Yu-Lien Tsai, Ming-Wei Lin.
Writing – review & editing: Chia-Chang Huang, Ming-Chih Hou, Han-Chieh Lin.
Footnotes
Abbreviations: CKD = chronic kidney disease, FABP = fatty acid-binding protein, NAFLD = non-alcoholic fatty liver disease, RBP = retinol binding protein.
How to cite this article: Tsai YL, Liu CW, Huang SF, Yang YY, Lin MW, Huang CC, Li TH, Huang YH, Hou MC, Lin HC. Urinary fatty acid and retinol binding protein-4 predict CKD progression in severe NAFLD patients with hypertension: 4-year study with clinical and experimental approaches. Medicine. 2020;99:2(e18626).
This work was supported (in part) by the Molecular and Genetic Imaging Core/National Research Program for Genomic Medicine at National Yang-Ming University. This work was supported by grants MOST106-2511-S-010-001-MY3 from the National Science Council, and V107C-022 and by the Taipei Veterans General Hospital.
The authors report no conflicts of interest.
References
- [1].Go AS, Chertow GM, Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. [DOI] [PubMed] [Google Scholar]
- [2].Sung KC, Wild SH, Byrne CD. Development of new fatty liver, or resolution of existing fatty liver, over five years of follow-up, and risk of incident hypertension. J Hepatol 2014;60:1040–5. [DOI] [PubMed] [Google Scholar]
- [3].Mantovani A, Zaza G, Byrne CD, et al. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: a systematic review and meta-analysis. Metabolism 2018;79:64–76. [DOI] [PubMed] [Google Scholar]
- [4].Cottone S, Mulè G, Nardi E, et al. C-reactive protein and intercellular adhesion molecule-1 are stronger predictors of oxidant stress than blood pressure in established hypertension. J Hypertens 2007;25:423–8. [DOI] [PubMed] [Google Scholar]
- [5].Zhang DW, Huang XZ, Wu JH, et al. Effects of intercellular adhesion molecule-1 on renal damage in spontaneously hypertensive rats. Ren Fail 2012;34:915–20. [DOI] [PubMed] [Google Scholar]
- [6].Jang HR, Kang D, Sinn DH, et al. Nonalcoholic fatty liver disease accelerates kidney function decline in patients with chronic kidney disease: a cohort study. Sci Rep 2018;8:4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lea JP, Nicholas SB. Diabetes mellitus and hypertension: key risk factors for kidney disease. J Natl Med Assoc 2002;94: 8 Suppl: 7S–15S. [PMC free article] [PubMed] [Google Scholar]
- [8].Singal AK, Hasanin M, Kaif M, et al. Nonalcoholic steatohepatitis is the most rapidly growing indication for simultaneous liver kidney transplantation in the United States. Transplantation 2016;100:607–12. [DOI] [PubMed] [Google Scholar]
- [9].Sinn DH, Kang D, Jang HR, et al. Development of chronic kidney disease in patients with non-alcoholic fatty liver disease: A cohort study. J Hepatol 2017;67:1274–80. [DOI] [PubMed] [Google Scholar]
- [10].Zhou Z, Chen H, Ju H, et al. Circulating retinol binding protein 4 levels in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Lipids Health Dis 2017;16:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Domingos MA, Moreira SR, Gomez L, et al. Urinary retinol-binding protein: relationship to renal function and cardiovascular risk factors in chronic kidney disease. PLoS One 2016;11:e0162782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mukai T, Egawa M, Takeuchi T, et al. Silencing of FABP1 ameliorates hepatic steatosis, inflammation, and oxidative stress in mice with nonalcoholic fatty liver disease. FEBS Open Bio 2017;7:1009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maeda K, Cao H, Kono K, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab 2005;1:107–19. [DOI] [PubMed] [Google Scholar]
- [14].Milner KL, van der Poorten D, Xu A, et al. Adipocyte fatty acid binding protein levels relate to inflammation and fibrosis in nonalcoholic fatty liver disease. Hepatology 2009;49:1926–34. [DOI] [PubMed] [Google Scholar]
- [15].Doi K, Noiri E, Sugaya T. Urinary L-type fatty acid-binding protein as a new renal biomarker in critical care. Curr Opin Crit Care 2010;16:545–9. [DOI] [PubMed] [Google Scholar]
- [16].Ferguson MA, Vaidya VS, Waikar SS, et al. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int 2010;77:708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chugh A, Bakris GL. Microalbuminuria: what is it? Why is it important? What should be done about it? An update. J Clin Hypertens 2007;9:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Okazaki Y, Furuhashi M, Tanaka M, et al. Urinary excretion of fatty acid-binding protein 4 is associated with albuminuria and renal dysfunction. PLoS One 2014;9:e115429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tanaka M, Furuhashi M, Okazaki Y, et al. Ectopic expression of fatty acid-binding protein 4 in the glomerulus is associated with proteinuria and renal dysfunction. Nephron Clin Pract 2014;128:345–51. [DOI] [PubMed] [Google Scholar]
- [20].Terra X, Auguet T, Broch M, et al. Retinol binding protein-4 circulating levels were higher in nonalcoholic fatty liver disease vs. histologically normal liver from morbidly obese women. Obesity (Silver Spring) 2013;21:170–7. [DOI] [PubMed] [Google Scholar]
- [21].Pallet N, Chauvet S, Chassé JF, et al. Urinary retinol binding protein is a marker of the extent of interstitial kidney fibrosis. PLoS One 2014;9:e84708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen CH, Hsieh TJ, Lin KD, et al. Increased unbound retinol-binding protein 4 concentration induces apoptosis through receptor-mediated signaling. J Biol Chemsitry 2012;287:9694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Allouch S, Munusamy S. Metformin attenuates albumin-induced alterations in renal tubular cells in vitro. J Cell Physiol 2017;232:3652–63. [DOI] [PubMed] [Google Scholar]
- [24].Alizadeh A, Mansour-Ghanaei F, Roozdar A, et al. Laboratory tests, liver vessels color doppler sonography, and fibroscan findings in patients with nonalcoholic fatty liver disease: An observation study. J Clin Imaging Sci 2018;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fattahi S, Kazemipour N, Hashemi M, et al. Alpha-1 antitrypsin, retinol binding protein and keratin 10 alterations in patients with psoriasis vulgaris, a proteomic approach. Iran J Basic Med Sci 2014;17:651. [PMC free article] [PubMed] [Google Scholar]
- [26].Jafar TH, Stark PC, Schmid CH, et al. Angiotensin-converting enzymne inhibition and progression of non-diabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001;135:73–87. [DOI] [PubMed] [Google Scholar]
- [27].Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–9. [DOI] [PubMed] [Google Scholar]
- [28].Bechtel U, Scheuer R, Landgraf R, et al. Assessment of soluble adhesion cleavage products (sICAM-1, sVCAM-1, sELAM-1) and complement cleavage products (sC4d, sC5b-9) in urine. Clinical monitoring of renal allograft recipients. Transplantation 1994;58:905–11. [DOI] [PubMed] [Google Scholar]
- [29].Lefere S, Van de Velde F, Devisscher L, et al. Serum vascular cell adhesion molecule-1 predicts significant liver fibrosis in non-alcoholic fatty liver disease. Int J Obes (Lond) 2017;41:1207–13. [DOI] [PubMed] [Google Scholar]
- [30].Machado MV, Gonçalves S, Carepa F, et al. Impaired renal function in morbid obese patients with nonalcoholic fatty liver disease. Liver Int 2012;32:241–8. [DOI] [PubMed] [Google Scholar]
- [31].Yun HR, Kim H, Park JT, et al. Korean Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD) Investigators. Obesity, metabolic abnormality, and progression of CKD. Am J Kidney Dis 2018;72:400–10. [DOI] [PubMed] [Google Scholar]
- [32].Bolignano D, Zoccali C. Effects of weight loss on renal function in obese CKD patients: a systematic review. Nephrol Dial Transplant 2013;iv82–98. [DOI] [PubMed] [Google Scholar]
- [33].Bianchi VE. Weight loss is a critical factor to reduce inflammation. Clin Nutr ESPEN 2018;28:21–35. [DOI] [PubMed] [Google Scholar]
- [34].Suh JB, Kim SM, Cho GJ, et al. Serum AFBP levels are elevated in patients with nonalcoholic fatty liver disease. Scand J Gastroenterol 2014;49:979–85. [DOI] [PubMed] [Google Scholar]
- [35].Reinehr T, Stoffel-Wagner B, Roth CL. Adipocyte fatty acid-binding protein in obese children before and after weight loss. Metabolism 2007;56:1735–41. [DOI] [PubMed] [Google Scholar]
- [36].Yu GI, Jun SE, Shin DH. Associations of VCAM-1 gene polymorphisms with obesity and inflammation markers. Inflamm Res 2017;66:217–25. [DOI] [PubMed] [Google Scholar]
- [37].Liu JJ, Yeoh LY, Sum CF, et al. SMART2D study. Vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1, is associated with diabetic kidney disease in Asians with type 2 diabetes. J Diabetes Compl 2015;29:707–12. [DOI] [PubMed] [Google Scholar]
- [38].Kargarfard M, Lam ET, Shariat A, et al. Effects of endurance and high intensity training on ICAM-1 and VCAM-1 levels and arterial pressure in obese and normal weight adolescents. Phys Sportsmed 2016;44:208–16. [DOI] [PubMed] [Google Scholar]
- [39].Ohse T, Inagi R, Tanaka T, et al. Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int 2006;70:1447–55. [DOI] [PubMed] [Google Scholar]
- [40].Gonçalves GL, Costa-Pessoa JM, Thieme K, et al. Intracellular albumin overload elicits endoplasmic reticulum stress and PKC-delta/p38 MAPK pathway activation to induce podocyte apoptosis. Sci Rep 2018;8:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yao F, Li Z, Ehara T, et al. Fatty Acid-Binding Protein 4 mediates apoptosis via endoplasmic reticulum stress in mesangial cells of diabetic nephropathy. Mol Cell Endocrinol 2015;411:232–42. [DOI] [PubMed] [Google Scholar]
- [42].Chen CH, Hsieh TJ, Lin KD, et al. Increased unbound Retinol-binding Protein 4 concentration induces apoptosis through receptor-mediated signaling. J Biol Chem 2012;287:9694–707. [DOI] [PMC free article] [PubMed] [Google Scholar]


