Abstract
Background:
Anterior vertebral body tethering to effect scoliosis correction in a growing spine has been shown to work with varying degrees of success. This report describes the mid-term results of this technique using a new device composed of a braided ultra-high molecular weight polyethylene (UHMWPE) cord anchored to bone screws applied without segmental compression.
Methods:
This was a single-center prospective observational study of an investigational device. Five female patients aged 9 to 12 years with thoracic scoliosis underwent thoracoscopic insertion of the UHMWPE tether. Radiographs and magnetic resonance imaging (MRI) were performed, and the Scoliosis Research Society (SRS)-22 was administered, preoperatively and at regular intervals after surgery, with a minimum of 4 years of follow-up.
Results:
All tethering devices spanning the end vertebrae (range, 7 to 8 vertebrae) were implanted successfully. Mean blood loss was 136 mL, and the mean operative time was 205 minutes. The mean preoperative main thoracic Cobb angle was 40.1°. Curve correction of the tethered segment ranged from 0% to 133.3% at 4 years. We observed greater correction in 2 patients with open triradiate cartilage (TRC), achieving full scoliosis correction at 2 years and 121.5% at 4 years. MRI showed improvement in periapical disc wedging morphology and 55% improvement of rotation at 3 years. There were 20 adverse events, of which 16 were mild and 4 were moderate in severity. The 4 moderate events of pneumonia, distal decompensation, curve progression, and overcorrection occurred in 3 patients, 2 of whom required fusion.
Conclusions:
Anterior vertebral body tethering resulted in scoliosis deformity correction in the coronal and axial planes, with preservation of curve flexibility. Actual correction by growth modulation was noted only in patients with open TRC, whereas curve stabilization was noted in patients with closed TRC. Overcorrection, curve progression, and distal decompensation are problems with this technique.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Curve progression in idiopathic scoliosis has been attributed to differential compressive loads in the concavity of the deformity causing diminished concave growth and accelerated convex growth. This leads to a vicious cycle of increased loading of the concave side and scoliosis progression1-4.
Treatment recommendations for progressive scoliosis include bracing and fusion surgery. Bracing prevents curve progression in some patients, but residual curve size is a predictor for pain and the perception of inferior health status5-8. Spinal fusion has been shown to correct the deformity and improve quality of life, but it involves major surgery, substantial financial cost, and loss of spinal motion. An ideal solution for treating early progressive curves involves non-fusion deformity correction while allowing spinal growth and motion. Some of these methods, which use growing-rod systems9-13 and expandable prosthetic ribs14,15, are primarily recommended for early-onset and non-idiopathic scoliosis.
Growth modulation by means of anterior vertebral body stapling to reverse the compression-force gradient across the apex of the deformity has been described1,16-18. Asymmetric inhibition of vertebral growth using posterior tethers19,20, and wedge-rod systems21 have also been used.
Animal models have shown the feasibility of using an anterolateral tether that is made of ultra-high molecular weight polyethylene (UHMWPE) and anchored to bone screws to modulate spine growth22-25. Clinical application of this method using off-label implants has shown promising but unpredictable early results26-29.
In the current report, we describe our initial experience using a novel anterolateral tethering device in patients with idiopathic scoliosis at risk of progression, with a minimum of 4 years of follow-up.
Materials and Methods
This was a single-center, Phase-2A pilot study involving 5 patients in whom a braided UHMWPE tether (MIScoli; DePuy Spine) was used in the surgical treatment of idiopathic scoliosis in the immature spine. This study received institutional review board approval.
Patient Assessment and Recruitment
From October 2010 to June 2016, 9 female patients with Lenke Type-1 scoliosis were consecutively recruited according to the inclusion and exclusion criteria (Table I). Candidates were given the option for brace treatment. Preoperative assessments included physical examination and standard full-length spine radiographs, radiographs of the left wrist/hand, and magnetic resonance imaging (MRI) of the entire spine.
TABLE I.
Inclusion and Exclusion Criteria for Scoliosis Tether Pilot Study
| Inclusion Criteria | Exclusion Criteria |
|
|
Device Description
The MIScoli device is made up of 2 components (Fig. 1). The tether component is a flat braided construct with a uniform width and is manufactured from UHMWPE. It is anchored to a vertebral-body component made of titanium alloy (Ti 61A-4V) and consisting of 3 parts: a staple, a bone screw, and a set screw. The 4-pronged staple has a diameter of 11 mm, and the length of the bone screw ranges from 22 to 44 mm, in 2-mm increments.
Fig. 1.
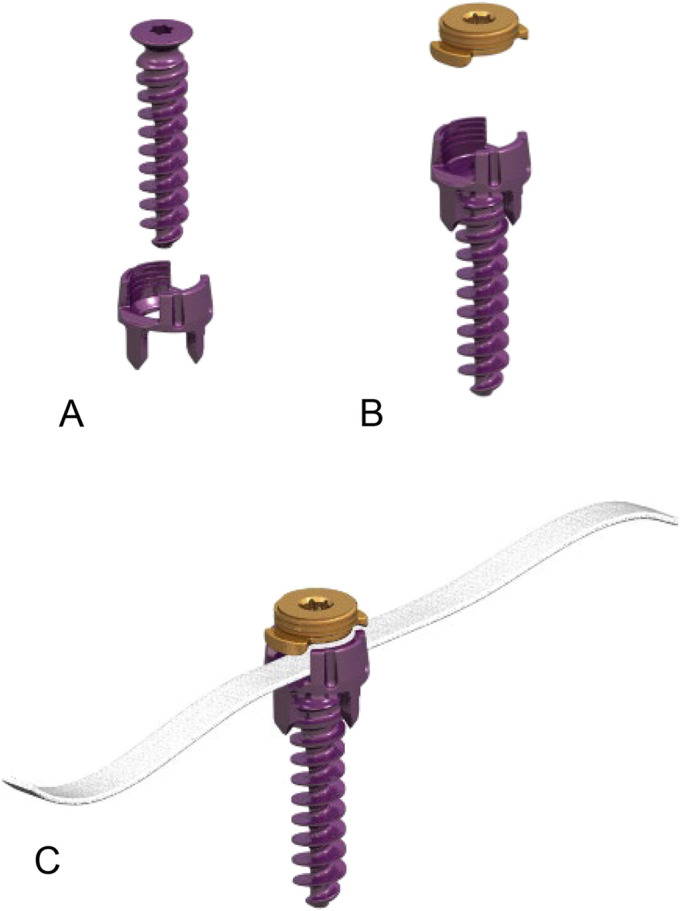
Figs. 1-A, 1-B, and 1-C The MIScoli tethering device, including the staple and bone screw (Fig. 1-A), set screw (Fig 1-B), and polyethylene tether (Fig. 1-C) (Reproduced with permission of DePuy Spine).
Operative Technique
Instrumentation levels followed the standard recommendation for anterior fusion, i.e., from superior to inferior end vertebrae. The surgical technique for thoracoscopic access to the thoracic spine for scoliosis surgery has been previously described30,31 and involves the use of 4 portals to access the thoracic cavity. One staple and 1 bone screw were placed at each level under image control. The UHMWPE tether was positioned onto the center of the staple yoke of the most cephalad tethered vertebra and secured with a set screw. The tether was then sequentially positioned onto the remaining segments, while slack in the tether was taken out by pulling on the tether by hand before the set screws were tightened. No segmental compression maneuvers between the vertebral bone screws to gain immediate curve correction were performed.
All patients followed our protocol for thoracoscopic-assisted scoliosis surgery. Follow-up visits were at 5 weeks and at 3, 6, 12, 18, 24, 30, 36, 42, and 48 months postoperatively or until skeletal maturity.
Outcome Parameters
Maturity was assessed on the basis of age, Risser stage triradiate cartilage (TRC) assessment, bone age by the Greulich and Pyle atlas32, and Sanders stage33. Patient outcome parameters included the Scoliosis Research Society (SRS)-22 questionnaire. Radiographic measures included Cobb angles of the main thoracic, proximal thoracic, and lumbar curves as well as Cobb angles of the tethered segments. Intervertebral disc wedging at the tethered levels was assessed on the posteroanterior radiographs. Magnetic resonance imaging (MRI) preoperatively and at 12, 24, and 36 months postoperatively was performed to assess implant position/placement, intervertebral disc morphology, and axial rotation of the apical vertebrae. All radiographs were assessed by an independent radiographic laboratory (Medical Metrics). Descriptive statistical analysis and subgroup analysis comparing patients with closed or open TRC were performed using SPSS (version 23; IBM). All adverse events were recorded.
Results
Five female patients (age range of 9 to 12 years) were recruited after screening 9 candidates; 4 of the 9 initially screened did not opt for tether placement and were excluded from the study. The TRC was open in 2 of the 5 patients (Patients 3 and 5) and closed in the other 3 patients. In 1 patient, menarche occurred 5 days prior to surgery (Sanders stage 5). The other 4 were premenarchal (Sanders stage 2). All patients were seen until skeletal maturity. Patient demographics and pre- and postoperative radiographic parameters are shown in Tables II and III and Figures 2 and 3.
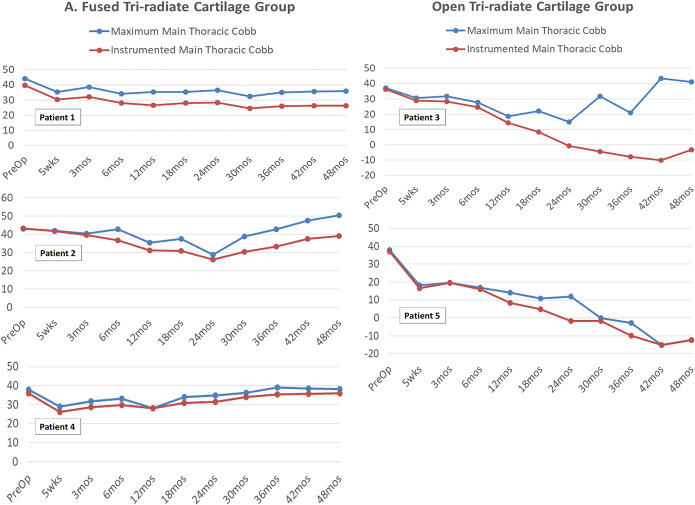
Fig. 2.
Pre- and postoperative Cobb angles (in degrees) of the main thoracic curve and the tethered segments of the 5 patients who underwent application of the tethering device.
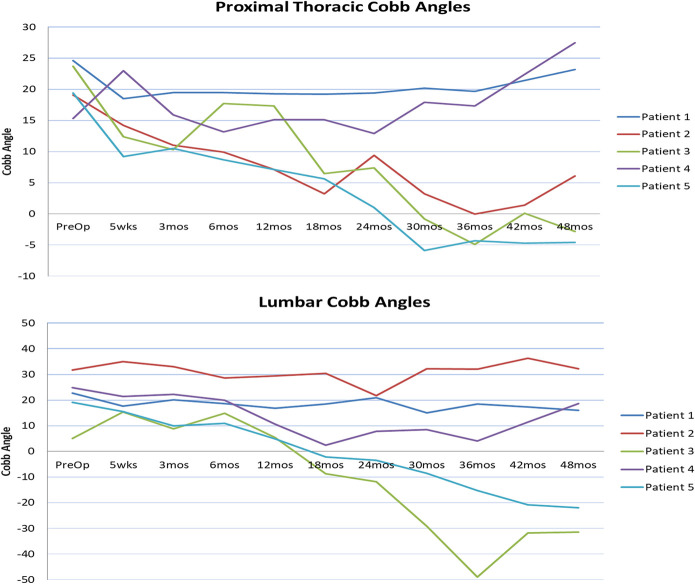
Fig. 3.
Pre- and postoperative Cobb angles (in degrees) of the proximal thoracic and lumbar curves.
TABLE II.
Patient Characteristics at the Time of Surgery
| Patient No. | Chronologic Age (yr + mo) | Skeletal Age (yr) | Sanders Stage | Menarche | Triradiate Cartilage | Curve Type | Tethered Segments |
| 1 | 12 + 2 | 13 | 5 | Yes* | Closed | Lenke 1AN | T5 to T12 |
| 2 | 11 + 11 | 13 | 2 | No | Closed | Lenke 1B- | T5 to T11 |
| 3 | 9 + 4 | 10 | 2 | No | Open | Lenke 1AN | T5 to T12 |
| 4 | 12 + 11 | 13 | 2 | No | Closed | Lenke 1AN | T6 to T12 |
| 5 | 12 + 4 | 12 | 2 | No | Open | Lenke 1A- | T6 to T12 |
Premenarchal at the time of surgical planning. Menarche for this patient occurred 5 days prior to the surgical date.
TABLE III.
Pre- and Postoperative Radiographic Measurements of the 5 Patients Who Underwent Application of the Tethering Device*
| Patient and Observation Period | MT Cobb Angle (deg) (% Change from Preop.) | Cobb Angle of Instrumented/Tethered Segments (deg) (% Change from Preop.) | Right Bending Cobb Angle (deg) | PT Cobb Angle (deg) (% Change from Preop.) | Lumbar Cobb Angle (deg) (% Change from Preop.) | Axial Rotation† (deg) |
| Patient 1 (closed TRC) | ||||||
| Preop. | 44.0 | 39.6 | 28.9 | 24.6 | 22.7 | 13.3 |
| 5 wk | 35.2 | 30.5 | 18.5 | 17.6 | ||
| 3 mo | 38.7 | 32.1 | 19.5 | 20.1 | ||
| 6 mo | 34.3 | 28.0 | 19.5 | 18.7 | ||
| 12 mo | 35.3 (19.8%) | 26.5 (33.1%) | 18.2 | 19.3 (21.5%) | 16.9 (25.6%) | 12.4 |
| 18 mo | 35.2 | 27.9 | 19.2 | 18.4 | ||
| 24 mo | 36.4 (17.3%) | 28.3 (28.5%) | 13.9 | 19.4 (21.1%) | 20.9 (7.9%) | 6.6 |
| 30 mo | 32.3 | 24.4 | 20.2 | 15.0 | ||
| 36 mo | 35.1 (20.2%) | 26.1 (34.1%) | 9.3 | 19.7 (19.9%) | 18.5 (18.5%) | 9.6 |
| 42 mo | 35.6 | 26.3 | 21.5 | 17.3 | ||
| 48 mo | 36.0 (18.2%) | 26.4 (33.3%) | 23.5 (4.5%) | 16.1 (29.1%) | ||
| Patient 2 (closed TRC) | ||||||
| Preop. | 43.2 | 43.5 | 30.0 | 19.1 | 31.8 | 10.8 |
| 5 wk | 42.1 | 41.9 | 14.2 | 35.0 | ||
| 3 mo | 40.5 | 39.8 | 11.0 | 33.1 | ||
| 6 mo | 42.9 | 36.7 | 9.9 | 28.6 | ||
| 12 mo | 35.4 (18.1%) | 31.2 (28.3%) | 25.7 | 7.1 (62.8%) | 29.5 (7.2%) | 6.5 |
| 18 mo | 37.6 | 30.9 | 3.2 | 30.4 | ||
| 24 mo | 28.9 (33.1%) | 26.3 (39.5%) | 23.9 | 9.4 (50.8%) | 21.7 (31.8%) | 3.8 |
| 30 mo | 38.9 | 30.5 | 3.2 | 32.3 | ||
| 36 mo | 42.9 (0.7%) | 33.5 (23.0%) | 22.3 | 0.0 (100%) | 32.1 (−0.9%) | 7.2 |
| 42 mo | 47.6 | 37.5 | 1.4 | 36.3 | ||
| 48 mo | 50.6 (−17.1%) | 39.1 (10.1%) | 6.1 (68.1%) | 32.3 (−1.6%) | ||
| 54 mo‡ | 58.0 (−34.3%) | 48.0 (−10.3%) | 5.4 (71.1%) | 43.9 (−38.1%) | ||
| Patient 3 (open TRC) | ||||||
| Preop. | 37.2 | 36.2 | 14.7 | 23.7 | 5.0 | 10.4 |
| 5 wk | 30.7 | 28.8 | 12.4 | 15.4 | ||
| 3 mo | 31.8 | 28.3 | 10.3 | 8.8 | ||
| 6 mo | 27.8 | 24.7 | 17.7 | 14.9 | ||
| 12 mo | 18.7 (49.7%) | 14.3 (60.5%) | 19.8 | 17.3 (27.0%) | 5.4 (−8.0%) | 2.1 |
| 18 mo | 22.0 | 8.5 | 6.5 | −8.7 | ||
| 24 mo | 14.9 (59.9%) | −0.7 (101.9%) | 4.2 | 7.4 (68.8%) | −11.8 (336%) | 6.4 |
| 30 mo | 31.7 | −4.5 | −0.8 | −29.1 | ||
| 36 mo§ | 21.0 (43.5%) | −7.9 (121.8%) | 5.7 | −4.9 (120.7%) | −49 (1,080%) | 5.2 |
| 42 mo | 43.5 | −10.0 | 0.1 | −31.7 | ||
| 48 mo§ | 41.1 (−10.5%) | −3.3 (109.1%) | −2.8 (111.8%) | −31.5 (730%) | ||
| Patient 4 (closed TRC) | ||||||
| Preop. | 37.9 | 36.0 | 25.2 | 15.3 | 24.8 | 10.0 |
| 5 wk | 29.0 | 26.1 | 23.0 | 21.5 | ||
| 3 mo | 31.9 | 28.8 | 15.9 | 22.3 | ||
| 6 mo | 33.1 | 29.9 | 13.2 | 20.0 | ||
| 12 mo | 28.2 (25.6%) | 28.0 (22.2%) | 13.0 | 15.1 (1.3%) | 10.6 (57.2%) | 4.5 |
| 18 mo | 34.1 | 31.0 | 15.1 | 2.5 | ||
| 24 mo | 34.8 (8.2%) | 31.4 (12.8%) | 8.2 | 12.9 (15.7%) | 7.9 (68.1%) | 5.4 |
| 30 mo | 36.2 | 33.9 | 17.9 | 8.5 | ||
| 36 mo | 39.1 (−3.2%) | 35.3 (1.9%) | 11.9 | 17.3 (−13.1%) | 4.1 (83.5%) | 0.5 |
| 42 mo | 38.6 | 35.7 | 22.4 | 11.4 | ||
| 48 mo | 38.1 (−0.5%) | 36.0 (0%) | 27.5 (−79.7%) | 18.7 (24.6%) | ||
| Patient 5 (open TRC) | ||||||
| Preop. | 38.0 | 37.2 | 29.4 | 19.4 | 19.1 | 5.4 |
| 5 wk | 18.2 | 16.5 | 9.2 | 15.5 | ||
| 3 mo | 19.5 | 19.5 | 10.5 | 10.0 | ||
| 6 mo | 16.8 | 16.0 | 8.7 | 11.0 | ||
| 12 mo | 14.2 (62.6%) | 8.3 (77.7%) | 23.7 | 7.1 (63.4%) | 4.9 (74.3%) | 4.3 |
| 18 mo | 10.8 | 4.8 | 5.6 | −2.1 | ||
| 24 mo | 12.0 (68.4%) | −1.7 (104.6%) | 28.0 | 1.0 (94.8%) | −3.4 (117.8%) | 3.2 |
| 30 mo | 0.0 | −1.6 | −5.9 | −8.6 | ||
| 36 mo | −2.8 (107.4%) | −10.0 (126.9%) | 29.3 | −4.3 (122.2%) | −15.2 (179.6%) | 0.0 |
| 42 mo | −15.2 | −15.2 | −4.7 | −20.8 | ||
| 48 mo | −12.4 (132.6%) | −12.4 (133.3%) | −4.6 (123.7%) | −21.9 (214.7%) |
Negative Cobb angles indicate reversal of curve, i.e., from right-sided to left-sided curve. MT = main thoracic, PT = proximal thoracic, and TRC = triradiate cartilage.
Measured from axial MRI scans of the apical vertebra with reference to the horizon.
Underwent posterior spinal fusion at 55 months for curve progression and distal decompensation.
Underwent tether removal at 38 months, for overcorrection and distal decompensation, and posterior spinal fusion at 52 months.
A mean of 7.4 spinal levels (range, 7 to 8 levels) received instrumentation. T5 and T12 were the most common cephalad and caudal instrumented segments, respectively. The mean operative time was 205 minutes (range, 189 to 243 minutes), with a mean blood loss of 136 mL (range, 80 to 200 mL). Hospital stay ranged from 4 to 5 days.
Thirty-seven staple-bone screw components were successfully implanted. Three screws in 3 patients were difficult to insert because of the small size of the most cephalad tethered vertebra. MRI assessment revealed good placement of all 37 staple-bone screw components, with no vertebral margin violations, disassembly, fracture, or pull-out at all time periods.
Coronal-Plane Assessment
The main thoracic (MT), proximal thoracic (PT), and lumbar curves before and after surgery for all 5 patients are presented in Table III and Figures 2 and 3. The MT curve improved from a preoperative mean of 40.1° (range, 37.2° to 44.0°) to 26.5° (range, 12.2° to 33.3°) in the immediate postoperative period. Subsequent postoperative corrections for each patient are shown in Table III and Figure 2.
The Cobb angles of the tethered segments were analyzed separately to assess the growth-modulation effect on these segments (red lines in Figure 2). We observed 2 correction patterns based on TRC characteristics (Table IV). The tethered segments in the 3 patients with closed TRC demonstrated a curve correction of 28.0% at 1 year, and a final correction of 14.9% at 4 years. Correction of the tethered segments was greater in the 2 patients with open TRC, with a 69.2% correction at 1 year, full scoliosis correction at 2 years, and overcorrection (121.5%) at 4 years. The magnitude of curve correction in these 2 patients was greatest at the time of TRC closure, coinciding with their growth spurt (Fig. 2-B). In those with closed TRC at the time of surgery, the curve returned to close to the immediate postoperative Cobb angle (Figs. 4 and 5), while those with open TRC continued to demonstrate correction (Fig. 6).
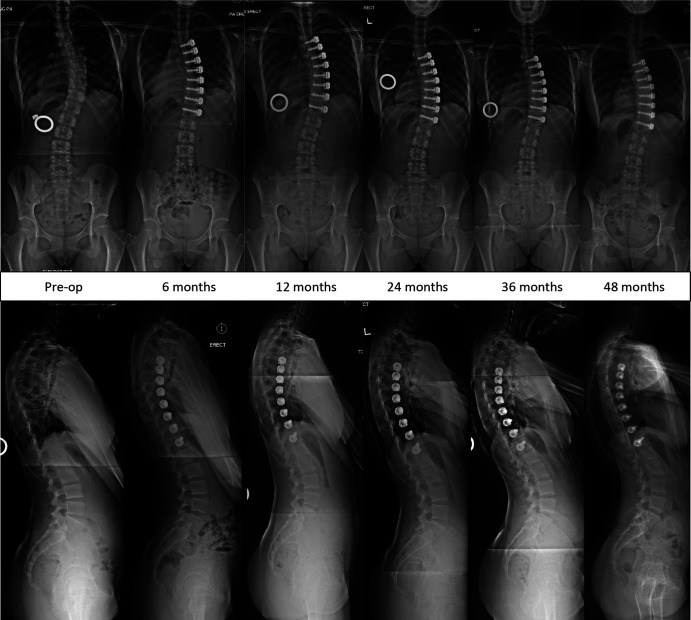
Fig. 4.
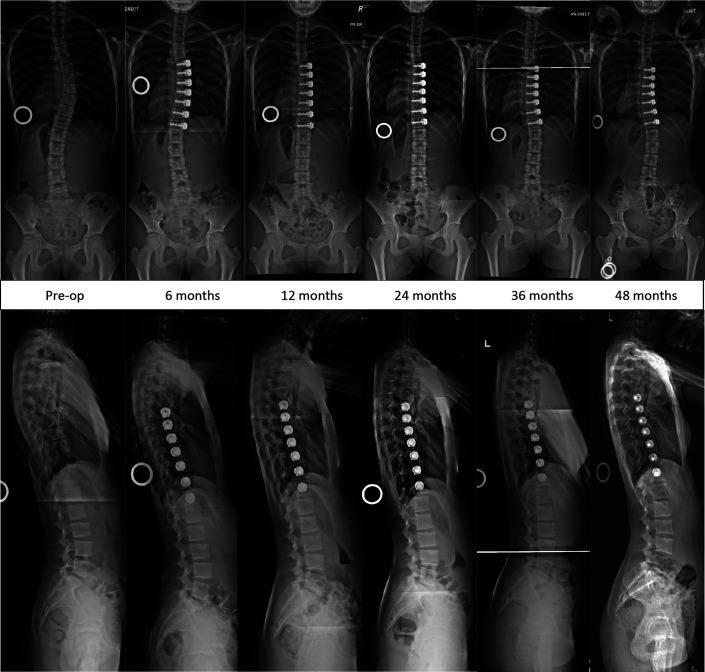
Images of Patient 1 (closed triradiate cartilage), who demonstrated stability of the coronal and sagittal correction of the tethered segments until 4 years.
Fig. 5.
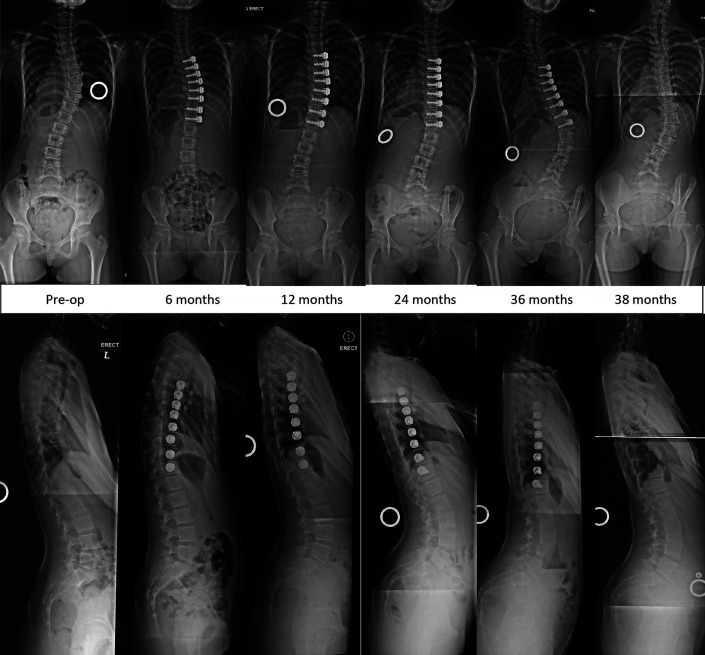
Images of Patient 4 (closed triradiate cartilage), who demonstrated stability of the coronal and sagittal correction of the tethered segments until 4 years.
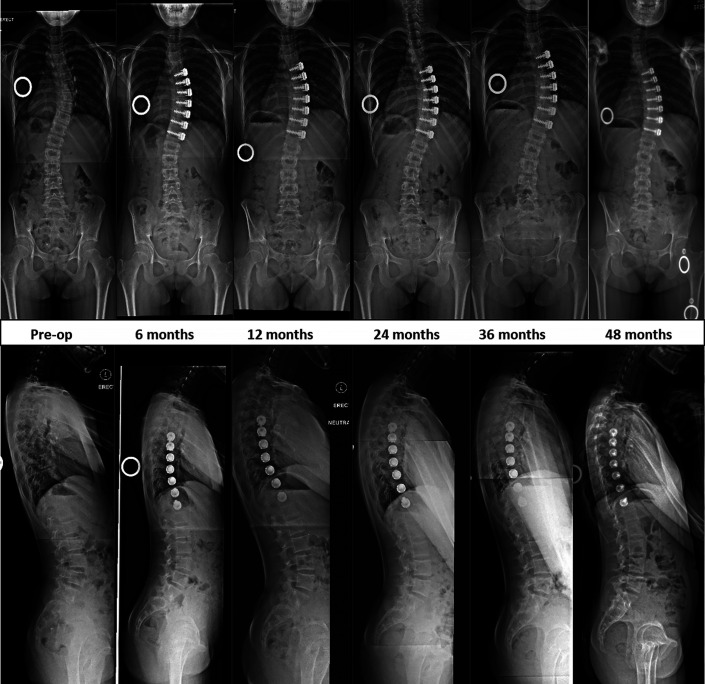
Fig. 6.
Images of Patient 5 (open triradiate cartilage). A tethering effect with progressive coronal correction was achieved at 2 years after tether application, with reversal of disc wedging in the tethered segments, followed by slight overcorrection until 4 years, without decompensation.
TABLE IV.
Cobb-Angle Improvement of the Tethered Segments After Surgery
| 1 Yr Postop. | 2 Yr Postop. | 3 Yr Postop. | 4 Yr Postop. | |
| Overall | 43.6% | 56.6% | 60.0% | 55.3% |
| Closed TRC* group | 28.0% | 28.0% | 20.4% | 14.9% |
| Open TRC* group | 69.2% | 103.3% | 124.5% | 121.5% |
TRC = triradiate cartilage.
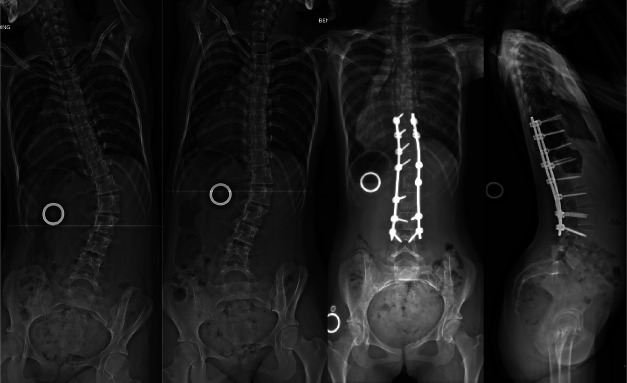
Two patients required additional surgical procedures. Patient 3 experienced overcorrection to −7.9° at 36 months. There was distal adding-on causing spinal imbalance, with the MT curve progressing to 21° (Fig. 7-A). She was the youngest patient and had open TRC at the time of surgery. Thoracoscopic removal of the tether was done at 38 months, followed by posterior fusion of the thoracolumbar curve 52 months after the index surgery (Fig. 7-B).
Images of Patient 3 (open triradiate cartilage)
Fig. 7-A.
A tethering effect with progressive coronal correction was achieved at 2 years after tether application, with reversal of disc wedging in the tethered segments, followed by overcorrection and distal decompensation requiring thoracoscopic removal of the tethering device at 38 months.
Fig. 7-B.
Side-bending images showing rigidity of the previously tethered segments and images following definitive posterior fusion performed at 52 months after the index surgery
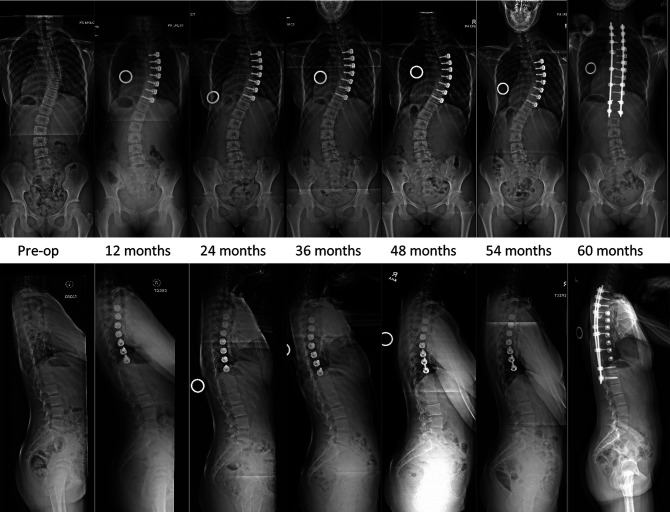
The other patient who required additional surgery (Patient 2) achieved modest correction until 24 months. The MT curve progressed thereafter, reaching 58° at 54 months, accompanied by distal decompensation of the lumbar curve to 44°. She underwent posterior thoracic fusion at 55 months without tether removal. Hers was the only case in this series with a Lenke “B” lumbar curve modifier preoperatively (Fig. 8).
Fig. 8.
Images of Patient 2 (closed triradiate cartilage), who experienced late curve progression and distal decompensation requiring posterior spinal instrumentation and fusion 5 years after tether application. The tether was not removed in this patient.
The PT curve improved from a preoperative mean of 20.4° (range, 15.3° to 24.6°) to 15.5° (range, 9.2° to 23.0°) in the immediate postoperative period. Subsequent postoperative PT curve corrections for each patient are shown in Table III and Figure 3. However, the trend toward spontaneous PT curve correction was noted in the patients with open TRC (mean of −3.7°).
The lumbar curve was unchanged from a preoperative mean of 20.7° (range, 5° to 31.8°) to 21.0° (range, 15.4° to 35.0°) in the immediate postoperative period. Individual curve corrections at subsequent time points are shown in Table III and Figure 3. At 4 years, the patients with open TRC at the time of surgery showed lumbar-curve reversal (mean of −26.7°), which reciprocated the MT curve overcorrection in this group. At 4 years, the lumbar curve correction for the patients with closed TRC was sustained, with the exception of that for Patient 2, in whom the lumbar curve increased, reciprocating the increase in the MT curve.
Sagittal-Plane Assessment and Curve Flexibility
There was no significant difference between the mean preoperative and 4-year postoperative thoracic kyphosis (16.0° compared with 17.8°) and lumbar lordosis (46.8° compared with 52.2°). Right side-bending analysis showed preservation of curve flexibility (preoperative range, 14.7° to 30.0°; latest postoperative range, 5.7° to 29.3°) (Table III).
Disc Height and Axial Rotation
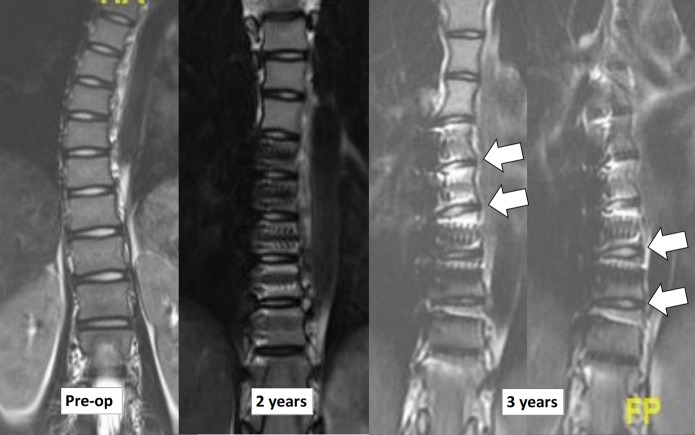
Preoperative measurements of the left and right disc heights showed wedge-shaped discs in the periapical segments, with smaller disc heights in the curve concavity. Postoperative improvement in periapical disc wedging morphology was noted particularly for the discs caudal to the apex (Fig. 9).
Fig. 9.
MRI of the thoracic spine (T2-weighted sequence) of Patient 5 before tether application and at 2 and 3 years postoperatively, showing preservation of intervertebral disc signals and a disc-wedging effect (arrows) after tether application.
Axial rotation of the apical segments of the scoliosis was assessed on the axial MRI images. The mean axial rotation decreased from 10° (range, 5.4° to 13.3°) preoperatively to 4.5° (range, 0° to 9.6°) at 36 months (Table III).
Adverse Events
There were 20 adverse events, of which 16 were mild and 4 were moderate in severity (Table V). The 4 moderate adverse events were observed in 3 patients. Patient 2 had curve progression and distal decompensation, whereas Patients 3 and 5 developed overcorrection and distal decompensation. Patient 3 also had difficult intubation due to small bronchial size and reactive airways. She developed community-acquired pneumonia during the second postoperative month, which was treated by intravenous antibiotics.
TABLE V.
Adverse Events
| Adverse Event | No. | Severity | Intervention |
| Fever (all subjects) | 7 | Mild | Medication |
| Postop. nausea (Patient 2) | 1 | Mild | Medication |
| Postop. vomiting (Patient 2) | 1 | Mild | Medication |
| Postop. hematuria (Patient 1) | 1 | Mild | Hydration |
| Reactive airways (Patient 3) | 1 | Mild | Pulmonary hygiene |
| Right pneumothorax (Patients 2 and 3) | 2 | Mild | Chest physiotherapy |
| Left/dependent lung pleural effusion (Patient 5) | 1 | Mild | Chest physiotherapy |
| Pneumonia (Patient 3) | 1 | Moderate | Antibiotics |
| Conjunctivitis (Patient 1) | 1 | Mild | None |
| Trunk listing (Patient 3) | 1 | Mild | Bracing |
| Overcorrection (Patients 3 and 5) | 2 | Moderate | Spinal fusion (Patient 3) |
| Curve progression/distal decompensation (Patient 2) | 1 | Moderate | Spinal fusion |
SRS-22 Questionnaire
The mean SRS-22 total score was 93.6, 89.4, 92.6, and 90.8 at the preoperative and 12, 24, and 36-month postoperative periods, respectively (p = 0.033 preoperative versus 36 months; higher score = better quality of life). An SRS-22 domain subanalysis showed improvement in self-image scores but did not reach significance (p = 0.301 preoperative versus 36 months). The mean satisfaction domain score decreased from 8.2 preoperatively to 7.0 at 12 months, but improved between 12 months and 36 months to 7.6 (p = 0.047).
Discussion
Spinal growth modulation with an anterolateral tether in immature patients with thoracic scoliosis resulted in mixed outcomes in this small series of patients followed for at least 4 years. While in some patients, the desired progressive improvement of the scoliosis occurred with growth, in other cases, curve progression occurred or the growth-modulating effect was so powerful that overcorrection resulted. Understanding the ideal patient characteristics and surgical methods will be critical for this technique to provide a reliable means of treating immature patients with scoliosis.
The search for a corrective non-fusion option in scoliosis led to the development of surgical spinal growth-modulation techniques that address the shortcomings of external bracing, such as noncompliance, and allow the application of corrective forces directly on the spine as opposed to indirectly through the chest wall. These techniques exploit the patients’ spinal growth and redirect it toward curve correction using the Hueter-Volkmann principle4. The desire (both of patients and surgeons) for such a non-fusion method must be tempered by the fact that much about this tethering approach remains to be understood.
Animal studies have shown that anterolateral UHMWPE tethers anchored to bone screws modulate spine growth22-25,34 and are thought to act as a passive anterior convex restraint to growth without the need to resort to epiphysiodesis, allowing correction by continued concave vertebral growth.
We performed a tethering procedure using a novel device in 5 immature girls by applying a UHMWPE tether on the curve convexity. To our knowledge, only 2 reports on tethering have described the results in a similar series of patients. Samdani et al. reported 70% correction from a mean preoperative angle of 44.2° and 44% improvement in axial rotation in 11 patients after 2 years28. Newton et al. reported 51% correction from a mean preoperative angle of 52°, with no significant change in thoracic kyphosis, in 17 patients at 2.5 years29. Our results are comparable with the findings of these reports using anterior tethering techniques: a mean 55.3% coronal correction at 4 years and 55% axial correction at 3 years. The surgical technique in the above 2 reports differed from ours in that those authors utilized segmental compression of the device to achieve initial curve correction, while in our study, the tension in the device was allowed to build as growth occurred to the point that the force would limit further convex growth.
Overcorrection is an undesirable outcome following anterior tethering that may result if “too small” a deformity is treated in a patient with “too much” growth remaining. While 1 patient in our series with open TRC was able to maintain coronal balance at 4 years despite overcorrection, the other patient developed overcorrection accompanied by distal decompensation that manifested as deterioration of the overall Cobb angle. This patient was the youngest (age 9 at the time of surgery) and the only patient with thoracolumbar kyphosis. The combination of large growth potential, main curve overcorrection, and the presence of distal junctional kyphosis could be important factors in the development of distal adding-on and decompensation in this patient. These issues were alluded to in a recent report on the use of growth modulation in patients <10 years of age35. In the study by Samdani et al., there were 2 cases of overcorrection requiring another operation, wherein the distal tension from the tether was released by unlocking the caudal 3 set screws28. In their series, Newton et al. observed 4 cases of complete or overcorrection requiring tether removal, and 4 cases of curve progression and/or adding-on requiring posterior fusion29.
The reason for late worsening and distal decompensation in Patient 2 is unclear. There was no acute angulation between screws to suggest tether breakage. Curve worsening could have been due to lumbar curve progression. Hers was the only case in this series with a Lenke “B” lumbar curve modifier preoperatively.
Our patient population was small owing to the investigative nature of the device. Nevertheless, this series of patients with a minimum of 4 years of follow-up demonstrates the potential for both successes and failures of this evolving technique.
The effect of the tether, used without segmental compression in our patients, can be summarized as follows: (1) Patients who had less growth remaining (closed TRC) had curve reduction that was mainly achieved from device implantation on a spine that had a smaller Cobb angle due to intraoperative patient position. In this group, the device prevented further progression, resulting in curve stability but with little overall additional growth modulation. These curves were at risk of progression given the curve size and remaining growth36-39. (2) Patients who were more immature (open TRC) had curve reduction that went on to complete correction/overcorrection. Progressive curve diminution was observed on reaching menarche and persisted until patients reached Risser stage 4. In this group, the device effected spinal growth modulation resulting in curve correction.
Attention to the optimal timing of tether application (based on skeletal maturity and estimates of growth remaining in combination with the size of the deformity), the magnitude of initial intraoperative active curve correction, and curve-pattern identification is likely necessary for consistent outcomes.
Our results suggest that if the tether is used in patients with less remaining growth, additional segmental compression during tether placement may be beneficial to achieve better correction. In very young and immature patients, additional segmental compression could be detrimental and may need to be avoided, as overcorrection may occur. The amount of tether tension (initial correction) is likely impacted by the curve size in addition to the remaining growth. The mild overcorrection in Patient 5 stabilized when she reached skeletal maturity 3 years following tether application, whereas leaving more slack between the tethered spinal segments in Patient 3 might have mitigated the strong overcorrection and subsequent decompensation. Developing algorithms to make predictions of the final outcome will require a much larger series of patients, to ultimately prevent the problems of overcorrection, curve progression, and distal decompensation associated with this technique, all of which may require additional surgical intervention.
Vertebral body tethering is a new growth-modulation procedure for the treatment of progressive thoracic scoliosis in the immature patient. Although 2 of the 5 patients experienced failure of treatment and required posterior fusion, 3 patients avoided fusion that was highly likely had they not been treated. Additional studies with more patients and longer follow-up will allow better evaluation of the indications and outcomes for this surgical procedure.
Footnotes
Investigation performed at National University Hospital, Singapore
Disclosure: The study sponsor, DePuy Spine, paid for the surgical, hospitalization, and outpatient attendance costs of all five patients. The surgeons and the institution involved in the study did not receive fees for performing the study. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one of the authors (P.O.N.) checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work, including with DePuy Spine, and “yes” to indicate that the author had a patent and/or copyright, planned, pending, or issued, broadly relevant to this work and including DePuy Spine as the licensee (http://links.lww.com/JBJSOA/A132).
References
- 1.Braun JT, Hoffman M, Akyuz E, Ogilvie JW, Brodke DS, Bachus KN. Mechanical modulation of vertebral growth in the fusionless treatment of progressive scoliosis in an experimental model. Spine. 2006. May 20;31(12):1314-20. [DOI] [PubMed] [Google Scholar]
- 2.Mente PL, Stokes IA, Spence H, Aronsson DD. Progression of vertebral wedging in an asymmetrically loaded rat tail model. Spine. 1997. Jun 15;22(12):1292-6. [DOI] [PubMed] [Google Scholar]
- 3.Perdriolle R, Becchetti S, Vidal J, Lopez P. Mechanical process and growth cartilages. Essential factors in the progression of scoliosis. Spine. 1993. Mar 1;18(3):343-9. [DOI] [PubMed] [Google Scholar]
- 4.Stokes IA, Spence H, Aronsson DD, Kilmer N. Mechanical modulation of vertebral body growth. Implications for scoliosis progression. Spine. 1996. May 15;21(10):1162-7. [DOI] [PubMed] [Google Scholar]
- 5.Heary RF, Bono CM, Kumar S. Bracing for scoliosis. Neurosurgery. 2008. Sep;63(3)(Suppl):125-30. [DOI] [PubMed] [Google Scholar]
- 6.Maruyama T. Bracing adolescent idiopathic scoliosis: a systematic review of the literature of effective conservative treatment looking for end results 5 years after weaning. Disabil Rehabil. 2008;30(10):786-91. [DOI] [PubMed] [Google Scholar]
- 7.Andersen MO, Christensen SB, Thomsen K. Outcome at 10 years after treatment for adolescent idiopathic scoliosis. Spine. 2006. Feb 1;31(3):350-4. [DOI] [PubMed] [Google Scholar]
- 8.Haefeli M, Elfering A, Kilian R, Min K, Boos N. Nonoperative treatment for adolescent idiopathic scoliosis: a 10- to 60-year follow-up with special reference to health-related quality of life. Spine. 2006. Feb 1;31(3):355-66; discussion 367. [DOI] [PubMed] [Google Scholar]
- 9.Akbarnia BA, Marks DS, Boachie-Adjei O, Thompson AG, Asher MA. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine. 2005. Sep 1;30(17)(Suppl):S46-57. [DOI] [PubMed] [Google Scholar]
- 10.Akbarnia BA, Breakwell LM, Marks DS, McCarthy RE, Thompson AG, Canale SK, Kostial PN, Tambe A, Asher MA; Growing Spine Study Group. Dual growing rod technique followed for three to eleven years until final fusion: the effect of frequency of lengthening. Spine. 2008. Apr 20;33(9):984-90. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy RE, Sucato D, Turner JL, Zhang H, Henson MA, McCarthy K. Shilla growing rods in a caprine animal model: a pilot study. Clin Orthop Relat Res. 2010. Mar;468(3):705-10. Epub 2009 Aug 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy R, Luhmann S, Lenke L. Greater than two year follow-up Shilla growth enhancing system for the treatment of scoliosis in children. Abstracts: The 2nd International Congress on Early Onset Scoliosis and Growing Spine, November 7-8, 2008, Montreal, Quebec, Chairman: Behrooz A. Akbarnia, MD. J Child Orthop. 2009;3(2):145-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouellet J. Surgical technique: modern Luqué trolley, a self-growing rod technique. Clin Orthop Relat Res. 2011. May;469(5):1356-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emans JB, Caubet JF, Ordonez CL, Lee EY, Ciarlo M. The treatment of spine and chest wall deformities with fused ribs by expansion thoracostomy and insertion of vertical expandable prosthetic titanium rib: growth of thoracic spine and improvement of lung volumes. Spine. 2005. Sep 1;30(17)(Suppl):S58-68. [DOI] [PubMed] [Google Scholar]
- 15.Hell AK, Campbell RM, Hefti F. The vertical expandable prosthetic titanium rib implant for the treatment of thoracic insufficiency syndrome associated with congenital and neuromuscular scoliosis in young children. J Pediatr Orthop B. 2005. Jul;14(4):287-93. [DOI] [PubMed] [Google Scholar]
- 16.Smith AD, Von Lackum WH, Wylie R. An operation for stapling vertebral bodies in congenital scoliosis. J Bone Joint Surg Am. 1954. Apr;36(2):342-8. [PubMed] [Google Scholar]
- 17.Betz RR, Kim J, D’Andrea LP, Mulcahey MJ, Balsara RK, Clements DH. An innovative technique of vertebral body stapling for the treatment of patients with adolescent idiopathic scoliosis: a feasibility, safety, and utility study. Spine. 2003. Oct 15;28(20):S255-65. [DOI] [PubMed] [Google Scholar]
- 18.Betz RR, D’Andrea LP, Mulcahey MJ, Chafetz RS. Vertebral body stapling procedure for the treatment of scoliosis in the growing child. Clin Orthop Relat Res. 2005. May;434:55-60. [DOI] [PubMed] [Google Scholar]
- 19.Lowe TG, Wilson L, Chien JT, Line BG, Klopp L, Wheeler D, Molz F. A posterior tether for fusionless modulation of sagittal plane growth in a sheep model. Spine. 2005. Sep 1;30(17)(Suppl):S69-74. [DOI] [PubMed] [Google Scholar]
- 20.Moal B, Schwab F, Demakakos J, Lafage R, Riviere P, Patel A, Lafage V. The impact of a corrective tether on a scoliosis porcine model: a detailed 3D analysis with a 20 weeks follow-up. Eur Spine J. 2013. Aug;22(8):1800-9. Epub 2013 Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betz RR, Cunningham B, Selgrath C, Drewry T, Sherman MC. Preclinical testing of a wedge-rod system for fusionless correction of scoliosis. Spine. 2003. Oct 15;28(20):S275-8. [DOI] [PubMed] [Google Scholar]
- 22.Newton PO, Fricka KB, Lee SS, Farnsworth CL, Cox TG, Mahar AT. Asymmetrical flexible tethering of spine growth in an immature bovine model. Spine. 2002. Apr 1;27(7):689-93. [DOI] [PubMed] [Google Scholar]
- 23.Newton PO, Farnsworth CL, Faro FD, Mahar AT, Odell TR, Mohamad F, Breisch E, Fricka K, Upasani VV, Amiel D. Spinal growth modulation with an anterolateral flexible tether in an immature bovine model: disc health and motion preservation. Spine. 2008. Apr 1;33(7):724-33. [DOI] [PubMed] [Google Scholar]
- 24.Newton PO, Upasani VV, Farnsworth CL, Oka R, Chambers RC, Dwek J, Kim JR, Perry A, Mahar AT. Spinal growth modulation with use of a tether in an immature porcine model. J Bone Joint Surg Am. 2008. Dec;90(12):2695-706. [DOI] [PubMed] [Google Scholar]
- 25.Newton PO, Farnsworth CL, Upasani VV, Chambers RC, Varley E, Tsutsui S. Effects of intraoperative tensioning of an anterolateral spinal tether on spinal growth modulation in a porcine model. Spine. 2011. Jan 15;36(2):109-17. [DOI] [PubMed] [Google Scholar]
- 26.Crawford CH, 3rd, Lenke LG. Growth modulation by means of anterior tethering resulting in progressive correction of juvenile idiopathic scoliosis: a case report. J Bone Joint Surg Am. 2010. Jan;92(1):202-9. [DOI] [PubMed] [Google Scholar]
- 27.Samdani AF, Ames RJ, Kimball JS, Pahys JM, Grewal H, Pelletier GJ, Betz RR. Anterior vertebral body tethering for immature adolescent idiopathic scoliosis: one-year results on the first 32 patients. Eur Spine J. 2015. Jul;24(7):1533-9. Epub 2014 Dec 16. [DOI] [PubMed] [Google Scholar]
- 28.Samdani AF, Ames RJ, Kimball JS, Pahys JM, Grewal H, Pelletier GJ, Betz RR. Anterior vertebral body tethering for idiopathic scoliosis: two-year results. Spine. 2014. Sep 15;39(20):1688-93. [DOI] [PubMed] [Google Scholar]
- 29.Newton PO, Kluck DG, Saito W, Yaszay B, Bartley CE, Bastrom TP. Anterior spinal growth tethering for skeletally immature patients with scoliosis: a retrospective look two to four years postoperatively. J Bone Joint Surg Am. 2018. Oct 3;100(19):1691-7. [DOI] [PubMed] [Google Scholar]
- 30.Liu GK, Kit WH. Video assisted thoracoscopic surgery for spinal conditions. Neurol India. 2005. Dec;53(4):489-98. [DOI] [PubMed] [Google Scholar]
- 31.Wong HK, Hee HT, Yu Z, Wong D. Results of thoracoscopic instrumented fusion versus conventional posterior instrumented fusion in adolescent idiopathic scoliosis undergoing selective thoracic fusion. Spine. 2004. Sep 15;29(18):2031-8; discussion 2039. [DOI] [PubMed] [Google Scholar]
- 32.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. Stanford University Press; 1959. [Google Scholar]
- 33.Sanders JO, Khoury JG, Kishan S, Browne RH, Mooney JF, 3rd, Arnold KD, McConnell SJ, Bauman JA, Finegold DN. Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am. 2008. Mar;90(3):540-53. [DOI] [PubMed] [Google Scholar]
- 34.Chay E, Patel A, Ungar B, Leung A, Moal B, Lafage V, Farcy JP, Schwab F. Impact of unilateral corrective tethering on the histology of the growth plate in an established porcine model for thoracic scoliosis. Spine. 2012. Jul 1;37(15):E883-9. [DOI] [PubMed] [Google Scholar]
- 35.Bumpass DB, Fuhrhop SK, Schootman M, Smith JC, Luhmann SJ. Vertebral body stapling for moderate juvenile and early adolescent idiopathic scoliosis: cautions and patient selection criteria. Spine. 2015. Dec;40(24):E1305-14. [DOI] [PubMed] [Google Scholar]
- 36.Sitoula P, Verma K, Holmes L, Jr, Gabos PG, Sanders JO, Yorgova P, Neiss G, Rogers K, Shah SA. Prediction of curve progression in idiopathic scoliosis: validation of the Sanders skeletal maturity staging system. Spine. 2015. Jul 1;40(13):1006-13. [DOI] [PubMed] [Google Scholar]
- 37.Tan KJ, Moe MM, Vaithinathan R, Wong HK. Curve progression in idiopathic scoliosis: follow-up study to skeletal maturity. Spine. 2009. Apr 1;34(7):697-700. [DOI] [PubMed] [Google Scholar]
- 38.Rowe DE, Bernstein SM, Riddick MF, Adler F, Emans JB, Gardner-Bonneau D. A meta-analysis of the efficacy of non-operative treatments for idiopathic scoliosis. J Bone Joint Surg Am. 1997. May;79(5):664-74. [DOI] [PubMed] [Google Scholar]
- 39.Nachemson AL, Peterson LE. Effectiveness of treatment with a brace in girls who have adolescent idiopathic scoliosis. A prospective, controlled study based on data from the Brace Study of the Scoliosis Research Society. J Bone Joint Surg Am. 1995. Jun;77(6):815-22. [DOI] [PubMed] [Google Scholar]