Abstract
To investigate the situation of antibiotic consumption and to assess the inappropriate use on pediatric inpatients of different types hospitals in Sichuan, China.
A cross-sectional survey of antibiotic prescriptions among hospitalized children aged 1month -14years were conducted from April 2018 to June 2018 in southwestern China. Antibiotic prescriptions were extracted from electronic records during hospitalization of each inpatient in five different types hospitals.
In this study, the antibiotic prescription rate of hospitalized children was 66.9% (1176/1758). Compared with tertiary children hospital (TC) (46.1%), general hospitals and non-tertiary children hospitals has higher rate of antibiotic prescription (almost 85%) (P < .001). 93.4% of inpatients received parenteral antibiotic. Overall, the most common antibiotics were Cefoperazone and enzyme inhibitor, Cefixime and Azithromycin. Lower respiratory tract infection (LRTI) was the leading reason for antibiotic consumption in pediatric wards (56.8%), followed by upper respiratory tract infection (URTI) (22.2%). For children with LRTI, Cephalosporins were heavy prescribed, especially broad-spectrum third-generation Cephalosporins (60.3%). The antibiotic prescription proportion of URTI in general hospitals and non-tertiary children hospitals (more than 18%) was higher than TC (8.1%) (P < .001).
There was inappropriate use of antibiotic in hospitalized children including overuse of parenteral administration, overprescribing of antibiotic on URTI and misuse of third-generation Cephalosporins in pediatric inpatients with LRTI. Compared with tertiary freestanding children hospital, the irrational antibiotic prescription of general hospitals and non-tertiary children hospitals were more serious. Management strategy should be implementer on quality improvement of antibiotic use.
Keywords: antibiotic prescriptions, appropriate, hospitalized children
1. Introduction
Antibiotic resistance is a global threat to public health and it has been worsened by the inappropriate of antibiotic in the increasing number and types of resistant bacteria. When comes to resistant microorganism especially to those resistant to multi-drug, the potential of antibiotics is declined and infection become incontrollable. In addition to the overgrowth of resistant microorganisms, inappropriate prescribing of antibiotics can lead to antibiotic-related toxicity and complications, gut-microbiome-mediated effects and immense healthcare expenditures.[1] Inappropriate antibiotic prescriptions are common in pediatric like misuse in viral respiratory tract infections, overuse of broad spectrum antibiotic in respiratory tract infections and urinary tract infections.[2–4] Reducing unnecessary use of antibiotics is an important step to decelerate antibiotic resistance. Rational Antimicrobial Stewardship program (ASP) should be forwarded based on the feedback of current inappropriate prescriptions and local resistance status to optimize the use of antibiotic and minimize bacteria resistance. Regional ASPs were implemented and have been proved effective in optimizing antibiotics use in children.[5–6] The majority of survey on pediatric antibiotic use were conducted in tertiary children's hospitals. However, there are plentiful underlying patients in pediatric wards of general hospital and secondary or primary children hospitals (Hospital PC). Study reported that there existed distinction in children guideline-concordant prescribing between different type hospitals.[7] Therefore, conducting a comprehensive survey in different hospital helps to understand the actual situation of local antibiotics use. This study evaluated the quality of antibiotic prescription and analyzed inappropriate consumption in different types of hospitals among hospitalized children in southwestern China for optimizing antimicrobial use and reducing antibiotic resistance.
2. Methods
A cross-sectional survey of hospitalized children antibiotic prescription was conducted in 5 different type hospitals from April 2018 to June 2018 in southwest China, in which several representative different levels hospitals were selected including: 1 tertiary children hospital (Hospital TC), 1 tertiary general hospital (Hospital TG), 1 second children hospital (Hospital SC), 1 second general hospital (Hospital SG), and 1 Hospital PC. Pediatric inpatients age with 1months-14 years were adopted in the period of selected weeks from every month (April 1–7, May 8–14, June 15–21) and the data of inpatients with antibiotic consumption were extracted during hospitalization. Outpatients, day-case patients, pediatric surgery ward and neonatal ward were excluded.
Data were extracted from electronic medical record by clinical doctors and pharmacists anonymously during hospitalization including patient demographic details, indication for antibiotics, route of antibiotic administration and type of antibiotics. According to anatomic systems, diagnosis of infection was categorized as respiratory tract infections, central nervous system infections, gastrointestinal tract infections, urinary tract infections, skin/soft tissue infections and cardiovascular infections. Respiratory tract infections were sub-grouped into upper respiratory infections (URTIs include acute otitis media, sinusitis, pharyngitis, tonsillitis, and laryngitis) and lower respiratory infections (LRTIs including acute bronchitis and pneumonia). Sepsis and unexplained fever, infection with malignancy and prophylaxis also were classified separately. Antibiotic were coded as J01 according to WHO Anatomical Therapeutic Chemical (ATC) classification system. Anti-tuberculosis medications were excluded.
Data were collected and handled in excel form (Microsoft Office 2011) and were analyzed by the SPSS 22.0. The prevalence and route of antibiotic were compared across different hospital types by the chi-square tests. P < .05 were considered statistically significant.
This study was approved by the Ethical Committee of West Second University Hospital. Informed consent was obtained from guardian or older children.
3. Results
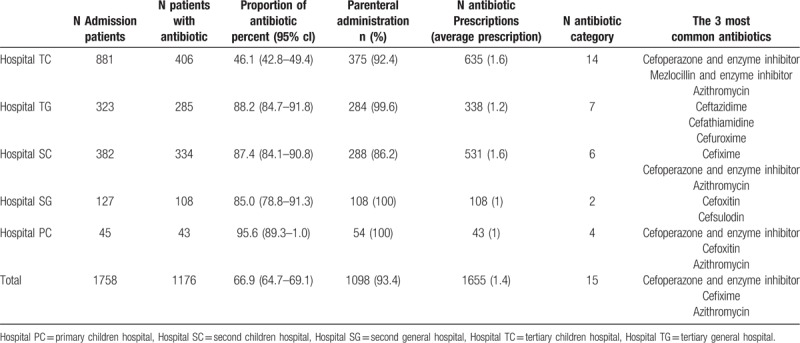
A total of 1758 inpatients were admission and 1176 (66.9%) were treated or prevented with antibiotic including 681 male and 495 female (1.38:1). The proportion of inpatients age with >1 month to <1 year, ≧1 year to <5 years, ≧5 years to <14 years were 20.1% (236), 54.3% (639), 25.6% (301), respectively. Table 1 shown the characteristics in hospitalized children treated with antibiotics in different types hospitals. There was obvious statistical difference about the rate of antibiotic among hospitals (P < .001), in which the percent of antibiotic use in TC (46.1%) was lower than that in general hospitals and non-tertiary children hospitals whose percent were more than 85%. Overall, 93.4% inpatients received parenteral antibiotics. Injected Cefuroxime, Azithromycin, Amoxicillin and enzyme inhibitor were used in Hospital TG, Hospital SC, Hospital SG, Hospital PG. During hospitalization, 1655 antibiotic prescriptions were prescribed in 1176 hospitalized patients with an average of 1.4 antibiotics per patient. The most prescriptions (8) was prescribed in a patient diagnosed as very severe pneumonia combined with respiratory failure in hospital TC. In addition, all patients with antibiotics were only prescribed in one antibiotic without combination and replacement during hospitalization of patients in Hospital SG and Hospital PG. The most common antibiotics were Cefoperazone and enzyme inhibitor, Cefixime and Azithromycin. Heterogeneity of antibiotic prescription were found among hospitals shown in Table 1.
Table 1.
The proportion of antibiotic in hospitalized children among hospitals.
According to ATC classification system, the composition of prescriptions was presented in Figure 1 including 15 antibiotic categories, of which 14 categories were used in TC. Cephalosporins were the most antibiotic, especially third-generation Cephalosporins. Furthermore, Macrolides was the second common category except for hospital SG, in which prescriptions only consisted of second-generation Cephalosporins and third-generation Cephalosporins.
Figure 1.
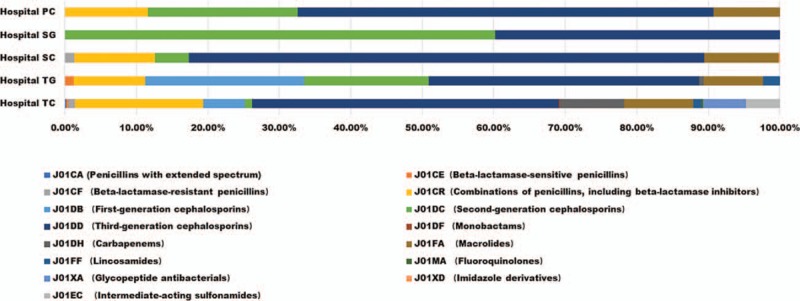
Antibiotic use of hospitalized children in difference hospitals.
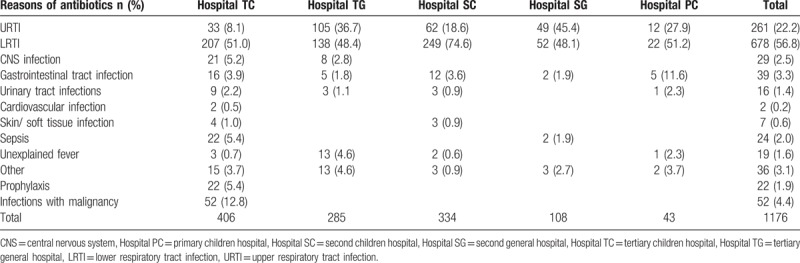
Different antibiotic categories were used largely due to different diseases and the bacterial resistance in hospitalized children among hospitals. The reasons of antibiotic prescriptions were shown in Table 2. The spectrum of disease was more diverse in hospital TC than that in other type hospitals. The conditions of Cardiovascular infections, prophylaxis and infection with malignancy individually occurred in hospital TC. As a whole, 56.8% and 22.2% of antibiotic prescriptions in pediatric inpatients were consumed in LRTIs and URTIs. There was statistical difference in the ratio of antibiotic on URTIs (P < .001). Compared with Hospital TC, higher rates were shown in other hospitals even up to 49% in Hospital SG.
Table 2.
The reasons of antibiotic prescription among hospitals.
LRTIs was the leading reason for antibiotic consumption in all pediatric wards of different type hospitals. The 678 children with LRTIs were prescribed with 969 antibiotic prescriptions. 84.6% of LRTIs children was young than 5 years. The composition of prescriptions was presented in Figure 2. Third-generation Cephalosporins (60.3%) and Macrolides (12.3%) was the most antibiotic category.
Figure 2.
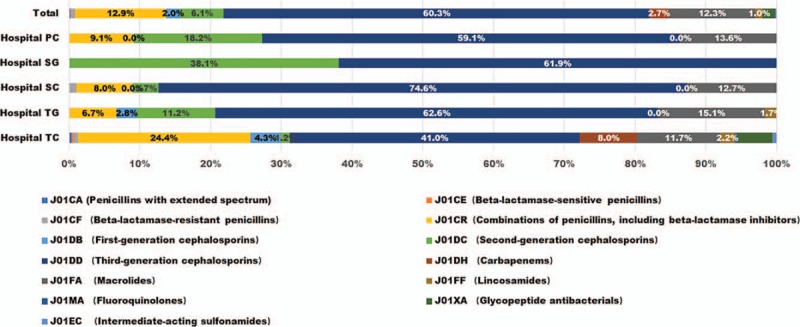
Antibiotic use of hospitalized children with LRTI in difference hospitals.
4. Discussion
In this study, a comparative data of antibiotic prescriptions in pediatric wards among different types hospitals were collected. The results revealed that 66.9% of hospitalized children were prescribed with antibiotics, which was higher than the percent of other studies have reported. Some point prevalence surveys reported 40.9% of pediatric inpatients were on antibiotic in UK,[8] 37% in Riga and 26.3% in Vilnius.[9] The data in those studies as mentioned above were collected on one selected day during hospitalization. In our study, however, all inpatients antibiotics information was extracted throughout the hospitalization. A cross sectional study in Ethiopia with a method similar to us shown that almost 74% of hospitalized children received antibiotic therapy,[10] which is higher than the proportion of this study. There are Geographical difference in antibiotic use of pediatric inpatients though they should be evaluated in the same way. The fact that higher rate of antibiotic use in Asia in children than that in Europe and North America is recognized.[11–12] In this study, the percent of antibiotic use in Hospital TC (46.1%), meet the Chinese national management strategy, in which the proportion of antibiotic prescription in hospitalized children does not exceed 60%.[13] But in other four hospitals including general hospitals or non-tertiary children hospitals almost 90% proportion prescribed antibiotic were far beyond the rule, which indicated overprescribing and irrational antibiotic utilization were obvious. This may be caused by poorer and suboptimal knowledge on antibiotic use of doctors and pediatric-specific resources, which has also been put forward in other reports.[7,14] Ongoing education programs on pediatric antibiotic manage for secondary, primary children care setting and general hospitals should be carried out for further improvement.
Parenteral route was widely administrated in this study and some antibiotics like Amoxicillin and enzyme inhibitor, Azithromycin, Cefuroxime were administrated intravenously in Hospital TG, Hospital SC, Hospital SG, Hospital PG even if they can be taken orally. Studies on antibiotic route shown that rate of exclusively oral antibiotics varied widely, ranging from 0 to almost 80% with the average 21.5% in hospitalized children across hospitals.[15] The parenteral route proportion (93.4%) of this study was amazing and inappropriate. Parenteral administration may indeed be preferable in certain situations, such as chosen antibiotics with limited oral bioavailability, complicated infections or central nervous system infection and children who cannot take oral medication. The route of administration depends on the type and severity of diseases, meanwhile the route also be affected by irrational social acceptance in hospitalized patients that intravenous drugs are more “effective” compared with oral antibiotics. Several randomized trials revealed that oral amoxicillin is equivalent in safety and effectiveness to parenteral penicillin for pediatric patients with community acquired pneumonia,[16–18] which is a most common infection in children. In addition, oral administration has potential to avert suffering of children, minimize the risk of hospital infection, reduce nursing burdens, shorten hospital stay and decrease medical expenses for many hospitalized children with common pediatric infections.[19] Early switch from parenteral to oral has been proposed to be a quality indicator for improving antibiotic prescribing on pediatric inpatients worldwide.[12] This study provides a strong evidence that multiple efforts should be made on overuse of parenteral route in Chinese hospitalized children.
This study found that a high proportion of antibiotic were prescribed for URTIs in pediatric inpatients, especially in general hospitals, in which the percentages were more than 30% suggests overprescribing. Actually, a majority of URTIs are caused by virus and do not require antibiotics.[20] Overuse of antibiotics is common in children with URTI including in china,[21–23] which might be affected by difficult distinguishing bacterial infections form viral URIS and insufficient knowledge of pediatrician and parents on antibiotic use for URTIs.[24–25] American Academy of Pediatric proposed that judicious antibiotic prescribing for URTIs in pediatrics should follow 3 principles:
-
(1)
determine the likelihood of bacterial infections according to clinical symptoms,
-
(2)
weigh benefits and harms of antibiotics on strict bacterial diagnosis,
-
(3)
choose advisable strategies based on the severity of diseases.[26]
Reducing unnecessary antibiotic use in pediatric URTIs may be another aspect we need pay attention for controlling inappropriate use of antibiotic in China.
Pneumonia is a major cause of childhood morbidity and mortality,[27] Empirical medications are always given to reduce serious complications before determining accurate pathogen. Results shown that LRTI was the leading reason for antibiotic consumption up to 56.8% and Cephalosporins were the most prescriptions, especially third-generation Cephalosporins (60.3%). According to the management of WHO that oral amoxicillin and parenteral ampicillin and gentamicin were recommended as first-line treatment for severe pneumonia,[28] the misuse of antibiotic was found in this study. In china, the common bacterial etiology of community-acquire pneumonia (CAP) among children under 5 years were Klebsiella pneumonia, Streptococcus pneumonia(SP), Escherichia coli, Staphylococcus aureus(SA), Haemophilus influenza (Hi),[29] and SP and Hi are the most common for hospitalized children.[30] At present, SP and Hi are high sensitive to penicillin, amoxicillin and Clavulanate separately according to the latest consensus in China.[31] Antibiotic de-escalation may be necessary according to bacteriological cultures and resistance in LRTI. Furthermore, the introduction of pneumococcal vaccines and Hi conjugate vaccines can effectively decrease the incidence of global pneumonia,[32–33] but only 25.2% of children received the vaccines in ShangHai, a developed city of China.[34] Therefore, increase vaccination may be another way to reduce the inappropriate use of antibiotics in LRTI.
Limitation: This is a short-term cross-sectional survey and data are only collected from April 2018 to June 2018, which may lead to the seasonal bias. However, to some extent it does reveal the general situation of current antibiotics use. Comprehensive investigation can be done and help us to understand the seasonal characteristics. Otherwise further attention can also be pay to other aspects of inappropriate antibiotic use like the dose and combined medication of antibiotics.
5. Conclusion
This study shown the inappropriate use of antibiotic in Chinese hospitalized children including overuse of antibiotic prescriptions, misuse of parenteral administration, overprescribing of antibiotic on URTI and the de-escalation requirement of third-generation Cephalosporins in pediatric inpatients with LRTI. The irrational use was more serious in general hospitals and non-tertiary children hospitals. Management strategy should be implemented on quality improvement of antibiotic use.
Author contributions
Conceptualization: Chaomin Wan, Yu Zhu.
Data curation: Ruixue Miao, Yun Zhao.
Formal analysis: Ruixue Miao, Zhiling Wang.
Funding acquisition: Ruixue Miao, Chaomin Wan.
Investigation: Ruixue Miao, Jialing Xia.
Methodology: Ruixue Miao, Chaomin Wan, Yu Zhu.
Project administration: Chaomin Wan.
Resources: Yun Zhao, Liling Zhang, Juan Liu, Jing Qin, Jialing Xia, Huiqiong Yan.
Software: Yu Zhu.
Supervision: Ruixue Miao, Chaomin Wan, Zhiling Wang, Yu Zhu.
Validation: Ruixue Miao, Yu Zhu, Yun Zhao, Liling Zhang, Juan Liu, Jing Qin, Huiqiong Yan.
Writing – original draft: Ruixue Miao.
Writing – review & editing: Chaomin Wan, Zhiling Wang.
Footnotes
Abbreviations: ASP = Antimicrobial Stewardship program, ATC = Anatomical Therapeutic Chemical, CAP = community-acquire pneumonia, Hi = Haemophilus influenza, Hospital PC = primary children hospital, Hospital SC = second children hospital, Hospital SG = second general hospital, Hospital TC = tertiary children hospital, Hospital TG = tertiary general hospital, LRTIs = lower respiratory infections, SP = Streptococcus pneumoniae, URTIs = upper respiratory infections.
How to cite this article: Miao R, Wan C, Wang Z, Zhu Y, Zhao Y, Zhang L, Liu J, Qin J, Xia J, Yan H. Inappropriate antibiotic prescriptions among pediatric inpatients in different type hospitals. Medicine. 2020;99:2(e18714).
This study was supported by The Fundamental Research Funds for Central Universities (No. 2012017yjsy196), The Pediatric Clinical Research Center Foundation of Sichuan Province, China (grant No. 2017-46-4), and National Science and Technology Major Project of China (grant No. 2018ZX10103-001).
The authors have no conflicts of interests to disclose.
References
- [1].Vangay P, Ward T, Gerber J, et al. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 2015;17:553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kronman MP, Zhou C, Mangione-Smith R. Bacterial prevalence and antimicrobial prescribing trends for acute respiratory tract infections. Pediatrics 2014;134:e956–65. [DOI] [PubMed] [Google Scholar]
- [3].Morgan JR, Carey KM, Barlam TF, et al. Inappropriate antibiotic prescribing for acute bronchitis in children and impact on subsequent episodes of care and treatment. Pediatr Infect Dis J 2019;38:271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Birgy A, Cohen R, Levy C, et al. Community faecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in French children. BMC Infect Dis 2012;12:315–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Godbout EJ, Pakyz AL, Daniel MJ, et al. Pediatric antimicrobial stewardship: state of the art. Curr Infect Dis Rep 2018;20:39. [DOI] [PubMed] [Google Scholar]
- [6].Smith MJ, Gerber JS, Hersh AL. Inpatient antimicrobial stewardship in pediatrics: a systematic review. J Pediatric Infect Dis Soc 2015;4:e127–35. [DOI] [PubMed] [Google Scholar]
- [7].Tribble AC, Ross RK, Gerber JS. Comparison of antibiotic prescribing for pediatric community-acquired pneumonia in children's and non-children's hospitals. JAMA Pediatr 2019;173:190–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gharbi M, Doerholt K, Vergnano S, et al. Using a simple point-prevalence survey to define appropriate antibiotic prescribing in hospitalised children across the UK. BMJ Open 2016;6:e012675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sviestina I, Usonis V, Gurksniene V, et al. Prescription of antibiotics in Riga and Vilnius tertiary children's hospitals. Eur J Hosp Pharm 2018;25:189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Genamo E, Bayisa H, Detemo R. Assessment of antibiotics use for hospitalized children in Butajira General Hospital, southern part of Ethiopia. Int J Pediatr 2019;7:8845–51. [Google Scholar]
- [11].Youngster I, Avorn J, Belleudi V, et al. Antibiotic use in children–a cross-national analysis of 6 countries. J Pediatr 2017;182:239–44. e1. [DOI] [PubMed] [Google Scholar]
- [12].Versporten A, Bielicki J, Drapier N, et al. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother 2016;71:1106–17. [DOI] [PubMed] [Google Scholar]
- [13].Department of Health, Ministry of Health. 2012 National Antimicrobial Drug Clinical Application Special Rehabilitation Program (Excerpt). Chinese Community Physician (Medical), 2012, 14(8). [Google Scholar]
- [14].Bai Y, Wang S, Yin X, et al. Factors associated with doctors’ knowledge on antibiotic use in China. Sci Rep 2016;6:23429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kronman MP, Hersh AL, Newland JG, et al. Getting over our inpatient oral antibiotic aversion. Pediatrics 2018;142:e20181634. [DOI] [PubMed] [Google Scholar]
- [16].Addo-Yobo E, Chisaka N, Hassan M, et al. Oral amoxicillin versus injectable penicillin for severe pneumonia in children aged 3 to 59 months: a randomised multicentre equivalency study. Lancet 2004;364:1141–8. [DOI] [PubMed] [Google Scholar]
- [17].Atkinson M, Lakhanpaul M, Smyth A, et al. Comparison of oral amoxicillin and intravenous benzyl penicillin for community acquired pneumonia in children (PIVOT trial): a multicentre pragmatic randomised controlled equivalence trial. Thorax 2007;62:1102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Agweyu A, Gathara D, Oliwa J, et al. Severe Pneumonia Study Group. Oral amoxicillin versus benzyl penicillin for severe pneumonia among kenyan children: A pragmatic randomized controlled noninferiority trial. Clin Infect Dis 2015;60:1216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cyriac JM, James E. Switch over from intravenous to oral therapy: a concise overview. J Pharmacol Pharmacother 2014;5:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hayden FG. Respiratory viral threats. Curr Opin Infect Dis 2006;19:169–78. [DOI] [PubMed] [Google Scholar]
- [21].Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 2009;302:758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dooling KL, Shapiro DJ, Van Beneden C, et al. Overprescribing and inappropriate antibiotic selection for children with pharyngitis in the United States, 1997-2010. JAMA Pediatr 2014;168:1073–4. [DOI] [PubMed] [Google Scholar]
- [23].Li J, Song X, Yang T, et al. A systematic review of antibiotic prescription associated with upper respiratory tract infections in China. Medicine 2016;95:e3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sa’ed HZ, Taha AA, Araj KF, et al. Parental knowledge, attitudes and practices regarding antibiotic use for acute upper respiratory tract infections in children: a cross-sectional study in Palestine. BMC Pediatr 2015;15:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hammour KA, Farha RA, Alsous M, et al. Evaluation of risk factors affecting parental knowledge and attitude toward antibiotic use in children with upper respiratory tract infections. Eur J Integr Med 2018;17:107–11. [Google Scholar]
- [26].Hersh AL, Jackson MA, Hicks LA, et al. Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatrics 2013;132:1146–54. [DOI] [PubMed] [Google Scholar]
- [27].Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016;388:3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].World Health Organization. Revised WHO classification and treatment of pneumonia in children at health facilities: evidence summaries. 2014. https://apps.who.int/iris/bitstream/handle/10665/137319/9789241507813_eng.pdf. [PubMed] [Google Scholar]
- [29].Ning G, Wang X, Wu D, et al. The etiology of community-acquired pneumonia among children under 5 years of age in mainland China, 2001-2015: a systematic review. Hum Vaccin Immunother 2017;13:2742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang Q, Guo Z, MacDonald NE. Vaccine preventable community-acquired pneumonia in hospitalized children in Northwest China. Pediatr Infect Dis J 2011;30:7–10. [DOI] [PubMed] [Google Scholar]
- [31].National Health and Wellness Committee of the People's Republic of China, State Administration of Traditional Chinese Medicine. Guidelines for the diagnosis and treatment of childhood community acquired pneumonia (2019 edition). 2019-2-11. [Google Scholar]
- [32].MacNeil JR, Cohn AC, Farley M, et al. Current epidemiology and trends in invasive Haemophilus influenzae disease—United States, 1989–2008. Clin Infect Dis 2011;53:1230–6. [DOI] [PubMed] [Google Scholar]
- [33].Griffin MR, Zhu Y, Moore MR, et al. U. S. Hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013;369:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wagner AL, Boulton ML, Sun X, et al. Perceptions of measles, pneumonia, and meningitis vaccines among caregivers in Shanghai, China, and the Health Belief Model: a cross-sectional study. BMC Pediatr 2017;17:143. [DOI] [PMC free article] [PubMed] [Google Scholar]


