Abstract
This retrospective study is to explore the clinicopathologic, immunophenotypic, and molecular genetic features of Waldeyer ring B-cell lymphoma (WR-BCL).
Tissue arrays from 65 WR-BCL cases were subjected to pathologic and immunophenotypic detections. Expression of Epstein–Barr virus-encoded small RNA (EBER) was detected by in situ hybridization. Interferon regulatory factor 4 (IRF4), BCL-2, BCL-6, and C-myelocytomatosis viral oncogeneav (MYC) gene abnormalities were investigated using interphase fluorescence in situ hybridization.
Among the 65 patients, there were 12 nasopharynx cases, 49 tonsil cases, and 4 tongue root cases. Moreover, there were 49 cases of diffuse large BCL (DLBCL) and 16 cases of follicular lymphoma (FL). More than 60% of the patients had Ann Arbor stage III/IV disease, with infiltrated neighboring organs, invaded spleens, and increased lactate dehydrogenase (LDH) levels. Tumor cells were positive for multiple myeloma antigen 1 (MUM1), BCL-2, BCL-6, and C-MYC. EBER expression was detected in lymphoma cells of 2 cases. Alteration frequencies of IRF4, BCL-2, BCL-6, and C-MYC were 24.6%, 32.3%, 27.7%, and 30.7%, respectively. Approximately 67.69% cases had stages 0 to II disease, while 32.31% cases had stage III disease. Five-year overall survival rate was 65.12%. Eastern Cooperative Oncology Group performance status (ECOG) score ≥2 was the only adverse factor for overall survival. IRF4/MUM1, C-MYC, and CD10 expressions were related to poor disease prognosis. WR-BCLs were largely dependent on ECOG, LDH, and bone marrow involvement. WR-DLBCL was associated with poor survival outcomes compared with WR-FL.
The WR-DLBCLs have distinct clinicopathologic features, with correlations between the IRF4/MUM1, C-MYC and CD10 expressions, ECOG, LDH, bone marrow involvement, and the disease prognosis.
Keywords: interferon regulatory factor 4/multiple myeloma antigen 1, Waldeyer ring, diffuse large B-cell lymphomas
1. Introduction
The interferon regulatory factor 4 gene (IRF4), also known as the multiple myeloma (MM) antigen 1 (MUM1), is widely expressed in the plasma cells, melanocytes, B cells, and activated T cells.[1] IRF4 protein is involved in several stages of B-cell development, including the differentiation of mature B cells into the antibody-secreting plasma cells.[2] IRF4 is downregulated in the t (6;14)(p25;q32) reciprocal translocation, and juxtaposed with the immunoglobulin heavy chain (IGH) gene locus in some cases of B-cell lymphomas (BCLs), including the diffuse large BCLs (DLBCLs),[3] chronic B-cell lymphoid leukemia,[4] splenic marginal zone lymphoma,[5] and Waldeyer ring (WR) DLBCL.
The WR represents one of the most common extranodal sites for the development of DLBCL. According to the specific clinical and/or pathologic features, WR-DLBCL should be classified as a variant of DLBCL, that is, the primary mediastinal large BCL.[6] Targeted therapies might aim at the genes involved in the recurrent chromosomal translocations. However, in contrast to the myeloid neoplasms and BCLs, translocations in most WR-DLBCL cases remain poorly understood.
In this study, the clinical outcomes of the patients with WR-BCL, WR-DLBCL, and WR-follicular lymphoma (FL) were analyzed and compared. The expression levels of IRF4 were evaluated, and the specific clinical or evolutionary features for the cases with IRF4 rearrangements were also investigated.
2. Materials and methods
2.1. Clinical data
The samples of paraffin-embedded BCLs were collected from patients with BCL. All tissue sections were reviewed using immunohistochemistry and rediagnosed by 2 independent lymphoma pathologists (Xinxia Li and Wenli Cui), according to the 2008 World Health Organization Classification of Tumors of Hematopoietic and Lymphoid Tissues. Inclusion criteria: cases diagnosed as DLBCL NOS (a neoplasm of medium or large B-lymphoid cells whose nuclei are the same size as, or large than those of normal macrophages or more than twice the size of those of normal lymphocytes, with a diffuse growth pattern, and at least 1 positive B-cell antibody, such as CD20, CD79a, or PAX5) of the Waldeyer ring, with sufficient clinical and immunohistochemical information were included.
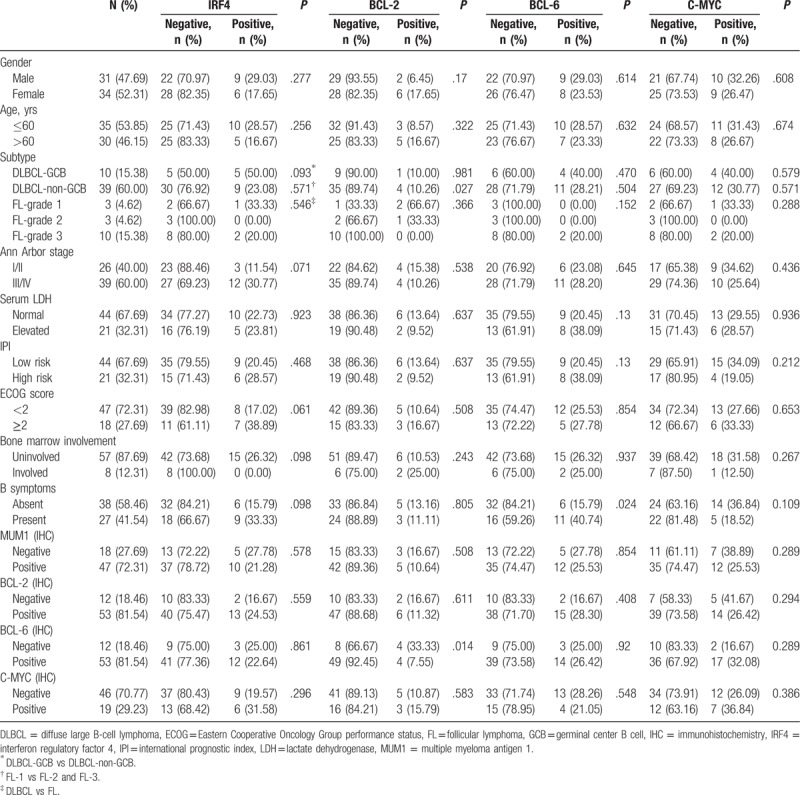
The follow-up items included the age, gender, lactate dehydrogenase (LDH) level, Ann Arbor stage, international prognostic index (IPI) score, Eastern Cooperative Oncology Group performance status (ECOG) score, B symptoms, and overall survival (OS) time. Then, the patients were assigned into the germinal center B (GCB) cell and non-GCB groups, respectively, according to the expression of CD10, Bcl-6, and MUM1.[7] The GCB subtypes included the CD10+ or CD10–, Bcl-6+, and MUM1– phenotypes, while the non-GCB subtypes included the CD10–, Bcl-6–, or Bcl-6+, and MUM1+ phenotypes (Table 1).
Table 1.
Comparison of characteristics between IRF4, BCL-2, BCL-6, and C-MYC expression and Waldeyer ring BCL.
Written informed consent was obtained from every patient and the study was approved by the ethics review board of the First Affiliated Hospital of Xinjiang Medical University.
2.2. Immunohistochemistry
Two tissue microarray (TMA) blocks were constructed using the tissue arrayer. For each case, there were 2 tumor cores of 0.6 mm obtained from the original paraffin blocks. The tissue blocks were cut into the 3-μm serial sections, which were used for the immunohistochemical analysis, according to the standard protocols.[8] The detected proteins included the MUM-1/IRF4 (EPR5653; Abcam, Cambridge, England, 1:100, cell nucleus), CD20 (L26, Gene, 1:150, cell membrane), CD5 (SP19, Zhongshan, 1:100, cell membrane), PAX-5 (SP34, Zhongshan, 1:50, cell nucleus), CD10 (56C6, Gene, 1:30, cell membrane), BCL-2 (56C6, Gene, 1:30, cell membrane), BCL-6 (GI191E/A8, Zhongshan, 1:80, cell nucleus), C-myelocytomatosis viral oncogeneav (MYC) (Y69, Zhongshan, 1:150, cell nucleus), and KI-67 (MIB-1, Gene, 1:150, cell nucleus). Briefly, after pretreatment with the 1 mM ethylene diamine tetraacetic acid buffer (pH 8.0; PT Module, LabVision, Fremont, CA) at 98°C for 30 minutes, the sections were stained with the mouse anti-human anti-IRF4 monoclonal antibody (MUM1p, 1:50; Dako, Carpinteria, CA), and the signals were detected using the Dual Link Envision+/DAB+ (Dako). Based on the percentage of positive staining, immunohistochemistry staining results were scored. In detail, cut-off point for CD10 protein was >30% of positive membranous staining on tumor cells; that for BCL-2 protein was >30% of positive cytoplasm staining on tumor cells; those for BCL-6 and MUM1 protein were >30% nuclear positivity on tumor cells. The positive expression was defined as ≥5% of tumor cell positive for staining, while the staining of <5% of all tumor cells (including no expression) was classified as negative expression.[7,9,10] The percentage of Ki-67 positive tumor cells was determined. According to the algorithm from Hans et al,[7] the samples were classified into the GCB-cell-like and non-GCB immunophenotypes.
2.3. Epstein–Barr virus-encoded small RNA in situ hybridization
The presence of Epstein–Barr virus (EBV) was detected by the in situ hybridization on the TMA with probes specific for the EBV-encoded small RNA (EBER) sequences (Dako Cytomation). The in situ hybridization was performed as previously described,[11] with the lymph glands containing infectious mononucleosis as positive control.
2.4. Fluorescent in situ hybridization analysis
The interphase fluorescent in situ hybridization (FISH) was performed on the TMA sections using the dual-color break apart rearrangement probes targeting the IRF4/6p25 (probes Z-2210-50; ZytoVision, Bremerhaven, Germany), MYC/8q24, BCL-2/18q21, and BCL-6/3q27 genes (probes 30-191096, 30-191018, 30-231050; Abbott AG, Abbott Park, IL), as previously described.[12,13] Results were analyzed and interpreted with the FISH 2.0 Software.[14,15] For each case, 10 fields of views (FOVs) were randomly selected, and 100 cells were counted from each FOV. The percentage of positive cells in these 10 FOVs was calculated. First, red and green signals were separated by >18% in the tumor cell nuclei. Second, the distance was greater than between the 2 signal points. Thirdly, the IRF4/6p25, MYC/8q24, BCL-2/18q21, and BCL-6/3q27 were considered as positive. Paraffin-embedded tissue sections were digested by 0.4% pepsin solution, hybridized with probe, and counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride. Totally 50 to 200 cells were analyzed for each case by the microscopist (MEL), with a minimum of 20 abnormal cells considered as abnormal.
2.5. Statistical analysis
The SPSS 19.0 statistical package was used for statistical analysis. Age, gender, ECOG score, Ann Arbor stage, B symptoms, LDH, and IPI were categorical variables. Pearson Chi-squared or Fisher exact test was used for the comparison of the categorical data. End points of interest were the OS (defined as the time interval from the randomization to the last follow-up or death due to cause). Survival functions were estimated using the Kaplan–Meier method, and the log-rank test was used for comparison. Significant factors affecting the OS in the univariate analysis were further examined by the Cox regression multivariate analysis. P < .05 was considered as statistically significant.
3. Results
3.1. Clinical characteristics of patients with WR-DLBCL
The clinical characteristics of the included patients are summarized in Table 1. In these 65 patients with primary WR-BCLs, there were 12 cases of nasopharynx, 49 cases of tonsil, and 4 cases of tongue root. For the subtype distribution, there were 49 DLBCL cases (75.38%) and 16 FL cases (24.62%) (Fig. 1 A, B).
Figure 1.
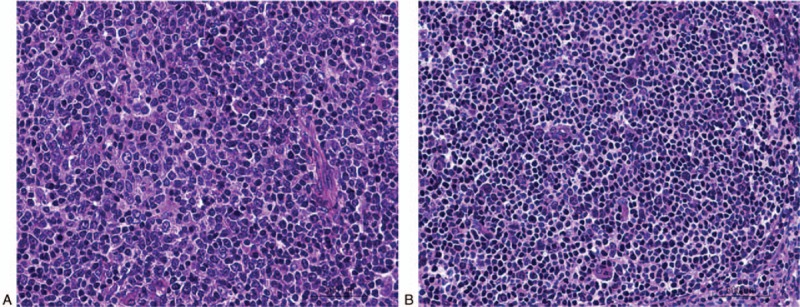
B-cell lymphomas in Waldeyer ring cases. Hematoxylin and eosin staining was performed to detect the B-cell lymphomas. (A) Waldeyer ring (WR) diffuse large B-cell lymphoma. (B) WR follicular lymphoma. (400×).
For the global immunophenotypic profile, out of the 49 cases, there was 10 GCB cases (20.41%) and 39 non-GCB cases (79.59%). In these patients, there were 31 males and 34 females (M/F ratio: 0.91/1), with a mean age of 58 years (ranging from 32 to 83 years). Moreover, in these patients, 26 patients (40.00%) were at stages I and II, while 39 patients (60.00%) were at stages III and IV. Furthermore, 8 patients (12.31%) showed bone marrow involvement. In addition, there were 43 patients (24 males and 19 females) with clinical follow-up data, with a median age of 60 years (ranging from 32 to 83 years). Of these 43 patients, 15 patients (34.88%) were at stage I or II, and 28 patients (65.12%) were stages III and IV. Moreover, 5 patients (11.63%) had bone marrow involvement. The aaIPI was available for 65 patients: 0 to 2 for 44 patients (67.69%), and ≥3 for 21 patients (32.31%). The majority of patients (64.62%, 42/65) received the cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like regimen as the induction treatment, while 23 patients could not receive the induction treatment due to the financial reasons or death.
3.2. Immunohistochemistry and EBV in situ hybridization
The expression percentages of CD20, CD5, CD10, BCL-2, BCL-6, C-MYC, IRF4/MUM1, and KI-67 were evaluated in these 65 patients by immunohistochemistry. The expression prevalences of different antigens were as follows: CD20 (100.00%, 65/65), CD5 (26.15%, 17/65), CD10 (83.08%, 54/65), BCL-2 (81.54%, 53/65), BCL-6 (81.54%, 53/65), and C-MYC (29.23%, 19/65) (Fig. 2A–F). Moreover, patients were divided into the IRF4-negative and -positive groups. According to this cut-off value, 72.31% of the patients (47/65) exhibited positive IRF4 expression, with the moderate or strong nuclear positiveness in the majority of the neoplastic cells in all the IRF4-positive cases (Fig. 2G). The expression of Ki-67 was high, which was much more than 40% (Fig. 2H). Furthermore, the EBER in situ hybridization was positive in the lymphoma cells in 2 of these 65 cases (Fig. 2I). The immunophenotypic profile was GCB in 10 of 49 cases (20.41%) and 39 non-GCB cases (79.59%).
Figure 2.
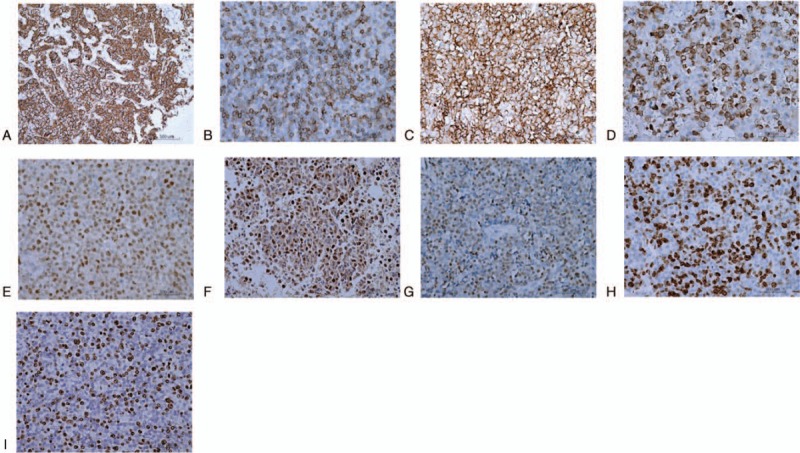
Immunohistochemical results with the EnVision method. (A–H) CD20 (A), CD5 (B), CD10 (C), B-cell lymphoma (BCL)-2 (D), BCL-6 (E), C-MYC (F), multiple myeloma antigen 1/interferon regulatory factor 4 (G), and KI-67 (H) expressions on oncocytes. (I) Epstein–Barr virus-encoded small RNA-positive oncocytes. Scale bar, 500 μm. MYC = myelocytomatosis viral oncogeneav.
3.3. Frequency of IRF4, BCL-2, BCL-6, and C-MYC translocations in patients with WR-BCLs
Evaluable FISH results of at least 2 loci were obtained for these 65 cases in the TMA. The results of IRF4, BCL-2, BCL-6, and C-MYC translocations in relation to all the other parameters are shown in Table 2. The IRF4 FISH was successful in all these 65 patients (100%). IRF4 translocation was observed in 15 patients out of the 65 patients (23.08%) (Fig. 3A), including 12 cases of DLBCL out of the 49 patients (24.49%) (including 6 GCB immunophenotype and 6 non-GCB) and 3 cases of FL out of the 16 patients (18.75%) (Table 1). In these patients, there were 10 patients positive for the IRF4/MUM1 expression. The clinical characteristics of these 15 patients with WR-BCL were successfully tested for the IRF4 translocation. Our results showed that the IRF4 rearrangement occurred more frequently among males, age of ≤60 years, DLBCL-GCB, stage III/IV, ECOG of ≥2, none bone marrow involvement, B symptoms, and positive for C-MYC expression (although without significant differences). Moreover, 8 cases of the 65 assessable patients (12.31%) (including 1 GCB, 4 non-GCB, and 3 FL cases) harbored the BCL-2 rearrangement (Fig. 3B), and these 6 cases positive for the BCL-2 expression. The BCL-2 rearrangement occurred more frequently on the FL-1 (P = .027). These cases occurred more frequently among females, age of ≤60 years, bone marrow involvement, B symptoms, and with C-MYC expression (although without significant differences). Furthermore, 7 cases of the 65 assessable patients (26.15%) (including 4 GCB, 11 non-GCB, and 2 FL cases) harbored the BCL-6 rearrangement (Fig. 3C), and there were 14 patients positive for the BCL-6 expression. The patients with B symptoms had more frequent BCL-6 rearrangement (P = .024). These cases occurred somewhat more frequently on the GCB, abnormal serum LDH, and IPI high risk (although without significant differences). In addition, 19 cases of the 65 assessable cases (29.31%) (including 4 GCB, 12 non-GCB, and 3 FL cases) harbored the C-MYC rearrangement (Fig. 3D), and there were 7 patients positive for the C-MYC expression. The patients with bone marrow involvement had more frequent C-MYC rearrangement (although without significant differences). Only 3 cases of the 42 cases (7.14%) harbored the BCL-2 rearrangement, 6 had BCL-6 rearrangement, and 5 had C-MYC rearrangement. Moreover, only one each was in association with BCL-2 and BCL-6 rearrangement.
Table 2.
Fluorescent in situ hybridization analysis of primary Waldeyer ring BCLs.
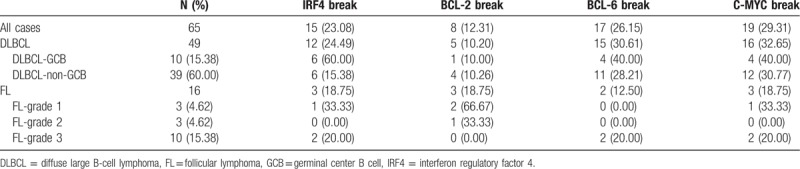
Figure 3.

Representative fluorescent in situ hybridization findings for patients with B-cell lymphomas. (A) Interferon regulatory factor 4 (IRF4) rearrangement and no IRF4 translocation. (B) B-cell lymphoma (BCL)-2 rearrangement and no BCL-2 translocation. (C) BCL-6 rearrangement and no BCL-6 translocation. (D) C-MYC rearrangement and no C-MYC translocation. MYC = myelocytomatosis viral oncogeneav.
3.4. Overall outcome for patients with WR-BCL
Clinical data were obtained retrospectively from the electronic medical records. There were 43 cases with updated survival information. The clinical features at the time of diagnosis were tested for the impacts on the outcome (Table 3). The average follow-up period was 29.7 months, with the median follow-up period of 16 months (ranging from 1 to 89 months), and 9 patients died within 1 year. The 5-year OS rate for the 43 patients was 65.12% (Fig. 4A). Totally 28 patients remained alive, and 22 patients were lost to the follow-up.
Table 3.
Disease-free survival of 43 patients with Waldeyer ring BCL based on prognostic factor grouping.
Figure 4.

Overall survival curves and influence of different factors on patients with Waldeyer ring B-cell lymphoma (WR-BCL). (A) The overall survival curves of these 43 patients with WR-BCL. (B) Survival curve showing the influences of PS (B), gender (C), TNM staging score (D), C-MYC expression (E), serum lactate dehydrogenase (F), bone marrow involvement on C-MYC gene expression (G), IPI (H), interferon regulatory factor 4 (IRF4) gene (I), BCL-2 gene (J), BCL-6 gene (K), and multiple myeloma antigen 1/IRF4 (L) gene expression in patients with WR-BCL. MYC = myelocytomatosis viral oncogeneav, PS = performance state.
The impacts of age, gender, ECOG score, Ann Arbor stage, B symptoms, LDH, and IPI on the survivals (OS) were studied. The ECOG score of ≥2 was associated with better OS (P = .001) (Fig. 4B), while the female gender, stage I/II, and C-MYC expression were associated with better OS, although without significant differences (P = .114, .274, and .775, respectively) (Fig. 4C–E). Abnormal serum LDH, C-MYC translocation, and higher aaIPI were correlated with shorter OS, although without significant differences (P = .092, .775, and .142, respectively) (Fig. 4F–H). Moreover, there were no significant differences in the IRF4, BCL-2, and BCL-6 translocation for the effects on the OS (Fig. 4I–L). The multivariate analysis showed that the IRF4/MUM1, C-MYC, and CD10 expressions were associated with poor survival outcomes in the WR-BCL group, which represented independent factors for OS (P = .018, .041, and .038, respectively) (Table 4). WR-BCLs were largely dependent on other prognostic factors, such as ECOG, LDH, and bone marrow involvement (P = .008, .030, and .012, respectively). WR-DLBCL was associated with worse survival outcomes, compared with WR-FL (P = .018).
Table 4.
Multivariate analysis of prognostic factors in relation to patient survival.

4. Discussion
In China, DLBCL is the most common subtype of non-Hodgkin lymphoma (NHL), accounting for 38% of all NHL cases.[16] Although DLBCL has been considered as a single entity in the WHO classification, the primary disease sites were associated with the particular clinicopathologic features and outcomes.[17] The WR comprises the lymphoid tissues arising in the nasopharynx, palatine tonsils, tongue base, soft palate, and oropharyngeal wall.[16] As the common sites for involvement among DLBCL in the head and neck, the WR involvement has been associated with more favorable clinicopathologic features and better outcome than the lymph node.[17] Based on specific anatomical, clinicopathologic, and genetic features, the WR-DLBCL should be classified as a variant of DLBCL. NHL-involving WRs have been associated with heterogeneous histologic distribution in both the pediatric and adult patients. Most of the NHL-involving WR cases are of the B-cell origin, with distinctive biologic and clinical behavior. However, the relationships among the clinical features, pathologic subtypes, and patient ages still remain unclear. In this study, 33 cases of the 36 WR-NHL cases were of the BCL type, and the subtypes varied among the pediatric and adult patients. The most common subtype in children was BL, followed by DLBCL and FL, while DLBCL was found to be predominant in adults, followed by FL.
In this study, the clinicopathologic and molecular genetic characteristics of primary WR-BCLs were investigated. Our study was performed based on a cohort of 65 patients with primary WR-BCLs, with updated survival information. Our results concerning the subtype distribution showed that there were 49 DLBCL (75.38%) and FL (24.62%) cases, including 43 cases with updated survival information. Based on these findings, for the first time, we demonstrate the presence of translocations of IRF4, BCL-2, BCL-6, and C-MYC in the WR-BCLs. Our results revealed the specific pathologic features of WR-DLBCLs that the WR-DLBCLs comprised the majority of tumors (79.59%) with the non-GCB phenotypic profile. Most unselected series of nodal and extranodal DLBCLs categorized according to the Hans algorithm report substantially higher proportions of lymphomas with non-GCB immunophenotype.[7,18] However, de Leval et al[6] have reported higher prevalence of tumors with GCB phenotype among WR-DLBCL cases than the nodal cases (61%, 74/122 vs 39%, 48/122).
As recently reported, the IRF4 translocations represent the primary molecular alterations in the subset of GCB-derived lymphomas. In our series, the IRF4 translocation was observed in 15 patients (23.08%, 15/65), including 12 DLBCL cases (24.49%, 12/49; 6 cases of 10 cases had GCB immunophenotype, and 6 cases of 39 case had non-GCB immunophenotype) and 3 FL cases (18.75%, 3/16), of which 10 cases were positive for the IRF4/MUM1 expression.
The WR-DLBCL with IRF4 rearrangement has unique morphologic features. For example, the neoplastic cells are medium sized to large, with chromatin which is more open than typically seen in centrocytes an small, basophilic nucleoli. Mitotic figures are infrequent, and a starry-sky pattern is absent. When a follicular pattern is present, the neoplastic follicles are large, with a back-to-back growth pattern and absent or attenuated mantle zones. The clinical characteristics of the 15 patients with WR-BCL subjected to the detection of IRF4 translocation. Our results showed that the IRF4 rearrangement occurred more frequently for the males subjects, age of ≤60 years, DLBCL-GCB, stage III/IV, ECOG of ≥2, nonbone marrow involvement, B symptoms, and C-MYC expression (although without significant differences).
In this study, positive results for BCL-2 detection were found in 81.54% cases, and the BCL-2 rearrangement frequency was 10.2%. In contrast with our findings, previous studies have reported that the BCL-2 rearrangement frequency was 20% to 30% for DLBCLs, which is strongly associated with the GCB immunophenotype.[19,20] However, our findings were consistent with the findings from de Leval et al.[6] In this study, our results showed that the BCL-2 rearrangement occurred more frequently on the FL-1 cases, with significant differences. Moreover, the BCL-2 rearrangement occurred more frequently in subjects of female, age of ≤60 years, bone marrow involvement, B symptoms, and C-MYC expression (although without significant differences). Taken together, these findings suggest that the BCL-2 expression is associated with poor prognosis. However, a recent study has suggested that the prognostic value of BCL-2 expression is restricted to the non-GCB tumors only.[21]
Our results showed that the MYC rearrangement was observed in 32.65% (16/49) WR-DLBCL cases and 18.75% (3/16) WR-FL cases, which was in contrast to the previous findings.[6] Moreover, the C-MYC rearrangement was related to the poor prognosis of WR-BCL cases, which was in line with the findings from Chen et al.[22] However, in contrast, the BCL-6 rearrangement was reported in 30.16% cases, in line with previous findings.[6] Moreover, the patients with B symptoms had more frequent BCL-6 rearrangement, with significant differences. These cases occurred more frequently for the cases with GCB, abnormal serum LDH, and IPI high risk (although without significant differences). Furthermore, our survival analysis showed that the WR-DLBCL group had higher OS rate than the WR-FL group (without significant differences). As previously described, the NHL-involving WR demonstrated more favorable outcomes for the diseases at primary stages compared to the nodal counterparts.[22] Similarly, in this study, our results of the univariate analysis of prognostic factors showed that, for the patients with WR-DLBCL, the ECOG score was significant factors affecting the OS. Multivariate analysis showed that the IRF4/MUM1 expression was associated with poor survival outcomes in WR-BCLs, implying that this gene might be a potential therapeutic target.[23]
This study has some limitations. First, the sample size is relatively small. Second, the follow-up time is relatively short. Third, using immunohistochemistry as diagnostic method has the limitation that this method is robust yet qualitative and objective, which cannot rule out human errors. Further studies with larger sample size and longer follow-up time are warranted.
In conclusion, our results showed that the IRF4/MUM1, C-MYC, and CD10 expressions were associated with poor survival outcomes in WR-BCLs, representing independent factors for the OS, which were largely dependent on other prognostic factors (such as ECOG, LDH, and bone marrow involvement). Compared with WR-FL, WR-DLBCL was associated with worse survival outcomes.
Author contributions
Conceptualization: Zhiping Ma, Xinxia Li, Wei Zhang, Wenli Cui.
Methodology: Zhiping Ma, Yi Shi, Xuelian Pang.
Software: Zhiping Ma, Yi Shi, Xuelian Pang.
Supervision: Zhiping Ma, Xinxia Li, Wei Zhang, Wenli Cui.
Writing – original draft: Zhiping Ma, Wenli Cui.
Writing – review & editing: Wenli Cui.
Footnotes
Abbreviations: DLBCL = diffuse large B-cell lymphoma, ECOG = Eastern Cooperative Oncology Group performance status, FFPE = formalin-fixed and paraffin-embedded, FISH = fluorescent in situ hybridization, FL = follicular lymphoma, GCB = germinal center B cell, IHC = immunohistochemistry, IPI = international prognostic index, LDH = lactate dehydrogenase, OS = overall survival, PFS = progression-free survival, TMA = tissue microarray, WR-BCL = Waldeyer ring B-cell lymphoma.
How to cite this article: Ma Z, Shi Y, Pang X, Li X, Cui W, Zhang W. Clinicopathologic features and prognostic analysis of Waldeyer ring B-cell lymphoma. Medicine. 2020;99:2(e18670).
This work was supported by the Health and Youth Medical Science Talents project of Xinjiang Uygur Autonomous Region (WJWY-201904), the National Natural Science Foundation of China (NSFC 81560035, 81360352 and 81660036), Science and Technology Talents Training Project of Xinjiang Uyghur Autonomous Region (qn2015bs011, 2018Q047), Science and Nature Foundation of Xinjiang Uyghur Autonomous Region (2014211C032 and 2019D01C286); and Resource Sharing Platform Construction of Science and Technology Department of the Autonomous Region (PT1802).
The authors have no conflicts of interest to disclose.
References
- [1].Falini B, Fizzotti M, Pucciarini A, et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood 2000;95:2084–92. [PubMed] [Google Scholar]
- [2].Lu R. Interferon regulatory factor 4 and 8 in B-cell development. Trends Immunol 2008;29:487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tamura A, Miura I, Iida S, et al. Interphase detection of immunoglobulin heavy chain gene translocations with specific oncogene loci in 173 patients with B-cell lymphoma. Cancer Genet Cytogenet 2001;129:1–9. [DOI] [PubMed] [Google Scholar]
- [4].Michaux L, Wlodarska I, Rack K, et al. Translocation t(1;6)(p35.3;p252): a new recurrent aberration in “unmutated” B-CLL. Leukemia 2005;19:77–82. [DOI] [PubMed] [Google Scholar]
- [5].Remstein ED, Law M, Mollejo M, et al. The prevalence of IG translocations and 7q32 deletions in splenic marginal zone lymphoma. Leukemia 2008;22:1268–72. [DOI] [PubMed] [Google Scholar]
- [6].de Leval L, Bonnet C, Copie-Bergman C, et al. Diffuse large B-cell lymphoma of Waldeyer's ring has distinct clinicopathologic features: a GELA study. Ann Oncol 2012;23:3143–51. [DOI] [PubMed] [Google Scholar]
- [7].Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–82. [DOI] [PubMed] [Google Scholar]
- [8].Oschlies I, Klapper W, Zimmermann M, et al. Diffuse large B-cell lymphoma in pediatric patients belongs predominantly to the germinal-center type B-cell lymphomas: a clinicopathologic analysis of cases included in the German BFM (Berlin-Frankfurt-Munster) multicenter trial. Blood 2006;107:4047–52. [DOI] [PubMed] [Google Scholar]
- [9].Mi HH, Park HY, Ko YH, et al. IRF4/MUM1 expression is associated with poor survival outcomes in patients with peripheral T-cell lymphoma. J Cancer 2017;8:1018–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Naz E, Mirza T, Danish F. Clinicopathologic evaluation of subgroups of diffuse large B cell lymphoma by immunohistochemistry. Asian Pac J Cancer Prev 2011;12:3335–9. [PubMed] [Google Scholar]
- [11].Saikia A, Raphael V, Shunyu NB, et al. Analysis of Epstein Barr virus encoded RNA expression in nasopharyngeal carcinoma in North-Eastern India: a chromogenic in situ hybridization based study. Iran J Otorhinolaryngol 2016;28:267–74. [PMC free article] [PubMed] [Google Scholar]
- [12].Misharina JA, Sitko VV, Klymenko SV, et al. MYC gene rearrangements detected by interphase fluorescence in situ hybridization in diffuse large B-cell lymphomas. Probl Radiac Med Radiobiol 2014;19:310–20. [PubMed] [Google Scholar]
- [13].Pemmaraju N, Gill J, Gupta S, et al. Triple-hit lymphoma. Proc (Bayl Univ Med Cent) 2014;27:125–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kanagal-Shamanna R, Medeiros LJ, Lu G, et al. High-grade B cell lymphoma, unclassifiable, with blastoid features: an unusual morphological subgroup associated frequently with BCL2 and/or MYC gene rearrangements and a poor prognosis. Histopathology 2012;61:945–54. [DOI] [PubMed] [Google Scholar]
- [15].Xu X, Zhang L, Wang Y, et al. Double-hit and triple-hit lymphomas arising from follicular lymphoma following acquisition of MYC: report of two cases and literature review. Int J Clin Exp Pathol 2013;6:788–94. [PMC free article] [PubMed] [Google Scholar]
- [16].Li X, Li G, Gao Z. The relative frequencies of lymphoma subtypes in China: a nationwide study of 10002 cases by the Chinese Lymphoma Study Group. Ann Oncol 2011;22:iv141. [Google Scholar]
- [17].Lopez-Guillermo A, Colomo L, Jimenez M, et al. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol 2005;23:2797–804. [DOI] [PubMed] [Google Scholar]
- [18].Ott G, Ziepert M, Klapper W, et al. Immunoblastic morphology but not the immunohistochemical GCB/nonGCB classifier predicts outcome in diffuse large B-cell lymphoma in the RICOVER-60 trial of the DSHNHL. Blood 2010;116:4916–25. [DOI] [PubMed] [Google Scholar]
- [19].Copie-Bergman C, Gaulard P, Leroy K, et al. Immuno-fluorescence in situ hybridization index predicts survival in patients with diffuse large B-cell lymphoma treated with R-CHOP: a GELA study. J Clin Oncol 2009;27:5573–9. [DOI] [PubMed] [Google Scholar]
- [20].Huang JZ, Sanger WG, Greiner TC, et al. The t(14;18) defines a unique subset of diffuse large B-cell lymphoma with a germinal center B-cell gene expression profile. Blood 2002;99:2285–90. [DOI] [PubMed] [Google Scholar]
- [21].Javeed I, Neppalli VT, George W, et al. BCL2 expression is a prognostic marker for the activated B-cell-like type of diffuse large B-cell lymphoma. J Clin Oncol 2006;24:961–8. [DOI] [PubMed] [Google Scholar]
- [22].Chen L, Al-Kzayer LF, Liu Y, et al. B-cell lymphomas involving Waldeyer's ring characterized by distinctive clinical and histopathological features: a comparison of pediatric to adult patients. Oncotarget 2017;8:11544–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Falini B, Fizzotti M, Pucciarini A, et al. Amonoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood 2000;95:2084–92. [PubMed] [Google Scholar]


