Abstract
The diverse collection of microorganisms that inhabit the gastrointestinal tract, collectively called the gut microbiota, profoundly influences many aspects of host physiology, including nutrient metabolism, resistance to infection and immune system development. Studies investigating the gut–brain axis demonstrate a critical role for the gut microbiota in orchestrating brain development and behavior, and the immune system is emerging as an important regulator of these interactions. Intestinal microbes modulate the maturation and function of tissue-resident immune cells in the CNS. Microbes also influence the activation of peripheral immune cells, which regulate responses to neuroinflammation, brain injury, autoimmunity and neurogenesis. Accordingly, both the gut microbiota and immune system are implicated in the etiopathogenesis or manifestation of neurodevelopmental, psychiatric and neurodegenerative diseases, such as autism spectrum disorder, depression and Alzheimer’s disease. In this review, we discuss the role of CNS-resident and peripheral immune pathways in microbiota–gut–brain communication during health and neurological disease.
The human gastrointestinal tract is colonized by trillions of microorganisms collectively termed the gut microbiota. These intestinal microbes regulate many aspects of host physiology, including immune system maturation and function1–3. In addition, there is increasing evidence that the gut microbiota regulate brain development, function and behavior4–6. Although several immunological consequences of the microbiota are well documented1–3, immunomodulation by the microbiota is emerging as an important pathway that orchestrates microbiota–gut–brain communication7.
Far from an immune-privileged site, the brain harbors resident immune cells that defend against infection and injury, while also supporting neurons in remodeling circuit connectivity and plasticity. Molecules traditionally acknowledged for their functions in the peripheral immune system are now being recognized for their importance in neurodevelopment. For example, cytokines, typically regarded as immunomodulatory signaling factors, are present in the developing brain, where they regulate neuronal differentiation and axonal pathfinding8; complement proteins, which tag and kill microorganisms and damaged cells for immune clearance, target synapses for pruning during neurodevelopment9; and major histocompatibility complex (MHC) class I proteins, which enable antigen presentation by immune cells, modulate neurite outgrowth and synaptic plasticity10. Together, these findings contribute to the redefinition of immunomodulatory proteins as regulatory factors in normal brain development. Conversely, several factors traditionally acknowledged for their functions in the CNS are becoming recognized for their roles in the immune system. Various leukocyte subtypes produce and respond to classical neurotransmitters, which regulate depolarization or hyperpolarization of neurons11–14. Stimulation of dopaminergic neurons in the ventral tegmental area, for example, results in widespread activation of peripheral innate and adaptive immune cells15. Bidirectional communication between the nervous and immune systems is further facilitated by the recently defined brain lymphatic system, which connects peripheral lymphatic tissues to the CNS, and by the blood-brain barrier, which regulates endothelial passage of immune cells and associated factors16,17.
Microbial colonization of the intestine has a significant impact on neurophysiology and behavior4–6. Compared to conventionally colonized controls, germ-free (GF) mice, which are raised under sterile conditions, or mice depleted of their intestinal microbiota with oral broad-spectrum antibiotics exhibit substantial alterations in behaviors and neuropathologies that are relevant to neurodevelopmental, psychiatric and neurodegenerative disorders6. Given the immunomodulatory properties of the gut microbiota, immune cell pathways within and peripheral to the CNS have been implicated as important mechanisms mediating microbial modulation of brain function and behavior7,18. In this review, we discuss roles for the gut microbiota as an integral mediator of neuroimmune interactions. We examine how microbial influences on the activation of peripheral innate and adaptive immune cells regulate responses to neuroinflammation, brain injury, autoimmunity and neurogenesis. We further assess evidence that neuroimmune modulation by the microbiota can contribute to the etiopathogenesis or manifestation of symptoms relevant to neurobehavioral and neurodegenerative disorders, such as autism spectrum disorder, anxiety depression, Alzheimer’s disease and Parkinson’s disease. Finally, we highlight immunological, neuroendocrine and neuronal interactions that coordinate communication between the intestinal microbiota and brain.
Development and function of brain resident immune cells
Microglia
Microglia are the most abundant resident immune cells in the brain, comprising 5–20% of glial cells19. Derived from yolk sac erythromyeloid progenitors19,20, microglia perform canonical functions of myeloid cells, including phagocytosis, antigen presentation, cytokine production and activation of inflammatory responses19,21. Their extensive cellular processes enable rapid immune surveillance and clearance of debris and infectious agents, taking an estimated 2–5 h to survey the entire brain with limited physical migration21,22. Microglial activation states span a wide spectrum ranging from proinflammatory to tissue protective23–25. During neurodevelopment, microglia tag and clear synapses for pruning, promote neuronal circuit wiring and produce cytokines and chemokines that guide neuronal differentiation19,24,26.
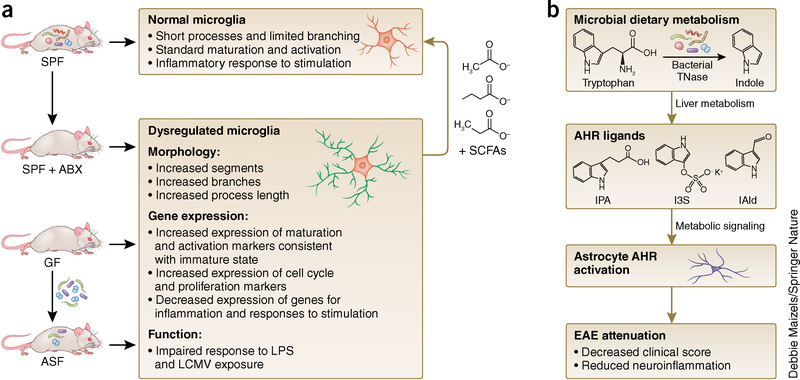
The microbiota influences microglial maturation and function (Fig. 1a). Compared to conventionally colonized controls, GF mice have increased numbers of immature microglia across gray and white matter of the cortex, corpus callosum, hippocampus, olfactory bulb and cerebellum27. Microglia in GF mice show longer processes and more branching, with elevated expression of colony stimulating factor 1 receptor (CSF1R), F4/80 and CD31, factors that decrease in expression with development toward an adult-stage phenotype27. Similar increases in immature microglia are observed after antibiotic treatment of conventional mice, though total microglia count remains unchanged27. This suggests differential effects of the microbiota on microglia, effects that depend on developmental timing and/or duration of microbial colonization. Consistent with this, gene expression differs between GF adult and newborn microglia when compared to their age-matched conventionally colonized controls20. Furthermore, several genes exhibit decreased expression in GF microglia, but more genes are downregulated in microglia from adult GF mice than in those from newborns20. The finding that microglia from newborn GF mice display altered gene expression profiles compared to those from newborn conventionally colonized mice suggests that the maternal microbiota may contribute to early life programming of microglial development.
Figure 1.
Effects of the microbiota on microglia and astrocyte biology. (a) Microglial maturation and function are affected by the presence or absence of a complex microbiota. Compared to conventionally colonized (specific pathogen–free, SPF) controls, mice reared in the absence of microbial colonization (germ free, GF) have microglia with abnormal morphology, altered gene expression and impaired functional response to stimulation. Similar microglial abnormalities are seen in microbiota-depleted mice, produced by antibiotic treatment of SPF mice (ABX) or colonization of GF mice with three bacterial taxa from a limited altered Schaedler flora (ASF) consortium (B. distasonis, L. salivarius, and Clostridium cluster XIV). Microglial abnormalities in GF mice are corrected by supplementation with short-chain fatty acids (SCFAs), products of bacterial fermentation. (b) Microbial metabolites bind to the aryl hydrocarbon receptor (AHR) in astrocytes, reducing symptoms of EAE. Type I interferon signaling in astrocytes diminishes inflammation and decreases EAE clinical scores, and this effect is reversed by antibiotic treatment. Particular tryptophan metabolites produced by the microbiota stimulate AHR and reduce EAE clinical scores. This suggests that the microbiota have a direct effect on AHR signaling and inflammation in astrocytes, which may have relevance to multiple sclerosis, where patients show decreased tryptophan-derived metabolites in sera. TNase, tryptophanase; IPA, indole-3-propionic acid; IAld, indole-3-aldehyde; I3S, indoxyl-3-sulfate.
Microglia from adult GF mice are also functionally impaired in response to challenge with lipopolysaccharide or lymphocytic choriomeningitis virus, displaying altered morphology and attenuated immune activation, including impaired induction of the proinflammatory cytokines interleukin (IL)-1β, IL-6 and tumor necrosis factor-α (TNF-α)20,27. These functional deficits align with the finding that naive adult microglia from GF mice have decreased expression of several genes relevant to interferon responses, innate responses to stimulation, viral defense and effector processes20.
While the precise mechanisms by which gut microbes influence brain microglia remain unclear, there appears to be specificity of microglial modulation to particular bacterial taxa. GF mice that were colonized with a minimal community of three bacterial species—Bacteroides distasonis, Lactobacillus salivarius and ASF (altered Schaedler flora) 356, a member of Clostridium cluster XIV—maintained abnormalities in microglia27. This suggests that microbial effects on microglia are not regulated by bacterial load in general, but require greater diversity of the gut microbiota or are conferred by particular microbial species and functions not represented by the minimal community tested.
Notably, GF-associated alterations in microglial morphology, abundance and gene expression are normalized by postnatal supplementation with short chain fatty acids (SCFAs), primary products of bacterial fermentation27,28, suggesting that SCFA-producing bacterial species may restore abnormalities in microglia seen in GF or antibiotic-treated mice. However, ASF 356 is reported to produce the SCFAs propionate and acetate29, despite its failure to correct microglial abnormalities after colonization in the three-member community. This suggests a need to examine levels of SCFAs achieved by microbial colonization relative to dosages that were supplemented exogenously. Consistent with a role for SCFAs in mediating effects of gut microbes on brain microglia, conventionally colonized mice harboring a deletion in G protein-coupled receptor 43 (GPR43), an SCFA receptor encoded by free fatty acid receptor 2 (FFAR2), exhibit abnormalities in microglia that are analogous to those seen in microbiota-depleted mice. As microglia do not express GPR43 directly27, whether the effects of the microbiota on microglia are mediated by direct signaling of SCFAs is unclear. Indeed, SCFAs are involved in a broad array of functions that influence gastrointestinal physiology, peripheral immunity, liver metabolism and blood-brain barrier integrity, which could indirectly contribute to effects on microglia. Future studies targeting specific bacterial taxa, particular SCFAs (acetate, propionate, butyrate) and conditional microglia-specific knockout of FFAR2 are warranted.
Whether the microbiota affect microglial function during early postnatal development is not known but could have important implications for brain development and behavior later in life. During early postnatal life, microglia are integral for synaptic pruning, tagging synapses for clearance with complement proteins on the basis of neural activity30. Early exposure of conventional mice to antibiotics results in elevated cecal levels of SCFAs compared to those in controls31, raising the possibility that alterations in SCFAs could influence microglia during early postnatal development. Several neurological disorders, including autism spectrum disorder, Parkinson’s disease, multiple sclerosis, anxiety, depression, stroke and amyotrophic lateral sclerosis, are all associated with both dysregulated microglia activity and dysbiosis of the gut microbiota (Table 1). Whether microbial effects on microglial maturation and function contribute to neurological symptoms are important questions for future study.
Table 1.
Reported alterations in the gut microbiota in neurological and psychiatric disorders
| Pathology type | Condition | Associated microbiota changes | Refs. |
|---|---|---|---|
| Autoimmune disease | Multiple sclerosis | Increased: Methanobrevibacter, Akkermansia | 135 |
| Decreased: Butyricimonas | |||
| Increased: Desulfovibroneceae | 136 | ||
| Decreased: Lachnospiraceae, Ruminococcaceae | |||
| Increased: Pseudomonas, Mycoplana, Haemophilus, Blautia, Dorea | 137 | ||
| Decreased: Parabacteroides, Adlercreutzia, Prevotella | |||
| Increased: Streptococcus, Eggerthella | 138 | ||
| Decreased: Clostridia clusters XIVa and IV, Bacteroidetes, Faecalibacterium | |||
| Increased: Ruminococcus | 139 | ||
| Decreased: Bacteroidaceae, Faecalibacterium | |||
| Neurodegenerative disorders | Parkinson’s disease | Increased: Blautia, Coprococcus, Roseburia, Proteobacteria | 140 |
| Decreased: Faecalibacterium, Prevotellaceae | |||
| Decreased: Prevotellaceae | 105 | ||
| Enterobacteriaceae correlate with motor impairments | |||
| Alzheimer’s disease | Association with bacterial and viral infection | 141 | |
| Injury | Spinal cord injury (SCI) | SCI with upper motor neuron bowel syndrome: decreased Pseudobutyrivibrio, Dialister, Megamonas | 142 |
| SCI with lower motor neuron bowel syndrome: decreased Roseburia, Pseudobutyrivibrio, Megamonas | |||
| SCI with neuropathic bladder: increased Klebsiella, Escherichia, Enterococcus | 143 | ||
| SCI with neuropathic bladder: decreased Lactobacillus, Corynebacterium, Staphylococcus, Streptococcus, Prevotella, Veillonella | |||
| Neuropsychiatric conditions | Major depressive disorder | Decreased: Bifidobacterium, Lactobacillus | 144 |
| Increased: Actinomycineae, Coriobacterineae, Lactobacillaceae, Streptococcaceae, Clostridiales, Eubacteriaceae, Lachnospiraceae, Ruminococcaceae, Erysipelotrichaceae | 86 | ||
| Decreased: Bacteroidaceae, Rikenellaceae, Lachnospiraceae, Acidaminococcaceae, Veillonellaceae, Sutterellaceae | |||
| Increased: Enterobacteriaceae, Alistipes, Acidaminococcaceae, Fusobacteriaceae, Porphyromonadaceae, Rikenellaceae | 145 | ||
| Decreased: Bacteroidaceae, Erysipelotrichaceae, Lachnospiraceae, Prevotellaceae, Ruminococcaceae and Veillonellaceae | |||
| Faecalibacterium negatively correlated with depressive symptoms | |||
| Anxiety disorder | Probiotic administration of B. longum and Lactobacillus helveticus decreased anxiety | 146 | |
| Probiotic administration of Lactobacillus casei, Lactobacillus acidophilus, L. rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, B. longum, Streptococcus thermophilus and Bifidobacterium lactis decreased anxiety | 147 | ||
| Autism | Increased: Lactobacillus, Desulfovibrio, Clostridium, cluster 1 | 148 | |
| Decreased: Bacteroides/Firmicutes, ratio | |||
| Increased: Sutterella | 149 | ||
| Increased: Clostridium, Bacteroides, Porphyromonas, Prevotella, Pseudomonas, Aeromonas, Enterobacteriaceae | 150 | ||
| Decreased: Enterococcus, Lactobacillus, Streptococcus, Lactococcus, Staphylococcus, Bifidobacteria | |||
| Increased: Lactobacillus | 151 | ||
| Decreased: Prevotella, Coprococcus, Veillonellaceae | |||
| Increased: Sutterella, Lachnospiraceae, Ruminococcaceae | 152 | ||
| Increased: Lactobacillus | 153 | ||
| Decreased: Bifidobacterium, Enterococcus | |||
| Increased: Bacteroidetes, Proteobacterium | 154 | ||
| Decreased: Actinobacterium, Bifidobacterium | |||
| Increased: Clostridium clusters I and II | 155 |
Astrocytes
Astrocytes are the most abundant glial cells in the brain and exhibit a diverse array of functions, including the regulation of blood-brain barrier integrity, ion gradient balance, neurotransmitter turnover, cerebral blood flow and nutrient transport32,33. Astrocytes integrate information from adjacent glial, neuronal, vascular and immune cells to regulate neural excitability34 and synapse formation35. Additionally, astrocytes are important for brain metabolism; as the main site of glycogen storage in the brain, astrocytes provide support when the brain is hypoglycemic33. Though they derive from neural progenitor cells and are not typically considered to be part of the CNS-resident immune system27, astrocytes perform immune-related functions, expressing pattern recognition receptors for detection of microbe-associated molecular patterns (MAMPs) and modulating neuroinflammatory responses through cytokine production and antigen presentation via MHC II36.
The gut microbiota modulates astrocyte activity via microbial metabolites that activate astrocyte aryl hydrocarbon receptors (AHR)37 (Fig. 1b). Type I interferon signaling in astrocytes attenuates inflammation and symptoms of experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis. This effect is mediated in part by activation of AHRs. Gut microbes metabolize dietary tryptophan to produce natural AHR ligands, including indole-3-aldehyde and indole-3-propionic acid38,39. Consistent with this notion, depletion of the gut microbiota using the antibiotic ampicillin decreases levels of the AHR agonist indoxyl-3-sulfate (I3S) and worsens EAE disease scores37. Supplementation of antibiotic-treated mice with the tryptophan metabolites I3S, indole-3-proprionic acid and indole- 3-aldehyde or with bacterial tryptophanases, which convert tryptophan to indole, improves EAE recovery. Similarly, peripheral administration of I3S or indole-3-aldehyde, or consumption of an indole-supplemented diet, improves EAE disease symptoms37. In human astrocytes, I3S treatment reduces expression of proinflammatory factors, including C-C motif chemokine ligand 2 (CCL2), tumor necrosis factor (TNF), nitric oxide synthase 2 (NOS2) and IL6 (ref. 37). While much remains to be explored with regards to particular microbial taxa that regulate astrocyte function, the gut bacterium Lactobacillus reuteri is of interest because it is known to produce indole-3-aldehyde from dietary tryptophan37,38. Collectively, these findings suggest that microbial metabolites of dietary tryptophan can modulate the inflammatory status of astrocytes, with important consequences for neuroinflammation.
Innate and adaptive immune cells in the CNS
Aside from glial subsets, innate and adaptive immune cells, including perivascular macrophages, CD4+ T and CD8+ T cells and mast cells, are also resident in the CNS21,26,40,41. While effects of the microbiota on these brain-resident immune cells have not been described, several studies revealed that the microbiota modulate peripheral myeloid cells, T cells and mast cells42, which share common hematopoietic progenitors with the brain subsets. For example, the microbiota are a major regulator of hematopoiesis and influence myeloid development in the embryonic yolk sac43, where microglial progenitors are believed to originate. Determining whether microbial effects on peripheral hematopoietic progenitors result in alterations in brain meningeal and/or tissue-resident immune cells would be important. Perivascular and choroid plexus macrophages would be of particular interest given their close localization to the vasculature21 and potential exposure to circulating factors that are regulated by the microbiota.
Peripheral immune cell responses and CNS interactions
CNS inflammation and injury
Intestinal bacteria are potent regulators of mucosal and systemic immune responses and contribute to the development of inflammatory disorders in the CNS (Fig. 2). In EAE, GF or antibiotic-treated mice exhibit reduced inflammation and disease scores compared to conventional mice44,45, suggesting a role for gut microbes in CNS inflammation. Studies investigating the contributions of specific members of the microbiota have revealed complex interactions between intestinal bacteria and CNS inflammation. Segmented filamentous bacteria are epithelial-associated bacteria that promote the development of IL-17A-producing T helper 17 (TH17) cells in the mouse small intestine46,47. Colonization of GF mice with these bacteria alone is sufficient to promote EAE compared to GF controls44, likely via induction of TH1748. Intestinal bacteria that promote immunoregulatory responses have been associated with protection against EAE. The human gut bacterium Bacteroides fragilis induces IL-10-producing regulatory T (Treg) cells in the mouse colon via capsular expression of polysaccharide A (PSA)49,50. While colonization of mice with wild-type B. fragilis reduces EAE severity, colonization with PSA-deficient B. fragilis restores EAE susceptibility45,51. Consistent with these findings, oral treatment of wild-type mice with PSA protects against CNS demyelination and inflammation through a Toll-like receptor (TLR)-2-dependent pathway52,53. Metabolites produced by the gut microbiota have also been implicated in protection from EAE. Treatment of mice with SCFAs reduces EAE and axonal damage through increased Treg differentiation54. In myelin oligodendrocyte glycoprotein (MOG)-specific T cell receptor (TCR) transgenic 2D2 mice, intestinal microbes promote a subset of autoreactive intraepithelial lymphocytes. These MOG-specific CD4+ lymphocytes are immunosuppressive and can protect wild-type mice from EAE in a lymphocyte activation gene (LAG)-3-dependent manner when adoptively transferred55. Altogether, gut bacteria influence the degree ofCNS inflammation through modulation of pro- and anti-inflammatorymucosal and systemic immune responses. Despite these findings, whether antigen-specific responses or bystander activation of immune cells by the microbiota is involved in regulation of CNS inflammation requires further investigation.
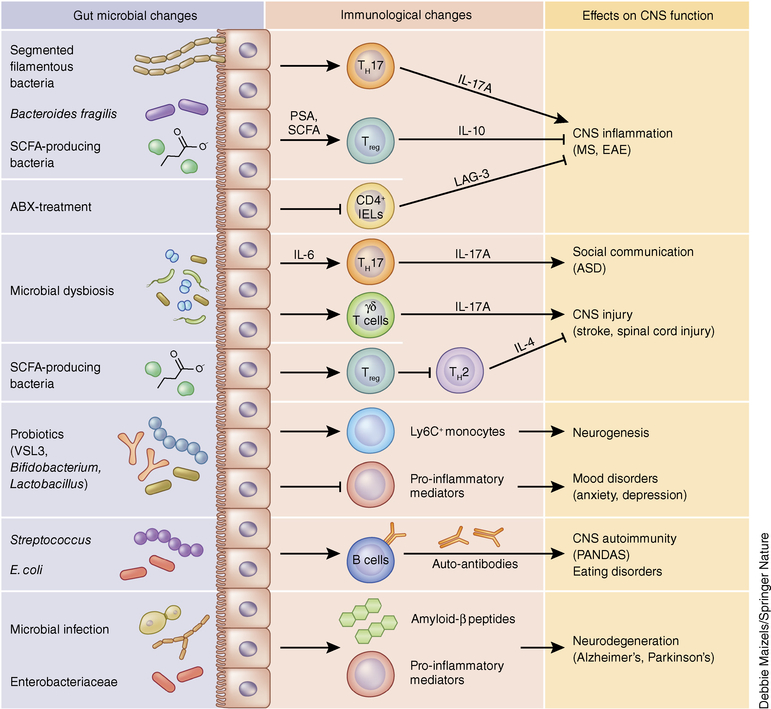
Figure 2.
Effects of the microbiota on peripheral immune cells and CNS function. Intestinal microbes in the gastrointestinal tract regulate peripheral immune responses, CNS function and behavior. Intestinal colonization by specific members of the microbiota is associated with activation of pro- or anti-inflammatory CD4+ T cell responses that regulate susceptibility to EAE. The material immune activation (MIA) model of ASD leads to microbial dysbiosis that is associated with elevated TH17 cell responses, which are sufficient to trigger social and behavioral defects. Mouse models of CNS injury (stroke, spinal cord injury) also appear to be regulated by microbiota–immune interactions. Oral administration of probiotics can elicit a population of Ly6C+ monocytes that increase hippocampal neurogenesis and enhance memory responses. Probiotics such as Bifidobacterium and Lactobacillus have potent anti-inflammatory properties that reduce behaviors associated with anxiety and depression. Furthermore, microbial infections can trigger antibody-dependent CNS autoimmunity and promote neurodegeneration. Collectively, regulation of peripheral immune responses is a critical pathway by which the intestinal microbiota and exogenous microbial challenges influence CNS function and behavior. ABX, antibiotic treatment of SPF mice; IELs, intraepithelial lymphocytes; LAG-3, lymphocyte activation gene-3.
Besides CNS inflammation, the microbiota also regulates the pathogenesis and resolution of CNS injury. In a mouse model of middle cerebral artery occlusion (MCAO)-induced ischemic brain injury, mice treated with the antibiotics amoxicillin and clavulanic acid show reduced infarct volume and improved sensorimotor function56. Neuroprotection is associated with reduced meningeal infiltration of small intestine IL-17+ γδ T cells, suggesting that intestinal bacteria regulate lymphocyte trafficking to the brain and immune responses to brain injury. In contrast, pretreatment with the antibiotics ciprofloxacin and metronidazole reduces survival following MCAO57, suggesting that particular microbial species cause differential effects on brain injury recovery. Consistent with a causal role for the microbiota in brain injury, MCAO results in intestinal dysbiosis, and transfer of the MCAO microbiota to GF animals leads to increased proinflammatory cytokine production in the intestine and brain and increased infarct volumes58,59. In addition, transfer of fecal microbiota from naive animals to MCAO mice reduces brain lesions volumes in wild-type but not Rag1−/− animals58, suggesting a critical role for the adaptive immune system in protection from stroke. Bacterial translocation and altered microbiota composition have been observed in a mouse model of spinal cord injury60. Mice pretreated with antibiotics demonstrate delayed locomotor recovery, suggesting a protective role for intestinal microbes in this model. Furthermore, administration of the probiotic cocktail VSL3 reduces lesion size and improves locomotor recovery, which is associated with increased Treg cells in the mesenteric lymph nodes. IL-4-producing CD4+ T cells in the CNS restore neuronal tissue homeostasis in this model61, suggesting a neuroprotective role for type 2 T helper (TH2) cells in CNS injury. However, the signals that promote tissue-protective TH2 cells in the CNS may be independent of the intestinal microbiota and involve the release of damage-associated molecules62. Altogether, these findings suggest that modulation of the peripheral immune system by the microbiota plays a critical role in the development of and recovery from CNS injury.
The humoral immune system and CNS autoimmunity
In addition to T cell–mediated mechanisms of CNS inflammation, B cells play an important role in the generation of CNS-reactive autoantibodies and the pathogenesis of neuroinflammatory disorders63,64. Intestinal microbes influence the development of behavioral disorders through modulation of humoral immune responses and antigen mimicry. Streptococcal infection is often associated with the generation of CNS-reactive antibodies that lead to behavioral abnormalities collectively known as pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). In the PANDAS animal model, where mice are immunized with group A β-hemolytic streptococci, mice develop behavioral abnormalities such as impaired motor coordination and repetitive rearing behavior65. Transfer of complete, but not IgG-depleted, sera from immunized mice is sufficient to induce behavioral deficits, suggesting that bacterial-infection-induced autoantibodies are critical to the development of PANDAS. Autoantibodies have also been implicated in the development of eating disorders, and intestinal bacteria appear to be involved in their generation. Antibodies produced in response to the heat shock protein ClpB derived from the bacterium Escherichia coli cross-react with α-melanocyte-stimulating hormone (α-MSH), a peptide critically involved in food intake66. Oral administration of wild-type but not ClpB-deficient E. coli results in the generation of ClpB- and α-MSH-reactive antibodies and decreased food intake. These findings suggest a role for intestinal-bacteria-induced autoantibodies in behavioral disorders and provide the framework for future studies.
Chronic inflammatory diseases
Chronic inflammatory diseases such as inflammatory bowel disease and chronic liver disease are commonly associated with impaired brain function and associated sickness behaviors67,68. Administration of probiotics can improve these symptoms by modulating peripheral immune pathways. In the bile duct ligation model of liver inflammation, mice routinely develop social disabilities, which were ameliorated following ingestion of the VSL3 probiotic cocktail69. Although probiotic treatment did not alter gut microbiota composition, it reduced the amount of circulating TNF-α and monocyte accumulation in the brain. Despite these findings, whether probiotics improve brain function by modulating peripheral immune responses requires further study.
Neurogenesis
Intestinal bacteria have been demonstrated to regulate fetal and adult neurogenesis. Bacterial cell wall components cross the maternal–fetal interface and activate TLR2, which leads to fetal cortical neuroproliferation and impaired cognitive function during adulthood70. These data suggest that the maternal microbiota could influence offspring neurogenesis and subsequent behavioral changes. Studies assessing hippocampal neurogenesis in adult GF mice reveal increased proliferation relative to conventional mice71. However, colonization of GF mice after weaning does not reduce neuronal cell proliferation to conventional levels, suggesting a critical period in which intestinal bacteria regulate hippocampal neurogenesis. In contrast, antibiotic treatment decreases hippocampal neurogenesis and spatial and object recognition, which can be restored by exercise and probiotic VSL3 treatment72. Both exercise and probiotics are associated with CNS monocyte expansion. Depletion of monocytes reduces neurogenesis while adoptive transfer of monocytes to antibiotic-treated mice increases neurogenesis, identifying circulating monocytes as a cellular mediator of microbiota–gut–brain communication. More studies are necessary to precisely define mechanisms of microbial–immune interactions in the context of neurodevelopment.
Associations with endophenotypes of neurological diseases
Social communication and autism spectrum disorder
The gut microbiota influence the development of features related to behavioral disorders such as anxiety, depression and autism, and recent studies have begun to elucidate the cellular and molecular pathways involved4,5. In the maternal immune activation (MIA) animal model of autism spectrum disorder (ASD), in which administration of the TLR3 agonist poly(I:C) in pregnant dams results in offspring with behavioral phenotypes similar to those observed in autism, MIA offspring exhibit increased intestinal permeability and alterations in the composition of the gut microbiota73. Oral administration of the human gut bacterium B. fragilis to MIA offspring during weaning ameliorates microbiota dysbiosis and significantly restores behavioral abnormalities during the open field exploration, prepulse inhibition, marble-burying and ultrasonic vocalization tests. However, PSA-deficient B. fragilis and some other Bacteroides members are also able to protect MIA offspring, suggesting that alterations of these behaviors is not due to PSA-specific modulation of mucosal and systemic immune responses. Instead, it was proposed that B. fragilis treatment alters the composition of serum metabolites and reduces the anxiety-associated molecule 4-ethylphenylsulfate. Further support for a link between intestinal dysbiosis and these symptoms is provided by the finding that a maternal high fat diet (MHFD) results in changes to offspring microbiota and abnormal social behavior74. Transfer of the control but not MHFD diet microbiota from offspring to GF mice improves their social impairments, suggesting a causal relationship between intestinal dysbiosis and these behavioral readouts. It was proposed that behavioral abnormalities in MHFD offspring are due to reduced expression of the neuropeptide oxytocin in the hypothalamus and reduced activity of the dopaminergic reward system. Collectively, these data suggest that the gut microbiota affect symptoms relevant to neurodevelopmental disorders through modulation of the host metabolome and neuropeptide production.
The host immune system, which is dynamically regulated by the gut microbiota, is also critical in the development of behavioral phenotypes in MIA offspring. MIA leads to elevated systemic levels of the proinflammatory cytokines IL-6 and IL-17A75,76. Consistent with a role for immune dysregulation in the development of behavioral abnormalities in MIA offspring, irradiation of the MIA offspring followed by bone marrow reconstitution from non-MIA mice reduces repetitive and anxiety-like behavior76. Administration of IL-6 in offspring is sufficient to trigger prepulse and latent inhibition deficiencies, and blockade of IL-6 but not interferon-γ, IL-1α or TNF-α in pregnant dams reduces behavioral deficits induced by MIA77. More recently, IL-17A was implicated as a critical inflammatory mediator in MIA. MIA offspring from mice lacking T cell–derived IL-17A are protected from developing impairments in the ultrasonic vocalization, sociability and marble-burying assays75. Furthermore, intraventricular IL-17A administration in fetuses is sufficient to elicit abnormalities in these behavioral tests. Given the function of IL-6 in promoting differentiation of TH17 cells, these data suggest that IL-6 and IL-17A act in concert to promote MIA-induced behavioral abnormalities. Since IL-17A and TH17 cells are regulated by the gut microbiota, these findings also raise the possibility that MIA-induced immune dysregulation is linked to intestinal dysbiosis. Further studies are required to address the contribution of intestinal bacteria in immune dysregulation and disease outcome in ASD.
Stress, anxiety and depression
Major depressive disorder (MDD) is a mood disorder generally diagnosed when patients experience a depressed mood or a loss of interest or pleasure in daily activities for more than 2 weeks. The precise etiology of depression is not well understood but involves stress-induced changes in neural circuitry and neuroendocrine pathways78, activation of the immune system79, and interactions between the host and microbiota5. Mice lacking a normal gut flora display reduced basal and stress-induced behavioral impairments associated with anxiety and depression, and these phenotypes are restored following intestinal colonization80. In response to dextran sodium sulfate (DSS)-induced intestinal inflammation, mice exhibit increased latency to step down from an elevated platform, a behavioral test measuring anxiety, which is ameliorated by probiotic administration of Bifidobacterium longum81. Similarly, in the maternal separation model of depression, rats display increased immobility during the forced swim test and elevated proinflammatory cytokines, which are restored to normal levels following treatment with the probiotic Bifidobacterium infantis82. Intestinal colonization with particular Lactobacillus strains produces antidepressive effects during steady state or in response to stress83,84. One study showed that maternal-separation-induced behavioral impairments in the stepdown, light-preference and tail suspension tests require the presence of a complete gut microbiota, which suggests a causal relationship between intestinal bacteria and depression-related endophenotypes85. Furthermore, humans with MDD harbor a microbiota distinct from that of healthy controls, and fecal transfer of the MDD microbiota to GF mice enhances immobility time during the forced swim and tail suspension tests and reduces center distance traveled in the open field exploration test86. These findings are consistent with those of a separate study, which demonstrated reduced microbiota diversity in patients with MDD relative to healthy controls87. Antibiotic-treated rats receiving fecal transplants from humans with MDD exhibit behavioral impairments in the sucrose preference, elevated plus maze and open field exploration tests87. These results collectively support the hypothesis that intestinal bacteria influence responses to physical and psychological stress. While the cellular and molecular pathways that mediate microbiota–gut–brain interactions in stress-associated disorders appear to involve modulation of the immune system82,88, host metabolism86 and/or vagal nerve stimulation81,84, detailed mechanisms are unclear. Given the immunomodulatory properties of intestinal bacteria and probiotics82,88, it is likely that alterations in immune homeostasis due to host–microbiota interactions can lead to changes in brain function through the hypothalamic–pituitary–adrenal (HPA) axis, a neuroendocrine, stress-sensing system that could be activated by proinflammatory cytokines89. Future studies using immunodeficient animals will be necessary to define the precise functions of the immune system in gut–brain communication in models of stress, anxiety and depression.
Neurodegeneration and aging
Alzheimer’s disease (AD) and Parkinson’s disease (PD) are neurodegenerative disorders with complex etiologies including age, genetic and environmental components. In AD, deposition of protein aggregates composed of amyloid-β peptide and tau in CNS tissues impairs cognitive function. However, the host-intrinsic and extrinsic factors that regulate these processes are unclear. Accumulating evidence suggests that pathogenic microbes may contribute to neurodegeneration. Bacterial90,91, viral92,93, fungal94 and parasitic95 infections targeting the CNS are associated with increased risk of AD. These infections likely promote chronic inflammatory responses in the CNS of susceptible individuals that could contribute to hallmarks of neurodegeneration such as synaptic degeneration and amyloidosis96. Bacterial infection induces amyloid-β peptide oligomerization, which has both antimicrobial and pathologic properties, proposing the idea that infection-induced amyloidosis may drive AD97. Aside from externally acquired microbes, one report demonstrated that mice representing a model of AD (APPPS1-Tg mice) derived under GF conditions exhibit reduced cerebral and serum amyloid-β levels98. These results are consistent with reduced plaque deposition in male APPSWE/PS1ΔE9 mice, which also model AD, treated with broad-spectrum antibiotics99. In support of a role for intestinal microbes in promoting Alzheimer’s disease, members of the host microbiota can regulate amyloidosis by producing their own amyloid peptides100. Additionally, manipulating the gut microbiota by antibiotic treatment or probiotic treatment alters learning and memory101,102. Despite these data, the role of microbiota-immune directed pathways in the development of AD requires further interrogation.
PD is a neurodegenerative motor disorder that is frequently associated with impaired gastric motility103 and elevated levels of α-synuclein in the intestine104, raising the possibility that the intestinal environment regulates the development of disease symptoms. One study revealed that the fecal microbiota of patients with PD had reduced abundance of Prevotellaceae compared to healthy controls while a subset of patients with severe postural instability and gait difficulty had elevated Enterobacteriaceae105, suggesting that these bacteria regulate motor function. Similar findings were reported in a separate study evaluating the gut microbiota in PD106. Patients also exhibit increased intestinal permeability and colonic inflammation107,108. Consistent with a potential role for microbes in promoting amyloidosis, the bacterial amyloid protein curli enhances α-synuclein aggregation in rats and worms109. Altogether, these data warrant further studies exploring the contributions of the gut microbiota–immune axis to the development of PD.
Neuroimmune pathways for microbial communication with the CNS
Bacteria-derived and host-derived products
Alterations in intestinal bacteria composition and signaling events in the gut can influence brain function and behavior through several mechanisms (Fig. 3). Activation of innate immune responses following exposure to microbial factors requires the recognition of MAMPs via pattern recognition receptors. The most well characterized family of pattern recognition receptors is TLRs, which are expressed not only on innate immune cells but also CNS-resident cell populations, including neurons and glial cells110. Since TLR ligands derived from the intestinal microbiota can be found systemically during chronic inflammatory disorders111, these products may be able to directly trigger innate immune pathways to affect CNS function. Although systemic lipopolysaccharide administration drives CNS inflammation by activating TLR4-expressing brain-resident cells112,113, experimental support for microbiota-derived MAMPs that trigger CNS inflammation is limited. However, one study demonstrated that peptidoglycan (PGN)-sensing molecules are highly expressed in the developing striatum and are reduced in expression in GF and antibiotic-treated neonates114. Mice deficient in PGN recognition protein 2 display increased sociability in the three-chamber social approach test, suggesting a role for PGN recognition in the CNS in social behavior.
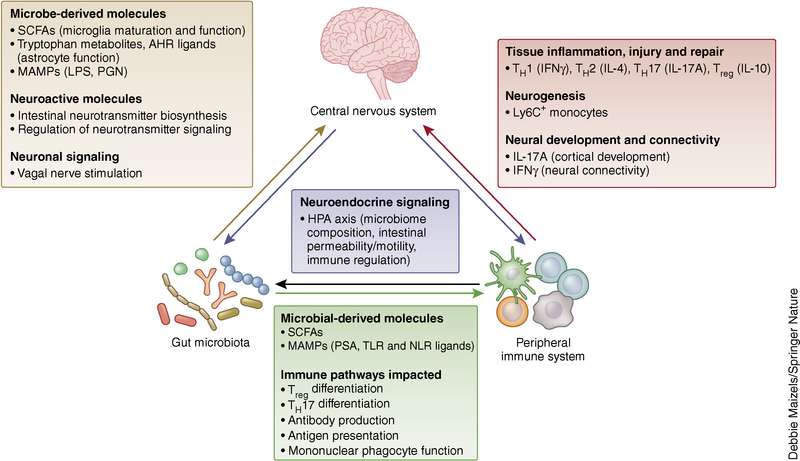
Figure 3.
Crosstalk between the microbiota, immune system and CNS. Interactions between the intestinal microbiota, peripheral immune system and CNS are essential for the maintenance of host health. Recognition of microbial derived products such as microbe-associated molecular patterns (MAMPs) and metabolic by-products of microbes (short chain fatty acids, SCFAs) activates distinct immune pathways throughout the host. The microbiota and immune system can independently or cooperatively regulate neurophysiology. Therefore, a prevailing theme in studies aimed at understanding the microbiota–gut–brain axis involves the role of intestinal microbes in modulating CNS function through CNS-resident and peripheral immune pathways. Biochemical changes in the CNS can also lead to altered microbial composition and immune cell responses through the HPA axis. Altogether, these findings suggest that the microbiota, immune system and CNS communicate bidirectionally. Future studies investigating the functional outcomes of these bidirectional interactions will inform the development of new therapeutic strategies for the treatment of neurological disorders. IFNγ, interferon gamma; LPS, lipopolysaccharide; NLR, Nod-like receptor; PGN, peptidoglycan; TLR, Toll-like receptor.
Intestinal bacterial colonization modulates the host metabolome, which influences CNS function27,37. Circulating metabolites can enter the CNS and directly affect neuroactivity37. Conversely, particular metabolites can regulate the function of peripheral immune cells3,115, which then can influence brain function, potentially through the recently described brain lymphatic network116. How immune cells coordinate gut–brain communication and whether microbe-derived metabolites can directly modulate CNS function are areas of intense investigation.
Neurotransmitters are of particular interest in light of studies demonstrating that the gut microbiota can modulate its production117,118 and that particular gut microbes can synthesize neurotransmitters de novo119. The majority of the host’s serotonin is derived from the gastrointestinal tract120, suggesting a role for the intestinal environment in modulating its synthesis. Consistent with this idea, GF mice have reduced levels of serum and colon serotonin compared to conventional controls39. Spore-forming bacteria in particular activate intestinal enterochromaffin cells to produce serotonin118. Furthermore, metabolites upregulated by spore-forming bacteria colonization as well as SCFAs can directly promote the production of serotonin and expression of the serotonin biosynthetic enzyme Tph1 in enterochromaffin cells117,118. Microbiota-dependent regulation of neurotransmitter pathways has also been observed for GABA84, norepinephrine121, dopamine121 and tryptamine122. Immune cells of both myeloid and lymphoid lineages express a wide range of neurotransmitter receptors and can respond to neurotransmitter stimulation by altering their inflammatory functions12. While these studies have direct implications for neurogastrointestinal health, future studies should also investigate the role of these microbe-mediated pathways in brain development, function and behavior.
Neuronal signaling
In addition to modulating intestinal production of neurochemicals, gut microbes can communicate with the CNS via stimulation of the vagus nerve, a bundle of parasympathetic motor and sensory fibers that conveys information from peripheral organs such as the gastrointestinal tract to the CNS. Treatment of wild-type mice with the bacterium Lactobacillus rhamnosus increases center exploration in the open field test and decreases immobility time in the forced swim test, and this effect is abolished following vagotomy84. Increased latency in the step-down test of anxiety induced by DSS colitis also depends on the vagus nerve81. Further, treatment with B. longum during DSS colitis does not ameliorate impairments in the step-down test in vagotomized mice, identifying a critical role for afferent vagal stimulation in bacteria-mediated changes in behavior. Conversely, the CNS transmits signals to the intestine through the efferent fibers of the vagal nerve. These neurons control intestinal motility, release of neurochemicals and the intestinal immune environment. Specifically, vagal nerve stimulation has been suggested to attenuate inflammatory responses through the neurotransmitter acetylcholine123, raising the possibility that the immunomodulatory properties of probiotic strains such as Lactobacillus and Bifidobacterium may depend on the vagus nerve. However, protection from DSS colitis conferred by L. rhamnosus and B. infantis was observed in both control and vagotomized mice124, suggesting that gut–brain communication via this pathway may be specific to certain bacterial strains and the inflammatory context. In addition to parasympathetic regulation, the sympathetic branch of the autonomic nervous system is also involved in intestinal homeostasis and modulates the intestinal immune environment59. Despite these findings, how intestinal microbes, sensory neurons and immune regulation are linked requires further study.
Neuroendocrine pathways
Biochemical changes in the brain can also lead to changes in intestinal physiology. One of the main pathways by which this occurs is the HPA axis. In response to physical or psychological stress, hormones released from the hypothalamus, pituitary and adrenal glands collectively influence multiple organ systems to allow the host to adapt to the environment. For example, activation of the stress response through the HPA axis can lead to changes in intestinal permeability125–128, motility129,130 and mucus production131,132. These events contribute to alterations in microbiota composition as demonstrated in mouse models of early-life and chronic stress85,133. Consistent with these findings, activation of the HPA axis affects immune cell responses. Glucocorticoids released by the HPA axis have profound anti-inflammatory influences on innate and adaptive immune cells but can also stimulate proinflammatory responses under certain conditions89,134. Stress-induced behavioral abnormalities may occur in a microbiota-dependent manner. Although maternal separation results in elevated serum corticosterone levels and increased acetylcholine release by the colon in both GF and conventional mice, it triggers behavioral alterations only in conventional mice85. Altogether, these findings support the idea that microbiota–gut–brain communication not only involves microbial modulation of brain function but also the reverse: CNS-induced biochemical changes result in altered intestinal biology, bacterial composition and immune function.
Concluding remarks
Accumulating evidence indicates that brain-resident and peripheral immune cells are critically involved in orchestrating microbiota–gut–brain communication. The gut microbiota regulate the developmentand function of microglia and astrocytes, which mediate neurophysiological processes including neural development, neurotransmission, CNS immune activation and blood-brain barrier integrity. The microbiota also modulate peripheral immune responses, with important consequences for brain inflammation, injury and behavior. Microbial influences on innate and adaptive immune activation in the intestine, fetal–maternal interface and systemic circulation are associated with symptoms of various neuroinflammatory, neurodegenerative and psychiatric disorders. Collectively, these findings establish the CNS and peripheral immune systems as important cellular mediators of communication across the microbiota–gut–brain axis. However, molecular mechanisms for how host–microbe interactions in the intestine remotely alter brain physiology remain largely unclear. Future investigations are necessary to define specific microbe-derived factors, immune effector functions and microbiota–immune pathways for modulating brain function and behavior. These studies will reveal fundamental biological insights for brain health, with the potential to inform the development of new microbial and immune-based therapeutic strategies for the treatment of neurological diseases.
ACKNOWLEDGMENTS
The authors are supported by funding from the NIH Director’s Early Independence award (5DP5OD017924 to E.Y.H.), Alfred P. Sloan Fellowship in Neuroscience (to E.Y.H.), NIH Ruth L. Kirschstein National Research Service Award (T32GM065823 to C.A.O.) and UCLA Life Sciences Division, Department of Integrative Biology & Physiology.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Belkaid Y & Hand TW Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Honda K & Littman DR The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Rooks MG & Garrett WS Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol 16, 341–352 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins SM, Surette M & Bercik P The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol 10, 735–742 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Cryan JF & Dinan TG Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci 13, 701–712 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Sampson TR & Mazmanian SK Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 17, 565–576 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reaa K, Dinan TG & Cryan JF The microbiome: a key regulator of stress and neuroinflammation. Neurobiol. Stress 4, 23–33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deverman BE & Patterson PH Cytokines and CNS development. Neuron 64, 61–78 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Stephan AH, Barres BA & Stevens B The complement system: an unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci 35, 369–389 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Elmer BM & McAllister AK Major histocompatibility complex class I proteins in brain development and plasticity. Trends Neurosci. 35, 660–670 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinstein LI, Revuelta A & Pando RH Catecholamines and acetylcholine are key regulators of the interaction between microbes and the immune system. Ann. NY Acad. Sci. 1351, 39–51 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Baganz NL & Blakely RD A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem. Neurosci 4, 48–63 (2013).This reference extensively reviews the role of serotonin signaling and serotonin uptake in immune cell function, highlighting serotonergic pathways that are intrinsic to innate and adaptive immune cells.
- 13.Ahern GP 5-HT and the immune system. Curr. Opin. Pharmacol 11, 29–33 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barragan A, Weidner JM, Jin Z, Korpi ER & Birnir B GABAergic signalling in the immune system. Acta Physiol. (Oxf.) 213, 819–827 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Ben-Shaanan TL et al. Activation of the reward system boosts innate and adaptive immunity. Nat. Med. 22, 940–944 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Erickson MA, Dohi K & Banks WA Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation 19, 121–130 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banks WA The blood-brain barrier in neuroimmunology: tales of separation and assimilation. Brain Behav. Immun 44, 1–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rook GA, Raison CL & Lowry CA Microbiota, immunoregulatory old friends and psychiatric disorders. Adv. Exp. Med. Biol 817, 319–356 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Nayak D, Roth TL & McGavern DB Microglia development and function. Annu. Rev. Immunol 32, 367–402 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matcovitch-Natan O et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 353, aad8670 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Nayak D, Zinselmeyer BH, Corps KN & McGavern DB In vivo dynamics of innate immune sentinels in the CNS. Intravital 1, 95–106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nimmerjahn A, Kirchhoff F & Helmchen F Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Bilbo SD & Schwarz JM The immune system and developmental programming of brain and behavior. Front. Neuroendocrinol 33, 267–286 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu X et al. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol 11, 56–64 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ransohoff RM A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci 19, 987–991 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Bogie JFJ, Stinissen P & Hendriks JJA Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol. 128, 191–213 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Erny D et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci 18, 965–977 (2015).This study demonstrates the critical role of the microbiome in modulating microglial development and maintenance, particularly how short-chain fatty acids promote microglial maturity.
- 28.Borre YE et al. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol. Med 20, 509–518 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Smith PM et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schafer DP et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho I et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khakh BS & Sofroniew MV Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18, 942–952 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen CJ, Massie A & De Keyser J Immune players in the CNS: the astrocyte. J. Neuroimmune Pharmacol 8, 824–839 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Rossi D Astrocyte physiopathology: at the crossroads of intercellular networking, inflammation and cell death. Prog. Neurobiol 130, 86–120 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Barres BA The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Dong Y & Benveniste EN Immune function of astrocytes. Glia 36, 180–190 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Rothhammer V et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med 22, 586–597 (2016).This study highlights how microbial metabolites of dietary tryptophan affect astrocytic inflammatory status, which modulates the severity of EAE.
- 38.Zelante T et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Wikoff WR et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 106, 3698–3703 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radjavi A, Smirnov I, Derecki N & Kipnis J Dynamics of the meningeal CD4+ T-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol. Psychiatry 19, 531–533 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribatti D The crucial role of mast cells in blood-brain barrier alterations. Exp. Cell Res 338, 119–125 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Forsythe P Microbes taming mast cells: implications for allergic inflammation and beyond. Eur. J. Pharmacol 778, 169–175 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Khosravi A et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 15, 374–381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee YK, Menezes JS, Umesaki Y & Mazmanian SK Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 108 (Suppl. 1), 4615–4622 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ochoa-Reparaz J et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol 183, 6041–6050 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Gaboriau-Routhiau V et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Ivanov II et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H & Kakuta S et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol 177, 566–573 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Round JL et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332, 974–977 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Round JL & Mazmanian SK Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 107, 12204–12209 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ochoa-Reparaz J et al. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J. Immunol. 185, 4101–4108 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Ochoa-Repáraz J et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 3, 487–495 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Wang Y et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat. Commun 5, 4432 (2014).Refs. 51–53 describe roles for Bacteroides fragilis derived PSA in neuroprotection from EAE through induction of regulatory T cell responses, highlighting the relationship between bacterially derived molecules, immune regulation and neuroinflammation.
- 54.Haghikia A et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43, 817–829 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Kadowaki A et al. Gut environment-induced intraepithelial autoreactive CD4+ T cells suppress central nervous system autoimmunity via LAG-3. Nat. Commun 7, 11639 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benakis C et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med 22, 516–523 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winek K et al. Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke 47, 1354–1363 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh V et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 36, 7428–7440 (2016).Refs. 56–58 describe a role for the gut microbiota in the development of brain injury in the MCAO mouse model of stroke. MCAO results in intestinal dysbiosis, which regulates disease outcome through modulation of the adaptive immune system.
- 59.Houlden A et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav. Immun 57, 10–20 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kigerl KA et al. Gut dysbiosis impairs recovery after spinal cord injury. J. Exp. Med 213, 2603–2620 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walsh JT et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J. Clin. Invest. 125, 2547 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gadani SP, Walsh JT, Smirnov I, Zheng J & Kipnis J The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron 85, 703–709 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M & Tedder TF Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest 118, 3420–3430 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pöllinger B et al. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J. Exp. Med 206, 1303–1316 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yaddanapudi K et al. Passive transfer of streptococcus-induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol. Psychiatry 15, 712–726 (2010). [DOI] [PubMed] [Google Scholar]
- 66.Tennoune N et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide α-MSH, at the origin of eating disorders. Transl. Psychiatry 4, e458 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D’Mello C & Swain MG Liver-brain interactions in inflammatory liver diseases: implications for fatigue and mood disorders. Brain Behav. Immun 35, 9–20 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Graff LA, Walker JR & Bernstein CN Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm. Bowel Dis 15, 1105–1118 (2009). [DOI] [PubMed] [Google Scholar]
- 69.D’Mello C et al. Probiotics improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain. J. Neurosci 35, 10821–10830 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Humann J et al. Bacterial peptidoglycan transverses the placenta to induce fetal neuroproliferation and aberrant postnatal behavior. Cell Host Microbe 19, 388–399 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ogbonnaya ES et al. Adult hippocampal neurogenesis is regulated by the microbiome. Biol. Psychiatry 78, e7–e9 (2015). [DOI] [PubMed] [Google Scholar]
- 72.Möhle L et al. Ly6Chi monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 15, 1945–1956 (2016).This study demonstrates that the gut microbiota promote hippocampal neurogenesis in adult mice through recruitment of monocytes to the CNS. However, ref. 74 observed that the gut microbiota inhibit this process, suggesting complex interactions between intestinal microbes and neurogenesis.
- 73.Hsiao EY et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buffington SA et al. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165, 1762–1775 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi GB et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016).This study demonstrates an important role for T cell–derived IL-17A in the development of behavioral abnormalities in the maternal immune activation mouse model of ASD, highlighting the relationship between immune dysregulation, neurophysiology and behavior.
- 76.Hsiao EY, McBride SW, Chow J, Mazmanian SK & Patterson PH Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl. Acad. Sci. USA 109, 12776–12781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith SE, Li J, Garbett K, Mirnics K & Patterson PH Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci 27, 10695–10702 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krishnan V & Nestler EJ The molecular neurobiology of depression. Nature 455, 894–902 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller AH & Raison CL The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol 16, 22–34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diaz Heijtz R et al. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 108, 3047–3052 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bercik P et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil 23, 1132–1139 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Desbonnet L et al. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170, 1179–1188 (2010). [DOI] [PubMed] [Google Scholar]
- 83.Arseneault-Bréard J et al. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 107, 1793–1799 (2012). [DOI] [PubMed] [Google Scholar]
- 84.Bravo JA et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 108, 16050–16055 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Palma G et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun 6, 7735 (2015). [DOI] [PubMed] [Google Scholar]
- 86.Zheng P et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21, 786–796 (2016). [DOI] [PubMed] [Google Scholar]
- 87.Kelly JR et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res 82, 109–118 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Desbonnet L, Garrett L, Clarke G, Bienenstock J & Dinan TG The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J. Psychiatr. Res 43, 164–174 (2008). [DOI] [PubMed] [Google Scholar]
- 89.Bellavance MA & Rivest S The HPA – immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol 5, 136 (2014).This study discusses the immunomodulatory effects of glucocorticoids released by activation of the HPA axis, a neuroendocrine pathway that allows host adaptation to physical and psychological stress.
- 90.Hammond CJ et al. Immunohistological detection of Chlamydia pneumoniae in the Alzheimer’s disease brain. BMC Neurosci 11, 121 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang WS et al. Association between Helicobacter pylori infection and dementia. J. Clin. Neurosci 21, 1355–1358 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Karim S et al. An association of virus infection with type 2 diabetes and Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 13, 429–439 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Lurain NS et al. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J. Infect. Dis 208, 564–572 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pisa D, Alonso R, Rábano A, Rodal I & Carrasco L Different brain regions are infected with fungi in Alzheimer’s disease. Sci. Rep 5, 15015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prandota J Possible link between Toxoplasma gondii and the anosmia associated with neurodegenerative diseases. Am. J. Alzheimers Dis. Other Demen 29, 205–214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Glass CK, Saijo K, Winner B, Marchetto MC & Gage FH Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumar DK et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med 8, 340ra72 (2016).This study demonstrates that bacterial infection promotes amyloid-β peptide aggregation as an antimicrobial response, raising the question of whether neurodegeneration in Alzheimer’s disease is causally associated with host responses to microbial infection.
- 98.Harach T et al. Reduction of Alzheimer’s disease beta-amyloid pathology in the absence of gut microbiota. Preprint at https://arxiv.org/abs/1509.02273 (2015). [Google Scholar]
- 99.Minter MR et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep 6, 30028 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chapman MR et al. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295, 851–855 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fröhlich EE et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun 56, 140–155 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang T et al. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef. Microbes 6, 707–717 (2015). [DOI] [PubMed] [Google Scholar]
- 103.Fasano A, Visanji NP, Liu LW, Lang AE & Pfeiffer RF Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 14, 625–639 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Shannon KM et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov. Disord 27, 709–715 (2012). [DOI] [PubMed] [Google Scholar]
- 105.Scheperjans F et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord 30, 350–358 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Keshavarzian A et al. Colonic bacterial composition in Parkinson’s disease. Mov. Disord 30, 1351–1360 (2015). [DOI] [PubMed] [Google Scholar]
- 107.Devos D et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis 50, 42–48 (2013). [DOI] [PubMed] [Google Scholar]
- 108.Forsyth CB et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6, e28032 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen SG et al. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci. Rep 6, 34477 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Crack PJ & Bray PJ Toll-like receptors in the brain and their potential roles in neuropathology. Immunol. Cell Biol 85, 476–480 (2007). [DOI] [PubMed] [Google Scholar]
- 111.Brenchley JM & Douek DC Microbial translocation across the GI tract. Annu. Rev. Immunol 30, 149–173 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chakravarty S & Herkenham M Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J. Neurosci 25, 1788–1796 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qin L et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55, 453–462 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arentsen T et al. The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol. Psychiatry 10.1038/mp.2016.182 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koh A, De Vadder F, Kovatcheva-Datchary P & Bäckhed F From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016). [DOI] [PubMed] [Google Scholar]
- 116.Louveau A et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).This study identifies a network of lymphatic vessels in the meningeal spaces of the CNS, challenging the idea that the brain lacks an organized immune surveillance system.
- 117.Reigstad CS et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29, 1395–1403 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yano JM et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lyte M Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 9, e1003726 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gershon MD & Tack J The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 (2007). [DOI] [PubMed] [Google Scholar]
- 121.Asano Y et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol 303, G1288–G1295 (2012). [DOI] [PubMed] [Google Scholar]
- 122.Williams BB et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 16, 495–503 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Borovikova LV et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000). [DOI] [PubMed] [Google Scholar]
- 124.van der Kleij H, O’Mahony C, Shanahan F, O’Mahony L & Bienenstock J Protective effects of Lactobacillus rhamnosus and Bifidobacterium infantis in murine models for colitis do not involve the vagus nerve. Am. J. Physiol. Regul. Integr. Comp. Physiol 295, R1131–R1137 (2008). [DOI] [PubMed] [Google Scholar]
- 125.Ait-Belgnaoui A et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 37, 1885–1895 (2012). [DOI] [PubMed] [Google Scholar]
- 126.Demaude J, Salvador-Cartier C, Fioramonti J, Ferrier L & Bueno L Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut 55, 655–661 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moussaoui N et al. Changes in intestinal glucocorticoid sensitivity in early life shape the risk of epithelial barrier defect in maternal-deprived rats. PLoS One 9, e88382 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lennon EM et al. Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10−/− mice. Inflamm. Bowel Dis 19, 712–719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gue M, Junien JL & Bueno L Conditioned emotional response in rats enhances colonic motility through the central release of corticotropin-releasing factor. Gastroenterology 100, 964–970 (1991). [DOI] [PubMed] [Google Scholar]
- 130.Gué M, Peeters T, Depoortere I, Vantrappen G & Buéno L Stress-induced changes in gastric emptying, postprandial motility, and plasma gut hormone levels in dogs. Gastroenterology 97, 1101–1107 (1989). [DOI] [PubMed] [Google Scholar]
- 131.Rubio CA & Huang CB Quantification of the sulphomucin-producing cell population of the colonic mucosa during protracted stress in rats. In Vivo 6, 81–84 (1992). [PubMed] [Google Scholar]
- 132.Da Silva S et al. Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: prevention by a probiotic treatment. Am. J. Physiol. Gastrointest. Liver Physiol 307, G420–G429 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Park AJ et al. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol. Motil 25, 733–e575 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hueston CM & Deak T The inflamed axis: the interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic-pituitary-adrenal axis. Physiol. Behav 124, 77–91 (2014). [DOI] [PubMed] [Google Scholar]
- 135.Jangi S et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun 7, 12015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tremlett H et al. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur. J. Neurol. 23, 1308–1321 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen J et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 6, 28484 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miyake S et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One 10, e0137429 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cantarel BL et al. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J. Investig. Med 63, 729–734 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Keshavarzian A et al. Colonic bacterial composition in Parkinson’s disease. Mov. Disord 30, 1351–1360 (2015). [DOI] [PubMed] [Google Scholar]
- 141.Bu XL et al. A study on the association between infectious burden and Alzheimer’s disease. Eur. J. Neurol 22, 1519–1525 (2015). [DOI] [PubMed] [Google Scholar]
- 142.Gungor B, Adiguzel E, Gursel I, Yilmaz B & Gursel M Intestinal microbiota in patients with spinal cord injury. PLoS One 11, e0145878 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fouts DE et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl. Med 10, 174 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aizawa E et al. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 202, 254–257 (2016). [DOI] [PubMed] [Google Scholar]
- 145.Jiang H et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun 48, 186–194 (2015). [DOI] [PubMed] [Google Scholar]
- 146.Messaoudi M et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 105, 755–764 (2011). [DOI] [PubMed] [Google Scholar]
- 147.Mohammadi AA et al. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 19, 387–395 (2016). [DOI] [PubMed] [Google Scholar]
- 148.Tomova A et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 138, 179–187 (2015). [DOI] [PubMed] [Google Scholar]
- 149.Wang L et al. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism 4, 42 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.De Angelis M et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One 8, e76993 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kang D-W et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 8, e68322 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Williams BL et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One 6, e24585 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Adams JB, Johansen LJ, Powell LD, Quig D & Rubin RA Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 11, 22 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Finegold SM et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16, 444–453 (2010). [DOI] [PubMed] [Google Scholar]
- 155.Parracho HMRT, Bingham MO, Gibson GR & McCartney AL Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 54, 987–991 (2005). [DOI] [PubMed] [Google Scholar]