Abstract
Background:
Cancer Survivorship Care Plans (“care plans”) often recommend an active lifestyle yet are rarely accompanied by programs to help patients enact the prescribed behavior change. As a step towards bridging this gap, this trial tested the feasibility of augmenting care planning with a multi-level physical activity intervention.
Methods:
Breast and colorectal cancer survivors were enrolled alongside a self-selected support partner (e.g., spouse, friend). Survivors received a care plan alone (Comparison Group) versus one augmented with a 12-week physical activity module (Intervention Group). For Intervention Group dyads, both members received a multi-component program including Fitbit trackers, with the survivor’s Fitbit linked to his/her electronic health record (EHR). Treating clinicians received periodic updates regarding the survivors’ physical activity. The primary outcome was ActiGraph-measured physical activity, analyzed using mixed models. Feedback questionnaires were administered to participants and clinicians at 12 weeks.
Results:
Survivors (n=50) were 54.4±11.2 years of age and 2.0±1.5 years post-diagnosis. Survivors in the Intervention Group increased moderate-to-vigorous intensity physical activity (MVPA) by 69±84 min/week vs. a 20±71 min/week decrease in the Comparison Group (p=.001). Likewise, daily steps increased by 1,470±1,881 vs. a 398±1,751 decrease (P=.002). Among responding clinicians, 100% looked at survivors’ activity data within the EHR at least once and 80% said it provided insight into their patients’ lifestyles.
Conclusions:
Augmenting a standard care plan with a multi-level, technology-based intervention increased physical activity among cancer survivors.
Implications for Cancer Survivors:
Technology-based approaches, including activity trackers, can be used by individuals to work towards an active lifestyle after cancer.
Keywords: Exercise, cancer survivorship, intervention, wearables, electronic health records, survivorship care plan
Introduction
Improved screening, detection, and early treatment have led to a large and growing population of colorectal and female breast cancer survivors that now includes approximately 4.5 million Americans [1, 2]. A growing body of evidence suggests that increased post-diagnosis physical activity decreases mortality risk in cancer survivors [3]. Several recent meta-analyses indicate that physical activity after breast cancer diagnosis is associated with up to 24% lower risk for recurrence, 41% lower risk of breast cancer mortality, and 48% lower risk of all-cause mortality [4–7]. Similarly, for colorectal cancer survivors, meta-analyses report up to 42% lower risk of all-cause mortality and 39% lower risk of colorectal cancer mortality [4, 8, 9]. In addition, multiple studies indicate that physical activity improves quality of life, fatigue, physical functioning, and anxiety among survivors [10–14].
Despite the overwhelming evidence for the beneficial effects of physical activity, only 20–30% of cancer survivors self-report meeting physical activity guidelines after completing treatment [15]. Objective data via accelerometry suggest the true prevalence of sufficient physical activity is lower, around 5% [16]. Physical activity interventions among cancer survivors are safe and feasible [17] but frequently utilize strategies that have low scalability (e.g., resource intensive, no clear mechanism for dissemination). A 2015 report highlighted this problem and identified the need for the development of sustainable and effective physical activity interventions that can be translated into clinical practice within academic and community settings [18].
The Institute of Medicine recommends that every cancer survivor receive survivorship care planning, which includes recommendations for follow-up, prevention, and health promotion, such as suggestions for healthy lifestyle changes [19, 20]. The care plan, which is typically delivered in a care planning session after completion of primary, active treatment, educates the survivor and establishes a portable document to facilitate transition of care [19–23]. It frequently includes lifestyle recommendations. However, it is well established that verbal or written recommendations alone are often insufficient for sustained physical activity change [24, 25].
A multi-level approach is more likely to enact sustained behavior change. Physical activity is a complex health behavior determined by the interaction of individual (e.g. self-efficacy, motivation), social, environmental, and policy-level factors [26]. Intervening at both the individual and interpersonal level can enhance behavior change by creating social support and reinforcing new norms within the family or social group [27]. Delivering the intervention alongside the care plan, relatively soon after the end of primary treatment, may capitalize on a phase when survivors are particularly amenable to change. Another potential benefit is that situating the intervention within the context of oncologic clinical care may increase the probability that survivors act upon the recommendations [28–31]. Consumer-based activity trackers, such as Fitbit, support daily self-monitoring and development of self-regulatory skills [24] and several trials have used these to promote activity in cancer survivors [32–34]. Additionally, the growing use and capabilities of the electronic health record (EHR) provide a novel opportunity to further link interventions with clinical care by integrating survivors’ Fitbit data with the health record. This integration may give survivors a heightened sense of accountability and reinforce that the clinical care team recognizes physical activity as an important component of healthy survivorship. In essence, we propose that the physical activity intervention does not need to be delivered by clinic personnel in order to be part of the survivor’s overall clinical care experience.
The objective of this pilot trial was to test the augmentation of care planning with a multi-level, technology-based physical activity intervention module. We hypothesized that the enhanced intervention approach would be feasible and deliver improvements in physical activity relative to care planning alone. This is important because physical activity interventions, when delivered in the context of care plans and standard oncologic care, have the potential to improve cancer outcomes and quality of life for millions of breast and colorectal cancer survivors.
Methods
This pilot trial tested (a) the feasibility of enrolling breast and colorectal cancer survivors and support partners into a 12-week randomized physical activity trial and (b) the effect of augmenting the care plan with a multi-level technology-based physical activity intervention on participants’ physical activity level. Data were collected from August 2016 to January 2018. All procedures were reviewed by the University of Wisconsin (UW) – Madison Health Sciences Institutional Review Board. Informed consent was obtained from all individual participants included in the study. The dataset generated and analyzed during the current study is available from the corresponding author on reasonable request.
Participants and recruitment.
This trial enrolled cancer survivor/support partner dyads. Inclusion criteria for cancer survivors were: (a) patient of the UW Health system, (b) 28–75 years of age, (c) diagnosed with Stage I-III colorectal cancer or female breast cancer within the past 5 years, and (d) finished with primary treatment (defined as having completed all definitive cancer surgery, (neo)adjuvant chemotherapy, and/or neo(adjuvant) radiation; survivors still receiving adjuvant endocrine or other targeted therapies were eligible). Cancer survivors were excluded if they (a) had evidence of recurrent or metastatic disease, (b) had previously received a care plan, (c) were performing >100 min/week of moderate-to-vigorous physical activity (MVPA), or (d) were not able to identify a support partner. Support partners could be any adult (age 18+) identified by the participant (e.g., spouse, friend, relative, co-worker). Additional eligibility criteria, which applied to both the cancer survivor and the support partner, were (a) ownership of a computer/tablet/smartphone, (b) regular use of the Internet, (c) ability to exercise safely, (d) fluency in English, and (e) willingness and ability to attend study visits.
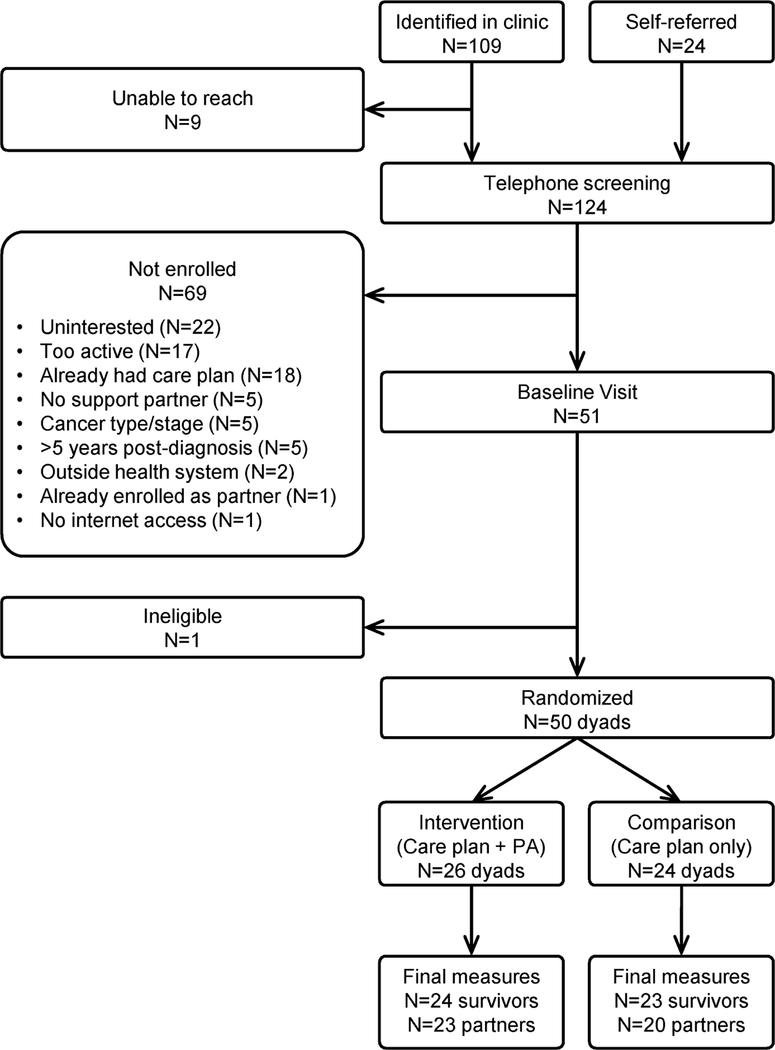
Survivors were recruited through the cancer clinics of the UW’s Carbone Comprehensive Cancer Center (UWCCC). Briefly, the study was introduced to the patient by a member of the clinic staff, and the patient was invited to sign a permission-to-contact form that allowed follow-up from the study team. Additionally, a small number of survivors self-referred to the study. After an initial telephone screening process conducted by the study team, eligible and interested participants (along with their identified support partners) were scheduled for a baseline visit at the UWCCC.
Randomization.
After completion of baseline measures, a computerized randomization scheme in REDCap [35] randomly assigned each dyad with equal probability to either the Intervention Group or the Comparison Group. Randomization was stratified by cancer type (breast vs. colorectal) and chemotherapy (yes/no).
Survivorship Care Planning.
The survivor was provided with the care plan near the time of randomization. Method of care plan delivery varied based on the survivor’s cancer type and time of primary treatment completion, given standard of care practices at UWCCC at the time of study initiation. Some breast cancer survivors who had recently completed primary treatment already had a care planning visit scheduled as part of their standard of care; they then reviewed the care plan with a provider in clinic during this visit [36, 37]. Survivors who did not have a care planning clinic visit scheduled, including colorectal cancer survivors and breast cancer survivors who were farther out from treatment, reviewed the care plan with a clinician over the phone. Care plans contained a written script on the benefits of physical activity for cancer survivors and physical activity recommendations of 150 min/week of moderate-to-vigorous intensity physical activity, consistent with the cancer survivorship recommendations from the American Cancer Society [38] and the American College of Sports Medicine [10]. Survivors received the same physical activity information in the care plan regardless of study group assignment.
Multi-level Technology-based Physical Activity Module (Intervention Group).
Dyads in the Intervention Group received a 12-week, multi-component intervention based on self-monitoring and the development of self-regulatory skills (i.e., goal-setting, frequent feedback, frequent review of goals), because these are the theory-based behavior change components most strongly-associated with successful increases in physical activity [24]. Participants were asked to gradually increase their level of MVPA to 150 min/week and their step count to 10,000 steps/day. All intervention components, other than the EHR integration, were provided to both the survivor and the support partner.
Fitbit tracker.
Each individual received a Fitbit tracker. Available models of wearable devices change quickly. Therefore, participants randomized early in the study received a Fitbit Charge HR; those randomized later received the Fitbit Charge 2, which is very similar.
Educational handbook.
This study-specific handbook was adapted from the handbook used in our previous trial of a Fitbit-based intervention in middle-aged and older women (the Active & Aware Study) [39, 40]. It contained information on the benefits of physical activity for cancer survivorship (this information was tailored to tumor type; breast cancer survivors and their support partners received a different version of the handbook than did colorectal cancer survivors and their support partners), content related to building self-efficacy and setting goals [24, 41], and detailed information regarding how to use the Fitbit and its website/app to work towards the physical activity goals (including step-by-step screenshots).
In-person instructional and goal-setting session.
The study coordinator set up each individual’s Fitbit and demonstrated how to download/install the software and use the Fitbit and website/app. Each participant’s baseline ActiGraph data was discussed and used to help guide participants in selecting appropriate individualized goals for the first week of the study. Survivors and support partners did not have to choose the same goals.
Social support.
Survivors and support partners were asked to assist each other in achieving and maintaining their activity goals. This could be through engaging in physical activity together, providing encouragement or accountability, helping a spouse with household tasks so they could find time to exercise, or other means as preferred by the dyad.
Email-based coaching.
The study coordinator sent individualized coaching e-mails to each survivor and support partner at 1, 2, 4, and 8 weeks. These emails were informed by each participant’s Fitbit data, which was tracked online via Fitabase (Small Steps, LLC). The study coordinator used these data to tailor e-mail coaching based on current personalized goals and recent progress, to help each individual set updated goals, and to provide suggestions and encouragement for individuals to support their dyad partner.
EHR integration.
An order was placed in each survivor’s EHR, which enabled Fitbit data to integrate into the EHR as part of a flowsheet. Once the order was placed, the survivor followed a simple process within his/her patient portal account to link the Fitbit to his/her EHR. Each survivor’s physical activity data (steps/day) was then viewable from the provider side of the EHR. Clinicians received notifications every 3 weeks to review the data and were asked to communicate with the survivor regarding progress.
Attention Control Components (Comparison Group)
Survivors and support partners assigned to the Comparison Group received the following components, which were intended to maintain engagement, reduce loss to follow-up, and reduce differences in the level of staff time and attention provided to each group.
Educational materials.
The Comparison Group received a printed copy of the 2015 US Dietary Guidelines for Americans [42]. This document focuses primarily on nutritional recommendations with only a brief section relating to physical activity.
Email contact.
Survivors and support partners received standardized e-mails at 1, 2, 4, and 8 weeks with information on healthy eating and stress management from the US Dietary Guidelines and American Heart Association. Physical activity coaching was not provided.
Measures
Participant characteristics.
Cancer survivors self-reported their demographics; cancer and treatment information was abstracted from the EHR to REDCap by the study coordinator. At the initial study visit, a standard stadiometer was used to measure the survivor and support partner’s height to the nearest 0.1 cm. Weight was measured on a digital scale to the nearest 0.1 kg. Because participants did not return for a study visit at 12 weeks (final measures were completed remotely), follow-up weight was obtained only for survivors and was abstracted from EHR using the clinic-measured weight closest to the date of the person’s completion of the study.
Physical activity.
At baseline and 12 weeks, each participant wore an ActiGraph GT3X+ accelerometer (ActiGraph, Pensecola, FL) on the hip during all their waking hours for 7 consecutive days. At both time points, data were downloaded and screened for completeness and irregularities. Participants were asked to re-wear the accelerometer if it was not worn for at least 10 hours per day for 5 days. Standard thresholds were used to aggregate data into minutes spent in sedentary, light, moderate, and vigorous activity [43]. Data was also collected on the amount of time spent in bouts of 10+ continuous minutes of moderate-to-vigorous activity, as this variable directly corresponded to the physical activity guidelines at the time data were collected.
Intervention feedback.
At 12 weeks, survivors and support partners completed questions evaluating various components of the study, including items specific to their study arm. The referring clinician for each survivor was sent a brief web-based questionnaire assessing the frequency with which they viewed their patients’ Fitbit data in the EHR and the helpfulness of the data. To reduce burden on the clinicians, this survey was sent once per clinician at the end of the trial, along with a list of the clinician’s patients who participated in the study. Thus, clinicians provided a general assessment of their use of the data, not a separate assessment specific to each patient on the study.
Statistical Analysis.
Characteristics of intervention vs. comparison group participants were compared using t-tests and chi-squared tests. The main analysis of physical activity outcomes was conducted using linear mixed models. As there were no significant differences between intervention groups at baseline, our primary models are unadjusted. However, we did conduct a sensitivity analysis adjusting for age and chemotherapy as covariates; this did not change the results on any outcome. As a large majority of participants had breast (vs. colorectal) cancer, it was not practical to conduct a sensitivity analysis controlling for tumor type. Because the small samples used in this feasibility trial affect the likelihood of observing significant effects, we also calculated effect sizes (Cohen’s d) for each of the physical activity outcomes. All analyses were performed in SAS 9.4 (SAS, Cary, NC).
Results
Participant characteristics.
Fifty cancer survivors and their support partners were enrolled between August 2016 and November 2017. Baseline characteristics are shown in Table 1. Cancer survivors were 29–73 years of age and, on average, 2.0 (SD=1.5) years past diagnosis. 32.0% had Stage 1 cancer; 50.0% had Stage 2, and 18.0% had Stage 3. Sixty-four percent had chemotherapy and 72.0% had radiation.
Table 1.
Baseline characteristics of cancer survivors.
| Total | Intervention | Comparison | ||
|---|---|---|---|---|
| Mean (SD) or N (%) | Mean (SD) or N (%) | Mean (SD) or N (%) | p-value | |
| N | 50 | 26 | 24 | |
| General characteristics | ||||
| Sex (% female) | 48 (96.0%) | 26 (100.0%) | 22 (91.7%) | .23 |
| Age | 54.4 (11.2) | 52.5 (12.2) | 56.5 (9.8) | .21 |
| BMI (kg/m2) | 32.2 (7.4) | 32.4 (6.2) | 33.4 (6.5) | .56 |
| Race/ethnicity | .37 | |||
| Non-Hispanic White | 47 (94.0%) | 25 (96.2%) | 22 (91.7%) | |
| Hispanic | 1 (2.0%) | 1 (3.9%) | 0 (0.0%) | |
| Black | 1 (2.0%) | 0 (0.0%) | 1 (4.2%) | |
| More than one race | 1 (2.0%) | 0 (0.0%) | 1 (4.2%) | |
| Physical activity | ||||
| MVPA min/week | 170 (131) | 174 (123) | 165 (142) | .80 |
| MVPA in 10+ min bouts | 15 (26) | 19 (29) | 11 (22) | .27 |
| Steps/day | 5,252 (2,237) | 5,318 (2115) | 5,181 (2405) | .83 |
| Cancer characteristics | ||||
| Tumor type (breast) | 45 (90.0%) | 25 (96.2%) | 20 (83.3%) | .18 |
| Years since diagnosis | 2.0 (1.5) | 1.9 (1.4) | 2.0 (1.7) | .72 |
| Stage | .08 | |||
| I | 16 (32.0%) | 11 (42.3%) | 5 (20.8%) | |
| II | 25 (50.0%) | 9 (34.6%) | 16 (66.7%) | |
| III | 9 (18.0%) | 6 (23.1%) | 3 (12.5%) | |
| Treatment | ||||
| Chemotherapy | 32 (64.0%) | 14 (53.9%) | 18 (75.0%) | .12 |
| Radiation | 36 (72.0%) | 20 (76.9%) | 16 (66.7%) | .42 |
Survivor physical activity.
The intervention module was associated with significant increases in accelerometer-measured physical activity (Table 2). Those in the Intervention Group increased their accumulated moderate-to-vigorous activity by 69 min/week (SD=84) compared to a decrease of 20 min/week (SD=74) in the Comparison Group (p=.0009). Moderate-to-vigorous intensity activity performed within 10-minute bouts increased by 69 min/week (SD=84) in the Intervention Group vs. a decrease of 6 min/week (SD=30) in the comparison group (p=.004). When examining intensity-specific variables, benefits were observed on moderate-intensity activity (p=.02) but there were no significant group-by-time differences for either light- or vigorous-intensity activity. The Intervention Group increased their daily steps by 1,470 (SD=1,881) per day compared to a decrease of 398 (SD=1,751) in the comparison group. Effect sizes were large (d>.80) for all outcomes except light-intensity activity (d=25; small effect) and total physical activity (d=.54; medium effect).
Table 2.
Physical activity changes in cancer survivors and support partners, as measured by the ActiGraph accelerometer.
| Survivors | ||||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Comparison | |||||||
| Baseline | 12 Weeks | Change | Baseline | 12 Weeks | Change | d | p-value | |
| Accumulated PA (min/wk) | N=26 | N=24 | N=24 | N=24 | N=23 | N=23 | ||
| Light | 1,258 (361) | 1,233 (441) | 15 (317) | 1286 (455) | 1200 (332) | −54 (224) | .25 | .41 |
| Moderate | 173 (121) | 224 (118) | 63 (88) | 162 (139) | 141 (105) | −19 (73) | 1.01 | .002 |
| Vigorous | 2 (2) | 7 (14) | 5 (14) | 3 (4) | 2 (3) | 0 (2) | 1.00 | .06 |
| Moderate + vigorous | 174 (123) | 230 (120) | 69 (84) | 165 (142) | 143 (107) | −20 (74) | 1.12 | .0009 |
| Total | 1,433 (459) | 1,463 (489) | 83 (317) | 1,451 (549) | 1343 (395) | −74 (266) | .54 | .08 |
| MVPA in bouts (min/wk) | 19 (29) | 69 (82) | 50 (84) | 11 (22) | 5 (17) | −6 (30) | .89 | .004 |
| Steps/day | 5,318 (2,115) | 6,697 (2,878) | 1,470 (1,881) | 5,181 (2,405) | 4,853 (1,703) | −398 (1,751) | 1.03 | .002 |
| Support Partners | ||||||||
| Intervention Group | Comparison Group | |||||||
| Baseline | 12 Weeks | Change | Baseline | 12 Weeks | Change | d | p-value | |
| Accumulated PA (min/wk) | N=26 | N=23 | N=23 | N=24 | N=21 | N=21 | ||
| Light | 1222 (395) | 1255 (378) | 18 (204) | 1197 (496) | 1092 (498) | −73 (261) | .39 | .17 |
| Moderate | 241 (122) | 302 (144) | 63 (146) | 154 (112) | 156 (116) | 10 (83) | .45 | .13 |
| Vigorous | 5 (9) | 2 (3) | −2 (10) | 3 (3) | 7 (15) | 5 (13) | .60 | .03 |
| Moderate + vigorous | 246 (126) | 304 (145) | 60 (151) | 156 (112) | 163 (122) | 14 (90) | .37 | .20 |
| Total | 1468 (485) | 1559 (452) | 79 (269) | 1354 (574) | 1256 (593) | −58 (307) | .10 | |
| MVPA in bouts (min/wk) | 34 (46) | 108 (114) | 72 (117) | 9 (20) | 13 (24) | 6 (23) | .79 | .007 |
| Steps/day | 6,199 (2,231) | 7,490 (2,462) | 1,298 (2,152) | 5,347 (2,802) | 5,194 (3,104) | −23 (1,971) | .64 | .03 |
Survivor weight changes.
Although not part of the specific aims, we did analyze changes in weight. Final weight data was abstracted from the EHR for 44 survivors (88%). The Intervention Group lost 1.8 kg (SD=2.8) (2.2% of starting weight) compared to a 0.5 kg (SD=3.0) loss (0.7% of starting weight) in the Comparison Group (p=.14). The effect size (d=.45) is considered small to medium.
Support-partner physical activity.
Although the primary goal of the study was to increase the survivors’ physical activity, we also analyzed ActiGraph data from the support partners. Relative to the Comparison Group, support partners in the Intervention Group significantly increased their moderate-to-vigorous intensity activity performed in 10-minute bouts (p=.007) and daily steps (p=.03). They decreased their vigorous-intensity activity relative to the Comparison Group; although statistically significant (p=.03), due to the very low prevalence of vigorous intensity activity in both groups, this does not reflect any meaningful change. Group-by-time interactions were not significant for other physical activity variables. Effect sizes were small or medium for all outcomes.
Support partners.
The majority of participants (63.6%) chose their spouse as a support partner; 22.7% chose a friend, 9.1% chose a relative, and 4.6% chose someone else. Over a third (36.4%) of survivors were in contact with their support partner specifically about physical activity 6–7 days per week; 22.7% were in contact 3–5 days/week, 22.7% 1–2 days/week, and 18.2% less than once per week. Dyad members supported each other’s physical activity by exercising together (59.1%), talking on the phone (36.4%), texting (22.7%), talking face-to-face (18.2%), emailing (9.1%), and adding each other to the leaderboard feature on the Fitbit website/app (9.1%).
Participant feedback regarding EHR integration.
Survivors rated the ease of linking their Fitbit to the patient health portal. Half (50.0%) rated the process as “very easy,” 18.2% rated it “somewhat easy,” 13.6% as “neither easy nor difficult” and 18.2% as “somewhat difficult.” No one rated it as “very difficult.” When asked whether their oncologist discussed physical activity with them during the 12-week study, 9.1% said that they had discussed it in person, 4.5% by phone, 63.6% by messages via the patient portal. Less than a third (31.8%) said that their oncologist did not discuss physical activity with them during the study.
Of those whose oncologist spoke with them about physical activity, 26.7% did so once, 53.3% did so twice, 20.0% did so 3 or more times. When asked about the degree to which it would be helpful to receive counsel and/or encouragement from their oncologist regarding physical activity, 4.6% said “very helpful,” 18.2% said “helpful,” 40.9% said “somewhat helpful,” 22.7% said “a little bit helpful,” and 13.6% said “not at all helpful.” However, when asked whether they would participate in a physical activity program if it were offered as a standard part of survivorship care, 77.3% said that they would have joined such a program, 22.7% were unsure, and no participants said that they would not have joined.
Satisfaction with physical activity section of care plan.
Overall, 69.6% of survivors (91.3% of Intervention Group and 47.8% of Comparison Group; p=.001) were “satisfied” or “very satisfied” with the standard physical activity information provided in the care plan document itself. Although participants commented that the information was “clear and informative” and that the “emphasis on how physical activity could have positive effect on health” was motivating, the lower satisfaction among comparison group participants was accompanied by comments such as “It was easy to ignore because I did not have to do anything with the information but read it,” and “I was hoping that there would be classes offered to get me started in physical activity or names of places that I could go to.”
Satisfaction with physical activity module.
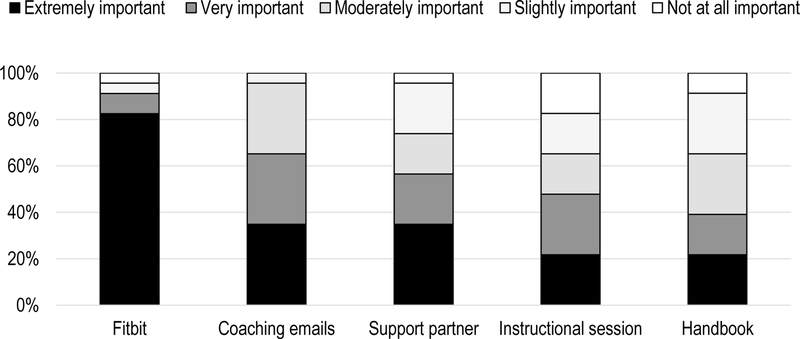
On measures of satisfaction, 73.9% of survivors in the Intervention Group reported that they were “extremely satisfied” with the intervention. An additional 17.4% were “somewhat satisfied” and 8.7% were “neither satisfied nor dissatisfied.” No participants were “somewhat dissatisfied” or “extremely dissatisfied” with the program. When asked about the importance of specific aspects of the physical activity module in helping to increase physical activity, 91.3% of participants said the Fitbit was “very important” or “extremely important. (Figure 2). The proportion of participants rating other module components as “very important” or “extremely important” was 65.2% for the coaching emails, 56.5% for the support partner, 47.8% for the in-person instructional session, and 38.1% for the intervention handbook. (The importance of any communications received from the clinician was not directly assessed.) Nearly half (43.5%) of participants reported that they logged into the Fitbit website more than once per day; 13.0% logged in daily, and 26.1% logged in 4–6 times per week, and 8.8% logged once per week or less.
Figure 2.
Participant ratings of importance of specific physical activity module components in helping to increase physical activity.
Clinician feedback.
Oncology physicians with patients on the study were asked about their usage of survivor step data. Each clinician was contacted at the end of the study, provided with a list of their patients who had been enrolled, and asked to provide a general assessment of their usage of the physical activity data (not to report their usage separately for each patient). Of the 8 clinicians contacted, 5 completed the survey. Of these, 100% said they looked at their patients’ PA data within the EHR at least once during the 12-week study (80% looked at it twice or more) and 80% said it provided at least some insight into their patients’ lifestyles.
Discussion
This pilot trial establishes the feasibility and short-term efficacy of augmenting care planning with a multi-level, technology-based physical activity module for breast and colorectal cancer survivors. Survivors who received this physical activity module achieved marked and statistically significant increases in their time spent in moderate and vigorous-intensity activity and in daily steps. Notably, they increased the accumulated moderate-to-vigorous intensity activity by over an hour per week, compared to a decrease of 20 minutes/week in the comparison group. This between-group difference of 89 min/week is substantial in consideration of the American Cancer Society’s recommendations for cancer survivors, which prescribe 150 min/week of MVPA [38]. The observed effect sizes further highlight the success of the module in achieving moderate-to-large increases in moderate-to-vigorous intensity activity and steps among survivors. Conversely, the lack of improvement among the comparison group confirms the insufficiency of providing lifestyle recommendations alone, without support to help survivors enact the prescribed changes.
Another important finding is that while each component of the module was perceived to contribute to increased physical activity, the fitness tracker itself was the key component. Since the publication of our Active and Aware study (one of the first published studies to use Fitbits as an intervention tool), numerous other trials have used Fitbits or other consumer-based fitness trackers to promote activity, including several studies in cancer survivors [32–34]. These indicate that, despite the aging of the US cancer survivorship population, there remains a strong and increasing role for wearables and other technologies to promote a healthy lifestyle [44, 45].
Despite the many opportunities for technology to support lifestyle changes in chronic disease populations, implementation remains a barrier [45]. Even the provision of a simple survivorship care plan has proven difficult in many oncology settings due to limited clinic staff and time. Given this, the addition of another component to care planning, specifically one that requires continued follow-up, would put additional strain on already limited resources [46, 47]. One potential strategy for improving the scalability of this approach is to provide the email-based coaching directly through the patient portal. This is supported by data from a study of intervention preferences in breast cancer survivors, 86% of whom said that they would be interested in receiving remote exercise coaching [48]. Likewise, over three-quarters of survivors in this study said they would have joined an exercise program if it had been offered to them as part of their clinical care. Therefore, technology-based approaches may be an appealing way to reach survivors, especially those for whom well-established supervised programs (e.g., Livestrong at the YMCA) [49] are not feasible due to work schedules, caregiving, or geographical or travel limitations.
Key strengths of this study include a randomized controlled design, an innovative intervention approach, the use of research-grade device-based physical activity measurement (the ActiGraph) for the primary behavior change outcome, and high retention across groups (94.0% of survivors and 88.0% of support partners at 12 weeks). Limitations include a sample that consisted of primarily non-Hispanic white breast cancer survivors, which, combined with a small sample size, precluded the possibility of examining the effect separately by tumor type or race/ethnicity. Additionally, because this pilot trial aimed only to test the efficacy of the module for adoption of physical activity over a relatively short-term intervention window, additional research would be needed to determine the efficacy of this approach for longer-term physical activity maintenance. Finally, the use of a traditional randomized controlled trial to test a multi-component intervention has some disadvantages. Namely, although our “package” of module components was efficacious, we are not able to discern the relative contribution of each component, whether any components are unneeded, or how one component might affect another. Other approaches, such as fractional factorial designs, are more suitable for answering these research questions [50].
In conclusion, our findings support the future exploration of intervention strategies that can be incorporated in the clinical context of survivorship care and include patient-generated physical activity data with the EHR. Additional research is needed to determine how to develop infrastructure that facilitates clinician and support staff’s ability to deliver a multi-component physical activity intervention within the clinical setting. Additionally, research is needed on how to improve the visualization and ease of using physical activity data within the health record so that clinicians and support staff can respond quickly and appropriately (e.g., a button that they can click to send a message of encouragement to a patient who has not met their physical activity goals) and how to identify resources to support the implementation and maintenance of lifestyle interventions.
Supplementary Material
Figure 1.
CONSORT diagram of recruitment of cancer survivors to a pilot physical activity trial.
Acknowledgements
The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Cadmus-Bertram’s time was supported by NIH grant 1K07CA178870.
Footnotes
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Wisconsin Health Sciences IRB (protocol 2015-1295) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2017–2018. Atlanta: American Cancer Society, Inc; 2017. [Google Scholar]
- 2.American Cancer Society. Colorectal Cancer Facts & Figures 2017–2019. Atlanta: American Cancer Society; 2017. [Google Scholar]
- 3.Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J, et al. The dose-response effect of physical activity on cancer mortality: Findings from 71 prospective cohort studies. Br J Sports Med. 2016;50(6):339–45. [DOI] [PubMed] [Google Scholar]
- 4.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann Oncol. 2014;25(7):1293–311. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med Oncol. 2011;28(3):753–65. [DOI] [PubMed] [Google Scholar]
- 6.Patterson R, Cadmus L, Emond J, Pierce J. Physical activity, diet, adiposity and breast cancer prognosis: A review of the epidemiologic literature. Maturitas. 2010;66(1):5–15. [DOI] [PubMed] [Google Scholar]
- 7.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635–54. [DOI] [PubMed] [Google Scholar]
- 8.Van Blarigan EL, Meyerhardt JA. Role of physical activity and diet after colorectal cancer diagnosis. J Clin Oncol. 2015;33(16):1825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Je Y, Jeon JY, Giovannucci EL, Meyerhardt JA. Association between physical activity and mortality in colorectal cancer: A meta-analysis of prospective cohort studies. Int J Cancer. 2013;133(8):1905–13. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–26. [DOI] [PubMed] [Google Scholar]
- 11.Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8:CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng Y, Huang M, Cheng AS, Zhou Y, So WK. Meta-analysis of the effects of exercise intervention on quality of life in breast cancer survivors. Breast Cancer. 2014;21(3):262–74. [DOI] [PubMed] [Google Scholar]
- 13.Peddle CJ, Au HJ, Courneya KS. Associations between exercise, quality of life, and fatigue in colorectal cancer survivors. Dis Colon Rectum. 2008;51(8):1242–8. [DOI] [PubMed] [Google Scholar]
- 14.Lynch BM, Cerin E, Owen N, Hawkes AL, Aitken JF. Prospective relationships of physical activity with quality of life among colorectal cancer survivors. J Clin Oncol. 2008;26(27):4480–7. [DOI] [PubMed] [Google Scholar]
- 15.Pinto BM, Ciccolo JT. Physical activity motivation and cancer survivorship. Recent Results Cancer Res. 2011;186:367–87. [DOI] [PubMed] [Google Scholar]
- 16.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. [DOI] [PubMed] [Google Scholar]
- 17.Stacey FG, James EL, Chapman K, Courneya KS, Lubans DR. A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. J Cancer Surviv. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Courneya KS, Rogers LQ, Campbell KL, Vallance JK, Friedenreich CM. Top 10 Research Questions Related to Physical Activity and Cancer Survivorship. Res Q Exerc Sport. 2015:1–10. [DOI] [PubMed] [Google Scholar]
- 19.Hewitt M, Ganz P. Implementing Cancer Survivorship Care Planning: Workshop Summary: National Academy Press; 2007. [Google Scholar]
- 20.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Translation. Washington, DC: Institute of Medicine; 2005. [Google Scholar]
- 21.Halpern MT, Viswanathan M, Evans TS, Birken SA, Basch E, Mayer DK. Models of Cancer Survivorship Care: Overview and Summary of Current Evidence. J Oncol Pract. 2014;11(1):319–27. [DOI] [PubMed] [Google Scholar]
- 22.Katon W, Von Korff M, Lin E, Walker E, Simon GE, Bush T, et al. Collaborative management to achieve treatment guidelines. Impact on depression in primary care. JAMA. 1995;273(13):1026–31. [PubMed] [Google Scholar]
- 23.Ayanian JZ, Zaslavsky AM, Arora NK, Kahn KL, Malin JL, Ganz PA, et al. Patients’ experiences with care for lung cancer and colorectal cancer: Findings from the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2010;28(27):4154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: A meta-regression. Health Psychol. 2009;28(6):690–701. [DOI] [PubMed] [Google Scholar]
- 25.Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy. Psychol Health. 2011;26(11):1479–98. [DOI] [PubMed] [Google Scholar]
- 26.Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJ, Martin BW, et al. Correlates of physical activity: Why are some people physically active and others not? Lancet. 2012;380(9838):258–71. [DOI] [PubMed] [Google Scholar]
- 27.Ball K, Jeffery RW, Abbott G, McNaughton SA, Crawford D. Is healthy behavior contagious: Associations of social norms with physical activity and healthy eating. Int J Behav Nutr Phys Act. 2010;7:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coa KI, Smith KC, Klassen AC, Caulfield LE, Helzlsouer K, Peairs K, et al. Capitalizing on the “teachable moment” to promote healthy dietary changes among cancer survivors: The perspectives of health care providers. Support Care Cancer. 2015;23(3):679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott AY, Mernitz H. Exercise and older patients: Prescribing guidelines. Am Fam Physician. 2006;74(3):437–44. [PubMed] [Google Scholar]
- 30.Patrick K, Pratt M, Sallis RE. The healthcare sector’s role in the U.S. National Physical Activity Plan. J Phys Act Health. 2009;6 Suppl 2:S211–9. [PubMed] [Google Scholar]
- 31.Weidinger KA, Lovegreen SL, Elliott MB, Hagood L, Haire-Joshu D, McGill JB, et al. How to make exercise counseling more effective: Lessons from rural America. J Fam Pract. 2008;57(6):394–402. [PubMed] [Google Scholar]
- 32.Gell NM, Grover KW, Humble M, Sexton M, Dittus K. Efficacy, feasibility, and acceptability of a novel technology-based intervention to support physical activity in cancer survivors. Support Care Cancer. 2017;25(4):1291–300. [DOI] [PubMed] [Google Scholar]
- 33.Hartman SJ, Natarajan L, Palmer BW, Parker B, Patterson RE, Sears DD. Impact of increasing physical activity on cognitive functioning in breast cancer survivors: Rationale and study design of Memory & Motion. Contemp Clin Trials. 2015;45(Pt B):371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips SM, Collins LM, Penedo FJ, Courneya KS, Welch W, Cottrell A, et al. Optimization of a technology-supported physical activity intervention for breast cancer survivors: Fit2Thrive study protocol. Contemp Clin Trials. 2018;66:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-- a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tevaarwerk AJ, Wisinski KB, Buhr KA, Njiaju UO, Tun M, Donohue S, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: A pilot study. J Oncol Pract. 2014;10(3):e150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hua A, Sesto ME, Zhang X, Wassenaar TR, Tevaarwerk AJ. Impact of survivorship care plans and planning on breast, colon, and prostate cancer survivors in a community oncology practice. J Cancer Educ. 2019; doi: 10.1007/s13187-018-1457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–74. [DOI] [PubMed] [Google Scholar]
- 39.Cadmus-Bertram L, Marcus BH, Patterson RE, Parker BA, Morey BL. Use of the Fitbit to measure adherence to a physical activity intervention among overweight or obese, postmenopausal women: Self-monitoring trajectory during 16 weeks. JMIR Mhealth Uhealth. 2015;3(4):e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cadmus-Bertram LA, Marcus BH, Patterson RE, Parker BA, Morey BL. Randomized trial of a Fitbit-based physical activity intervention for women. Am J Prev Med. 2015;49(3):414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 42.U.S. Department of Health and Human Services and U.S. Department of Agriculture (2015). 2015 – 2020 Dietary Guidelines for Americans. 8th Edition. Available at https://health.gov/dietaryguidelines/2015/guidelines/.
- 43.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–81. [DOI] [PubMed] [Google Scholar]
- 44.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips SM, Cadmus-Bertram L, Rosenberg D, Buman MP, Lynch BM. Wearable technology and physical activity in chronic disease: Opportunities and challenges. Am J Prev Med. 2018;54(1):144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birken SA, Raskin S, Zhang Y, Lane G, Zizzi A, Pratt-Chapman M. Survivorship care plan implementation in US cancer programs: a national survey of cancer care providers. J Cancer Educ. 2018; doi: 10.1007/s13187-018-1374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tevaarwerk AJ, Sesto ME. Continued challenges to the adoption and implementation of survivorship care plans. J Oncol Pract. 2018;14(10):573–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillips SM, Conroy DE, Keadle SK, Pellegrini CA, Lloyd GR, Penedo FJ, et al. Breast cancer survivors’ preferences for technology-supported exercise interventions. Support Care Cancer. 2017;25(10)3243–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irwin ML, Cartmel B, Harrigan M, Li F, Sanft T, Shockro L, et al. Effect of the LIVESTRONG at the YMCA exercise program on physical activity, fitness, quality of life, and fatigue in cancer survivors. Cancer. 2017;123(7):1249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): New methods for more potent eHealth interventions. Am J Prev Med. 2007;32(5 Suppl):S112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.