Abstract
Introduction:
Standard care interventions to reduce children’s tobacco smoke exposure (TSE) may not be sufficient to promote behavior change in underserved populations. A previous study demonstrated short-term efficacy of an experimental counseling intervention, “Family Rules for Establishing Smokefree Homes (FRESH)” compared with standard care on boosting low-income children’s TSE reduction and maternal smoking at 16-week end of treatment (EOT). This study tested long-term post-treatment efficacy of this treatment through 12-month follow-up.
Study design:
This study was a two-arm RCT.
Setting/participants:
Maternal smokers (N=300) not seeking cessation treatment were recruited from low-income, urban communities. Participants exposed their <4-year-old children to tobacco smoke daily. Data collection and analyses occurred from 2006 to 2018.
Intervention:
The FRESH behavioral intervention included two home visits and seven phone sessions. FRESH used cognitive behavioral skills training, support, problem solving, and positive social reinforcement to facilitate adoption of increasingly challenging TSE-protection behaviors. No nicotine-replacement therapy or medication was provided.
Main outcome measures:
Primary outcomes were child cotinine (TSE biomarker) and reported TSE from EOT through 12 months post-treatment. Secondary outcome was bioverified maternal smoking cessation.
Results:
Compared with controls, children in FRESH had significantly lower cotinine (β= −0.31, p<0.01) and lower maternal-reported TSE (β= −1.48, p=0.001) through the 12-month follow-up. A significant effect of time (β= −0.03, p=0.003) reflected a post-treatment decrease in cotinine. There was no treatment X time interaction, suggesting the treatment effect was sustained post-treatment. Compared with controls, FRESH mothers maintained significantly greater odds of quitting from EOT through 12-month follow-up (OR=8.87, 95% CI=2.33, 33.75).
Conclusions:
Study results with a sample of underserved maternal smokers demonstrated that the short-term effect of FRESH counseling at 16-week EOT was maintained through 12 months post-treatment—for both bioverified child TSE reduction and maternal smoking cessation. Smokers in low-income communities demonstrate elevated challenges to success in standard smoking treatment. FRESH follow-up results suggest high potential value of more-intensive behavioral intervention for vulnerable smokers.
INTRODUCTION
Tobacco use and tobacco smoke exposure (TSE) are associated with cancer, respiratory diseases, and cardiovascular diseases.1-4 TSE includes secondhand smoke from burning tobacco and exhaled smoke, as well as residual nicotine and tobacco contaminants (“thirdhand smoke”) that build up and re-disperse within indoor environments.5,6 TSE accounts for 42,000 non-smoker deaths annually in the U.S.2 and at least 600,000 deaths per year globally.8 Among children, TSE is a leading cause of preventable disease and death9 and is associated with numerous health issues, such as sudden infant death syndrome, respiratory diseases, and increased infections.3,9,10 In recent decades, policies restricting smoking in worksite and public spaces have facilitated significant decreases in tobacco use and TSE in the U.S.9,11-13 However, these reductions have not been uniform across subpopulations, resulting in TSE disparities among low-income groups.7 In the U.S., the smoking prevalence in impoverished populations is almost twice as high as that in higher-income groups,14 and TSE rates are greatest in vulnerable populations such as children, non-Hispanic blacks, and individuals living in poverty.9,15 Most public policies do not directly affect residential smoking restrictions, which may lead to increased risk of TSE consequences among the youngest exposed children who spend most of their time inside at home and in close proximity to their smoking parents. One promising exception is the new U.S. Department of Housing and Urban Development regulation restricting residential smoking in low-income housing. It is too early to judge effects prior to full implementation of the regulation and subsequent assessment of child TSE.
Maternal smoking is the primary source of TSE among young and underserved children.15-18 Women encounter greater barriers to cessation19-22 and are less successful at quitting than men— disparities that are magnified among women of childbearing age.23-27 Because low-income female smokers are less likely than others to respond favorably to existing evidence-based cessation treatments,28,29 a growing number of studies have tested the efficacy of harm reduction interventions aimed at child TSE reduction as the primary goal of smoking treatment. However, current standard TSE reduction interventions (e.g., brief healthcare provider advice) have typically shown limited effectiveness.30
A systematic review shows two limitations of previous child TSE-reduction trials, including lack of bioverified outcomes31 and limited long-term follow-up examining maintenance of TSE outcomes, shortcomings that are even more evident in studies targeting vulnerable populations and parental smokers.32,33 Low-income smokers are more likely to live in subsidized multiunit rentals, which can be susceptible to TSE sources outside the immediate residence34 and have elevated thirdhand tobacco smoke residue from previous residents.35 Moreover, low-income smokers report lower stigma around smoking behaviors than other smokers36 and may experience less social pressure to create smokefree environments for their children. Not surprisingly, brief advice, health education, and self-help strategies have limited effect on changing residential contingencies and norms affecting smoking and behavior change.
The need to improve maternal smoking and child TSE reduction interventions in vulnerable populations remains a significant public health priority.15,37 Interventions targeting these populations require more-intensive behavior change strategies. For example, skills training must address unique barriers women and low-income smokers face when attempting to modify smoking. Interventions could be enhanced by guiding maternal smokers’ efforts to shift residential smoking norms toward the unacceptability of indoor smoking, and acceptability of protecting children from TSE.38,39 Moreover, interventions that focus on maternal TSE reduction could include elements that boost smokers’ confidence in smoking behavior change that, in turn, could lead to a quit attempt.40 Thus, more-intensive interventions are warranted.
This study was designed to examine long-term, 12-month post-treatment effects of an experimental counseling intervention, Family Rules for Establishing Smokefree Homes (FRESH]), on bioverified child TSE and maternal smoking cessation. The FRESH trial targeted low-income maternal smokers interested in learning how to protect their children from TSE without the expectation of quitting smoking during treatment. Analyses of short-term, end-of-treatment (EOT) outcomes after 16 weeks of treatment demonstrated preliminary efficacy of FRESH behavioral counseling compared with standard care control treatment in reducing bioverified child exposure and promoting bioverified maternal cessation.41 The current paper presents the long-term results of the FRESH trial. The a priori hypotheses of this study were that the significant main effect of treatment observed at EOT (16 weeks) would be sustained as a linear function over time from EOT through 12-month follow-up.
METHODS
The trial was approved by the Temple University IRB (protocol #7152). Data collection and analyses occurred from 2006 to 2018. The study was a two-arm RCT. Randomization used small blocks of random length stratified by child sex, race, and recruitment site. The project biostatistician generated the allocation sequence, which was concealed from the project team. At in-home baseline, participants completed consent and structured interviewer-administered assessments, and provided a child urine sample. After the 16-week intervention period, assessment staff (blind to assignment) conducted in-home assessments at EOT, 3 months post-EOT, and 12-months post-EOT. Outcome measures included child urine cotinine (TSE biomarker), reported TSE from self and all sources, and bioverified 7-day point prevalence cessation. This report focused on post-treatment outcome assessments (EOT through two follow-ups). Greater detail of the study methods and protocols highlighted in this section can be found in the short-term outcome paper.41
Study Population
Recruitment targeted maternal smokers with young children in low-income, urban communities. Systematic recruitment methods included advertising in free newspapers, on public transit, in neighborhood stores, and in community clinics serving target communities. Eligible mothers smoked five or more cigarettes per day, and had a child aged <4 years who was exposed to at least two maternal cigarettes per day in the same room. Respondents were excluded if they were pregnant, currently diagnosed or treated for severe mental illness, or non-English speaking.
Interventions
Participants were randomized to the experimental intervention (FRESH Behavioral Counseling), or an enhanced standard care intervention (Control). Content in the two interventions were equivalent, including TSE-related health education and guidance in evidence-based, cognitive behavioral strategies for smoking behavior change emphasizing child TSE protections. All trial participants received “no smoking” signs and printed behavioral contracts with instructions to discuss treatment goals among family members and post signed contracts in the house (techniques adapted from applied behavioral analysis to facilitate adoption and adherence to newly agreed-upon residential smoking restrictions).42,43 Materials also included structured information to facilitate skills for smoking behavior change in the context of identified environmental stressors and unique barriers to behavior change. All participants received information about accessible smoking-cessation services, medications, and low- or no-cost nicotine-replacement therapy (NRT); however, the trial did not provide medication or NRT.
Between the two groups, intervention modes and processes differed. The Control group received a treatment binder with all print materials, brief advice, and overview of materials and encouragement to discuss materials with other home residents. The FRESH Counseling group received the same materials as Controls plus 16 weeks of behavioral counseling (Master’s degree–level counselors maintained ≥95% protocol fidelity.) The FRESH intervention included two in-home sessions during the first 6 weeks of treatment and up to seven phone sessions. Using an individualized treatment approach with short-term goal setting, counselors focused on mothers’ efforts toward child TSE reduction while providing social reinforcement (e.g., praise) and guidance toward incrementally more challenging smoking behavior change. For example, the first week would include initiation of stimulus control and coaching (e.g., assisting with placement of no smoking signs, problem solving barriers to protecting children) to help mothers begin to avoid smoking in self-designated smokefree zones where children play, eat, or sleep. Counselors used motivational interviewing techniques to boost motivation to modify smoking to protect children, and coaching to enhance residential social support for child TSE protection. Counselors facilitated completion of behavioral contracts with family members’ signatures designating their commitment to a smokefree home. In subsequent weeks, skills training and problem solving would continue, while counselors encouraged sequentially more ambitious short-term goals for enhancing TSE reduction (e.g., eliminating indoor smoking among residents… then TSE from visitors.) This process was intended to build mothers’ self-efficacy in smoking behavior change, including the eventual consideration of a smoking quit attempt.
Measures
Three outcome measures were obtained at EOT (16 weeks), and both 3 and 12 months post-treatment:
Child urine cotinine was the primary outcome.44 Staff followed previous study collection and storage protocols.45,46 Assays were completed using high-performance liquid chromatography–mass spectrometry.47 The lowest detectible level was 0.1 ng/mL.
Maternal-reported TSE from her own smoking and TSE from all sources were collected with interviewer-administered, timeline follow-back methods used previously.48,49 TSE was operationalized as mean cigarettes per day to which the child was exposed in the same room as a smoker during the 7 days prior to assessment.
Bioverified 7-day point prevalence quit status was confirmed with maternal saliva cotinine (high-performance liquid chromatography–mass spectrometry). Non-verified quit status as well as self-reports of “not quit” were coded as quit=0.
The independent variable was treatment assignment coded 0=Control group, 1=FRESH.
Control variables included baseline demographics, smoking- and exposure-related behaviors, home environment, and psychosocial factors. Race was recoded as binary (i.e., 1=African American, 0=non-African American). Marital status was coded as 0=single or 1=married or living with a partner. Child age was dichotomized as 0= <12 months old and 1=12–48 months old given the likelihood that infants have more frequent maternal contact exposure than toddlers. Nicotine dependence was assessed using the Fagerström Test of Nicotine Dependence (FTND).50 Other smokers living in home was dichotomized as 1=other smokers or 0=mother is the only smoker. Home smoking restrictions were coded as 1=total indoor smoking ban or 0=no to some smoking restrictions. The Center for Epidemiologic Studies Depression Scale (CES-D) was used to assess depressive symptoms.51 The sum of life events mothers reported experiencing in the 3 months prior to assessment represented stressful life events.52 Mean smoking-related weight concerns was measured with a four-item assessment with a 6-point response scale (0=not at all, 5=very much) used in previous research.53 The total number of pediatric provider visits for TSE-related illnesses (e.g., respiratory symptoms, ear infections) in the previous 3 months as well as recall of TSE health messaging from providers and other sources (e.g., media) were collected.54 Also, baseline exposure variables related to outcomes of interest (baseline cotinine, baseline maternal TSE, and baseline all source TSE) were included in their respective multivariable analyses.
Statistical Analysis
Analyses of child TSE followed an intention-to-treat approach where all randomized participants were included in the outcome analyses. Initial analyses generated descriptive statistics, QQ plots, and histograms to examine variable distributions. CES-D, life events, weight concerns, total doctor visits, and FTND scores were standardized. Two cotinine values were below 0.1 ng/mL at EOT and 12-month follow-up, respectively. To be consistent with the publication of short-term outcomes,41 the authors included the original assayed values in the data set (0.00 and 0.05). The distribution of child cotinine values was not normal; however, it did follow a log-normal distribution. For the child cotinine analysis, a log-normal (i.e., Gaussian family but a log link) mixed effects model was fit using the SAS glmmix procedure to the untransformed cotinine values. The model included a random intercept and was estimated using an MMPL (maximum marginal expansions pseudo likelihood), a pseudo likelihood method approximating maximum likelihood estimation (SAS, version 9.2). The following baseline covariates were included in the child cotinine model: child cotinine, FTND, CES-D, baseline other smokers in home, life events, support, marital status, child age, total doctor visits, and home smoking ban. In addition, the model included the time-varying covariates total household ban and other smokers in the home besides the participant. Two models of reported TSE (from self and all sources) were fit using a generalized linear mixed model with a logit link using the glmer function and the Laplace Approximation. The maternal TSE model included baseline FTND, total doctor visits, total home smoking ban, and other smokers in home as covariates. The all-source TSE model included baseline total pediatrician visits, total home smoking ban, and other smokers in home as covariates. Initially, baseline FTND was included in this latter model, but the model failed to converge so it was removed. For each outcome, the authors tested for over-dispersion and there was none. All models included a random intercept as well as effects for the intervention and time (across the three follow-up time points in months). Authors also tested for intervention X time interactions. When the interaction was not significant, this term was removed from the final model to improve parsimony and model fit. The secondary outcome, binary bioverified quit, was assessed by change in quit status using a binary generalized linear mixed model including a treatment X time interaction, a random intercept, and a logit link function. This model included standardized data from baseline assessments of weight concerns, total doctor visits, and FTND as covariates. The generalized linear mixed model was run using SPSS, version 24.
RESULTS
The previously published short-term outcome results41 included detailed demographic baseline data showing no between-group differences in baseline demographic, smoking, or exposure characteristics except that there were significantly more single mothers in the Control group (86.9%) than in Counseling (76.1%). The sample included 87.3% African Americans, 11.7% Caucasians, and 1.0% Native American or Asian, with 8.5% identifying as Latina. More than two thirds of the sample was unemployed and single, and nearly 60% completed at least a high school education. The sample reported mean CES-D scores >19 and an average of approximately nine current stressful life events, suggesting elevated distress.
Baseline smoking and exposure characteristics suggest that mothers were moderate-to-heavy smokers for this target population (a daily average of 12 cigarettes with a mean FTND just below 5.0). Mothers reported that their target child was exposed to a daily average of approximately five of her cigarettes in addition to approximately nine cigarettes per day from other sources.
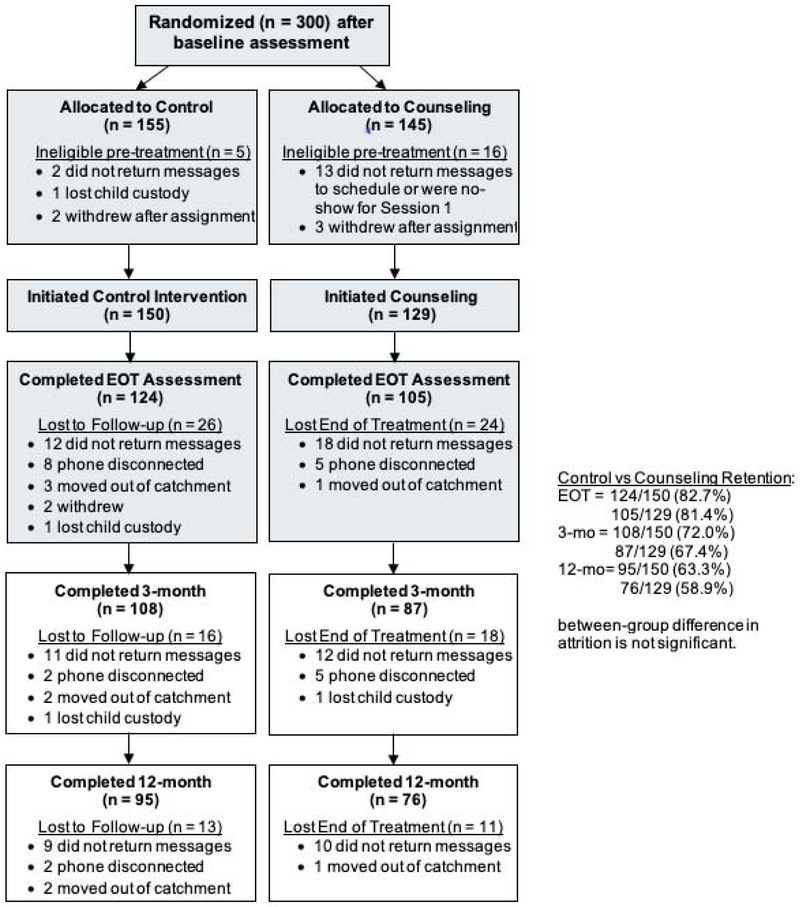
Half of the sample lived with another smoker, and nearly half of the sample reported living in a home with at least some indoor smoking restrictions (7% reported having a total indoor smoking ban). Children in the sample showed high levels of baseline cotinine [(arithmetic mean=36.23 ng/mL, SD=121.73 ng/mL; geometric mean=16.22 ng/mL, SE=7.08 ng/mL)], characteristic of young children living in low-income, urban environments and within homes that are likely to have high degrees of thirdhand tobacco smoke contamination. Figure 1 shows participant flow from randomization. Enrollment ended when the goal of 300 participants were randomized. Retention from EOT through follow-up was similar in both groups. Such retention is consistent with clinical trials research with similar low-income, highly distressed populations. There were no reported adverse events during the trial.
Figure 1.
Participant flow through follow-up assessments.
Note: Shaded participants flow previously published in the American Journal of Preventive Medicine.41
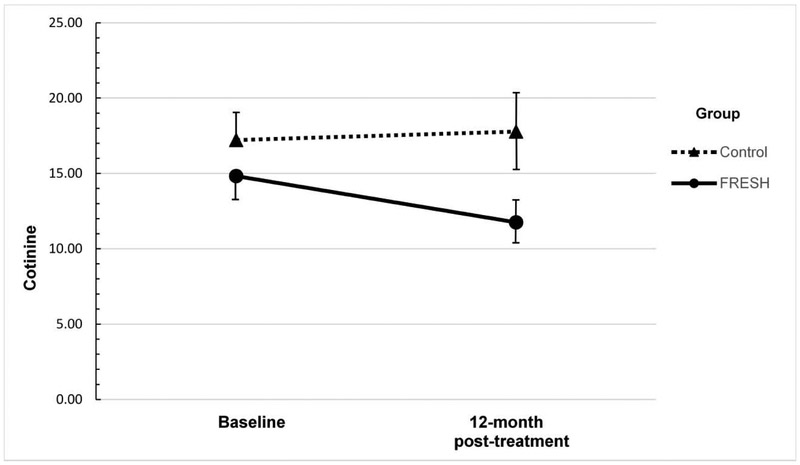
Results of the repeated measures analysis of child cotinine appear in Table 1. There was a statistically significant effect of intervention on cotinine levels (β= −0.31, SE=0.13, p=0.01): Controlling for baseline cotinine and other covariates, participants in the FRESH Counseling group had children with lower cotinine levels from EOT assessment through 12-month follow-up than Controls. There was also a significant effect of months reflecting a decrease in the entire sample’s cotinine levels from EOT through follow-up (β= −0.03, SE=0.01, p=0.003). Testing for an intervention X month interaction showed a non-significant interaction term that did not contribute to the model. Therefore, the final model does not include an interaction term. Three covariates also had a significant effect on child cotinine over time. These terms included baseline child cotinine level as well as the time-varying covariates capturing other smokers in the home, and whether homes had an indoor smoking ban. Figure 2 used back-transformed geometric means to illustrate the change in child cotinine by group from baseline to 12-month follow-up. There was a statistically significant effect of the intervention on child TSE from maternal smoking (β= −1.48, SE=0.45, p=0.001): Children in FRESH were less likely to be exposed to tobacco smoke from mom than Control children. There was no effect of time and no time X treatment interaction. Thus, the treatment effect was maintained over time. Among the covariates, there were statistically significant effects of FTND and total doctor visits. The analysis of factors affecting TSE from all sources showed no effect of treatment or time. Only the number of pediatrician visits predicted exposure in the “all sources of exposure” model (Table 2).
Table 1.
Factors Affecting Child Cotinine Through Follow-up
| Predictor | Beta | SE |
|---|---|---|
| Treatment group (1=counseling) | −0.31** | 0.13 |
| Time (in months) | −0.03** | 0.01 |
| Baseline cotinine | 0.002*** | 0.001 |
| Marital status (1=yes) | −0.13 | 0.16 |
| Child age (1= <12 months old) | 0.16 | 0.13 |
| Baseline FTND (standardized) | 0.06 | 0.06 |
| Baseline CESD (standardized) | −0.05 | 0.07 |
| Baseline total TSE health messages received | −0.02 | 0.06 |
| Baseline stressful events (standardized) | 0.05 | 0.06 |
| Baseline social support (standardized) | −0.11 | 0.07 |
| Baseline other smokers in home | 0.55*** | 0.13 |
| Time varying other smokers in homea | 0.08 | 0.13 |
| Baseline home smoking ban | −0.50 | 0.24 |
| Time varying home smoking bana | −0.54*** | 0.13 |
Note: Boldface indicates statistical significance (**p<0.01; ***p<0.001).
Time varying covariates are calculated as a vector of the same baseline variable at end of treatment, 3- and 12-month follow-ups.
FTND, Fagerstrom Test for Nicotine Dependence; CESD, Center for Epidemiologic Studies Depression Scale; TSE, Tobacco Smoke Exposure.
Figure 2.
Geometric mean child cotinine between groups.
FRESH, Family Rules for Establishing Smokefree Homes.
Table 2.
Factors Affecting Reported Child Tobacco Smoke Exposure
| Predictor | Exposure from mom | Exposure - all sources |
|---|---|---|
| Beta (SE) | Beta (SE) | |
| Treatment group (1=counseling) | −1.48*** (0.45) | −0.87 (0.53) |
| Time (in months) | 0.02 (0.02) | 0.02 (0.03) |
| Baseline TSEa | 0.04 (0.06) | 0.02 (0.03) |
| Baseline other smokers in home | 0.33 (0.43) | 0.87 (0.54) |
| Baseline home smoking ban | −1.14 (0.83) | −0.37 (1.01) |
| Baseline total pediatrics visits (standardized) | −0.58* (0.23) | −0.60* (0.29) |
| Baseline FTND (standardized) | 0.46* (0.23) | 0.32 (0.27) |
Note: Boldface indicates statistical significance (*p<0.05; **p<0.01).
Baseline TSE=exposure from mom in left column; from all sources in right column. TSE, Tobacco Smoke Exposure; FTND, Fagerstrom Test for Nicotine Dependence.
There was a large, significant effect of FRESH treatment on repeated measures of bioverified abstinence controlling for baseline nicotine dependence, number of pediatrician visits, and weight concerns. Specifically, participants in FRESH had significantly greater odds of being quit compared with the Control group (OR=8.87, 95% CI=2.33, 33.75). The largest difference in bioverified quit status between FRESH Counseling and Control groups was at EOT (13.8% vs 1.9%). There was neither a significant main effect of time nor a significant time X intervention interaction. Thus, the FRESH treatment effect on quit status was maintained through 12 months post-treatment (12.3% vs 6.1%) even though the control group demonstrated an increase in bioverified quits during the post-treatment period.
DISCUSSION
These results support the primary hypothesis that the effects of FRESH behavioral counseling on 16-week, short-term child TSE reduction would be sustained through the 12-month, post-treatment period. This study is among the first to show with a vulnerable sample of maternal smokers that, compared with an active standard care control intervention, behavioral counseling led to long-term, post-treatment reductions in bioverified child TSE. The effect of FRESH Counseling on reported child TSE from maternal smoking was consistent with the child cotinine results, further supporting the hypothesis that FRESH counseling would boost mothers’ TSE protection efforts relative to the Control condition. The significant between-group difference in smoking quit rates supported the secondary hypothesis that the short-term differences in quit rates at EOT would be sustained through 12 months post treatment. Thus, it appears that focusing vulnerable maternal smokers’ efforts on reducing their child’s TSE not only helps mothers learn how to protect their children from exposure, but also may facilitate their subsequent effort to quit smoking.
The effect of FRESH counseling on long-term maintenance of child TSE reduction is indeed notable and highly encouraging, particularly given its potential to reduce tobacco-related disparities among underserved smokers. Nevertheless, the 12-month mean and range of cotinine values suggests an opportunity for improving the magnitude of the long-term, post-treatment effect. Study results point to areas future interventions could address. First, nicotine use is a chronic relapsing disorder. Thus, providing ongoing treatment contact would be appropriate, given that effective treatments of other chronic conditions (e.g., diabetes) are typically not withdrawn. Also, there are additional strategies, such as monetary contingencies based on behavioral economics that could augment the FRESH intervention strategies designed to shape social contingencies and family-level norms around child TSE protections. Alternatively, the null effect of treatment on TSE from all sources suggests that expanded strategies are needed to help expand mothers’ skills with child TSE protections beyond their homes and their own smoking. Even though many FRESH intervention mothers may have successfully overcome obstacles to TSE reduction related to the residential-level social milieu, reducing all sources of TSE is even more challenging. Thus, extending FRESH treatment beyond 16 weeks could foster greater skills development to enable mothers to tackle a wider array of TSE-related contexts. Additionally, intervention intensity could be increased with multilevel interventions designed to create synergistic effects (e.g., integrating smokefree public housing policies and TSE messaging) with residential-level skills training and support for families with smokers to create smokefree homes and neighborhoods). Recent studies55-57 have begun to test multilevel interventions that could potentiate efficacy of TSE reduction counseling by adding advice and support from trusted providers (e.g., primary care physicians, community health workers). A trusted healthcare provider’s advice could increase motivation to initiate and maintain engagement in more-intensive treatment components (e.g., counseling) that are necessary to promote skills for smoking behavior change.
The sustained effect of FRESH counseling on long-term bioverified quit status was another highlight of the results for a number of reasons. First, eligible participants were invited to enroll in the trial even if they were not seeking cessation treatment or planning to quit smoking at enrollment—they could simply focus on a treatment goal of creating a smokefree home and protecting their child from TSE. It is remarkable to see this effect in a non–cessation treatment seeking population known to have greater challenges to quitting smoking than the general population of smokers. Also, NRT was not provided in this trial, and few participants reported obtaining it from outside sources. The results suggest that mothers’ TSE reduction efforts may facilitate smoking cessation. Offering NRT and withdrawal medications when mothers consider quitting would likely enhance the magnitude of the treatment effect beyond what was observed in this trial. Given that quit attempts would eliminate TSE from maternal smoking, improving treatment effects on cessation would also improve TSE outcomes.
Limitations
Targeting a specific vulnerable population for enrollment in this trial limits generalizability of results beyond this population. Similarly, this study’s target population experiences elevated concerns about biomarker assessment as well as psychosocial challenges and nuisance barriers to long-term repeated measures assessment compared with broader populations. Although there was no evidence of between-group differences in retention, the conservative analytic plan (e.g., coding missing quit status as “not quit”) attempted to address potential selection effects. Given that this high-risk population consistently demonstrates low response to standard smoking-cessation intervention, any trial that demonstrates associations between intervention and outcomes in vulnerable populations still provides valuable information for future research and practice, even when considering attrition.58 Also, the FRESH intervention was relatively intensive. Because intensive behavioral counseling requires more resources than the provision of self-help materials and brief advice, critics often argue that more-intensive behavioral smoking interventions are not sustainable. However, more-intensive behavioral intervention may be necessary to promote smoking behavior change in the target population59,60 to compete with the oversaturation of industry advertising and other corporate efforts that contribute to the elevated smoking rates in underserved communities. Moreover, there are other public health programs that include intensive behavioral intervention with home visits. For example, the Nurse–Family Partnership includes 30 home visits with low-income parents to address a spectrum of child health and developmental issues. Early intervention and prevention efforts of the Nurse–Family Partnership reduce costs associated with future utilization of government subsidized health services.61 By comparison, the FRESH approach seems appropriate and potentially valuable when comparing the alternative long-term consequences of TSE and maternal smoking.
Future behavioral theory–driven studies could test social and behavioral mechanisms that facilitate both TSE reduction and maternal cessation. FRESH counseling was designed to boost incremental behavioral, social, and environmental changes by enhancing self-control skills in smoking contexts, social support, and skills to shape home-level child TSE protection, and problem solving around challenges with children’s TSE harm reduction. Perhaps these efforts boosted smokers’ self-efficacy with smoking behavior change, shaped family-level social norms that maintain TSE reduction, and enhanced mothers’ motivation to quit smoking. Similarly, understanding more about potential moderators of smoking behavior change, such as baseline levels of nicotine dependence, could also inform future optimization strategies that could further improve smoking outcomes.
CONCLUSIONS
The FRESH RCT demonstrated long-term, 12-month post-intervention effects of behavioral counseling on bioverified TSE and cessation outcomes in a vulnerable population of maternal smokers. This is one of the first studies to show both long-term, sustained TSE reduction and cessation effects. Compared with brief advice and self-help materials, a more-intensive counseling intervention emphasizing protection of children from TSE can promote reductions in child exposure and facilitate mothers to smoking-cessation success. As vulnerable populations continue to be subject to more-intensive industry exposure to tobacco products and demonstrate less success in standard smoking interventions, the outcomes of this trial suggest high potential value in investing in more-comprehensive smoking interventions to promote TSE protection and smoking cessation. Future research testing optimization strategies, multilevel intervention, and quitline implementation would build on these findings.
ACKNOWLEDGMENTS
This study was funded by grants from the NIH, National Cancer Institute awarded to Collins (CA105183, CA93756) that supported all operations related to the trial and from Temple University indirect cost recovery funds to Collins that enabled the final waves of cotinine assays. The sponsors had no role in study design, data collection, analysis or interpretation, or writing the manuscript.
The investigators thank the many research assistants and health counselors that worked on this trial.
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults — United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311 (2):183–192. 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 3.HHS. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: HHS; 2006. [PubMed] [Google Scholar]
- 4.HHS. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Atlanta, GA: HHS; 2014. www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf. Accessed January 3, 2019. [Google Scholar]
- 5.Matt GE, Quintana PJE, Destaillats H, et al. Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect. 2011;119(9):1218–1226. 10.1289/ehp.1103500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob P, Benowitz NL, Destaillats H, et al. Thirdhand smoke: new evidence, challenges, and future directions. Chem Res Toxicol. 2017;30(1):270–294. 10.1021/acs.chemrestox.6b00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Max W, Sung H-Y, Shi Y. Deaths from secondhand smoke exposure in the United States: economic implications. Am J Public Health. 2012;102(11):2173–2180. 10.2105/ajph.2012.300805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Öberg M, Woodward A, Jaakkola MS, Peruga A, Prüss-Ustün A. Global estimate of the burden of disease from second-hand smoke. Geneva, Switzerland: World Health Organization Press; 2010. [Google Scholar]
- 9.Homa DM, Neff LJ, King BA, et al. Vital signs: disparities in nonsmokers’ exposure to secondhand smoke--United States, 1999–2012. MMWR Morb Mortal Wkly Rep. 2015;64(4):103–108. [PMC free article] [PubMed] [Google Scholar]
- 10.Metsios GS, Flouris AD, Angioi M, Koutedakis Y. Passive smoking and the development of cardiovascular disease in children: a systematic review. Cardiol Res Pract. 2011;2011:587650 10.4061/2011/587650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC, Smoking and Tobacco Use, Fact Sheet. Smoke-Free Policies Reduce Secondhand Smoke Exposure.www.cdc.gov/tobacco/data_statistics/fact_sheets/secondhand_smoke/protection/shs_exposure/index.htm. Published 2018. Accessed August 26, 2019. [Google Scholar]
- 12.McNabola A, Gill LW. The control of environmental tobacco smoke: a policy review. Int J Environ Res Public Health. 2009;6(2):741–758. 10.3390/ijerph6020741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fichtenberg CM, Glantz SA. Effect of smoke-free workplaces on smoking behaviour: systematic review. 2002;325:188 10.1136/bmj.325.7357.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamal A, Phillips E, Gentzke AS, et al. Current cigarette smoking among adults — United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(2): 53–59. 10.15585/mmwr.mm6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stiby AI, Macleod J, Hickman M, Yip VL, Timpson NJ, Munafò MR. Association of maternal smoking with child cotinine levels. Nicotine Tob Res. 2013; 15(12):2029–2036. 10.1093/ntr/ntt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvis MJ, Russell MA, Feyerabend C, et al. Passive exposure to tobacco smoke: saliva cotinine concentrations in a representative population sample of non-smoking schoolchildren. Br Med J (Clin Res Ed). 1985;291(6500):927–929. 10.1136/bmj.291.6500.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh GK, Siahpush M, Kogan MD. Disparities in children’s exposure to environmental tobacco smoke in the United States, 2007. Pediatrics. 2010;126(1):4–13. 10.1542/peds.2009-2744. [DOI] [PubMed] [Google Scholar]
- 18.Max W, Sung H-Y, Shi Y. Who is exposed to secondhand smoke? Self-reported and serum cotinine measured exposure in the U.S., 1999–2006. Int J Environ Res Public Health. 2009;6(5): 1633–1648. 10.3390/ijerph6051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nademin ME, Napolitano MA, Xanthopoulos MS, Fava JL, Richardson E, Marcus B. Smoking cessation in college-aged women: a qualitative analysis of factors important to this population. Addict Res Theory. 2010;18(6):649–666. 10.3109/16066351003660601. [DOI] [Google Scholar]
- 20.Collins BN, Nair US. Women and smoking In: Spiers MV, Geller PA, Kloss JD, eds. Women’s Health Psychology. New York, NY: Wiley; 2013:123–148. [Google Scholar]
- 21.Napolitano MA, Lloyd-Richardson EE, Fava JL, Marcus BH. Targeting body image schema for smoking cessation among college females: rationale, program description, and pilot study results. Behav Modif. 2011;35(4):323–346. 10.1177/0145445511404840. [DOI] [PubMed] [Google Scholar]
- 22.Munafò MR, Clark TG, Johnstone EC, Murphy MFG, Walton RT. The genetic basis for smoking behavior: a systematic review and meta-analysis. Nicotine Tob Res.2004;6(4):583–597. 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- 23.Saladin ME, Gray KM, Carpenter MJ, LaRowe SD, DeSantis SM, Upadhyaya HP. Gender differences in craving and cue reactivity to smoking and negative affect/stress cues. Am J Addict. 2012;21(3):210–220. 10.1111/j.1521-0391.2012.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM, CDC. Trends in smoking before, during, and after pregnancy - Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. MMWR Surveill Summ. 2009;58(4):1–29. [PubMed] [Google Scholar]
- 25.Melvin C, Gaffney C. Treating nicotine use and dependence of pregnant and parenting smokers: an update. Nicotine Tob Res. 2004;6(suppl 2):S107–S124. 10.1080/14622200410001669231. [DOI] [PubMed] [Google Scholar]
- 26.Kahn RS, Certain L, Whitaker RC. A reexamination of smoking before, during, and after pregnancy. Am J Public Health. 2002;92(11): 1801–1808. 10.2105/ajph.92.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiscock R, Bauld L, Amos A, Fidler JA, Munafo M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci. 2012;1248(1): 107–123. 10.1111/j.1749-6632.2011.06202.x. [DOI] [PubMed] [Google Scholar]
- 28.Kulak JA, Cornelius ME, Fong GT, Giovino GA. Differences in quit attempts and cigarette smoking abstinence between whites and African Americans in the United States: literature review and results from the International Tobacco Control US Survey. Nicotine Tob Res. 2016;18(suppl 1):S79–S87. 10.1093/ntr/ntv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu SS, Kodl MM, Joseph AM, et al. Racial/ethnic disparities in the use of nicotine replacement therapy and quit ratios in lifetime smokers ages 25 to 44 years. Cancer Epidemiol Biomarkers Prev. 2008; 17(7): 1640–1647. 10.1158/1055-9965.epi-07-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JF, Monroe AD. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics. 2001;108(l):18–24. 10.1542/peds.108.1.18. [DOI] [PubMed] [Google Scholar]
- 31.Baxi R, Sharma M, Roseby R, et al. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane Database Syst Rev. 2014;(0):CD001746 10.1002/14651858.CD001746.pub3. [DOI] [PubMed] [Google Scholar]
- 32.Rosen F, Myers V, Winickoff J, Kott J. Effectiveness of interventions to reduce tobacco smoke pollution in homes: a systematic review and meta-analysis. Int J Environ Res Public Health. 2015;12(12):16043–16059. 10.3390/ijerph121215038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen LJ, Noach MB, Winickoff JP, Hovell MF. Parental smoking cessation to protect young children: a systematic review and meta-analysis. Pediatrics. 2012;129(1):141–152. 10.1542/peds.2010-3209. [DOI] [PubMed] [Google Scholar]
- 34.King BA, Babb SD, Tynan MA, Gerzoff RB. National and state estimates of secondhand smoke infiltration among U.S. multiunit housing residents. Nicotine Tob Res. 2013; 15(7): 1316–1321. 10.1093/ntr/nts254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Northrup TF, Jacob P, Benowitz NL, et al. Thirdhand smoke: state of the science and a call for policy expansion. Public Health Rep. 2016;131(2):233–238. 10.1177/003335491613100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stuber J, Galea S, Link BG. Smoking and the emergence of a stigmatized social status. Soc Sci Med. 2008;67(3):420–430. https://doi.Org/10.1016/j.socscimed.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mbulo L, Palipudi KM, Andes L, et al. Secondhand smoke exposure at home among one billion children in 21 countries: findings from the Global Adult Tobacco Survey (GATS). Tob Control. 2016;25(e2):e95–e100. 10.1136/tobaccocontrol-2015-052693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alamar B, Glantz SA. Effect of increased social unacceptability of cigarette smoking on reduction in cigarette consumption. Am J Public Health. 2006;96(8):1359–1363. 10.2105/ajph.2005.069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hovell MF, Hughes SC. The behavioral ecology of secondhand smoke exposure: a pathway to complete tobacco control. Nicotine Tob Res. 2009; 11(11):1254–1264. 10.1093/ntr/ntp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosen LJ, Myers V, Hovell M, Zucker D, Noach MB. Meta-analysis of parental protection of children from tobacco smoke exposure. Pediatrics. 2014;133(4):698–714. 10.1542/peds.2013-0958. [DOI] [PubMed] [Google Scholar]
- 41.Collins BN, Nair US, Hovell MF, et al. Reducing underserved children’s exposure to tobacco smoke: a randomized counseling trial with maternal smokers in underserved communities: effective strategies to reduce young children’s SHS exposure. Am J Prev Med. 2015;49(4): 534–544. 10.1016/j.amepre.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008;27(3):379–387. https://doi.Org/10.1037/0278-6133.27.3.379. [DOI] [PubMed] [Google Scholar]
- 43.Sayegh CS, Huey SJ, Zara EJ, Jhaveri K. Follow-up treatment effects of contingency management and motivational interviewing on substance use: a meta-analysis. Psychol Addict Behav. 2017;31(4):403–414. 10.1037/adb0000277. [DOI] [PubMed] [Google Scholar]
- 44.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996; 18(2):188–204. 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 45.Hovell MF, Zakarian JM, Matt GE, Hofstetter CR, Bernert JT, Pirkle J. Effect of counselling mothers on their children’s exposure to environmental tobacco smoke: randomised controlled trial. BMJ. 2000;321:337 10.1136/bmj.321.7257.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niedbala RS, Haley N, Kardos S, Kardos K. Automated homogeneous immunoassay analysis of cotinine in urine. J Anal Toxicol. 2002;26(3): 166–170. https://doi.Org/10.1093/jat/26.3.166. [DOI] [PubMed] [Google Scholar]
- 47.Bernert JT, McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers*. J Anal Toxicol. 2000;24(5):333–339. https://doi.Org/10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- 48.Matt GE, Hovell MF, Zakarian JM, Bernert JT, Pirkle JL, Hammond SK. Measuring secondhand smoke exposure in babies: the reliability and validity of mother reports in a sample of low-income families. Health Psychol. 2000;19(3):232–241. https://doi.Org/10.1037//0278-6133.19.3.232. [DOI] [PubMed] [Google Scholar]
- 49.Matt GE, Wahlgren DR, Hovell MF, et al. Measuring environmental tobacco smoke exposure in infants and young children through urine cotinine and memory-based parental reports: empirical findings and discussion. Tob Control. 1999;8(3):282–289. 10.1136/tc.8.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9): 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 51.Radloff LS. A CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. 10.1177/014662167700100306. [DOI] [Google Scholar]
- 52.Collins B, Nair U, Shwarz M, Jaffe K, Winickoff J. SHS-related pediatric sick visits are linked to maternal depressive symptoms among low-income African American smokers: opportunity for intervention in pediatrics. J Child Fam Stud. 2013;22(7):1013–1021. 10.1007/s10826-012-9663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collins B, Nair U, Hovell MH, Audrain-McGovern J. Smoking-related weight concerns among underserved, black maternal smokers. Am J Health Behav. 2009;33(6):699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lavery A, Nair US, Bass S, Collins BN. The influence of health messaging source and frequency on maternal smoking and child exposure among low-income mothers. J Commun Healthc. 2016;9(3):200–209. 10.1080/17538068.2016.1231858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collins BN, Lepore SJ, Winickoff JP, et al. An office-initiated multilevel intervention for tobacco smoke exposure: a randomized trial. Pediatrics. 2018;141(suppl 1):S75–S86. 10.1542/peds.2017-1026K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collins BN, Lepore SJ. Babies Living Safe & Smokefree: randomized controlled trial of a multilevel multimodal behavioral intervention to reduce low-income children’s tobacco smoke exposure. BMC Public Health. 2017;17:249 10.1186/s12889-017-4145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lepore SJ, Collins BN, Coffman DL, et al. Kids Safe and Smokefree (KiSS) multilevel intervention to reduce child tobacco smoke exposure: long-term results of a randomized controlled trial. Int J Environ Res Public Health. 2018; 15(6): 1239 10.3390/ijerph15061239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gustavson K, von Soest T, Karevold E, Røysamb E. Attrition and generalizability in longitudinal studies: findings from a 15-year population-based study and a Monte Carlo simulation study. BMC Public Health. 2012;12:918 10.1186/1471-2458-12-918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cokkinides VE, Ward E, Jemal A, Thun MJ. Under-use of smoking-cessation treatments. Am J Prev Med. 2005;28(1):119–122. https://doi.Org/10.1016/j.amepre.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 60.Burns EK, Deaton EA, Levinson AH. Rates and reasons: disparities in low intentions to use a state smoking cessation quitline. Am J Health Promot. 2011;25(5 suppl):S59–S65. 10.4278/ajhp.100611-quan-183. [DOI] [PubMed] [Google Scholar]
- 61.Olds DL. The nurse–family partnership: an evidence-based preventive intervention. Infant Ment Health J. 2006;27(1):5–25. 10.1002/imhj.20077. [DOI] [PubMed] [Google Scholar]