Abstract
Background/Aims
There is insufficient quality data to recommend the use of herbs for the treatment of acute bronchitis. Small number of randomized trials of plant extracts for this purpose were determined to be low quality and there are concerns for the safety. HL301 is a combined product of seven medicinal plants. In the present study, we tried to evaluate the efficacy and safety of HL301 for the treatment of acute bronchitis with a randomized, double-blind, placebo-controlled, multicenter trial design.
Methods
A total of 166 patients with acute bronchitis were randomized to receive placebo or HL301 (600 mg/day) for 7 days. The primary endpoint was change in bronchitis severity score (BSS) from baseline visit (visit 2) to the end of treatment (visit 3). Other efficacy variables were the change of each component of the BSS (cough, sputum, dyspnea, chest pain, and crackle) with treatment, response rate, improvement rate, satisfaction rate and number of rescue medications taken.
Results
Changes in the BSS from visit 2 to visit 3 were higher in the HL301 group than in the placebo group both in the full analysis set (4.57 ± 1.82 vs. 3.15 ± 3.08, p < 0.01) and in the per protocol set (4.62 ± 1.81 vs. 3.30 ± 3.03, p < 0.01). Four BSS components (cough, sputum, dyspnea, and chest pain) improved more with HL301 treatment than with placebo treatment. Participants treated with HL301 showed higher response, improvement, and satisfaction rates and less use of rescue medication than the placebo group.
Conclusions
HL301 (600 mg/day) was effective and safe for symptomatic treatment of acute bronchitis.
Keywords: HL301, Acute bronchitis, Bronchitis severity score, Herb
INTRODUCTION
The efficacy and safety of herbs for symptomatic treatment of bronchitis is not well established due to insufficiency of qualified data. In a previous study, we reported the efficacy and safety of HL301, a product containing seven herbs for the treatment of acute bronchitis (AB) and acute exacerbation of chronic bronchitis (AECB) [1]. During the review of the manuscript and in the process of reporting the results of the study to the Korean Food and Drug Administration, we received a common comment concerning the combined grouping of patients with AB and AECB. As is well known, AB and AECB are very different diseases in causative pathogens, treatment modalities, and outcomes. AB is usually caused by viral infection [2]. Common clinical symptoms are cough, with or without the production of sputum, which lasts from several days to weeks. It is typically self-limited, resolving within 1 to 3 weeks. Treatment of AB is focused on supportive care and antibiotics are generally not necessary for the great majority of patients [3]. On the other hand, 70% to 80% of acute exacerbations of chronic obstructive pulmonary disease (AE COPD) develop due to respiratory infections and bacterial infections trigger one-third to one-half of COPD exacerbations [4]. Systemic corticosteroids and antibiotics play important roles in the treatment of moderate or severe AE COPD [5]. Although we enrolled patients with a mild form of AE COPD in the previous study, duration of symptoms and clinical responses rate must have been different between AB and AE COPD. On considering the pitfall of the previous study, we decided to evaluate the efficacy and safety of HL301 solely focusing on patients with AB.
In the first investigation of HL301 on humans, we evaluated a broad range of doses from 600 to 1,800 mg/day. In this new trial, which was designed as a phase 2b study, we were able to focus on the most effective, minimum dose of 600 mg/day. In addition, we strictly selected patients who had developed symptoms of AB very recently. Therefore, we only enrolled the patients who had developed symptoms of AB within 48 hours of their baseline visit.
METHODS
Study design
This was a phase 2b, randomized, double-blind, placebo-controlled, multicenter (seven university-affiliated hospitals of South Korea) study to investigate the efficacy and safety of HL301 compared with a placebo in patients with AB. This study consisted of a screening visit (visit 1), baseline visit (visit 2), a 7-day treatment period, and an end of treatment visit (visit 3). Visit 1 and 2 were performed on the same day or separated by as long as 3 days. Visit 3 was scheduled to occur 7 to 10 days after the second visit. This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was approved by the Institutional Review Board at each center (1812-003-311). Written informed consent was obtained from eligible patients who voluntarily agreed to participate in the trial. This trial was registered at ClinicalTrials.gov (NCT03309800).
Patient population
In the present study, we recruited patients with AB. AB was defined as the development of a cough, sputum, or related symptoms within 2 days of the baseline visit (visit 2). Major eligibility criteria were (1) male or female between 19 to 80 years of age and (2) total bronchitis severity score (BSS) of five points or more at the screening visit (visit 1) and baseline visit (visit 2) due to AB. The BSS was based on the sum of severity ratings of 0 (absent), 1 (mild), 2 (moderate), 3 (severe), or 4 (very severe) for the five most important features associated with bronchitis, namely, cough, sputum, dyspnea, chest pain, and crackle, (3) symptoms of AB that developed within 48 hours of the baseline visit.
The major exclusion criteria were patients (1) with respiratory or systemic diseases that required treatment with systemic antibiotics, (2) who had taken systemic corticosteroids or immunosuppressants within 4 weeks of the baseline visit, (3) who had taken antibiotics, anti-viral agents, angiotensin converting enzyme inhibitors, or inhaled corticosteroids within two weeks of the baseline visit, (4) who had been prescribed beta-agonists, anti-cholinergics, methylxanthines, antihistamines, anti-tussive, mucolytics, or herbs with anti-tussive/mucolytic effects within 2 days of the baseline visit, (5) who had a history of drug abuse, (6) who had impaired renal (creatinine clearance less than 30 mL/min) or hepatic function (liver enzymes over three times of the upper normal value), and (7) who were smokers of over 15 cigarettes/day.
Eligible patients were allocated randomly either to HL301 600 mg/day (300 mg twice a day) or to placebo by 1:1 ratio. Random number was generated with predetermined block size (either 4 or 6) by the use of SAS version 9.4 program (SAS Institute Inc., Cary, NC, USA). Random number was labeled onto box surface of investigative product in sequence. The list of random number sequence was delivered to each participating institute. On the day of baseline visit, enrolled participant was assigned with random number according to the list of sequence. Attending pharmacist delivered the designated investigative product to the participant with the same random number.
Study drug and rescue medication
The study medication (HL301) is a drug modified from Chung-Sang-Bo-Ha-Tang (CSBHT), which has been used to treat chronic pulmonary diseases in Korea for centuries. HL301 is consisted of seven species of medicinal plants, the root of Rehmannia glutinosa, the cortex of Paeonia suffruticosa, the fruit of Schizandra chinensis, the root of Asparagus cochinchinensis, seeds of Prunus armeniaca, the root of Scutellaria baicalensis, and the root of Stemona sessilifolia with a dry weight ratio of 8:4:4:4:3:3:2 [6,7]. The placebo had same outlook and weight with the investigative product, which enabled double-blinding of the present study both to investigators and participants. Investigational products (HL301 and placebo) were provided by Hanlim Pharm. Co. Ltd., Seoul, Republic of Korea.
Acetaminophen 650 mg was allowed as a rescue medicine when necessary (fever more than 39°C or unbearable pain) with a limit of 6 tablets/day and 18 tablets/week. Except acetaminophen, the intake of other analgesics, antihistamines, beta-agonists, anti-cholinergics, central nervous system stimulants, anti-tussives, mucolytics, or herbs containing any of seven components of HL301 was not allowed during the whole study period. Other medications that participants have been taking before the enrollment were allowed to take as long as attending physicians decided they would not affect the symptoms of AB.
Outcome assessment
The primary outcome of efficacy was change in BSS between visit 2 and visit 3 (BSS at visit 2 to BSS at visit 3). Other efficacy variables were the difference in each component of the BSS (cough, sputum, dyspnea, chest pain, and crackle) between visit 2 and visit 3, response rate (the percentage of participants whose BSS was below 3 points at visit 3 or whose BSS at visit 2 decreased by more than 7 points at visit 3), improvement rate (the percentile of participants whose response to treatment was rated as ‘complete recovery’ or ‘major improvement’ by investigators at visit 3), participant satisfaction (at visit 3, the participants’ response toward the investigative products was classified into five categories: ‘very dissatisfied,’ ‘dissatisfied,’ ‘neutral,’ ‘satisfied,’ and ‘very satisfied’), and tablets taken as rescue medication during the treatment period. The safety outcome criteria consisted of the number, type and severity of adverse events (AEs).
Sample size and statistics
The average decrease in BSS from baseline was expected to be 3.57 ± 2.85 points with placebo and 4.63 ± 2.33 points with active treatment based on the previous study [1]. Minimally important difference of BSS change from visit 3 to visit 2 was not designated. A target population of 168 patients (84 per group) was estimated to provide ≥ 80% power with a two-tailed level of significance of 5% taking into account a dropout rate of 10%.
Efficacy was analyzed for both the full analysis set (FAS) and per protocol set (PPS). FAS was defined as all randomized patients who took at least one dose of study medication and were available for at least one measure of efficacy evaluation. The PPS consisted of participants who fulfilled all visit schedules with a drug compliance ≥ 70%. Safety results were summarized descriptively for all randomized patients who took at least one dose of study medication. The last-observation-carried-forward (LOCF) method was not used for missing data in the present study.
Continuous variables are presented as the mean ± standard deviation. Categorical data were presented as number (%). Intergroup comparison of demographics and safety variables was performed by t test or Wilcoxon rank sum test. Intergroup comparison of categorical data was performed using the chi-square test or Fisher’s exact test. Intragroup analysis between baseline (visit 2) and end of treatment (visit 3) was done with a paired t test or Wilcoxon’s signed rank test.
RESULTS
Participants enrollment
A total of 167 patients were screened for participation in the study. After exclusion of one participant by the exclusion criteria, 166 were randomized. Five participants from the placebo group (two for withdrawal of consent, one by exclusion criteria, one for poor compliance to drug, and one for an error in randomization) and two participants from the HL301 group (due to withdrawal of consent) were excluded from the FAS (Fig. 1).
Figure 1.

Flow chart of participant enrollment. Targeted population was 168 patients (84 per group) taking into account of 10% dropout rate. FAS, full analysis set; PPS, per protocol set.
Characteristics of participants
Of the 166 participants in the FAS, 92 (55.4%) were male and 74 (44.6%) were female. There was no significant difference in median age or comorbidities between the two groups at the baseline visit (Table 1). Compliance to the investigative product was over 97% both in the HL301 group and in the placebo group.
Table 1.
Baseline characteristics of participants
| Variable | Placebo | HL301 | All | p value |
|---|---|---|---|---|
| Number (FAS) | 85 | 81 | 166 | |
| Sex, male:female | 45:40 | 47:34 | 92:74 | 0.51 |
| Age, yr | 38.53 ± 10.93 | 35.99 ± 11.71 | 37.29 ± 11.35 | 0.15 |
| Heavy smokera | 0 | 0 | 0 | NA |
| Comorbidities | 32 (37.65) | 25 (30.86) | 57 (34.34) | 0.35 |
| Respiratory | 10 | 7 | 17 | |
| Infection | 6 | 10 | 16 | |
| Metabolic | 5 | 9 | 14 | |
| Musculoskeletal | 7 | 4 | 11 | |
| Gastrointestinal | 7 | 3 | 10 | |
| Vascular | 3 | 7 | 10 | |
| Dermatologic | 5 | 4 | 9 | |
| Others | 24 | 14 | 38 |
Values are presented as mean ± SD or number (%).
FAS, full analysis set; NA, not available.
Those who smoke > 15 cigarettes/day.
Outcomes
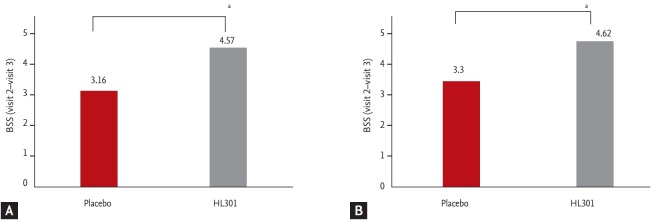
The primary outcome, the change in BSS between visit 2 and visit 3 was higher in the HL301 group than in the placebo group both in the FAS (4.57 ± 1.82 vs. 3.15 ± 3.08, p < 0.001) and in the PPS (4.62 ± 1.81 vs. 3.30 ± 3.03, p < 0.001) (Fig. 2).
Figure 2.
The difference in bronchitis severity score (BSS) from baseline visit (visit 2) to the end of treatment visit (visit 3). (A) The full analysis set and (B) the per protocol set. ap < 0.001.
Four components of the BSS (cough, sputum, dyspnea, and chest pain) were improved more with HL301 treatment than with placebo treatment both in the FAS and PPS (Table 2). On the while, the difference in crackle between visit 2 and visit 3 was higher in placebo group than in HL301 group both in the FAS (0.25 ± 0.53 vs. 0.12 ± 0.37, p = 0.040) and the PPS (0.25 ± 0.54 vs. 0.13 ± 0.37, p = 0.047).
Table 2.
Difference in each component of the bronchitis severity score between baseline visit (visit 2) and the end of treatment (visit 3) in the placebo and HL301 groups
| Variable | FAS |
PPS |
||||
|---|---|---|---|---|---|---|
| Placebo (n = 85) | HL301 (n = 81) | p value | Placebo (n = 80) | HL301 (n = 79) | p value | |
| Cough | 1.37 ± 1.29 | 1.77 ± 0.88 | 0.0253 | 1.39 ± 1.25 | 1.77 ± 0.89 | 0.0496 |
| Sputum | 1.02 ± 1.20 | 1.58 ± 1.06 | 0.0017 | 1.09 ± 1.20 | 1.61 ± 1.06 | 0.0037 |
| Dyspnea | 0.09 ± 0.50 | 0.31 ± 0.52 | 0.0038 | 0.10 ± 0.49 | 0.32 ± 0.52 | 0.0038 |
| Chest pain | 0.47 ± 0.77 | 0.79 ± 0.63 | 0.0005 | 0.48 ± 0.76 | 0.80 ± 0.63 | 0.0007 |
| Crackle | 0.25 ± 0.53 | 0.12 ± 0.37 | 0.0402 | 0.25 ± 0.54 | 0.13 ± 0.37 | 0.0470 |
Values are presented as mean ± SD.
FAS, full analysis set; PPS, per protocol set.
A response rate was indicated by the percentage of patients whose BSS was below 3 points at visit 3 or whose BSS at visit 2 decreased by more than 7 points at visit 3. Patients treated with HL301 showed a higher response rate than those treated with placebo both in the FAS (82.72% vs. 58.82%, p < 0.001) and the PPS (84.81% vs. 61.25%, p < 0.001) (Fig. 3).
Figure 3.
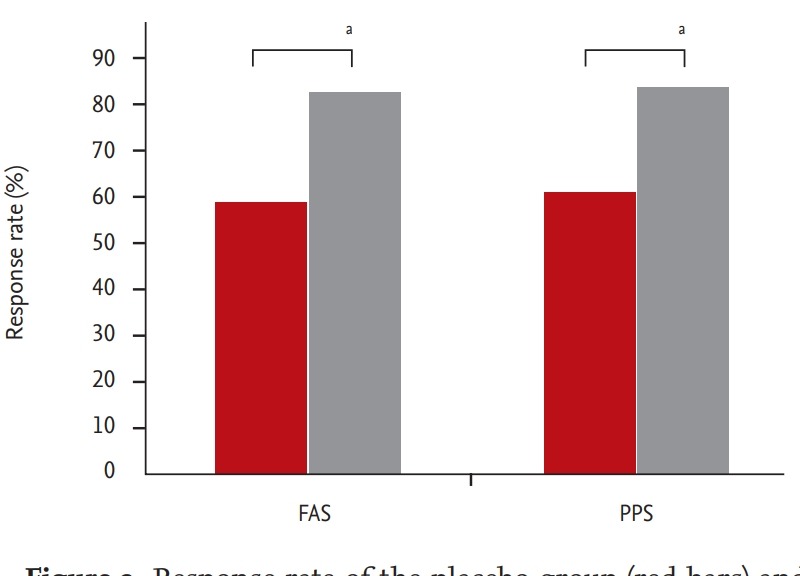
Response rate of the placebo group (red bars) and HL301 group (gray bars) in the full analysis set (FAS) and the per protocol set (PPS). Response rate indicates the percentile of patients whose bronchitis severity score (BSS) was below 3 points at visit 3 or whose BSS at visit 2 decreased by more than 7 points at visit 3. ap < 0.005.
Investigators divided the improvement states of AB into five categories at visit 3, which were ‘complete recovery,’ ‘major improvement,’ ‘slight to moderate improvement,’ ‘no change,’ and ‘deterioration.’ Complete recovery and major improvement were considered to be improved states. Whereas, the other three categories were regarded as not-improved states. Patients treated with HL301 showed a higher improvement rate than those treated with placebo in both the FAS and the PPS (Table 3).
Table 3.
Improvement of acute bronchitis at the end of treatment (visit 3)
| Variable | FAS |
PPS |
||||
|---|---|---|---|---|---|---|
| Placebo | HL301 | p value | Placebo | HL301 | p value | |
| Number | 85 | 81 | 0.0020 | 80 | 79 | 0.0022 |
| Improveda | 40 (47.06) | 56 (69.14) | 39 (48.75) | 56 (70.89) | ||
| Not-improvedb | 45 (52.94) | 25 (30.86) | 41 (51.25) | 23 (29.11) | ||
Values are presented as number (%).
FAS, full analysis set; PPS, per protocol set.
Improved: ‘complete recovery’ and ‘major improvement.’
Not-improved: ‘slight to moderate improvement,’ ‘no change,’ and ‘deterioration.’
As for participants’ satisfaction toward the investigative products, HL301-treated participants gave a higher satisfaction rate (satisfied + very satisfied/dissatisfied + very dissatisfied) than the placebo-treated group both in the FAS and the PPS (Table 4).
Table 4.
Participant responses toward investigative products at visit 3
| Variable | FAS |
PPS |
||||
|---|---|---|---|---|---|---|
| Placebo | HL301 | p value | Placebo | HL301 | p value | |
| Number | 85 | 81 | < 0.0001 | 80 | 79 | 0.0013 |
| Very dissatisfied | 9 (10.59) | 1 (1.23) | 4 (5.00) | 0 | ||
| Dissatisfied | 15 (17.65) | 2 (2.47) | 16 (20.00) | 5 (6.33) | ||
| Neutral | 20 (23.53) | 33 (40.74) | 21 (26.25) | 18 (22.78) | ||
| Satisfied | 32 (37.65) | 26 (32.10) | 30 (37.50) | 33 (41.77) | ||
| Very satisfied | 9 (10.59) | 19 (23.46) | 9 (11.25) | 23 (29.11) | ||
Values are presented as number (%).
FAS, full analysis set; PPS, per protocol set.
Rescue medication was used in five patients from the HL301 group and in 15 patients from the placebo group in the FAS (number of tablets used: 1.80 ± 0.84 vs. 5.27 ± 4.80, p = 0.121). In the PPS, rescue medication was used in five patients from the HL301 group and in 13 patients from the placebo group (number of tablets used: 1.80 ± 0.84 vs. 5.92 ± 4.84, p < 0.001) (Fig. 4).
Figure 4.
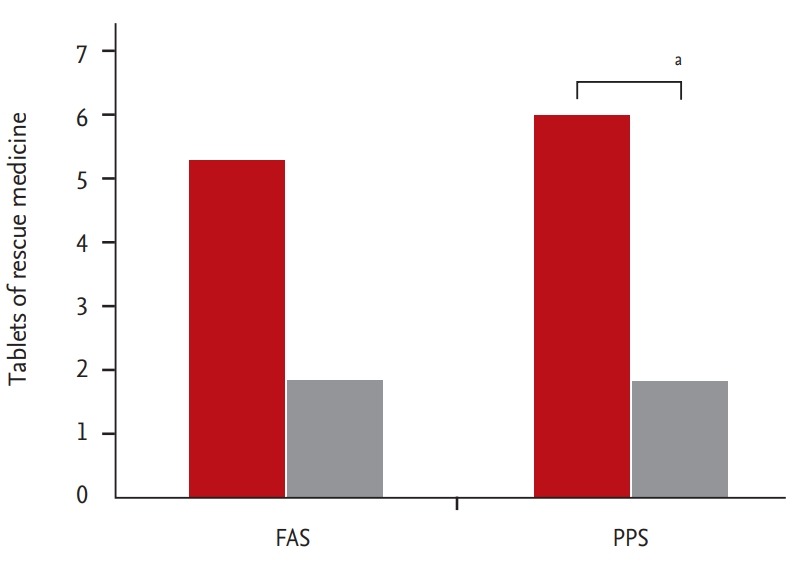
Tablets of rescue medication taken during the study period in the placebo group (red bars) and in the HL301 group (gray bars) in the full analysis set (FAS) and the per protocol set (PPS). ap < 0.01.
Adverse events
In the FAS, four out of the 85 participants in the placebo group and one out of 81 participants in the HL301 group reported AEs during the trial and the difference was not statistically significant (Table 5). None of the AEs required discontinuation of the study drug or dropout from the trial. In addition, there were no reports of mortality among participants during the trial period.
Table 5.
Adverse events in the safety set
| Variable | Placebo | HL301 | p value |
|---|---|---|---|
| Number | 85 | 81 | |
| Number of AEs | 4 (4.71) | 1 (1.23) | 0.3681 |
| AEs with possible relation to study drug | 3 (3.53) | 0 | 0.2459 |
| Number of SAEs | 0 | 0 | NA |
| AEs lead to withdrawal of the study drug | 0 | 0 | NA |
Values are presented as number (%).
AE, adverse event; SAE, severe adverse event; NA, not available.
DISCUSSION
CSBHT has been used to treat chronic pulmonary diseases in Korea for centuries. In chronic airway inflammation model of mice, CSBHT was as effective as dexamethasone at moderately reducing airway inflammation [6]. CSBHT contains 18 species of medicinal plants, which made it difficult to standardize for herbal formula. HL301 (presented as PM014 in previous articles) is a modified drug that contains seven species of medicinal plants from the 18 herbs found in CSBHT [7]. HL301 was effective on allergic airway inflammation, lipopolysaccharide-induced acute lung injury, and cigarette smoke-induced COPD-like lung inflammation in animal model [7-9]. HL301 treatment, as high as 3,000 mg/kg/day for 13 weeks did not result in any systemic or toxicologically significant changes [10].
In the previous study, which was the first human trial of HL301, we evaluated the efficacy and safety of HL301 for the treatment of AB and AECB in a randomized, double-blind, placebo-controlled, multicenter trial design [1]. Three different doses of HL301 (600, 1,200, and 1,800 mg/day) were effective in decreasing the total BSS compared to placebo in AB and AECB patients. Among the five parameters included in the BSS of the previous study, cough was the only significant, individual parameter that supported the efficacy of HL301. In addition, participants who were treated with HL301 had a better chance of achieving symptomatic improvement compared to placebo-treated patients. As the first human trial, the previous study demonstrated the efficacy and safety of HL301 for treatment of AB and AECB.
However, there were several limitations of the previous trial, the most important of which was the combined grouping of patients with AB and AECB. During review of the manuscript and in the reporting the results of the study to the Korean Food and Drug Administration, this issue was commonly raised. Therefore, the present study focused on the evaluation of HL301 solely in patients with AB.
In the present study, HL301 (600 mg/day) was effect ive in decreasing the total BSS in patients with AB both in the FAS and the PPS compared to the placebo (Fig. 1). In the previous trial, ‘cough’ was the only significant, individual parameter of the BSS that supported the efficacy of HL301. However, the present study showed that four components of the BSS (cough, sputum, dyspnea, and chest pain) were improved more with HL301 treatment than with placebo treatment both in the FAS and the PPS (Table 2). Ironically, the difference in crackle between visit 2 and visit 3 was higher in the placebo group than in the HL301 group. When we checked the baseline value of ‘crackle’ at visit 2, it was higher in the placebo group than in the HL301 group both in the FAS (0.29 ± 0.57 vs. 0.12 ± 0.37, p < 0.05) and the PPS (0.30 ± 0.58 vs. 0.13 ± 0.37, p < 0.05). Therefore, it was not the placebo effect, but the difference in the ‘crackle’ at the baseline visit that made a significant difference in the ‘crackle’ component.
It is important to note that participants treated with HL301 showed higher rates of response (Fig. 3), improvement (Table 3), and satisfaction (Table 4) and less use of rescue medication (Fig. 4) than the placebo group. Higher response, improvement, and satisfaction rates were observed both in the FAS and in the PPS with participants treated with HL301. However, less use of rescue medication with the HL301 group was statistically significant only in the PPS. The PPS consisted of participants who complete the visit schedule with a drug compliance ≥ 70%. Therefore, reduced use of rescue medicine must have been more apparent in the PPS than in the FAS.
Considering these findings, a 600 mg/day dose of HL301 was effective in the symptomatic treatment of AB. It is noteworthy that a 600 mg/day dose, applied in the present study was the minimum dose of HL301 in the previous trial, which evaluated three different doses (600, 1,200, and 1,800 mg/day). More interestingly, we were able to achieve a more significant improvement in four components of the BSS (cough, sputum, dyspnea, and chest pain) with a 600 mg/day dose of HL301 treatment. The design of the present study, which made strict selection of patients with very recent onset of AB (symptoms of AB within 2 days before visit 2) could have highlighted the efficacy of HL301.
Although the evidence of the efficacy of HL301 looks more convincing in the present trial, there are many challenges to overcome before introduction of HL301 into practical utilization. First, we made a strict selection of patients with AB who had developed symptoms very recently. In the near future, it will be necessary to evaluate whether HL301 is also effective in the late stages of AB or in other chronic respiratory diseases. Second, there are many other alternative agents that are effective in the symptomatic control of AB. Future study needs to focus on the relative efficacy of HL301 compared to preexisting drugs with similar effects. Third, HL301 is composed of seven medicinal herbs. Some herbs may have real efficacy, while others may not. Identification of essential components of HL301 would be necessary in the future.
Still, it is important to note that we were able to present a promising therapeutic agent for the symptomatic treatment of AB. As is well known, AB is one of the most common diseases leading to outpatient department visits and cough is the most common symptoms of AB [11,12]. Treatment of AB is focused on the assurance of patients and supportive care. Although antibiotics provide little benefit for AB in primary care [13], they are greatly overused for this disease in Korea [14,15] and elsewhere [16]. Among the factors associated with the prescription of antibiotics for AB, fever and sputum were associated with higher odds of antibiotics prescription [17]. We hope that the introduction of new effective agents such as HL301 will be helpful for counteracting trends in the misuse or overuse of antibiotics in the treatment of AB by attenuating symptoms of AB.
In conclusion, HL301, even at a low dose (600 mg/day) was well tolerated and effective in the treatment of AB with recent onset.
KEY MESSAGE
1. HL301 (600 mg/day) was effective in decreasing the total bronchitis severity score in patients with acute bronchitis compared to the placebo.
2. HL301 (600 mg/day) treatment was well tolerated and there was not statistically significant difference in adverse events compared to placebo.
3. HL301 is safe and effective in the treatment of acute bronchitis with recent onset.
Footnotes
This study was commissioned and financially supported by Hanlim Pharm. Co. Ltd. Seoul, Republic of Korea.
REFERENCES
- 1.Park MJ, Rhee CK, Kim YH, et al. Efficacy and safety of HL301 in the treatment of acute bronchitis and acute exacerbation of chronic bronchitis: a phase 2, randomized, double-blind, placebo-controlled, multicenter study. Curr Med Res Opin. 2017;33:919–925. doi: 10.1080/03007995.2017.1295030. [DOI] [PubMed] [Google Scholar]
- 2.Clark TW, Medina MJ, Batham S, Curran MD, Parmar S, Nicholson KG. Adults hospitalised with acute respiratory illness rarely have detectable bacteria in the absence of COPD or pneumonia; viral infection predominates in a large prospective UK sample. J Infect. 2014;69:507–515. doi: 10.1016/j.jinf.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris AM, Hicks LA, Qaseem A, High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425–434. doi: 10.7326/M15-1840. [DOI] [PubMed] [Google Scholar]
- 4.Nseir S, Cavestri B, Di Pompeo C, et al. Factors predicting bacterial involvement in severe acute exacerbations of chronic obstructive pulmonary disease. Respiration. 2008;76:253–260. doi: 10.1159/000139611. [DOI] [PubMed] [Google Scholar]
- 5.Nair S, Thomas E, Pearson SB, Henry MT. A randomized controlled trial to assess the optimal dose and effect of nebulized albuterol in acute exacerbations of COPD. Chest. 2005;128:48–54. doi: 10.1378/chest.128.1.48. [DOI] [PubMed] [Google Scholar]
- 6.Roh GS, Seo SW, Yeo S, et al. Efficacy of a traditional Korean medicine, Chung-Sang-Bo-Ha-Tang, in a murine model of chronic asthma. Int Immunopharmacol. 2005;5:427–436. doi: 10.1016/j.intimp.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Lee H, Kim Y, Kim HJ, et al. Herbal formula, PM014, attenuates lung inflammation in a murine model of chronic obstructive pulmonary disease. Evid Based Complement Alternat Med. 2012;2012:769830. doi: 10.1155/2012/769830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung KH, Choi HL, Park S, et al. The effects of the standardized herbal formula PM014 on pulmonary inflammation and airway responsiveness in a murine model of cockroach allergen-induced asthma. J Ethnopharmacol. 2014;155:113–122. doi: 10.1016/j.jep.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Jung KH, Haam KK, Park S, et al. The standardized herbal formula, PM014, ameliorated cigarette smoke-induced lung inflammation in a murine model of chronic obstructive pulmonary disease. BMC Complement Altern Med. 2013;13:219. doi: 10.1186/1472-6882-13-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung HS, Lee H, Bae H. Thirteen-week study of PM014 subchronic oral toxicity in rats. Evid Based Complement Alternat Med. 2014;2014:189673. doi: 10.1155/2014/189673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee CK, Jung JY, Lee SW, et al. The Korean cough guideline: recommendation and summary statement. Tuberc Respir Dis (Seoul) 2016;79:14–21. doi: 10.4046/trd.2016.79.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon JW, Moon JY, Kim SH, et al. Korean version of the Cough Symptom Score: clinical utility and validity for chronic cough. Korean J Intern Med. 2017;32:910–915. doi: 10.3904/kjim.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Little P, Stuart B, Moore M, et al. Amoxicillin for acute lower-respiratory-tract infection in primary care when pneumonia is not suspected: a 12-country, randomised, placebo-controlled trial. Lancet Infect Dis. 2013;13:123–129. doi: 10.1016/S1473-3099(12)70300-6. [DOI] [PubMed] [Google Scholar]
- 14.Park JY, Park S, Lee SH, et al. Microorganisms causing community-acquired acute bronchitis: the role of bacterial infection. PLoS One. 2016;11:e0165553. doi: 10.1371/journal.pone.0165553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S, Oh KC, Kim KS, et al. Role of atypical pathogens and the antibiotic prescription pattern in acute bronchitis: a multicenter study in Korea. J Korean Med Sci. 2015;30:1446–1452. doi: 10.3346/jkms.2015.30.10.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Little P, Rumsby K, Kelly J, et al. Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomized controlled trial. JAMA. 2005;293:3029–3035. doi: 10.1001/jama.293.24.3029. [DOI] [PubMed] [Google Scholar]
- 17.McKay R, Mah A, Law MR, McGrail K, Patrick DM. Systematic review of factors associated with antibiotic prescribing for respiratory tract infections. Antimicrob Agents Chemother. 2016;60:4106–4118. doi: 10.1128/AAC.00209-16. [DOI] [PMC free article] [PubMed] [Google Scholar]