Abstract
A high heterologous expression of an alkaline pectate lyase (APL) pelNK93I in E. coli was obtained through optimizing the lactose feeding and fed-batch fermentation. The highest soluble APL activity produced by E. coli BL21 (pET22b-pelNK93I) was 10,181 U/mL which is the highest level so far. On this basis, to improve the extracellular yield of APL, optimized glycine feeding was used to achieve elevated extracellular production of pelNK93I. The highest extracellular APL activity produced by E. coli BL21 (pET22b-pelNK93I) was 6357 U/mL which was also relatively higher than that in previous reports. The final productivity of APL was 282.8 U/mL/h in the fermentation of E. coli BL21 (pET22b-pelNK93I) in a 10 L fermenter. Thus the current study has provided a cost-effective method for the over-expression and preparation of alkaline pectate lyase pelNK93I for its industrial applications. Moreover, pelNK93I (4 U/mL) used for bioscouring increased cottonseed husk removal and radial capillary effect of cotton fabric by 37.63% and 47.06%, respectively, making it a promising enzyme in green textile technology.
Keywords: pelNK93I, Fed-batch fermentation, Glycine feeding, Cottonseed husk removal, Enzymatic pretreatment
Introduction
Alkaline pectate lyase (EC 4.2.2.2) (APL) catalyzes the cleavage of α-1, 4-glycosidic bonds of pectin polymer to generate shorter unsaturated oligogalacturonates (Kamijo et al. 2019; Kohli and Gupta 2015). Recently, APL has attracted extensive attention for its broad use in industries, such as food processing, plant fiber degumming, wastewater treatment, and textiles (Chiliveri and Linga 2014; Salim et al. 2017; Sharma et al. 2019; Wu et al. 2017). Especially in the textile industry, APL as a key enzyme is used in mild bioscouring pretreatment processes with decreased environmental pollution and energy consumption, while the traditional chemical treatment methods are carried out under high pH and temperature conditions with high costs in energy and effluent treatment (Abdulrachman et al. 2017; Chen et al. 2007; Khan et al. 2018). However, due to the slim profits of the textile industry, the production cost of APL restricts its application in the bio-textile industry. APLs produced by Bacillus subtilis, Pichia pastoris or Aspergillus niger currently on the market are usually of food grade for application in food and fodder and were not suitable for bio-treatment of textile due to its high price (Kohli and Gupta 2015). Hence, to improve APL’s application for bio-treatment of textile, it is crucial to further increase its fermentation yield and reduce production cost.
Many characterized APLs have been explored and reported to date (Guo et al. 2019; Wang et al. 2010; Yu et al. 2017; Zhou et al. 2017b). High-level expressions of APL in recombinant E. coli have been reported more and more recently (Wang et al. 2015; Zhou et al. 2017a; Zhuge et al. 2007). E. coli as one of the most common hosts for the expression of recombinant proteins has remarkable advantages in the production of APL such as simple molecular manipulation, faster growth rate, and lower cost fermentation culture (Chen 2012; Choi and Lee 2004). Wang et al. (2015) used mild continuous lactose feeding strategy to receive extracellular and total APL activities of 4478 U/mL and 5337 U/mL, respectively. Zhou et al. (2017a) have reported that the total activity of recombinant alkaline pectate lyase BacPelA reached 8378 U/mL by high-cell-density cultivation in fed-batch fermentation with productivity of 239.4 U/mL/h using E. coli as host. While extracellular APL activity was only 587.9 U/mL, the reprocess to obtain intracellular proteins was complex and expensive. Recently, various approaches have been adopted to promote extracellular production of recombinant proteins in E. coli, including genetic engineering of the host with manipulations of transport pathways or co-expressing of molecular chaperones (Ni and Chen 2009), modifying the expressed protein by fusion with carrier proteins (Qian et al. 2008), and culturing strategies by varying the medium compositions, cultural conditions, nutritional feeding designs, induction modes and utilizations of media addition (Cheng et al. 2011; Fang et al. 2011). Among these, glycine feeding has been particularly confirmed to be a promising method. During cell growth, glycine can replace l-alanine and d-alanine in peptidoglycan as the cell wall is synthesized along with continuous propagation of cells and leads to a very loose cell wall (Li et al. 2010), thereby increasing the permeability of cells and improving the protein secretion.
In this study, we received a high-level expression of an alkaline pectate lyase pelNK93I, both in total and extracellular activity using the recombinant strain E. coli BL21 (pET22b-pelNK93I). Through combining lactose feeding and glycine feeding in the optimum condition, the total APL activity was up to 10,181 U/mL and the extracellular APL activity was up to 6357 U/ml, representing the highest yield of APL reported to date. Moreover, according to its remarkable enhancement in cottonseed husk removal and bioscouring, the alkaline pectate lyase pelNK93I was a promising enzyme to apply in enzymatic textile treatment.
Materials and methods
Bacterial strains, media and feeding solutions
The recombinant strain E. coli BL21 (pET22b-pelNK93I) harboring the mutant gene of pelN was constructed previously (Li et al. 2014; Zhou et al. 2017c). One site mutant K93I of pectate lyase (pelN) from Paenibacillus sp. 0602 enhanced the specific activity by 1.75-fold. The accession number of the protein sequence of pelN in NCBI was AGM38211.1. The gene fragment of pelNK93I without signal peptide encoding sequence was amplified by PCR using primers pelN-F and pelN-R, and inserted into the expression plasmid pET-22b (+) with the method of Li et al. (2014). Seed cultures were grown in LB medium that contained 10.0 g/L tryptone, 5.0 g/L yeast extract, 10.0 g/L NaCl and 100 μg/mL ampicillin. Shake-flask cultures were grown in TB medium that contained 5.0 g/L glycerol, 12.0 g/L tryptone, 24.0 g/L yeast extract, 16.4 g/L K2HPO4, 2.3 g/L KH2PO4 and 100 μg/mL ampicillin. The fermentation medium for cultivation in a 10 L fermentor contained 20 g/L glucose, 30 g/L yeast extract powder (FM902), 8 g/L (NH4)2SO4, 3 g/L KH2PO4, 1 g/L MgSO4·7H2O, 0.02 g/L FeSO4·7H2O, 0.02 g/L MnSO4·7H2O and 100 μg/mL ampicillin, pH 7.0. The fed-batch feeding solution contained 500.0 g/L glucose. The induction solution had a concentration of 200.0 g/L lactose. Glycine feeding was performed with a solution that contained 100.0 g/L glycine.
Shake-flask culture conditions
The seed culture medium was prepared by incubating 20 μL of frozen glycerol stock (-80 °C) in a 100 mL shake flask (containing 20 mL of LB medium) at 37 °C and 220 rpm for 12 h. Then 400 μL of seed culture was inoculated into a 250 mL shake flask containing 20 mL of TB medium, and subsequently incubated at 37 °C and 220 rpm. After 3.5 h of cultivation, protein expression was induced by 20 g/L lactose at different temperatures (20 °C, 25 °C, 30 °C, 37 °C).
Bioreactor culture conditions
In a 10 L fermentor, 10% (v/v) concentration of inoculum was inoculated into 4 L of fermentation medium for cultivation at 37 °C. When the dissolved oxygen (DO) starts to go up, glucose feeding with an initial rate of 5 g/L/h was started, constantly adjusting the feeding rate to keep the specific growth rate of cells at 0.25 h−1 (Wang et al. 2015) and the concentration of glucose was less than 5 g/L. During the entire process, the DO level was kept at 30% by controlling the cascading impeller speed between 400 and 800 rpm and the air flow rate at 400 mL/h. The pH was kept at 7.0 by addition of 25% NH4OH or lactic acid when required. Antifoam was added manually when it was necessary.
Cell fractionation and SDS-PAGE assay
The cultivation broth was centrifuged at 13,800g for 5 min. The culture supernatant that comprised the extracellular soluble protein fraction was harvested to detect extracellular pectate lyase. The precipitate was washed twice with NaH2PO4–Na2HPO4 buffer solution (pH 7.0). After that the precipitate was resuspended with the same buffer and treated with ultrasonication at 50 Hz for 5 min in ice water. The disrupted mixture was centrifuged at 13,800g for 5 min. The supernatant was the intracellular soluble protein fraction and the disrupted pellet was the intracellular denatured protein fraction as inclusion body. The recombinant proteins in each fraction were determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under denatured conditions. Electrophoresis was performed with a 5% stacking gel and a 12% separating gel. The protein bands were visualized by staining with Coomassie Brilliant Blue R-250 dye.
Enzyme assay
To analyze the APL activity, 20 μL of the diluted enzyme solution was added to 400 μL of 0.2% pectin (Sigma Chemical Co. type P7276) in 50 mmol/L glycine–NaOH buffers (with 50 μmol/L CaCl2) at pH 9.0. The reaction mixture was incubated at 60 °C for 10 min, and the reaction was terminated by adding 600 μL of 30 mmol/L H3PO4. The product yields were detected by using an ELISA (BioTek, Epoch2TC). One standard enzyme unit was defined as the yield of 1 μmol unsaturated polygalacturonic acid per minute with a molar extinction coefficient of 4600.0 L/(mol cm) (Wang et al. 2015; Zhou et al. 2017a, 2017c).
Enzymatic desizing and scouring treatment of cotton fabric
Cotton fabric (textile number: 20*16; density: 128*60) with an area of 20 cm*20 cm was clipped for enzymatic treatment. The complex enzyme solution (1 L) consisted of amylase (5 U/ml), APL (2 U/ml, 3 U/ml, 4 U/ml and 5 U/ml) and penetrant JFC (fatty alcohol-polyoxyethylene ether) (5 g/L). First of all, cotton fabrics were immersed in the complex enzyme solution for 3 s and then placed in a steamer at 60 °C for 60 min. After that, the cotton fabrics were taken out from the steamer and rinsed with hot water (95 °C) four times. For the next step, 80% of the water was forced out from the cotton fabrics by a diminutive roller fabric press machine (Rapid® Labortex CO., LTD). The pressed cotton fabrics were immersed in the oxygen bleaching solution (5 g/L H2O2, stabilizer 15 g/L and JFC 2 g/L) for 3 s. Then the cotton fabrics were placed in a steamer at 100 °Cs for 60 min. After that, the cotton fabrics were taken out from the steamer and rinsed with hot water (95 °C) four times. In the end, water of the cotton fabrics was forced out by a diminutive roller fabric press machine and then ironed to dry. The final dry cotton fabrics were used to count the remaining cottonseed husks and measure their capillary effect. The removal rate of cottonseed husk is the percentage of the remaining numbers of cottonseed husks to the original numbers of cottonseed husks in the cotton fabrics. The radial capillary effect of the treated cotton fabric is the vertical rise length of water from the bottom of a cotton fabric strip (20 cm long in radial direction, 5 cm wide in zonal direction) in 30 min.
SEM assay for the degradation of cotton seed husk by pelNK93I
5 g of cottonseed husks were washed twice with water and transferred to 200 ml buffers of 0.05 M Gly–NaOH (pH 9.0) including 4 U/ml of alkaline pectate lyase pelNK93I. The mixture was incubated at 60 °C for 60 min. The controls had the same treatment without the addition of APL. SEM was used to observe the surface morphology of the untreated and enzyme-treated cottonseed husks. The samples were coated with a 200-Å gold layer using a vacuum sputterer (Zhou et al. 2015), and samples were then observed by SEM using Hitachi SU8010 (Hitachi, Japan).
Sequence information
The nucleotide sequence of pelN and the amino acid sequence of pelN could be searched in the GenBank database under accession Nos. KC351190 and AGM38211, respectively.
Results and discussion
Effect of the induction temperature on the alkaline pectate lyase production in the shake flask range
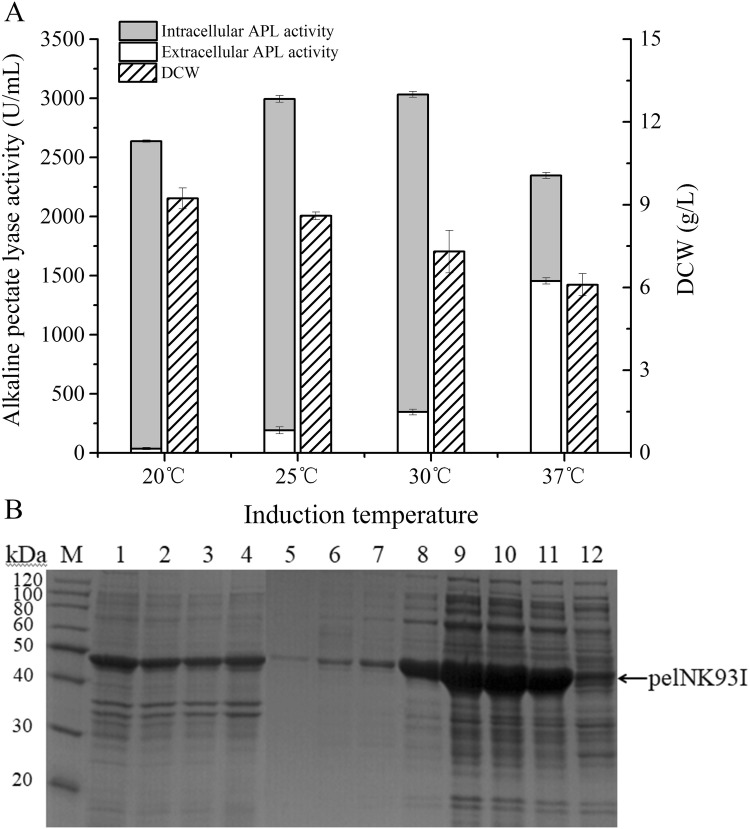
The induction temperature is an important factor relating to the growth rate of cells and the folding of recombinant proteins in recombinant E. coli cells. In this work, cell growth and recombinant enzyme production of E. coli BL21 (pET22b-pelNK93I) at four different induction temperatures from 20 to 37 °C were compared. The highest dry cell weight was up to 9.23 g/L at 32 h cultivation in TB medium when E. coli BL21 (pET22b-pelNK93I) was induced at 20 °C (Fig. 1a). The biomass decreases with the increase in induction temperature (Fig. 1a), probably because the E.coli cells have higher growth rates at relatively higher culture temperatures. When the E.coli cells were cultivated at 22 °C for 32 h, there was a relatively lower percentage of autolyzed cells to obtain the highest dry cell weight at 32 h fermentation (Fig. 1a). While at higher culture temperatures, part of the cells lysed to death at 32 h cultivation. For this reason, we can find relatively higher extracellular alkaline pectate lyase (APL) activity at higher induction temperatures (Fig. 1a). At 20 °C induction, little extracellular APL activity was determined, while at 37 °C induction temperature the extracellular APL activity was up to 62% of its total activity (Fig. 1a). However, the highest total soluble APL activities were produced at relatively lower induction temperatures (25 °C and 30 °C) (Fig. 1a). From the SDS-PAGE analysis, we found that the protein production of E. coli BL21 (pET22b-pelNK93I) was consistent with the APL activity at various induction temperatures (Fig. 1b). At induction temperature of 37 °C, the amount of inclusion bodies was the highest also because of the higher growth rate (Fig. 1b). These results indicated that the most suitable induction temperature for pelNK93I expression was 30 °C, at which there was a balance between cell growth and recombinant enzyme production.
Fig. 1.
Cell growth and pelNK93I production at different induction temperatures. a The cell growths and alkaline pectate lyase activities determined under different induction temperatures. Extracellular alkaline pectate lyase activity (white), intracellular alkaline pectate lyase activity (gray), biomass weight of dry cells (slash); b SDS-PAGE analysis of the recombinant alkaline pectate lyase (pelNK93I) production with different induction temperatures. Lane M: molecular weight of marker standard. Lanes 1–4: inclusion bodies produced by E. coli BL21 (pET22b-pelNK93I) at different induction temperatures (37 °C, 30 °C, 25 °C, 20 °C); lanes 5–8: extracellular soluble proteins produced by E. coli BL21 (pET22b-pelNK93I) at different induction temperatures (20 °C, 25 °C, 30 °C, 37 °C); lanes 9–12: intracellular soluble proteins produced by E. coli BL21 (pET22b-pelNK93I) at different induction temperatures (20 °C, 25 °C, 30 °C, 37 °C)
Selection of candidate induction point for high-level production of pelNK93I in fed-batch fermentation of a 10 L fermentor
First of all, the nutrient feeding strategy was critical to fermentor cultivation because it affected the metabolic pathway fluxes, the cell density and consequently the specificproductivity of recombinant proteins. In this work, a real-time control for the specific growth rate of cells and the glucose concentration was applied to the fed-batch fermentation of E. coli BL21 (pET22b-pelNK93I) in a 10 L fermentor during the glucose feeding stage. In the fermentation, over accumulation of acetate (> 2 g/L) which will result in inhibition of cell growth and formation of recombinant protein should be avoided (Wang et al. 2015). Cells yield acetate when a high specific growth rate of cells, DO limitation, or high-level carbon source concentration (and so on) are reached (Wang et al. 2015). Therefore, exponential feeding should be carried out and acetate formation prevented by controlling the specific growth rate of the cells at 0.25 h−1 (below the threshold of the growth rate for acetate formation, which was ∼ 0.32 h−1) (Wang et al. 2015). Using this feeding strategy could balance the cell growth rate and acetate accumulation to obtain a higher yield of recombinant proteins. So this feeding strategy was employed in all fermentor cultivations of this study. On this basis, to determine the optimal point to start induction, lactose was added at three different culture points (DCW of 15 g/L, 25 g/L and 35 g/L) in a 10 L fermentor at a constant feeding rate of 0.5 g/L/h at the temperature of 30 °C. When the induction started at the DCW of 35 g/L, the biomass was remarkably higher than that of the other two conditions after 12 h cultivation (Fig. 2a). However, the high biomass of culture was not necessary for the production of recombinant proteins. Contrary to the biomass, when inducting at the DCW of 35 g/L both total and extracellular activity of pelNK93I keep the lowest value during the whole fermentation process (Fig. 2b, c), because when the DCW is up to 35 g/L the cell growth entersinto the stationary phase. The cell metabolism and substance transport level off in this growth phase that cause cells being insensitive to inducers and low synthesis level of foreign proteins. Besides, with induction at the DCW of 15 g/L and 25 g/L, the highest total APL activities were up to 6456 U/ml and 8176 U/ml, respectively (Fig. 2b), because the expression of heterologous proteins can affect the growth of the host cells by imposing a metabolic burden, decreasing growth rate and affecting cell density (Cheng et al. 2011; Wang et al. 2015). So due to earlier induction at a DCW of 15 g/L, the biomass of the host cells were too less to express high concentrations of heterologous proteins and lead to APL activity in the late fermentation period that increased slowly (Fig. 2b). Thus, comparing the three induction points, induction at the DCW of 25 g/L not only led to the highest total APL activity, but also released the highest level of extracellular APL activity (2841 U/mL), 1.6 and 3.7 times higher than that inducted at the DCW of 15 g/L and 35 g/L ,respectively (Fig. 2c). It means that the optimum induction point (DCW of 25 g/L) could result in the balance between cell growth and recombinant protein production (and extracellular release), which facilitates maximum synthesis of recombinant proteins.
Fig. 2.

Effect of different induction points on the cell growth and pelNK93I production. a Comparison of the time profiles for biomass (DCW); b comparison of the total alkaline pectate lyase activity; c comparison of the extracellular alkaline pectate lyase activity. Solid square represents induction at DCW of 15 g/L, solid circle represents induction at DCW of 25 g/L and solid triangle represents induction at DCW of 35 g/L
The optimum lactose feeding rate for high-level production of pelNK93I in fed-batch fermentation of a 10 L fermentor
Because of the lower cost and less effect on cell growth, lactose was a better inducer in the industrial manufacture of recombinant proteins compared with IPTG. To investigate the effect of lactose feeding rate on APL production, expression was induced by adding lactose at constant feeding rates of 0.5, 1.0 and 1.5 g/L/h at the DCW of 25 g/L in a 10 L fermentor and the temperature was controlled at 30 °C. The growth profile of E. coli BL21 (pET22b-pelNK93I) was not significantly affected by different lactose feeding rates (Fig. 3a). According to the APL activity profiles, both total APL activity and extracellular APL activity reached the relatively highest value at the lactose feeding rate of 1.0 g/L/h (Fig. 3b, c). At this condition, the highest total APL activity was up to 10,181 U/ml after 36 h fermentation (Fig. 3b), while extracellular activity of APL was only 3298 U/mL at the time of 36 h fermentation (Fig. 3c). The total APL activity obtained in this work is 21% higher than that (8378.2 U/ml) of BacPelA (Zhou et al. 2017a) reported previously. However, the secretion rate of pelNK93I was only 32.4%, which is remarkably lower than that (83.9%) of PL reported by Wang et al. (2015). So the extracellular APL activity produced by E. coli BL21 (pET22b-pelNK93I) still needs to be further improved.
Fig. 3.
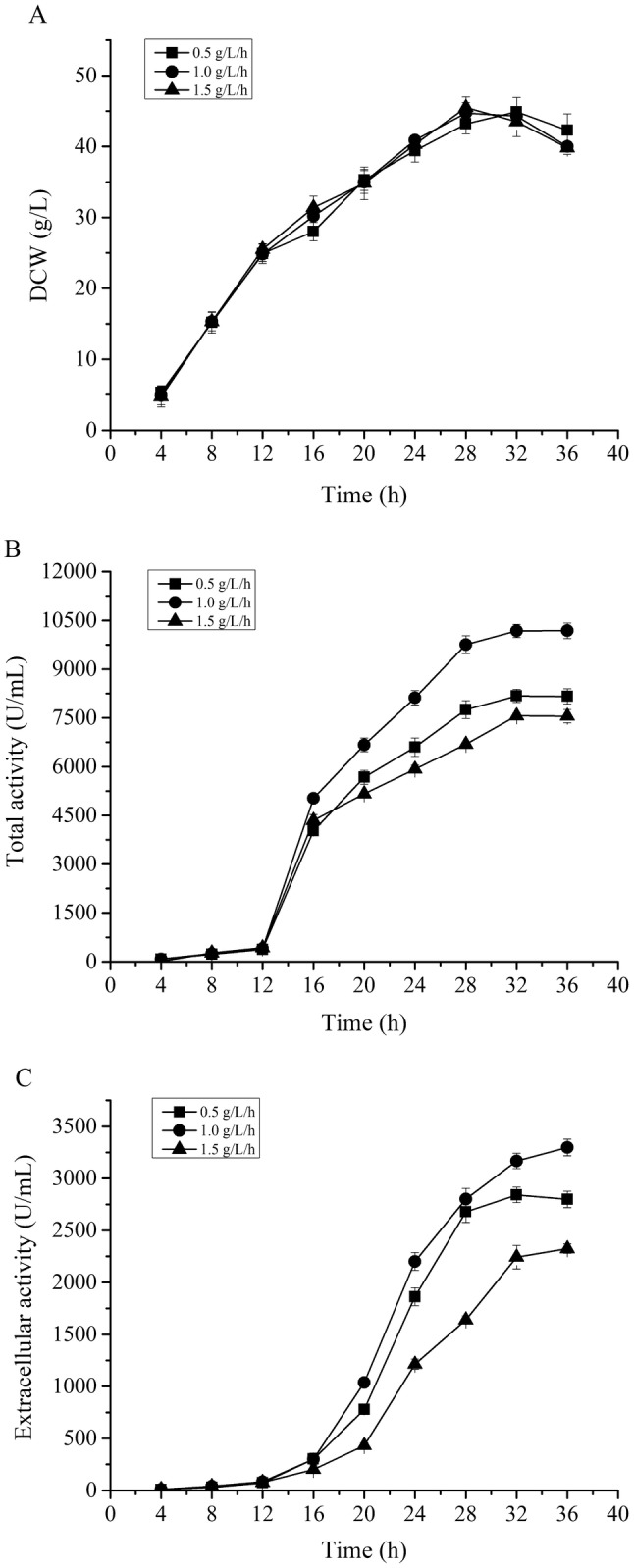
Effect of different lactose feeding rates on the cell growth and pelNK93I production. a Comparison of the time profiles for biomass (DCW); b comparison of the total alkaline pectate lyase activity; c comparison of the extracellular alkaline pectate lyase activity. Solid square represents lactose feeding rate of 0.5 g/L/h, solid circle represents lactose feeding rate of 1.0 g/L/h and solid triangle represents lactose feeding rate of 1.5 g/L/h
Enhanced extracellular production of pelNK93I by optimizing glycine feeding rate
Generally, high-level extracellular production of recombinant proteins, which could simplify the extraction and purification processes, has an absolute advantage in large-scale industrial production (Su et al. 2012). Thus, on the basis of the above optimum lactose feeding condition, to further enhance the extracellular proportion of the recombinant alkaline pectate lyase pelNK93I, a strategy of glycine feeding was adopted in this work. During cell growth, glycine can replace l-alanine and d-alanine in peptidoglycan as the cell wall is synthesized, along with continuous propagation of cells and lead to a very loose cell wall, thereby increasing the permeability of cells and improving the protein secretion (Li et al. 2010). As in a previous report, the supplementation of 1% glycine was optimally carried out at the middle of the exponential growth phase to enhance the extracellular production of the recombinant proteins (Yu et al. 2016). We started to add glycine at the middle of the exponential growth phase (12 h fermentation). Three glycine feeding amounts (0.5 g/L, 1.0 g/L and 1.5 g/L) were selected to enhance the extracellular APL activity in this work. The results indicated that glycine supplementation resulted in impaired cell growth (Fig. 4a), which adversely affected the overall recombinant protein production compared to no-glycine feeding (Fig. 4b). When the glycine feeding amount was 1.0 g/L, the highest extracellular APL activity was up to 6357 U/mL, while the corresponding total APL activity was 8876 U/mL (Fig. 4b, c). Therefore, although the total APL activity was a little lower than that (10,181 U/ml) with no no-glycine feeding, the high-level extracellular APL activity of pelNK93I was 41.6% higher than that (4478 U/ml) of PL (Wang et al. 2015) and the highest extracellular production of APL to date. The extracellular APL protein content was 0.5–1 g/L and accounted for 71–75% of the total protein content after 36 h fermentation. Finally, the productivity of APL was 282.8 U mL−1 h−1 in the fermentation of E. coli BL21 (pET22b-pelNK93I) in a 10 L fermenter. It demonstrated good potential for scale-up to industrial production of APL.
Fig. 4.
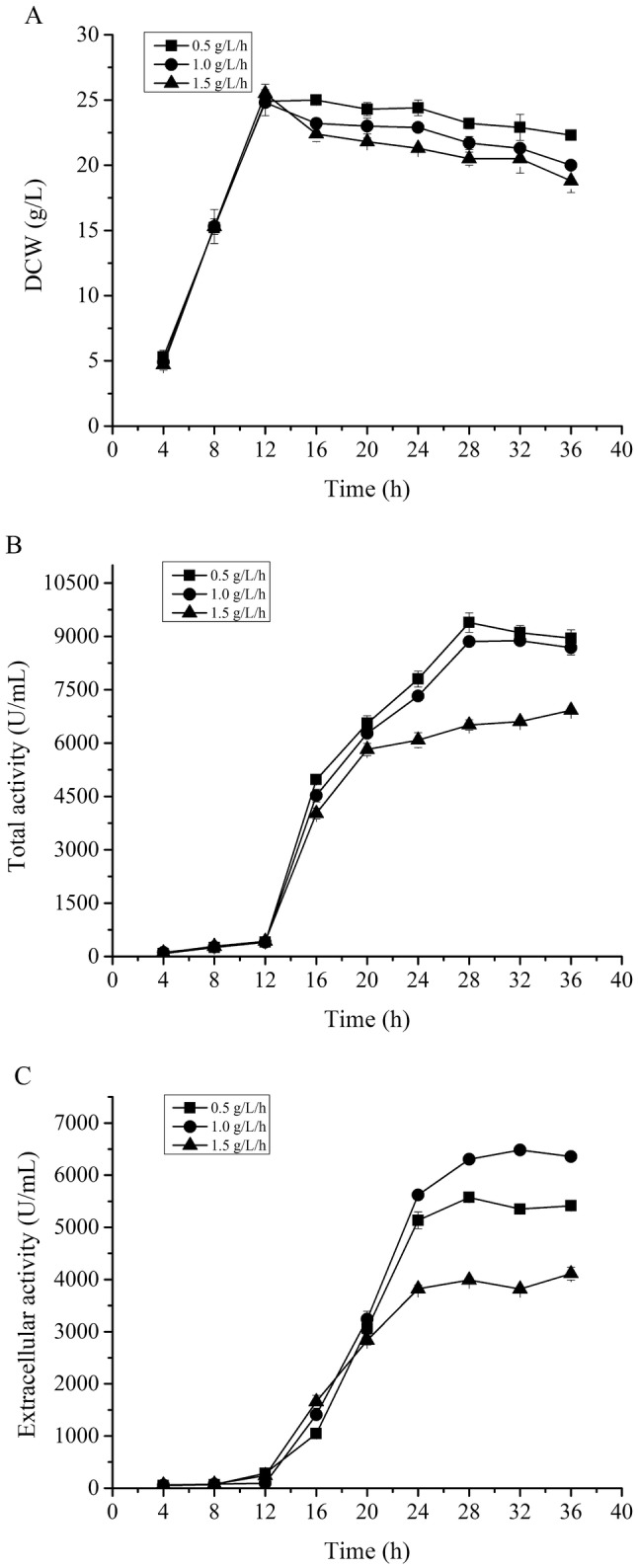
Effect of different glycine feeding rates on cell growth and pelNK93I production. a Comparison of the time profiles for biomass (DCW); b comparison of the total alkaline pectate lyase activity; c comparison of the extracellular alkaline pectate lyase activity. Solid square represents glycine feeding amount of 0.5 g/L, solid circle represents glycine feeding amount of 1.0 g/L and solid triangle represents glycine feeding amount of 1.5 g/L. The recombinant strain E. coli BL21 (pET22b-pelNK93I) started induction at the DCW of 25 g/L with lactose feeding rate of 1.0 g/L/h under 30 °C
Effects of pelNK93I on enzymatic pretreatment of cotton fabric and degradation of cottonseed husk
Generally, alkaline pectate lyase has ability to degrade the natural pectin composition both on the cottonseed husk and cotton fabric, which could further promote the removal of cottonseed husk and enhance the capillary effect of cotton fabric, respectively. While the enzymatic pretreatment of cotton fabric before dyeing usually contains desizing and scouring processes, combining these two processes could enhance the treatment efficiency. Thus, we used the complex enzymes which contain amylase (5 U/ml) and alkaline pectate lyase to pretreat the cotton fabric. Compared with the commercial alkaline pectate lyase (Bioprep3000L), pelNK93I contributed to higher removal rate of cottonseed husk by 5.10% and higher radial capillary effect of fabric by 13.33% at the same dosage (Table 1). With combined treatment of 5 U/ml amylase and 4 U/ml pelNK93I for 30 min, the removal rate of cottonseed husk and radial capillary effect of fabric were 37.63% and 1.47 times higher than that of the control (with only amylase treatment), respectively. This high-level scoured effects of cotton fabric make the enzymatic pretreatment of cotton fabric a more promising and environmental process. Moreover, through analysis of the SEM images, the cottonseed husk surface treated with pelNK93I showed rougher, looser and more hydrophilicity than the control (Fig. 5). Thus, we verify that the alkaline pectate lyase (pelNK93I) could degrade the pectin composition on the surface of cottonseed husks to make it fragile to remove by washing. In a word, the alkaline pectate lyase pelNK93I is a promising candidate for enzymatic treatment of cotton fabric.
Table 1.
Effect of pelNK93I on the bio-treatment of cotton fabric
| Enzymes | Amylase | Amylase + alkaline pectate lyasea | Amylase + pelNK93I (2 U/ml) | Amylase + pelNK93I (3 U/ml) | Amylase + pelNK93I (4 U/ml) | Amylase + pelNK93I (5 U/ml) |
|---|---|---|---|---|---|---|
| Number of cottonseed husk (before treatment) | 93 ± 4 | 96 ± 4 | 95 ± 3 | 93 ± 5 | 99 ± 6 | 95 ± 4 |
| Number of cottonseed husk (after treatment) | 35 ± 3 | 8 ± 1 | 8 ± 3 | 3 ± 1 | 0 | 0 |
| Removal rate of cottonseed husk | 62.37% | 91.67% | 91.58% | 96.77% | 100% | 100% |
| Capillary effect (radial direction, cm/30 min) | 8.5 ± 0.3 | 10.5 ± 0.4 | 9.6 ± 0.3 | 11.9 ± 0.5 | 12.5 ± 0.5 | 12.3 ± 0.4 |
aAn alkaline pectate lyase (Bioprep3000L) purchased from novozymes with the dosage of 3 U/ml
Data are presented as mean ± SD (n = 3)
Fig. 5.
SEM images of cottonseed husk surface treated by alkaline pectate lyase pelNK93I; a, c, e treated by Tris–HCl buffer at pH 9.0 and 60 °C for 60 min; b, d, f: treated by 4 U/ml pelNK93I at pH 9.0 and 60 °C for 60 min; a, b: × 400 magnification; c, d: × 800 magnification; × 2000 magnification
Conclusion
In this work, the optimum induction temperature (30 °C), optimum induction point (DCW of 25 g/L) and optimum lactose feeding rate (1.0 g/L/h) were determined successively for high production of alkaline pectate lyase pelNK93I in E. coli, resulting in the highest total APL activity of 10,181 U/mL in a 10 L fermentor. Then extracellular APL activity was up to 6357 U/mL at a glycine feeding rate of 1.0 g/L/h. pelNK93I showed high degradation capacity for the cottonseed husks, both on cotton fabric and solitarily. Therefore, pelNK93I with high extracellular yield is promisingly used in large-scale bioscouring of the textile industry.
Acknowledgements
The authors thank Dr. Zhanping Zhou and Jiangning Song for their kind donation of the strain E. coli BL21 (pET22b-pelNK93I). This work was financially supported by the National Natural Science Fund of China (Grant 31701534), the Tianjin outstanding talent training program, the Tianjin Science and Technology Planning Project (Grant 14ZCZDSY00157 and 15PTCYSY00020) and Yantai Marine economy innovation development demonstration project (Grant YHCX-SW-L-201703).
Compliance with ethical standards
Conflict of interest
The authors have declared no conflict of interest.
Footnotes
Jie Zhen and Ming Tan authors contributed equally to this work.
Contributor Information
Yanhe Ma, Email: ma_yh@tib.cas.cn.
Hongchen Zheng, Email: zheng_hc@tib.cas.cn.
Hui Song, Email: song_h@tib.cas.cn.
References
- Abdulrachman D, et al. Heterologous expression of Aspergillus aculeatus endo-polygalacturonase in Pichia pastoris by high cell density fermentation and its application in textile scouring. BMC Biotechnol. 2017;17:15. doi: 10.1186/s12896-017-0334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Rachel. Bacterial expression systems for recombinant protein production: E. coli and beyond. Biotechnology Advances. 2012;30(5):1102–1107. doi: 10.1016/j.biotechadv.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang Q, Hua ZZ, Du GC. Research and application of biotechnology in textile industries in China. Enzyme Microbial Technol. 2007;40:1651–1655. doi: 10.1016/j.enzmictec.2006.07.040. [DOI] [Google Scholar]
- Cheng Jing, Wu Dan, Chen Sheng, Chen Jian, Wu Jing. High-Level Extracellular Production of α-Cyclodextrin Glycosyltransferase with recombinantEscherichia coliBL21 (DE3) Journal of Agricultural and Food Chemistry. 2011;59(8):3797–3802. doi: 10.1021/jf200033m. [DOI] [PubMed] [Google Scholar]
- Chiliveri Swarupa Rani, Linga Venkateswar Rao. A novel thermostable, alkaline pectate lyase from Bacillus tequilensis SV11 with potential in textile industry. Carbohydrate Polymers. 2014;111:264–272. doi: 10.1016/j.carbpol.2014.04.065. [DOI] [PubMed] [Google Scholar]
- Choi JH, Lee SY. Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl Microbiol Biotechnol. 2004;64:625–635. doi: 10.1007/s00253-004-1559-9. [DOI] [PubMed] [Google Scholar]
- Fang S, Li J, Liu L, Du G, Chen J. Overproduction of alkaline polygalacturonate lyase in recombinant Escherichia coli by a two-stage glycerol feeding approach. Bioresour Technol. 2011;102:10671–10678. doi: 10.1016/j.biortech.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Guo Fenfen, Li Xuezhi, Zhao Jian, Li Guanxi, Gao Peike, Han Xiaolong. Optimizing Culture Conditions by Statistical Approach to Enhance Production of Pectinase from Bacillus sp. Y1. BioMed Research International. 2019;2019:1–10. doi: 10.1155/2019/8146948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo Junya, Sakai Kiyota, Suzuki Hiromitsu, Suzuki Kengo, Kunitake Emi, Shimizu Motoyuki, Kato Masashi. Identification and characterization of a thermostable pectate lyase from Aspergillus luchuensis var. saitoi. Food Chemistry. 2019;276:503–510. doi: 10.1016/j.foodchem.2018.10.059. [DOI] [PubMed] [Google Scholar]
- Khan MM, Choi YS, Kim YK, Yoo JC. Immobilization of an alkaline endopolygalacturonase purified from Bacillus paralicheniformis exhibits bioscouring of cotton fabrics. Bioprocess Biosyst Eng. 2018;41:1425–1436. doi: 10.1007/s00449-018-1971-7. [DOI] [PubMed] [Google Scholar]
- Kohli P, Gupta R. Alkaline pectinases: a review biocatalysis and agricultural. Biotechnology. 2015;4:279–285. doi: 10.1016/j.bcab.2015.07.001. [DOI] [Google Scholar]
- Li Z, Gu Z, Wang M, Du G, Wu J, Chen J. Delayed supplementation of glycine enhances extracellular secretion of the recombinant alpha-cyclodextrin glycosyltransferase in Escherichia coli. Appl Microbiol Biotechnol. 2010;85:553–561. doi: 10.1007/s00253-009-2157-7. [DOI] [PubMed] [Google Scholar]
- Li Xiaoman, Wang Huilin, Zhou Cheng, Ma Yanhe, Li Jian, Song Jiangning. Cloning, expression and characterization of a pectate lyase from Paenibacillus sp. 0602 in recombinant Escherichia coli. BMC Biotechnology. 2014;14(1):18. doi: 10.1186/1472-6750-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y, Chen R. Extracellular recombinant protein production from Escherichia coli. Biotechnol Lett. 2009;31:1661–1670. doi: 10.1007/s10529-009-0077-3. [DOI] [PubMed] [Google Scholar]
- Qian ZG, Xia XX, Choi JH, Lee SY. Proteome-based identification of fusion partner for high-level extracellular production of recombinant proteins in Escherichia coli. Biotechnol Bioeng. 2008;101:587–601. doi: 10.1002/bit.21898. [DOI] [PubMed] [Google Scholar]
- Salim Abdalla Ali, Grbavčić Sanja, Šekuljica Nataša, Stefanović Andrea, Jakovetić Tanasković Sonja, Luković Nevena, Knežević-Jugović Zorica. Production of enzymes by a newly isolated Bacillus sp. TMF-1 in solid state fermentation on agricultural by-products: The evaluation of substrate pretreatment methods. Bioresource Technology. 2017;228:193–200. doi: 10.1016/j.biortech.2016.12.081. [DOI] [PubMed] [Google Scholar]
- Sharma D, Sharma G, Mahajan R. Development of strategy for simultaneous enhanced production of alkaline xylanase-pectinase enzymes by a bacterial isolate in short submerged fermentation cycle. Enzyme Microb Technol. 2019;122:90–100. doi: 10.1016/j.enzmictec.2018.12.008. [DOI] [PubMed] [Google Scholar]
- Su Lingqia, Chen Sheng, Yi Li, Woodard Ronald W, Chen Jian, Wu Jing. Extracellular overexpression of recombinant Thermobifida fusca cutinase by alpha-hemolysin secretion system in E. coli BL21(DE3) Microbial Cell Factories. 2012;11(1):8. doi: 10.1186/1475-2859-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang Y, Zhang D, Li J, Hua Z, Du G, Chen J. Enhancement of cell viability and alkaline polygalacturonate lyase production by sorbitol co-feeding with methanol in Pichia pastoris fermentation. Bioresour Technol. 2010;101:1318–1323. doi: 10.1016/j.biortech.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Wang Huilin, Li Xiaoman, Ma Yanhe, Song Jiangning. Process optimization of high-level extracellular production of alkaline pectate lyase in recombinant Escherichia coli BL21 (DE3) Biochemical Engineering Journal. 2015;93:38–46. doi: 10.1016/j.bej.2014.08.020. [DOI] [Google Scholar]
- Wu C, Xiao Y, Lin W, Li J, Zhang S, Zhu J, Rong J. Aqueous enzymatic process for cell wall degradation and lipid extraction from Nannochloropsis sp. Bioresour Technol. 2017;223:312–316. doi: 10.1016/j.biortech.2016.10.063. [DOI] [PubMed] [Google Scholar]
- Yu Zhenxiao, Zheng Hongchen, Zhao Xingya, Li Shufang, Xu Jianyong, Song Hui. High level extracellular production of a recombinant alkaline catalase in E. coli BL21 under ethanol stress and its application in hydrogen peroxide removal after cotton fabrics bleaching. Bioresource Technology. 2016;214:303–310. doi: 10.1016/j.biortech.2016.04.110. [DOI] [PubMed] [Google Scholar]
- Yu Ping, Zhang Yishu, Gu Donglu. Production optimization of a heat-tolerant alkaline pectinase from Bacillus subtilis ZGL14 and its purification and characterization. Bioengineered. 2017;8(5):613–623. doi: 10.1080/21655979.2017.1292188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Cheng, Ye Jintong, Xue Yanfen, Ma Yanhe. Directed Evolution and Structural Analysis of Alkaline Pectate Lyase from the Alkaliphilic Bacterium Bacillus sp. Strain N16-5 To Improve Its Thermostability for Efficient Ramie Degumming. Applied and Environmental Microbiology. 2015;81(17):5714–5723. doi: 10.1128/AEM.01017-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Cheng, Xue Yanfen, Ma Yanhe. Cloning, evaluation, and high-level expression of a thermo-alkaline pectate lyase from alkaliphilic Bacillus clausii with potential in ramie degumming. Applied Microbiology and Biotechnology. 2017;101(9):3663–3676. doi: 10.1007/s00253-017-8110-2. [DOI] [PubMed] [Google Scholar]
- Zhou M, Wu J, Wang T, Gao L, Yin H, Lu X (2017b) The purification and characterization of a novel alkali-stable pectate lyase produced by Bacillus subtilis PB1. World J Microbiol Biotechnol 33:190. 10.1007/s11274-017-2357-8 [DOI] [PubMed]
- Zhou Z, et al. Structure-based engineering of a pectate lyase with improved specific activity for ramie degumming. Appl Microbiol Biotechnol. 2017;101:2919–2929. doi: 10.1007/s00253-016-7994-6. [DOI] [PubMed] [Google Scholar]
- Zhuge Bin, Du Guo-Cheng, Shen Wei, Zhuge Jian, Chen Jian. Efficient secretory expression of an alkaline pectate lyase gene from Bacillus subtilis in E. coli and the purification and characterization of the protein. Biotechnology Letters. 2007;29(3):405–410. doi: 10.1007/s10529-006-9249-6. [DOI] [PubMed] [Google Scholar]