Abstract
Objective
Avoiding a catastrophic outcome may be a more realistic goal than achieving functional independence in the treatment of acute stroke in octogenarians. This study aimed to investigate predictors of catastrophic outcome in elderly patients after an endovascular thrombectomy with an acute anterior circulation large vessel occlusion (LVO).
Materials and Methods
Data from 82 patients aged ≥ 80 years, who were treated with thrombectomy for acute anterior circulation LVO, were analyzed. The association between clinical/imaging variables and catastrophic outcomes was assessed. A catastrophic outcome was defined as a modified Rankin Scale score of 4–6 at 90 days.
Results
Successful reperfusion was achieved in 61 patients (74.4%), while 47 patients (57.3%) had a catastrophic outcome. The 90-day mortality rate of the treated patients was 15.9% (13/82). The catastrophic outcome group had a significantly lower baseline diffusion-weighted imaging-Alberta stroke program early CT score (DWI-ASPECTS) (7 vs. 8, p = 0.014) and a longer procedure time (42 minutes vs. 29 minutes, p = 0.031) compared to the non-catastrophic outcome group. Successful reperfusion was significantly less frequent in the catastrophic outcome group (63.8% vs. 88.6%, p = 0.011) compared to the non-catastrophic outcome group. In a binary logistic regression analysis, DWI-ASPECTS (odds ratio [OR], 0.709; 95% confidence interval [CI], 0.524–0.960; p = 0.026) and successful reperfusion (OR, 0.242; 95% CI, 0.071–0.822; p = 0.023) were independent predictors of a catastrophic outcome.
Conclusion
Baseline infarct size and reperfusion status were independently associated with a catastrophic outcome after endovascular thrombectomy in elderly patients aged ≥ 80 years with acute anterior circulation LVO.
Keywords: Acute ischemic stroke, Mechanical thrombectomy, Acute large vessel occlusion, Octogenarian, Nonagenarian, Outcome predictor
INTRODUCTION
A meta-analysis of individual patient-level data from five randomized controlled trials showed that patients aged ≥ 80 years could benefit from endovascular thrombectomy compared to standard medical therapy (1). However, the efficacy of thrombectomy is still unsatisfactory in elderly patients compared to that in younger patients in ‘real-world’ practice. A multicenter retrospective study from seven comprehensive stroke centers in the United States showed that old age (≥ 80 years) was significantly associated with poorer outcomes (modified Rankin Scale [mRS] 3–6; 79% vs. 56%), postprocedural hemorrhage (42% vs. 28%), and mortality (38% vs. 20%) compared to younger counterparts (2). This finding suggests that a more refined selection criteria for endovascular thrombectomy is needed for octogenarians with acute large vessel occlusion (LVO).
The 90-day mRS score is used to evaluate post-treatment outcomes after endovascular thrombectomy in acute stroke. In general, a good outcome has been defined as functional independence (mRS scores of 0–2) at 90 days. However, considering a lower expectation of functional independence in elderly patients and the fact that elderly patients are more likely to have medical comorbidities, pre-stroke disability, reduced neuronal plasticity, and cognitive dysfunction compared to younger patients, an mRS score of 3 could be considered as a favorable outcome in octogenarians (3,4,5). A multicenter, international survey also showed that an mRS score of 3 was considered as an acceptable outcome by approximately 80% of physicians who were involved in treating patients with severe stroke (6). In this regard, understanding the predictors of catastrophic outcome (mRS 4–6) after endovascular thrombectomy may help in making treatment decisions and improve treatment outcomes in elderly patients. Although some studies have addressed the predictors of functional independence after thrombectomy in elderly patients (7,8,9,10,11), predictors of catastrophic outcomes have not been fully investigated. Therefore, this study aimed to investigate the predictors of catastrophic outcome after endovascular thrombectomy in elderly patients aged ≥ 80 years with acute anterior circulation LVO.
MATERIALS AND METHODS
Study Population
From January 2011 to July 2016, a total of 380 patients presenting with acute anterior circulation stroke underwent endovascular thrombectomy at a comprehensive stroke center. The clinical and radiologic data were prospectively collected and stored in a stroke database at our hospital. Of these, data from 82 patients aged 80 years and older were retrieved and retrospectively analyzed for this study. The institutional ethics committee approved this retrospective analysis and waived the requirement for informed consent based on the study design.
Endovascular Treatment
Inclusion criteria for endovascular thrombectomy were as follows: femoral artery puncture started within 6 hours of symptom onset, National Institutes of Health Stroke Scale (NIHSS) score ≥ 4 at admission, pre-stroke mRS score ≤ 2, no intracranial hemorrhage detected on the pretreatment brain CT, and intracranial large vessel (intracranial internal carotid artery [ICA] and M1 and M2 segments of the middle cerebral artery [MCA]) occlusion confirmed on catheter angiography. Endovascular therapy was performed under local anesthesia. Thrombectomy with a Solitaire stent or a Trevo stent was performed as the front-line technique. When stent-retriever thrombectomy failed to achieve successful reperfusion within three attempts, contact aspiration thrombectomy was performed with a Penumbra reperfusion catheter or intermediate catheter (12). Successful reperfusion was defined as a modified thrombolysis in cerebral ischemia (m-TICI) grade of 2b or 3 on the final angiogram (13). Carotid angioplasty and stenting were performed before intracranial endovascular thrombectomy when a tandem occlusion was found at the proximal cervical segment of the ICA. A balloon angioplasty, with or without stenting, was performed when severe (≥ 70%) underlying intracranial stenosis was found at the arterial occlusion site (14).
All patients underwent non-contrast CT before, immediately after, and 24 hours after endovascular therapy. In the post-treatment CT, intracranial hemorrhage was classified as either hemorrhagic infarction or parenchymal hemorrhage, according to the European Cooperative Acute Stroke Study II criteria (15). Symptomatic hemorrhage was defined as any intracranial hemorrhage associated with neurological deterioration (NIHSS scale increase of ≥ 4 points). An experienced neuroradiologist retrospectively reviewed the pre-treatment CT images and recorded the Alberta stroke program early CT score (ASPECTS). Stroke subtype was determined at the time of discharge based on the Trial of ORG 10172 in the Acute Stroke Treatment classification (16). Clinical outcomes were evaluated during an outpatient visit or via telephone interview 90 days after stroke. A catastrophic outcome was defined as an mRS score of 4–6.
Diffusion-Weighted Imaging
All patients underwent diffusion-weighted imaging (DWI) before endovascular thrombectomy according to the stroke protocol of our institution. DWI examinations were performed with a 1.5T unit (Signa HDxt; GE Healthcare, Milwaukee, WI, USA). DWI sequences were obtained on the axial plane using a single-shot spin-echo echo-planar imaging technique with the following parameters: repetition time 9000 ms, echo time 80 ms, section thickness 4 mm, intersection gap 0 mm, field of view 260 × 260 mm, and b-values 0 and 1000 s/mm2. The DWI-ASPECTS was retrospectively assessed by two neuroradiologists who were blinded to patients' information. Conclusions were reached by consensus in cases of disagreement.
Statistical Analysis
Statistical analyses were performed with SPSS software (version 23.0; IBM Corp., Armonk, NY, USA). We compared differences in baseline and procedural characteristics between patients with catastrophic outcomes (mRS 4–6) and those with non-catastrophic outcomes (mRS 0–3). The χ2 test or Fisher's exact test was used to compare categorical variables, and the Mann-Whitney U test was used to compare continuous variables. Next, a binary logistic regression analysis was performed to identify independent predictors for a catastrophic outcome. Variables with a p value < 0.05 in the univariate analysis were included in a multivariate logistic regression model. P values < 0.05 were considered statistically significant.
RESULTS
Data from 82 patients (28 men and 54 women; median age, 83 years; age range, 80–95 years) with anterior circulation stroke were analyzed. Of these, 56 patients had occlusions of the MCA and 26 had occlusions of the ICA. Thirty-five eligible patients (42.7%) received an intravenous recombinant tissue plasminogen activator before endovascular therapy. The median baseline DWI-ASPECTS was 7, and baseline NIHSS score was 15. Underlying intracranial atherosclerotic stenosis was found at the occlusion site in six (7.3%) patients. Eleven patients (13.4%) had a tandem occlusion in the cervical segment of the ICA. The stroke subtype was cardioembolism in 52 patients (63.4%), large artery atherosclerosis in 15 patients (18.3%), and undetermined in 15 patients (18.3%). Overall, successful reperfusion was achieved in 61 patients (74.4%). Forty-seven patients (57.3%) had a 90-day catastrophic outcome (mRS 4–6) while 35 patients (42.7%) had a 90-day non-catastrophic outcome (mRS 0–3). Parenchymal hemorrhage occurred in 6.1% of patients (5/82) and symptomatic hemorrhage in 3.7% (3/82). The 90-day mortality rate was 15.9% (13/82).
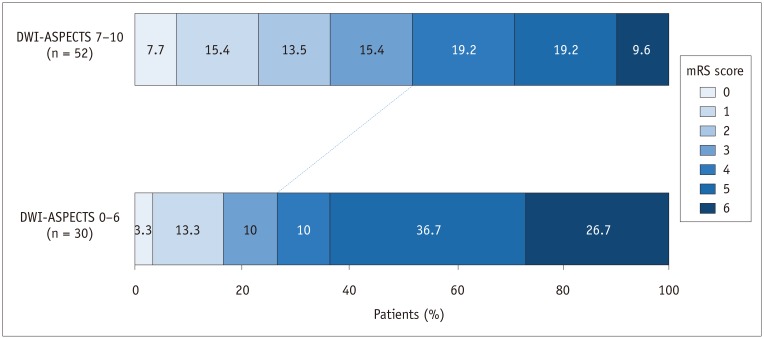
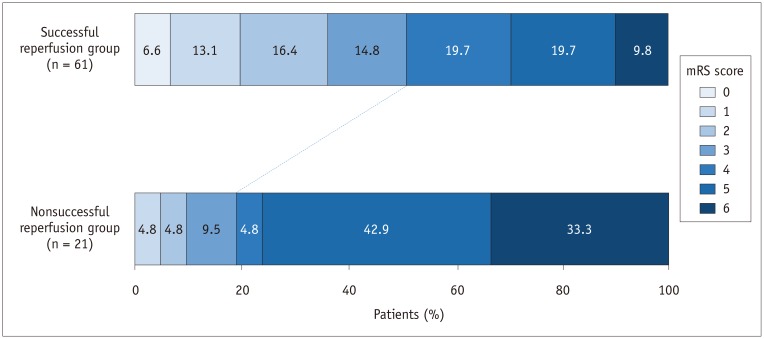
Comparisons of clinical and procedural characteristics between patients with catastrophic outcomes and those with non-catastrophic outcomes are presented in Table 1. The catastrophic outcome group had a significantly lower baseline DWI-ASPECTS (7 vs. 8, p = 0.014) and longer procedure time (42 minutes vs. 29 minutes, p = 0.031) compared to the non-catastrophic outcome group. When DWI-ASPECTS were dichotomized into the two groups (scores of 7–10 vs. 0–6), 48.1% (25/52) of patients with a DWI-ASPECTS ≥ 7 and 73.3% (22/30) of patients with a DWI-ASPECTS < 7 had a catastrophic outcome. This difference was statistically significant (p = 0.026) (Fig. 1). Successful reperfusion was significantly less frequent in the catastrophic outcome group (63.8% vs. 88.6%, p = 0.011) (Fig. 2). There were no differences in other baseline or procedural characteristics between the two groups. In a binary logistic regression analysis adjusted for potential confounders (DWI-ASPECTS, procedure time, and successful reperfusion), DWI-ASPECTS (per 1-point increase; odds ratio [OR], 0.709; 95% confidence interval [CI], 0.524–0.960; p = 0.026) and successful reperfusion (OR 0.242; 95% CI 0.071–0.822; p = 0.023) were independent predictors of catastrophic outcome at 90 days (Table 2).
Table 1. Comparison of Characteristics between Patients with 90-Day Catastrophic Outcome (mRS 4–6) and Those with Non-Catastrophic Outcome (mRS 0–3).
| Variables | Total (n = 82) | mRS 4–6 (n = 47) | mRS 0–3 (n = 35) | P |
|---|---|---|---|---|
| Age, yrs | 83 (81–86) | 82 (80–86) | 83 (81–86) | 0.524 |
| Age > 85 yrs | 22 (26.8) | 13 (27.7) | 9 (25.7) | 0.844 |
| Sex, female | 54 (65.9) | 34 (72.3) | 20 (57.1) | 0.151 |
| Risk factors | ||||
| Hypertension | 65 (79.3) | 38 (80.9) | 27 (77.1) | 0.682 |
| Diabetes mellitus | 21 (25.6) | 12 (25.5) | 9 (25.7) | 0.985 |
| Dyslipidemia | 20 (24.4) | 9 (19.1) | 11 (31.4) | 0.200 |
| Atrial fibrillation | 51 (62.2) | 31 (37.8) | 20 (57.1) | 0.416 |
| Smoking | 12 (14.6) | 10 (21.3) | 2 (5.7) | 0.062 |
| Coronary artery disease | 12 (14.6) | 6 (12.8) | 6 (17.1) | 0.579 |
| Previous stroke or TIA | 22 (26.8) | 12 (25.5) | 10 (28.6) | 0.759 |
| Congestive heart failure | 4 (4.9) | 2 (4.3) | 2 (5.7) | 1.000 |
| Intravenous thrombolysis | 35 (42.7) | 23 (48.9) | 12 (34.3) | 0.185 |
| Occlusion sites | 0.963 | |||
| MCA | 56 (68.3) | 32 (68.1) | 24 (68.6) | |
| ICA | 26 (31.7) | 15 (31.9) | 11 (31.4) | |
| DWI-ASPECTS | 7 (6–8) | 7 (5–8) | 8 (7–9) | 0.014 |
| DWI-ASPECTS ≥ 7 | 52 (63.4) | 25 (53.2) | 27 (77.1) | 0.026 |
| CT-ASPECTS | 9 (8–10) | 9 (8–9) | 9 (8–10) | 0.367 |
| Time to treatment, min | 210 (160–285) | 220 (165–285) | 195 (150–285) | 0.189 |
| Procedure time, min | 37 (25–53) | 42 (30–55) | 29 (20–50) | 0.031 |
| Time to final reperfusion, min | 257 (195–329) | 277 (203–332) | 227 (180–320) | 0.091 |
| Baseline NIHSS score | 15 (12–16) | 15 (12–18) | 15 (12–16) | 0.101 |
| Underlying severe ICAS | 6 (7.3) | 1 (2.1) | 5 (14.3) | 0.079 |
| Cervical ICA tandem occlusion | 11 (13.4) | 7 (14.9) | 4 (11.4) | 0.751 |
| Stroke etiology | 0.557 | |||
| Cardioembolism | 52 (63.4) | 32 (68.1) | 20 (57.1) | |
| Large artery atherosclerosis | 15 (18.3) | 8 (17.0) | 7 (20.0) | |
| Undetermined | 15 (18.3) | 7 (14.9) | 8 (22.9) | |
| Successful reperfusion | 61 (74.4) | 30 (63.8) | 31 (88.6) | 0.011 |
| Subarachnoid hemorrhage | 12 (14.6) | 8 (17.0) | 4 (11.4) | 0.543 |
| Hemorrhagic infarction | 16 (19.5) | 11 (23.4) | 5 (14.3) | 0.303 |
| Parenchymal hemorrhage | 5 (6.1) | 4 (8.5) | 1 (2.9) | 0.387 |
| Symptomatic hemorrhage | 3 (3.7) | 3 (6.4) | 0 | 0.257 |
Values are presented as n (%) or median (interquartile range). ASPECTS = Alberta stroke program early CT score, DWI = diffusion-weighted imaging, ICA = internal carotid artery, ICAS = intracranial atherosclerotic stenosis, MCA = middle cerebral artery, mRS = modified Rankin Scale, NIHSS = National Institutes of Health Stroke Scale, TIA = transient ischemic attack
Fig. 1. Distribution of mRS scores at 90 days, according to DWI-ASPECTS, after thrombectomy in elderly patients with acute anterior circulation large vessel occlusion.
DWI-ASPECTS = diffusion-weighted imaging Alberta stroke program early CT score, mRS = modified Rankin Scale
Fig. 2. Distribution of mRS scores at 90 days, according to reperfusion status, after thrombectomy in elderly patients with acute anterior circulation large vessel occlusion.
Table 2. Univariate and Multivariate Binary Logistic Regression Analysis for Predictors of Catastrophic 90-Day Outcome (mRS 4–6).
| Variables | Unadjusted OR | 95% CI | P | Adjusted OR | 95% CI | P |
|---|---|---|---|---|---|---|
| DWI-ASPECTS, per 1-point increase | 0.690 | 0.509–0.936 | 0.017 | 0.709 | 0.524–0.960 | 0.026 |
| DWI-ASPECTS ≥ 7 | 0.337 | 0.127–0.893 | 0.029 | - | - | - |
| Procedure time | 1.023 | 0.999–1.047 | 0.052 | - | - | - |
| Successful reperfusion | 0.228 | 0.069–0.755 | 0.016 | 0.242 | 0.071–0.822 | 0.023 |
CI = confidence interval, OR = odds ratio
DISCUSSION
Because of a graying society, the proportion of octogenarians among patients treated with endovascular thrombectomy due to acute LVO is steadily increasing. Currently, there is no age limitation for thrombectomy in patients with acute anterior circulation LVO. Most guidelines recommend that patient age > 80 should not be used as a contraindication for endovascular thrombectomy (17,18). Following a stroke due to intracranial LVO at a very old age, mRS 3 could be considered a reasonable functional outcome as these patients are still able to ambulate without assistance. Avoiding a catastrophic outcome may be a more realistic goal than achieving functional independence in treatment of acute stroke in octogenarians. In this context, we investigated the predictors of a catastrophic outcome after thrombectomy in elderly patients, which had not yet been reported. As a result, we found that the initial infarct size measured by the DWI-ASPECTS and reperfusion status were independently associated with a catastrophic outcome after thrombectomy in elderly patients aged ≥ 80 years with acute anterior circulation LVO.
Our study suggests that successful reperfusion is a strong negative predictor of catastrophic outcome after thrombectomy in elderly patients (OR = 0.242, p = 0.023). Moreover, a catastrophic outcome was significantly less frequent in patients with successful reperfusion compared to those with unsuccessful reperfusion (49.2% [n = 30/61] vs. 81% [n = 17/21]). Our results are well in line with those from a study by Imahori et al. (19) who demonstrated a significant association between reperfusion status and functional outcome in elderly patients with anterior circulation LVO. They found that the rate of a good outcome (mRS 0–2 at 90 days) was not significantly different between patients aged ≥ 80 years and those aged < 80 years (65% vs. 68%) when complete reperfusion (m-TICI 3) was achieved after stent-retriever thrombectomy (19). This finding suggests that elderly patients can have similar rates of a good outcome to those of younger counterparts if complete reperfusion can be achieved with endovascular thrombectomy. Recently, Koizumi et al. (9) reported the outcomes after endovascular thrombectomy in 78 elderly patients aged ≥ 80 years and showed that 43.5% (27/62) of patients with successful reperfusion had a good outcome. They also found that successful reperfusion was one of the independent predictors of a good outcome (OR = 1.40, p = 0.0081) (9). The results of previous studies and our study highlight the importance of achieving successful reperfusion to avoid catastrophic outcomes in elderly stroke patients who are undergoing thrombectomy.
Our study also confirmed that the initial infarct size is a strong determinant of functional outcome after thrombectomy in elderly patients. In the present study, baseline DWI-ASPECTS was significantly associated with a catastrophic outcome. Catastrophic outcomes occurred more frequently in patients with DWI-ASPECTS scores of < 7 compared to those with scores of ≥ 7 (73.3% [n = 22/30] vs. 48.1% [n = 25/52], p = 0.026). This finding supports the notion that infarct size threshold for a favorable outcome after endovascular therapy decreases with increasing age. Ribo et al. (20) reported that the target cut-off infarct volume for predicting a good outcome (mRS ≤ 2) was 49 mL for patients < 70 years, 32.5 mL for patients 70–79 years, and 15.2 mL for patients ≥ 80 years. Danière et al. (21) also suggested that baseline imaging selection criteria may need to be adapted according to age, with a smaller infarct size cut-off for elderly patients. The DWI or CTP assessment with clinical mismatch in the triage of wake-up and late presenting strokes undergoing neurointervention with Trevo (DAWN) study also adopted this concept of age-dependent infarct size threshold for patient selection. The DAWN study included patients aged ≥ 80 years if they had a baseline infarct volume of ≤ 21 mL, while including patients < 80 years if they had an infarct volume of ≤ 51 mL (22).
This study has several limitations. This study is a single center retrospective study with a relatively small number of patients. In addition, post-stroke medical comorbidities such as post-stroke pneumonia were not assessed in the present study. Elderly patients are more likely to have a wide spectrum of post-stroke comorbidities that could affect outcomes compared to younger patients. Future studies should be performed to evaluate post-stroke medical comorbidities as potential variables in the analysis.
In conclusion, baseline infarct size and reperfusion status were independently associated with catastrophic outcome after endovascular thrombectomy in elderly patients aged ≥ 80 years with acute anterior circulation LVO. Our results may help to identify elderly stroke patients who are likely to benefit from thrombectomy and to emphasize the importance of successful reperfusion to avoid a catastrophic outcome after thrombectomy.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 2.Alawieh A, Starke RM, Chatterjee AR, Turk A, De Leacy R, Rai AT, et al. Outcomes of endovascular thrombectomy in the elderly: a ‘real-world’ multicenter study. J Neurointerv Surg. 2019;11:545–553. doi: 10.1136/neurintsurg-2018-014289. [DOI] [PubMed] [Google Scholar]
- 3.Chandra RV, Leslie-Mazwi TM, Mehta BP, Yoo AJ, Simonsen CZ. Clinical outcome after intra-arterial stroke therapy in the very elderly: why is it so heterogeneous? Front Neurol. 2014;5:60. doi: 10.3389/fneur.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen JE, Gomori JM, Leker RR. Stent retriever-based thrombectomy in octogenarians. Interv Neurol. 2016;5:111–117. doi: 10.1159/000446795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilditch CA, Nicholson P, Murad MH, Rabinstein A, Schaafsma J, Pikula A, et al. Endovascular management of acute stroke in the elderly: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2018;39:887–891. doi: 10.3174/ajnr.A5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neugebauer H, Creutzfeldt CJ, Hemphill JC, 3rd, Heuschmann PU, Jüttler E. DESTINY-S: attitudes of physicians toward disability and treatment in malignant MCA infarction. Neurocrit Care. 2014;21:27–34. doi: 10.1007/s12028-014-9956-0. [DOI] [PubMed] [Google Scholar]
- 7.Kurre W, Aguilar-Pérez M, Niehaus L, Fischer S, Schmid E, Bäzner H, et al. Predictors of outcome after mechanical thrombectomy for anterior circulation large vessel occlusion in patients aged ≥80 years. Cerebrovasc Dis. 2013;36:430–436. doi: 10.1159/000356186. [DOI] [PubMed] [Google Scholar]
- 8.Lima A, Haussen DC, Rebello LC, Dehkharghani S, Grossberg J, Grigoryan M, et al. Endovascular therapy for large vessel stroke in the elderly: hope in the new stroke era. Cerebrovasc Dis. 2016;42:421–427. doi: 10.1159/000446852. [DOI] [PubMed] [Google Scholar]
- 9.Koizumi S, Ota T, Shigeta K, Amano T, Ueda M, Matsumaru Y, et al. Onset to reperfusion time was not important in mechanical thrombectomy for elderly patients: a retrospective multicenter study in Tama area, Tokyo. Cerebrovasc Dis. 2018;46:89–96. doi: 10.1159/000492867. [DOI] [PubMed] [Google Scholar]
- 10.Barral M, Lassalle L, Dargazanli C, Mazighi M, Redjem H, Blanc R, et al. Predictors of favorable outcome after mechanical thrombectomy for anterior circulation acute ischemic stroke in octogenarians. J Neuroradiol. 2018;45:211–216. doi: 10.1016/j.neurad.2018.01.055. [DOI] [PubMed] [Google Scholar]
- 11.Slawski DE, Salahuddin H, Shawver J, Kenmuir CL, Tietjen GE, Korsnack A, et al. Mechanical thrombectomy in elderly stroke patients with mild-to-moderate baseline disability. Interv Neurol. 2018;7:246–255. doi: 10.1159/000487333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon W, Kim SK, Park MS, Baek BH, Lee YY. Predictive factors for good outcome and mortality after stent-retriever thrombectomy in patients with acute anterior circulation stroke. J Stroke. 2017;19:97–103. doi: 10.5853/jos.2016.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization working group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon W, Kim SK, Park MS, Kim BC, Kang HK. Endovascular treatment and the outcomes of atherosclerotic intracranial stenosis in patients with hyperacute stroke. Neurosurgery. 2015;76:680–686. doi: 10.1227/NEU.0000000000000694. [DOI] [PubMed] [Google Scholar]
- 15.Larrue V, von Kummer R, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32:438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 16.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Mokin M, Ansari SA, McTaggart RA, Bulsara KR, Goyal M, Chen M, et al. Society of NeuroInterventional Surgery. Indications for thrombectomy in acute ischemic stroke from emergent large vessel occlusion (ELVO): report of the SNIS standards and guidelines committee. J Neurointerv Surg. 2019;11:215–220. doi: 10.1136/neurintsurg-2018-014640. [DOI] [PubMed] [Google Scholar]
- 18.Jayaraman MV, McTaggart RA. Endovascular treatment of anterior circulation large vessel occlusion in the elderly. Front Neurol. 2018;8:713. doi: 10.3389/fneur.2017.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imahori T, Tanaka K, Arai A, Shiomi R, Fujiwara D, Mori T, et al. Mechanical thrombectomy for acute ischemic stroke patients aged 80 years or older. J Stroke Cerebrovasc Dis. 2017;26:2793–2799. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.060. [DOI] [PubMed] [Google Scholar]
- 20.Ribo M, Flores A, Mansilla E, Rubiera M, Tomasello A, Coscojuela P, et al. Age-adjusted infarct volume threshold for good outcome after endovascular treatment. J Neurointerv Surg. 2014;6:418–422. doi: 10.1136/neurintsurg-2013-010786. [DOI] [PubMed] [Google Scholar]
- 21.Danière F, Lobotesis K, Machi P, Eker O, Mourand I, Riquelme C, et al. Patient selection for stroke endovascular therapy--DWI-ASPECTS thresholds should vary among age groups: insights from the RECOST study. AJNR Am J Neuroradiol. 2015;36:32–39. doi: 10.3174/ajnr.A4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–12. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]