Abstract
Educational attainment is associated with cognition among older adults, but this association is complex and not well understood. While associated with better cognition among healthy adults, more education predicts faster decline in older adults with cognitive impairment. Education may influence cognitive functioning through mechanisms involving brain maintenance (BM: reduced age-related pathology) or cognitive reserve (CR: altered pathology-cognition association). We examined evidence for each mechanism by quantifying main and interaction effects of education within a well-studied pathway involving systolic blood pressure (SBP), white matter hyperintensities (WMH), and episodic memory in two samples without dementia at baseline (total N =1,136). There were no effects of education on SBP or WMH, suggesting a lack of evidence for BM. In the sample less likely to progress to dementia, education attenuated the effect of WMH on memory at baseline. In the sample more likely to progress to dementia, education exacerbated this effect at baseline. These moderations provide evidence for a CR mechanism and are consistent with previous findings of faster decline once CR is depleted.
Keywords: cognitive aging, white matter hyperintensities, cognitive reserve, brain maintenance, episodic memory, moderation
1. Introduction
A wealth of data indicates that higher educational attainment is associated with better late-life cognitive functioning and reduced risk of dementia. One meta-analysis of available observational evidence estimated that having fewer years of education is associated with roughly twice the risk of dementia (Meng & D’Arcy, 2012), though this study was limited by inconsistent operationalization of education groups. Even more compelling evidence that increased educational attainment leads to reduced dementia risk comes from natural experiments and Mendelian randomization studies (Glymour et al., 2008; Nguyen et al., 2016; Okbay et al., 2016; Branigan et al., 2013; Ostergaard et al., 2015; Huang & Zhou, 2013). While many studies ascertained and quantified associations between education and cognition among older adults, fewer studies have investigated physiological processes affected by education that could underlie its apparent cognitive benefits. For example, it is not yet fully understood which components of the pathogenic pathways involved in age-related cognitive decline may be influenced by education.
A useful framework for approaching these questions can be found among the theoretical concepts of brain maintenance and cognitive reserve (Barulli & Stern, 2013). Brain maintenance refers to a relative lack of age-related brain pathology (e.g., white matter hyperintensities), which reflects individual differences in disease susceptibility (Nyberg et al., 2012). Reduced disease susceptibility could reflect an upstream protective effect of education on the development of risk factors for brain pathology. However, it may also reflect a direct protective effect of education on brain pathology independent of health risk factors and/or reduced negative impact of these risk factors on the accumulation of brain pathology. Finally, cognitive reserve refers to differences in cognitive and neural processes that sustain cognition in the face of brain pathology and is often exemplified by a weaker negative association between brain pathology and cognition (Barulli & Stern, 2013; Stern et al., 2018). Recently, cognitive reserve was operationalized as residual variance in episodic memory after accounting for brain and demographic variables (Reed et al., 2010; Zahodne et al., 2013; Zahodne et al., 2015). This operational definition was validated by demonstrating that residual episodic memory variance moderates associations between brain pathology and cognition cross-sectionally (Reed et al., 2010; Zahodne et al., 2015) and longitudinally (Zahodne et al., 2015; Bettcher et al., under revision), which is considered to be the strongest evidence for cognitive reserve (Stern et al., 2018). The current study uses this conceptual framework to investigate whether the role of education in cognitive aging reflects cognitive reserve versus another mechanism (i.e., brain maintenance). This question is pertinent given the continued use of education as a static proxy for reserve (Pettigrew & Soldan, 2019). (Education is considered static because the vast majority of older adults achieved their highest level of formal education by mid-life, so a variable reflecting educational attainment is not likely to fluctuate over the course of late life.)
In order to tease apart brain maintenance and cognitive reserve, the current study focused on one well-studied life course pathway in cognitive aging, in which a health risk factor has been linked to a marker of brain pathology that, in turn, has been linked to increased dementia risk. Specifically, we examined the role of education in a pathway involving systolic blood pressure (SBP), white matter hyperintensities (WMH), and episodic memory performance. Higher SBP, particularly when measured in mid-life, has been consistently linked to greater WMH in older adults (Dufouil et al., 2001; Rosano et al., 2015; Takami et al., 2012; Sierra, 2011). While this association is commonly interpreted as reflecting vascular mechanisms, it is also possible that it is driven by other pathways to injury, such as immune changes and neuroinflammation. In addition to consistent evidence for a link between SBP and WMH, we chose SBP as the primary health risk factor in this study because it is commonly measured in cohort studies of cognitive aging, shows substantial variability, and predicts both episodic memory (Gifford et al., 2013) and incident Alzheimer’s disease (AD) (Qiu, Winblad & Fratiglioni, 2005).
In turn, WMH volume has been shown to independently predict incident AD (Mortamais et al., 2014; Brickman et al., 2015; Birdsill et al., 2014). WMH volume is also negatively associated with episodic memory in older adult samples (Maillard et al., 2012; Ku et al., 2011; Parks et al., 2011; Fujishima et al., 2014), as well as an age-heterogeneous adult sample (Habes et al., 2016). We chose episodic memory as the cognitive outcome in this study because episodic memory impairment is typically the most prominent core deficit in preclinical AD (Bäckman, Small & Fratiglioni, 2001). Clinical AD has been linked to both vascular and non-vascular pathologies (Lee et al., 2016; Hane, Lee & Leonenko, 2017), with the majority of cases characterized by mixed pathologies (Barnes et al., 2015). Because episodic memory performance is, in part, influenced by genetic (e.g., APOE genotype; Lim et al., 2017; Han et al., 2007), sociodemographic (e.g., gender; Pauls, Petermann & Lepach, 2013), and health (e.g., diabetes; Pappas, Andel, Infurna & Seetharaman, 2017) factors, these variables were included as covariates in the current study. These and other factors may contribute to episodic memory via WMH, as well as through other brain pathologies (e.g., amyloid deposition; Sperling et al., 2013).
Current understanding of the protective effects of education on cognition is complicated by the well-replicated finding that more education is associated with better cognitive outcomes in healthy older adults, but faster cognitive decline in older adults with mild cognitive impairment (MCI) or dementia (Amieva et al., 2014; Scarmeas et al., 2006; Stern et al., 1999; Mungas et al., 2018). Another issue complicating the study of education as a protective factor is selection due to study recruitment, loss to follow up, study procedures, or death (Weuve et al., 2015). Indeed, aggregate associations between education and health decrease with age, which is often attributed to the strong inverse association between education and mortality (Dupre, 2007). Thus, interpreting associations between education and cognitive aging outcomes requires a consideration of age and disease stage, as well as selection bias, which is greater in samples with more attrition. These complexities highlight the value of using multiple, heterogeneous samples to study processes of brain maintenance and cognitive reserve.
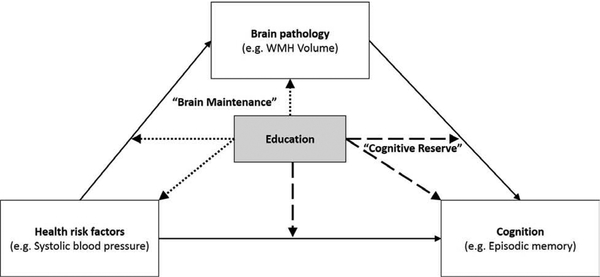
The aims of the current study were to (1) locate the protective effects of education along a pathogenic pathway in cognitive aging; and (2) examine whether patterns of association differ across two non-overlapping samples of older adults examined at different stages of a longitudinal epidemiological study. Within a single cross-sectional moderated mediation model, we simultaneously estimated main effects of education on (a) SBP, (b) WMH, and (c) episodic memory performance, as well as moderating effects of education on associations between (d) SBP and WMH and (e) WMH and episodic memory performance separately in the two samples. As depicted in Figure 1, negative main effects of education on SBP or WMH, and/or a weaker association between SBP and WMH, would provide evidence for brain maintenance. A positive main effect of education on episodic memory, or a weaker association between WMH and episodic memory, would provide evidence for cognitive reserve.
Figure 1.
Schematic of “brain maintenance” and “cognitive reserve.” Solid lines correspond to direct and indirect pathways. Dotted lines correspond to pathways that would provide evidence for brain maintenance. Dashed lines correspond to pathways that would provide evidence for Cognitive reserve.
2. Material and Methods
2.1. Participants
The older adults in this study participated in the Washington Heights-Inwood Columbia Aging Project (WHICAP), a prospective, community-based longitudinal study of aging and dementia in northern Manhattan. Full descriptions of study procedures and the larger WHICAP sample have been published previously (Tang et al., 2001; Manly et al., 2005). In brief, participants were identified from among Medicare-eligible residents of the geographic region of northern Manhattan in recruitment waves beginning in 1992, 1999, and 2009. Participants were eligible for recruitment in 1992 if they were age 65 or older, were fluent in English or Spanish, were willing to be visited for an in-person interview and examination, and were willing to be followed longitudinally. Eligibility criteria were the same in 1999 and 2009, except that participants were also excluded if they reported a dementia diagnosis or had serious memory complaints at screening. Participant follow-up occurred at approximate 24-month intervals. In the current study, education was operationalized as the highest year of education completed, ranging from 0 to 20.
2.1.1. 2005 Scan Sample
Starting in 2005, 769 active WHICAP participants who were initially enrolled during recruitment phases beginning in 1992 or 1999 and who were classified as dementia free at their most recent study visit received structural MRI. The subset of 623 participants included in the current study met the following inclusion criteria: (1) underwent at least partial neuropsychological evaluation at the time of their MRI, (2) did not meet criteria for dementia based on this neuropsychological evaluation, (3) had complete data on predictors (i.e., age, sex, education, chronic disease burden, APOE-ε4 status), and (4) had processed MRI data at the time of the current study. Characteristics of the 2005 scan sample are provided in Table 1. At the time of their initial enrollment in WHICAP, participants recruited in 1992 who were included in the current study were younger (71.95 versus 77.01; t(169.09) = 12.76; p < 0.001), reported fewer chronic diseases (0.81 versus 1.01; t(1179) = 2.13; p = 0.03), and had higher memory scores (0.40 versus −0.22; t(66.87) = −7.30; p < 0.001), compared with participants recruited in 1992 who were not included in the current study. At the time of their initial enrollment in WHICAP, participants recruited in 1999 who were included in the current study were younger (74.73 versus 77.64; t(1094.70) = 9.57; p < 0.001), were more likely to be non-Hispanic White (31.30% versus 29.14%; χ2(1) = 14.13; p = 0.003), reported more years of education (11.22 versus 9.95; t(892.793) = −5.38; p < 0.001), reported fewer chronic diseases (2.09 versus 2.41; t(921.53) = 4.49; p < 0.001), had higher memory scores (0.37 ve6rsus 0.04; t(1155.92) = −9.32; p < 0.001), and were less likely to have MCI (18.59% versus 23.65%; χ2(1) = 5.41; p = 0.02), compared with participants recruited in 1992 who were not included in the current study.
Table 1.
Characteristics of the two samples
| 2005 scan sample N=623 |
2011 scan sample N=513 |
p | |||
|---|---|---|---|---|---|
| Mean or % |
SD | Mean or % |
SD | ||
| Age (years) | 80.07 | 5.55 | 73.90 | 5.68 | <0.001 |
| APOE-ε4(%) | 25.68 | - | 32.36 | - | 0.013 |
| Sex (% female) | 67.74 | - | 56.73 | - | <0.001 |
| Race and ethnicity (%) | 0.192 | ||||
| Non-Hispanic White | 28.89 | - | 31.38 | - | |
| Non-Hispanic Black | 34.99 | - | 37.62 | - | |
| Hispanic | 36.12 | - | 30.99 | - | |
| Education (years; 0–20) | 10.82 | 4.72 | 12.82 | 4.47 | <0.001 |
| Chronic disease burden (count; 1–13) | 2.08 | 1.40 | 2.17 | 1.34 | 0.241 |
| Systolic blood pressure (mmHg) | 142.18 | 21.56 | 135.48 | 19.00 | <0.001 |
| WMH (cm3) | 3.55 | 5.80 | 5.34 | 6.89 | NA |
| Episodic memory (composite) | 0.12 | 0.74 | 0.47 | 0.70 | <0.001 |
| Prevalent MCI (%) | 23.93 | - | 17.97 | - | 0.015 |
| Number of visits before scan | 1.68 | 0.07 | 0.48 | 0.02 | <0.001 |
| Number of visits after scan | 1.46 | 0.06 | 0.83 | 0.04 | <0.001 |
| Years of follow-up after scan | 4.85 | 4.03 | 2.80 | 2.31 | <0.001 |
| Incident dementia (%) | 12.20 | - | 0.01 | - | <0.001 |
2.1.2. 2011 Scan Sample
Beginning in 2011, 552 newly-recruited WHICAP participants were invited to undergo high resolution structural MRI. The subset of 513 participants included in the present study met inclusion criteria identical to those used for the 2005 scan sample, as listed above. Characteristics of the 2011 scan sample are provided in Table 1. At the time of their initial enrollment in WHICAP, participants included in the current study were younger (72.99 versus 75.95; t(1108.65) = 10.14; p < 0.001), were less likely to be women (56.73% versus 70.17%; χ2(1) = 31.82; p < 0.001), were more likely to be non-Hispanic White (31.38% versus 17.64%; χ2(1) = 140.01; p < 0.001), reported more years of education (12.82 versus 10.08; t(1017.66) = −11.48; p < 0.001), reported fewer chronic diseases (2.81 versus 3.11; t(2113) = 3.72; p < 0.001), and had higher memory scores (0.50 versus 0.21; t(1031.70) = −7.95; p < 0.001), but were more likely to carry at least one APOE-ε4 allele (32.36% versus 25.02%; χ2(1) = 31.82; p < 0.001), compared to the 1,616 newly-recruited participants who were not included in the current study.
2.2. Systolic Blood Pressure
Standardized resting blood pressure measurements were made using the Dinamap Pro 100 (Critikon Co, Tampa, Florida). The cuff was placed on the right arm while the participant was seated, and recordings were obtained every 3 minutes across 9 minutes. The average of the three measurements was used in the current study.
2.3. Magnetic Resonance Imaging
2.3.1. 2005 Scan Sample
Magnetic resonance images were obtained on a 1.5T Philips Intera scanner at Columbia University Medical Center between 2005 and 2007. T1-weighted (repetition time = 20 ms, echo time = 2.1 ms, field of view 240 cm, 256 × 160 matrix, 1.3 mm slice thickness) and T2-weighted fluid attenuated inversion recovery (FLAIR; repetition time = 11,000 ms, echo time = 144.0 ms, inversion time = 2800 ms, field of view 25 cm, 2 nex, 256 × 192 matrix with 3 mm slice thickness) images were acquired in the axial orientation. WMH volumes were derived using previously-described procedures (Brickman, Muraskin, & Zimmerman, 2009; Brickman, et al., 2011; Brickman et al., 2012). Whole-brain WMH volumes were quantified from T2-weighted FLAIR images. In brief, images were skull stripped, and a Gaussian curve was fit to map voxel intensity values. Voxels above a standardized study-specific threshold of the image mean were labeled as WMH. Labeled images were also visually inspected and corrected if errors were detected. Total WMH volume was log-transformed prior to analysis.
2.3.2. 2011 Scan Sample
Magnetic resonance images were obtained on a 3.0T Philips Achieva scanner at Columbia University Medical Center between 2011 and 2014. T1-weighted (repetition time = 6.6 ms, echo time = 3.0 ms, field of view 256 cm, 256 × 256 matrix, 1.0 mm slice thickness) and T2-weighted FLAIR (repetition time = 8,000 ms, echo time = 332.0 ms, inversion time = 2400 ms, field of view = 240 × 240 × 180 mm with 1.2 mm slice thickness) images were acquired in the axial orientation. WMH volumes were derived using procedures identical to those used in the 2005 sample, as described above.
2.4. Episodic memory
Participants were interviewed and tested in their preferred language (English or Spanish). Factor analysis has shown that episodic memory is a distinct domain assessed by the WHICAP neuropsychological battery and is invariant across English and Spanish speakers (Siedlecki et al., 2010). In the current study, memory was assessed on the same day as the blood pressure assessment and, on average, 8 days after the MRI (median = −5 days). Memory measures include total immediate recall, delayed recall, and delayed recognition scores from the Selective Reminding Test (Buschke and Fuld, 1974). To compute an episodic memory composite score, raw scores on each of the three trial types were converted to z-scores using means and standard deviations from the first study visit in the larger WHICAP sample, and resultant z-scores were averaged.
After each visit, dementia diagnoses were made by a consensus of neurologists and neuropsychologists based on Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition criteria (American Psychiatric Association, 1987) using all available neuropsychological and interview data. Structural MRI data were not used for consensus diagnoses. MCI is operationalized in WHICAP using the following criteria: (1) presence of a memory complaint, (2) objective impairment in at least one cognitive domain based on the average of scores on neuropsychological measures within that domain and a 1.5 SD cutoff using corrections for age, years of education, ethnicity, and sex and based on previously established norms, (3) essentially preserved activities of daily living, (4) no diagnosis of dementia at the consensus conference, and (5) preserved general cognitive function (Manly et al., 2008).
2.5. Covariates
Moderated mediation models controlled for age, age squared, sex, race and ethnicity, chronic disease burden, and APOE-ε4 status. Age was operationalized as age in years at the time of the neuropsychological assessment. Sex was dichotomized with men as the reference group. Race and ethnicity were dummy-coded into non-Hispanic Black, Hispanic, and non-Hispanic White, with non-Hispanic White as the reference group. In line with previous published studies in WHICAP, chronic disease burden was the sum of the presence/absence of the following 13 conditions: diabetes, heart disease, stroke, arthritis, chronic obstructive pulmonary disease (COPD), thyroid disease, liver disease, renal disease, ulcer, peripheral vascular disease, cancer, Parkinson’s disease, essential tremor. Given that each of these conditions may be differently associated with WMH and memory, a sensitivity analysis was conducted to confirm that the pattern of results did not differ if only those conditions that were significantly associated with WMH or memory within each scan sample were included as separate dichotomous covariates. In the 2005 scan sample, conditions included in this sensitivity analysis were stroke and cancer. In the 2011 scan sample, conditions included in this sensitivity analysis were diabetes, COPD, thyroid disease, peripheral vascular disease, and essential tremor. Genotyping in WHICAP has been described previously (Mayeux et al., 1995). APOE-ε4 status was a dichotomous variable representing the presence of at least one APOE-ε4 allele.
2.6. Statistical Analyses
Descriptive statistics and scan sample comparisons (e.g., chi square tests, independent samples t-tests, Cox regression) were conducted in SPSS 24 (IBM Corp., Armonk, NY). Moderated mediation models were conducted in Mplus version 8 (Muthén & Muthén, Los Angeles, CA). Separate models were conducted in the 2005 and 2011 scan samples. Within each scan sample, episodic memory composite scores were regressed onto WMH volume and SBP, and WMH volume was regressed onto SBP within the same model. The indirect effect of SBP on episodic memory through the mediator (i.e., WMH volume) was defined as the product of the SBP-WMH association and the WMH-episodic memory association. The direct effect of SBP on episodic memory was defined as the association between SBP and episodic memory, independent of WMH.
Within the same model, all three variables of interest (SBP, WMH, episodic memory) were regressed onto educational attainment and all covariates. Thus, education was allowed to be both an upstream exposure that could affect episodic memory directly, as well as indirectly through its influence on SBP and WMH. In addition, two multiplicative interaction terms (for education and SBP and for education and WMH volume) were computed to test for potential moderation of the direct and indirect effects of SBP on episodic memory by educational attainment. Thus, education was also modeled as a moderator. To evaluate whether education moderates the indirect effect of SBP on episodic memory through WMH, WMH volume was regressed onto the product of education and SBP, and episodic memory was regressed onto the product of education and WMH volume. With regard to education moderation of the SBP direct effect, episodic memory was also regressed onto the product of education and SBP. Covariates were included in all moderated mediation models.
3. Results
3.1. Differences between 2005 and 2011 scan samples
Table 1 summarizes characteristics of the 2005 and 2011 scan samples. Compared to participants scanned in 2005, participants scanned in 2011 were younger, were more likely to carry at least one copy of the APOE-ε4 allele, were less likely to be women, reported more years of education, had lower average SBP, scored better on episodic memory tests, were less likely to have MCI at the time of their MRI, had been participating in the longitudinal study for less time when they underwent MRI, completed fewer follow-up visits between their MRI and the time of the current analyses, and were less likely to experience incident dementia between their MRI and 2018. These differences remained significant after controlling for age, with the exception of APOE-ε4 frequency and MCI prevalence. The cohorts were similar in terms of racial and ethnic composition and burden of chronic diseases at the time of the MRI scan.
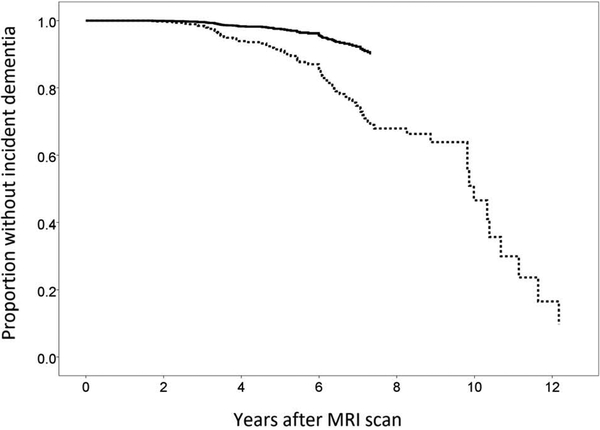
Figure 2 displays the results of a survival analysis comparing incident dementia across the two scan samples, controlling for age at the time of the MRI scan, age squared, and years of follow-up. Among the 623 participants in the 2005 sample, 451 had available data at a first follow-up visit (28–24 months later), 295 had available data at a second follow-up visit, 204 had available data at a third follow-up visit, 83 had available data at a fourth follow-up visit, and 19 had available data at a fifth follow-up visit. Among the 513 participants in the 2011 sample, 421 had available data at a first follow-up visit, 201 had available data at a second follow-up visit, and 33 had available data at a third follow-up visit. Compared to participants scanned in 2005, participants scanned in 2011 had a lower rate of incident dementia (hazard ratio = 0.28; 95% CI: 0.11 to 0.68).
Figure 2.
Incident dementia by sample. Depicted curves control for age at MRI scan and length of follow-up (i.e., number of study visits after MRI scan)
3.2. Estimated education effects in the 2005 scan sample
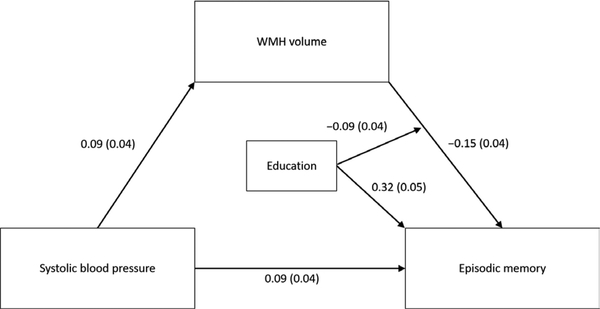
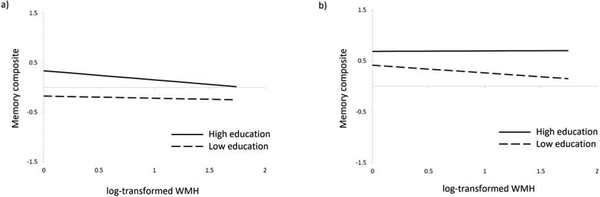
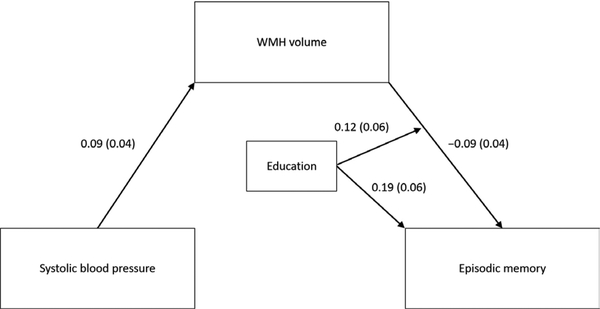
In an initial mediation model estimating all possible effects of education, there were no main effects of education on SBP (β = −0.03; SE = 0.05; p = 0.53) or WMH volume (β = −0.02; SE = 0.05; p = 0.64) and no significant interactions between education and SBP on WMH volume (β = 0.00; SE = 0.04; p = 0.98) or episodic memory (β = −0.00; SE = 0.04; p = 0.97), so these paths were removed from the model. Results of the final model estimated in the 2005 sample are shown in Table 2 and Figure 3. Higher SBP was associated with greater WMH volume. In turn, greater WMH volume was associated with lower episodic memory. As depicted in Figure 4a, education modified the association between WMH and episodic memory such that individuals with higher education showed a stronger negative association. Education was also positively associated with episodic memory. Controlling for WMH volume, education, and all covariates, higher SBP was associated with better episodic memory. This pattern of results did not change when the chronic disease burden covariate was replaced with separate dichotomous indicators of stroke and cancer, the only two conditions significantly associated with WMH or memory in this scan sample.
Table 2.
Standardized regression results from 2005 mediation model
| β | SE | p | |
|---|---|---|---|
| SBP on | |||
| Age | 0.05 | 0.04 | 0.295 |
| Age squared | −0.07 | 0.04 | 0.090 |
| Female | 0.04 | 0.04 | 0.298 |
| Black | 0.03 | 0.05 | 0.590 |
| Hispanic | 0.07 | 0.05 | 0.175 |
| Chronic diseases | −0.06 | 0.04 | 0.118 |
| APOE-ε4 | 0.00 | 0.04 | 0.958 |
| WMH on | |||
| Age | 0.19 | 0.04 | <0.001 |
| Age squared | −0.02 | 0.04 | 0.683 |
| Female | −0.01 | 0.04 | 0.849 |
| Black | 0.25 | 0.05 | <0.001 |
| Hispanic | 0.14 | 0.05 | 0.003 |
| Chronic diseases | 0.02 | 0.04 | 0.703 |
| APOE-ε4 | 0.02 | 0.04 | 0.653 |
| SBP | 0.09 | 0.04 | 0.022 |
| Memory on | |||
| Age | −0.24 | 0.04 | <0.001 |
| Age squared | −0.05 | 0.04 | 0.213 |
| Female | 0.18 | 0.04 | <0.001 |
| Black | −0.19 | 0.04 | <0.001 |
| Hispanic | −0.03 | 0.05 | 0.629 |
| Chronic diseases | −0.01 | 0.04 | 0.853 |
| APOE-ε4 | −0.03 | 0.04 | 0.466 |
| Education | 0.32 | 0.05 | <0.001 |
| SBP | 0.09 | 0.04 | 0.013 |
| WMH | −0.15 | 0.04 | <0.001 |
| Education × WMH | −0.09 | 0.04 | 0.031 |
Note. SBP = Systolic blood pressure; WMH = White matter hyperintensities
Figure 3.
Results of moderated mediation model in 2005 sample. Estimates correspond to standardized regression coefficients (standard errors). For simplicity, nonsignificant paths (i.e., main effects of education on systolic blood pressure and WMH volume; interactions between education and systolic blood pressure) were excluded. Model controlled for age, sex, race and ethnicity, chronic disease burden, and APOE-ε4 status.
Figure 4.
Education by white matter hyperintensity volume interactions in the (a) 2005 sample and (b) 2011 sample. In each panel, low and high education correspond to 1 SD below and above the mean within each sample. In the 2005 sample, low and high education correspond to 6 and 16 years, respectively. In the 2011 sample, low and high education correspond to 8 and 17 years, respectively. Note. WMH = white matter hyperintensities.
3.3. Estimated education effects in the 2011 scan sample
In an initial mediation model estimating all possible effects of education, there were no main effects of education on SBP (β = −0.05; SE = 0.06; p = 0.42) or WMH volume (β = −0.07; SE = 0.06; p = 0.20) and no significant interactions between education and SBP on WMH volume (β = 0.02; SE = 0.05; p = 0.71) or episodic memory (β = −0.07; SE = 0.04; p = 0.11), so these paths were removed from the model. Results of the final model estimated in the 2011 scan sample are shown in Table 3 and Figure 5, Higher SBP was associated with greater WMH volume. In turn, greater WMH volume was associated with lower episodic memory. As depicted in Figure 4b, education modified the association between WMH and episodic memory such that individuals with higher education showed a weaker negative association. Education was also positively associated with episodic memory. Controlling for WMH volume, education, and all covariates, SBP was not meaningfully associated with episodic memory. This pattern of results did not change when the chronic disease burden covariate was replaced with separate dichotomous indicators of diabetes, COPD, thyroid disease, peripheral vascular disease, and essential tremor, which were the only conditions significantly associated with WMH or memory in this scan sample.
Table 3.
Standardized regression results from 2011 mediation model
| β | SE | p | |
|---|---|---|---|
| SBP on | |||
| Age | 0.07 | 0.07 | 0.329 |
| Age squared | −0.05 | 0.07 | 0.446 |
| Female | 0.05 | 0.04 | 0.254 |
| Black | 0.14 | 0.05 | 0.006 |
| Hispanic | 0.05 | 0.05 | 0.369 |
| Chronic diseases | 0.01 | 0.04 | 0.804 |
| APOE-ε4 | −0.12 | 0.04 | 0.006 |
| WMH on | |||
| Age | 0.14 | 0.07 | 0.043 |
| Age squared | −0.03 | 0.07 | 0.715 |
| Female | −0.07 | 0.04 | 0.117 |
| Black | 0.04 | 0.05 | 0.389 |
| Hispanic | −0.06 | 0.05 | 0.221 |
| Chronic diseases | 0.06 | 0.04 | 0.157 |
| APOE-ε4 | 0.03 | 0.04 | 0.566 |
| SBP | 0.09 | 0.04 | 0.048 |
| Memory on | |||
| Age | −0.22 | 0.06 | <0.001 |
| Age squared | 0.04 | 0.06 | 0.533 |
| Female | 0.17 | 0.04 | <0.001 |
| Black | −0.14 | 0.05 | 0.004 |
| Hispanic | −0.10 | 0.06 | 0.069 |
| Chronic diseases | −0.06 | 0.04 | 0.134 |
| APOE-ε4 | −0.08 | 0.04 | 0.053 |
| Education | 0.19 | 0.06 | 0.003 |
| SBP | −0.02 | 0.04 | 0.646 |
| WMH | −0.09 | 0.04 | 0.019 |
| Education × WMH | 0.12 | 0.06 | 0.025 |
Note. SBP = Systolic blood pressure; WMH = White matter hyperintensities
Figure 5.
Results of moderated mediation model in 2011 sample. Estimates correspond to standardized regression coefficients (standard errors). For simplicity, nonsignificant paths (i.e., main effects of education on systolic blood pressure, WMH, and episodic memory, main effect of systolic blood pressure on episodic memory, interactions between education and systolic blood pressure) were excluded. Model controlled for age, sex, race and ethnicity, chronic disease burden, and APOE-ε4 status.
3.4. Racial and Ethnic Differences
Sensitivity analyses that included interaction terms were conducted in order to test whether the identified education effects differed across the three racial ethnic groups represented in this study: Hispanic, non-Hispanic Black, and non-Hispanic White. Because the current study may not have been adequately powered to detect certain interactions (e.g., three-way interaction between race, education, and WMH), parameter estimates from fully stratified models were also examined. The only education effect that differed significantly between groups was the main effect of education on episodic memory in the 2005 sample (β= −0.42; SE = 0.18; p = 0.020). Though positive and significant in all groups, the association between education and episodic memory was weaker in the Hispanic group than in the non-Hispanic White group in the 2005 sample only. Fully stratified models presented in Supplementary Table 1 revealed consistent parameter estimates across the three groups in both 2005 and 2011, though some of these estimates did not reach statistical significance in these relatively smaller subsamples.
4. Discussion
This study examined the effects of education on the pathway between blood pressure (SBP), white matter hyperintensities (WMH), and episodic memory in two non-overlapping samples of racially and ethnically diverse older adults recruited at different phases of a longitudinal study (Figures 2 and 3). Results common to both samples were that higher SBP was associated with greater WMH volume, which in turn was associated with lower episodic memory, and higher education was independently associated with higher episodic memory. In both samples, education was not associated with WMH volume or SBP (i.e., the upstream predictor of WMH). Therefore, these results do not provide evidence that education promotes brain maintenance.
In contrast, the moderating effects of education on the association between WMH and episodic memory differed across the two scan samples in a manner consistent with theoretical models of cognitive reserve, as well as recent longitudinal findings (Mungas et al., 2018). Specifically, education weakened the association between WMH and episodic memory in the 2011 scan sample but strengthened the association in the 2005 sample, which likely had higher degrees of pathology given that they were older, had higher SBP, performed worse on memory tests, were more likely to have prevalent MCI, and exhibited faster progression to dementia after MRI. This pattern of results may suggest that the protective effect of education is limited to earlier stages of age-related pathology. In later stages, on the other hand, exacerbation of the negative cognitive effects of brain pathology may reflect the depletion of cognitive reserve. In a recent study that systematically examined the moderating effect of education on verbal episodic memory across a spectrum of gray matter atrophy, education appeared protective when gray matter change was minimal (i.e., gray matter change that was 0.1 SD slower than average in the sample), inconsequential when gray matter atrophy was moderate (i.e., standardized gray matter change = −0.3), and disadvantageous when gray matter atrophy was more severe (standardized gray matter change = −0.6) (Mungas et al., 2018).
In sum, these findings suggest that the role of education is more consistent with cognitive reserve than brain maintenance, at least with regard to the pathway linking SBP and WMH to episodic memory. Together, these results demonstrate challenges in studying the dynamic construct of cognitive reserve using static proxy measures and highlight issues of study design and selection. They also suggest that disease stage should be taken into account when interpreting the predictive utility of education.
The finding that education buffered the negative impact of WMH on cognitive functioning in the younger (2011) sample scanned nearer the time of study recruitment is consistent with several previous studies (Dufouil et al., 2003; Mortamais et al., 2014; Nebes et al., 2006). According to the theory of cognitive reserve, life experiences such as higher educational attainment may increase older adults’ ability to maintain episodic memory in the face of advancing brain pathology by altering the functional characteristics of brain networks (Barulli & Stern, 2013). Specifically, education may improve the efficiency and/or capacity of the primary brain network(s) involved in episodic memory. Education may also enhance the brain’s flexibility, for example by facilitating the recruitment of alternate networks to support or supplant primary networks when they become compromised or overtaxed (i.e., compensation). Indeed, evidence from task-based functional neuroimaging studies points to differential network expression as a function of education and other proxies for cognitive reserve (Stern et al., 2005; Scarmeas et al., 2003). In a recent fMRI study of older adults with high levels of WMH, education was positively associated with the recruitment of additional brain networks not expressed by younger adults during a working memory task (Fernández-Cabello et al., 2016).
The absence of a significant association between education and WMH volume in either sample contrasts with a recent European study that found a significant negative association between education and WMH volume (Habes et al., 2016). However, that study included an extremely wide age range (20–90), making it difficult to compare to the current study of older adults. Nonetheless, the current pattern of results provides greater support for cognitive reserve than brain maintenance with regard to the protective effects of education on the pathway involving SBP, WMH, and episodic memory. It is also important to note that while the absence of significant associations between education and WMH or its antecedent (i.e., SBP) in the current study argues against a brain maintenance process, it is still possible that education protects against other types of neuropathology, such as amyloid plaques. However, findings regarding the association between education and amyloid have been mixed (negative association: Landau et al., 2012; Yasuno et al., 2015; no association: Del Ser et al., 1999; Vemuri et al., 2012).
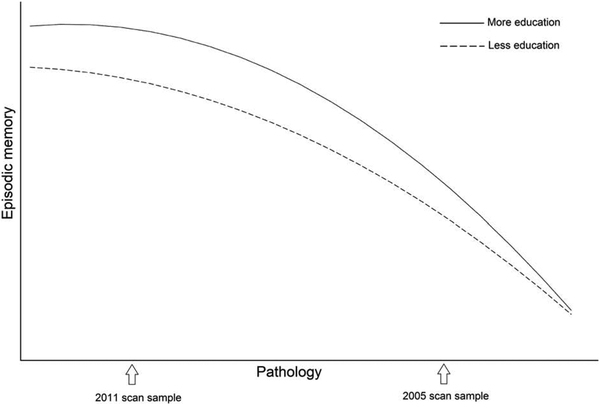
Our finding of opposite moderating effects of education on the association between WMH and episodic memory in the two scan samples (i.e., buffering in the 2011 sample but exacerbating in the 2005 sample) may reflect known differences in the effects of education at different stages of dementia, in line with theoretical models of the effects of cognitive reserve (Stern 2010) that suggest a “compression of morbidity” or maintenance of cognition for a longer period followed by a more precipitous decline. Specifically, individuals in the 2005 scan sample, on average, may have been further into a prodromal stage of dementia than the 2011 sample. Indeed, the 2005 sample comprised a larger proportion of individuals who met criteria for MCI at the time of their MRI scan, compared with the 2011 sample. They also exhibited faster progression to dementia after undergoing MRI, controlling for their older age and longer follow-up period. While education is known to delay the onset of cognitive decline, it also predicts faster disease progression in older adults with MCI or dementia (Amieva et al., 2014; Scarmeas et al., 2006; Stern et al., 1999). This compression of cognitive morbidity indicates that the protective effects of education may diminish as brain pathology advances, as depicted in the theoretical model shown in Figure 6.
Figure 6.
Theoretical rendering of cognitive reserve, modified from Barulli & Stern (2013). Earlier in the process of age-related disease (i.e., left half of Figure 6), individuals with more education are predicted to show a weaker association (i.e., shallower slope) between pathology (all types) and episodic memory than individuals with less education, in line with the findings in the 2011 scan sample. As pathology continues to accumulate (i.e., right half of Figure 6), individuals with more education are predicted to show a stronger association (i.e., steeper slope) between pathology and episodic memory than individuals with less education, in line with the findings in the 2005 scan sample. This model is also consistent with the findings of Brickman et al., (2011) in that, at any given level of cognition (i.e., at any point on the y-axis), individuals with more education are predicted to have more advanced age-related pathology than individuals with less education.
A recent longitudinal study in a sample of older adults exhibiting a wide range of brain atrophy rates provides empirical support for the theoretical model depicted in Figure 6. Specifically, education buffered the association between gray matter volume loss and episodic memory among individuals with slow rates of atrophy and exacerbated this association among individuals with fast rates of atrophy (Mungas et al., 2018). Importantly, the model of compression of cognitive morbidity predicted by the theory of cognitive reserve shown in Figure 6 still allows for a positive main effect of education on cognitive performance, as observed for the 2005 scan sample in the current study. This theoretical model is also consistent with results from a previous analysis of WMH and cognition in the 2005 WHICAP scan sample, in which better performance on tests of speed and language was associated with greater WMH volume after controlling for education and reading ability (Brickman et al., 2011). At any given level of cognition (i.e., at any point on the y-axis), individuals with more education are predicted to have more advanced age-related pathology than individuals with less education, as indicated by the right shift corresponding to more education.
Another explanation for why more education was associated with a stronger negative association between WMH and episodic memory in the 2005 scan sample is that this sample may have been more highly selected than the 2011 scan sample due to mortality and study attrition. In terms of the potential for mortality selection, the 2005 scan sample was significantly older than the 2011 scan sample. In terms of the potential for study attrition to contribute to differential sample selection in the 2005 and 2011 scan samples, the 2005 scan sample comprised participants enrolled in 1992 or 1999 who remained in the study and dementia-free for at least six years, while the 2011 scan sample comprised newly-enrolled, dementia-free participants. Consistent with the possibility that the 2005 scan sample was more highly selected, the prevalence of the APOE-ε4 allele was lower in the 2005 scan sample than in the 2011 scan sample.
Low education is a powerful predictor of mortality (Hummer & Hernandez, 2013; Galiea et al., 2011), attrition (Gottesman et al., 2014), and dementia (Ott et al., 1995). Therefore, lower levels of education may be a marker of resilience among people who live to older ages, continue study participation, and remain free of dementia (i.e., are more highly selected). Thus, the weaker negative impact of WMH on episodic memory among individuals with less education compared to individuals with more education in the 2005 scan sample may not reflect a causal effect of education. Selection bias may also underlie the positive direct effect of SBP on episodic memory in the 2005 scan sample, as individuals most susceptible to the negative effects of high blood pressure may have been more likely to die, leave the study, or develop dementia prior to 2005. However, it is also possible that this positive direct effect of SBP on episodic memory is causal, as elevated blood pressure may protect against the negative cognitive impact of chronic hypoperfusion in frail older adults (Reutenberg et al., 2005). Finally, it is also possible that sample differences could reflect cohort differences, as there was an approximate 10-year difference in the average birth year across the two samples. However, it is notable that birth cohort differences are often explained in terms of differences in education and health, which were included in the current models.
Limitations of this study include the use of different MRI scanners for the 2005 and 2011 scan samples (i.e., 1.5T and 3.0T, respectively), which precluded our ability to directly compare brain characteristics of the two samples. The numerically higher mean WMH volume in the 2011 sample compared to the 2005 sample likely reflects differences in sensitivity related to field strength (Stankiewicz et al., 2011; Wattjes et al., 2006). Of note, we used identical procedures for quantifying WMH to maximize the comparability of the stratified models. Additional concordance in study catchment area, recruitment methods, neuropsychological procedures, and study personnel also help to minimize sample differences. Strengths of the study include the racial, ethnic, and educational diversity of the sample, which increases the generalizability of results. Indeed, sensitivity analyses indicated that the pattern of effects were similar across Hispanic, non-Hispanic Black, and non-Hispanic White subgroups, though there was some evidence that more education was less strongly associated with better episodic memory among Hispanics tested in 2005. This difference may reflect differences in the educational experiences of Hispanics in WHICAP, as most were educated outside of the United States. Another important strength of this study is the inclusion of both cognitive and brain metrics, which allowed us to simultaneously test for brain maintenance and cognitive reserve simultaneously within a single model.
5. Conclusions
This large study of racially, ethnically, and educationally diverse older adults provides evidence that education may be a component of (and proxy for) cognitive reserve along the pathway from SBP to WMH volume to episodic memory. Results did not support a brain maintenance role for education in this pathway. The protective effect of education may be limited to earlier stages of age-related pathology, while showing a reverse effect in later stages in line with the depletion of cognitive reserve. Differing directions for the moderating effect of education on the WMH-episodic memory link highlight the importance of considering the timing of MRI during the course of a longitudinal study due to attrition and/or the dynamic nature of cognitive reserve.
Supplementary Material
6. Acknowledgements
Data collection and sharing for this project was supported by the Washington Heights-Inwood Columbia Aging Project (WHICAP, P01AG07232, R01AG037212, RF1AG054023) funded by the National Institute on Aging (NIA). This manuscript has been reviewed by WHICAP investigators for scientific content and consistency of data interpretation with previous WHICAP Study publications. We acknowledge the WHICAP study participants and the WHICAP research and support staff for their contributions to this study. This publication was also supported by the NIA (R01AG054520, R00AG047963, R01AG031563, R00AG053410), the Advanced Psychometrics Methods in Cognitive Aging Research Conference (R13AG030995), and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no conflicts of interest.
References
- Amieva H, Mokri H, Le Goff M, Meillon C, Jacqmin-Gadda H, Foubert-Samier A, Orgogozo JM, Stern Y, Dartigues JF, 2014. Compensatory mechanisms in higher-educated subjects with Alzheimer’s disease: a study of 20 years of cognitive decline. Brain 2014;137(Pt 4):1167–1175. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Small BJ, Fratiglioni L: Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain 2001; 124(Pt1):96–102. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Leurgans S, Aggarwal NT, Shah RC, Arvanitakis Z, et al. Mixed pathology is more likely in black than white decedents with Alzheimer’s disease. Neurology 2015;85:528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci 2013;17:502–509. doi: 10.1016/j.tics.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsill AC, Koscik RL, Jonaitis EM, Johnson SC, Okonkwo OC, … Bendlin BB. Regional white matter hyperintensities: aging, Alzheimer’s disease risk, and cognitive function. Neurobiol Aging 2014;35:769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branigan AR, McCallum KJ, Freese J. Variation in the Heritability of Educational Attainment: An International Meta-Analysis. Social Forces 2013;92(1):109–140. [Google Scholar]
- Brickman AM, Siedlecki KL, Muraskin J, Manly J, Luchsinger JA, Yeung LK, Brown TR, DeCarli C, Stern Y. White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiol Aging 2011;32:1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Zahodne LB, Guzman VA, Narkhede A, Meier IB … Mayeux R. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer’s disease incidence. Neurobiol Aging 2015;36:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caamano-Isorna F, Corral M, Montes-Martinez A, Takkouche B. Education and dementia: a meta-analytic study. Neuroepidemiology 2006;26(4):226–232. doi: 10.1159/000093378 [DOI] [PubMed] [Google Scholar]
- Deary IJ, Johnson W. Intelligence and education: causal perceptions drive analytic processes and therefore conclusions. Int J Epidemiol 2010;39(5):1362–1369. doi: 10.1093/ije/dyq072 [DOI] [PubMed] [Google Scholar]
- Del Ser T, Hachinski V, Merskey H, Munoz DG. An autopsy-verified study of the effect of education on degenerative dementia. Brain 1999;122(Pt 12):2309–2319. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Alpérovitch A, Tzourio C. Influence of education on the relationship between white matter lesions and cognition. Neurology 2003;60:831–836. [DOI] [PubMed] [Google Scholar]
- Dufouil C, de Kersaint-Gilly A, Besançon V, Levy C, Auffray E, … Tzourio C. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI cohort. Neurology 2001;56:921–926. [DOI] [PubMed] [Google Scholar]
- Dupre ME. Educational differences in age-related patterns of disease: reconsidering the cumulative disadvantage and age-as-leveler hypotheses. J Health and Soc Beh 2007;48:1–15. [DOI] [PubMed] [Google Scholar]
- Fernández-Cabello S, Valls-Pedret C, Schurz M, et al. White matter hyperintensities and cognitive reserve during a working memory task: a functional magnetic resonance imaging study in cognitively normal older adults. Neurobiol Aging 2016;48:23–33. [DOI] [PubMed] [Google Scholar]
- Fujishima M, Maikusa N, Nakamura K, Nakatsuka M, Matsuda H, Meguro K. Mild cognitive impairment, poor episodic memory, and late-life depression are associated with cerebral cortical thinning and increased white matter hyperintensities. Front Aging Neurosci 2014;6:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea S, Tracy M, Hoggatt KJ, DiMaggio C, Karpati A. Estimated deaths attributable to social factors in the United States. Am J Public Health 2011;101:1456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford KA, Badaracco M, Liu D, Tripodis Y, Gentile A, Lu Z, Palmisano J, Jefferson AL. Blood pressure and cognition among older adults: a meta-analysis. Arch Clin Neuropsychol 2013;28:649–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour MM, Kawachi I, Jencks CS, Berkman LF. Does childhood schooling affect old age memory or mental status? Using state schooling laws as natural experiments. J Epidemiol Community Health 2008;62(6):532–537. doi: 10.1136/jech.2006.059469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin C America’s graduation from high school: The evolution and spread of secondary schooling in the twentieth century. Journal of Economic History 1998;58:345–374. doi: 10.1017/S0022050700020544 [DOI] [Google Scholar]
- Gottesman RF, Rawlings AM, Sharrett AR et al. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC neurocognitive study. Am J Epidemiol 2014;179:956–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habes M, Erus G, Toledo JB, Zhang Y, Bryan N, … Davatzikos C. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 2016;139:1164–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, et al. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol Aging 2007;28:2398–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane FT, Blee BY, Leonenko Z. Recent progress in Alzheimer’s disease research, part 1: Pathology. J Alzheimers Dis 2017;57:1–28. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Alonso A, Logroscino G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology 2008;19:448–450. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhou Y. Effects of education on cognition at older ages: evidence from China’s Great Famine. Soc Sci Med 2013;98:54–62. doi: 10.1016/j.socscimed.2013.08.021 [DOI] [PubMed] [Google Scholar]
- Hummer RA, Hernandez EM. The effect of educational attainment on adult mortality in the United States. Popul Bull 2013; 68:1–16. [PMC free article] [PubMed] [Google Scholar]
- Ku BD, Na DL, Moon SY, Kim SY, Seo SW, … Han SH. Neuropsychological correlates of the proportional impact of white matter hyperintensities on mild to moderate dementia: the MRI 300 study. Dement Geriatr Cogn Disord 2011;31:397–405. [DOI] [PubMed] [Google Scholar]
- Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O’Neil JP, … Jagust WJ. Association of lifetime cognitive engagement and low beta-amyloid deposition. Arch Neurol 2012;69(5):623–629. doi: 10.1001/archneurol.2011.2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Viqar F, Zimmerman ME, Narkhede A, Tosto G, Benzinger TL, … Brickman AM; Dominantly Inherited Alzheimer Network. Ann Neurol 2016;79:929–939. Doi: 10.1002/ana.24647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Williamson R, Laws SM, Villemagne VL, Bourgeat P, Fowler C, et al. Effect of APOE genotype on amyloid deposition, brain volume, and memory in cognitively normal older individuals. J Alzheimers Dis 2017;58:1293–1302. [DOI] [PubMed] [Google Scholar]
- Maillard P, Carmichael L, Fletcher E, Reed B, Mungas D, DeCarli C. Coevolution of white matter hyperinsities and cognition in the elderly. Neurology 2012;79:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol 2008;63:494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Touradji P, Tang MX, Stern Y. Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol 2003;25:680–690. [DOI] [PubMed] [Google Scholar]
- Meng X, D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One 2012;7(6):e38268. doi: 10.1371/journal.pone.0038268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M, Artero S, Ritchie K. White matter hyperintensities as early and independent predictors of Alzheimer’s disease risk. J Alzheimers Dis 2014;42:S393–S400. [DOI] [PubMed] [Google Scholar]
- Mortamais M, Portet F, Brickman AM, Provenzano FA, Muraskin J, … Artero S. Education modulates the impact of white matter lesions on the risk of mild cognitive impairment and dementia. Am J Geriatr Psychiatry 2014;22:1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Gavett B, Fletcher E, Tomaszewski Farias S, DeCarli C, Reed B. Education amplifies brain atrophy effect on cognitive decline: implications for cognitive reserve. Neurobiology of Aging 2018;68:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebes RD, Meltzer CC, Whyte EM, Scanlon JM, Halligan EM, … Dekosky ST. The relation of white matter hyperintensities to cognitive performance in the normal old: education matters. Neurospychol Dev Cogn B Aging Neuropsychol Cogn 2006;13:326–340. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Tchetgen Tchetgen EJ, Kawachi I, Gilman SE, Walter S, Liu SY, … Glymour MM Instrumental variable approaches to identifying the causal effect of educational attainment on dementia risk. Ann Epidemiol 2016;26(1):71–76.e71–73. doi: 10.1016/j.annepidem.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Lovden M, Riklund K, Lindenberger U, Backman L. Memory aging and brain maintenance. Trends Cogn Sci 2012;16(5):292–305. doi: 10.1016/j.tics.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Okbay A, Beauchamp JP, Fontana MA, Lee JJ, Pers TH, Rietveld CA., … Benjamin DJ. Genome-wide association study identifies 74 loci associated with educational attainment. Nature 2016;533(7604):539–542. doi: 10.1038/nature17671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard SD, Mukherjee S, Sharp SJ, Proitsi P, Lotta LA, Day F, … Scott RA. Associations between Potentially Modifiable Risk Factors and Alzheimer Disease: A Mendelian Randomization Study. PLoS Med 2015;12(6): e1001841; discussion e1001841. doi: 10.1371/journal.pmed.1001841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Breteler MM, van Harskamp F, Claus JJ, van der Cammen TJ, Grobbee DE, Hofman A. Prevalence of Alzheimer’s disease and vascular dementia: association with education. The Rotterdam study. BMJ 1995; 310: 970–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas C, Andel R, Infurna FJ, Seetharaman S. Glycated haemoglobin (HbA1c), diabetes and trajectories of change in episodic memory performance. J Epidemiol Community Health 2017;71:115–120. [DOI] [PubMed] [Google Scholar]
- Parks CM, Iosif AM, Farias S, Reed B, Mungas D, DeCarli C. Executive function mediates effects of white matter hyperintensities on episodic memory. Neuropsychologia 2011;49:2817–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls F, Petermann F, Lepach AC. Gender differences in episodic memory and visual working memory including the effects of age. Memory 2013;21:857–874. [DOI] [PubMed] [Google Scholar]
- Pettigrew C, Soldan A. Defining cognitive reserve and implications for cognitive aging. Curr Neurol Neurosci Rep 2019;19:1 Doi: 10.1007/s11910-019-0917-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 2005;4:487–499. [DOI] [PubMed] [Google Scholar]
- Reed BR, Mungas D, Tomaszewski Farias S, Harvey D, Beckett L, Widaman K, et al. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain 2010;133:2196–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Abebe KZ, Aizenstein HJ, Boudrea R, Jennings JR, … Newman AB. Longitudinal systolic blood pressure characteristics and integrity of white matter tracts in a cohort of very old black and white adults. Am J Hypertension 2015;28:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol 2005; 57: 789–794. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Albert SM, Manly JJ, Stern Y. Education and rates of cognitive decline in incident Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2006;77:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Anderson KE, Hilton J, Flynn J, et al. Cognitive reserve modulates functional brain responses during memory tasks: a PET study in healthy young an delderly subjects. NeuroImage 2003;19:1215–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra C Associations between ambulatory blood pressure parameters and cerebral white matter lesions. Int J Hypertens 2011; 478710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Johnson KA, Doraiswamy PM, Reiman EM, Fleisher AS et al. Amyloid deposition detected with florbetapir F 18 ((18)F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiol Aging 2013;34:822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz JM, Glanz BI, Healy BC, Arora A, Neema M et al. Brain MRI lesion load at 1.5T and 3T versus clinical status in Multiple Sclerosis. J Neuroimaging 2011;21:e50–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, and the Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup. Whitepaper: Defining and investigating cognitive reserve, brain reserve and brain maintenance. Alzheimer Dementia 2018. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in ad is related to education and occupation: cognitive reserve? Neurology 1999;53:1942–1947. [DOI] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, … van Heertum R. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex 2005;15:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami T, Yamano S, Okada S, Sakuma M, Mormoto T, … Saito Y. Major risk factors for the appearance of white-matter lesions on MRI in hypertensive patients with controlled blood pressure. Vasc Health Risk Manag 2012;8:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Roberts RO, Lowe VJ, … Jack CR, Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann Neurol 2012;72(5):730–738. doi: 10.1002/ana.23665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattjes MP, Lutterbey GG, Harzheim M, Geiseke J, Träber F, et al. Higher sensitivity in the detection of inflammatory brain lesions in patients with clinically isolated syndromes suggestive of multiple sclerosis using high field MRI: an intraindivdiual comparison of 1.5 T with 3.0 T. Eur Radiol 2006;16:2067–2073. [DOI] [PubMed] [Google Scholar]
- Weuve J, Proust-Lima C, Power MC, Gross AL, Hofer SM, … Glymour MM, Dufouil C. Guidelines for reporting methodological challenges and evaluating potential bias in dementia research. Alzheimer Dementia 2015;11:1098–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuno F, Kazui H, Morita N, Kajimoto K, Ihara M, Taguchi A, … Kishimoto T. Low amyloid-beta deposition correlates with high education in cognitively normal older adults: a pilot study. Int J Geriatr Psychiatry 2015;30(9):919–926. doi: 10.1002/gps.4235 [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Azar M, Brickman AM, Glymour MM. Racial disparities in cognitive performance in mid- and late adulthood: Analyses of two cohort studies. J Am Geriatr Soc 2016;64:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Brickman AM, Narkhede A, Griffith EY, Guzman VA, et al. Is residual memory variance a valid method for quantifying cognitive reserve? A longitudinal application. Neuropsychologia 2015;77:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Brickman AM, Siedlecki KL, Decarli C, Stern Y. Quantifying cognitive reserve in older adults by decomposing episodic memory variance: replication and extension. J Int Neuropsychol Soc 2013;19:854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.