Abstract
Methods for the analysis of steroids have long been of interest due to the multiple uses for such methods in medical applications, sports monitoring, and environmental science. The analysis of steroids involves inherent analytical hurdles due to their low biological concentrations, poor ionization efficiencies, and frequent occurrence of isomerism. One analytical technique that has been recently applied to steroid analysis is ion mobility spectrometry (IMS). While previous work has focused on the use of metal adduction and multimer formation to enhance separation through IMS analysis coupled to mass spectrometry (MS), this work furthers this approach by coupling IMS-MS with liquid chromatography (LC). Three different LC methods with varying tradeoffs between chromatographic resolution and run time were developed, with one of these achieving a resolution above 1.5 for all steroid isomers. These results also indicate that the coupling of LC to IMS-MS can increase the overall resolution of steroid isomers relative to what can be achieved by either LC or IMS alone. Furthermore, the use of LC and IMS in concert can allow for a more rapid analysis of steroid isomers than can be achieved by LC-MS alone. Finally, the IMS dimension provided for measurements of ion-neutral collision cross sections (CCSs), which were found to be in good agreement with previously reported measurements. Thus, this approach provides three complementary quantitative parameters (retention time, CCS, and mass-to-charge ratio) that can contribute the identification of analytes. Overall, the work presented here demonstrates the potential of coupling LC, IMS, and MS for the analysis of isomeric steroid hormones.
Keywords: Steroids, Liquid Chromatography, Ion Mobility Spectrometry, Mass Spectrometry
1. Introduction
Steroids are a class of hormones that are synthesized from cholesterol and are involved in cellular signaling, primarily by acting on nuclear receptors [1]. Steroids have become a biomolecular class of increasing interest for rapid analysis due to their usage as performance enhancing drugs, as biomarkers, and as emerging contaminants [2–11]. However, steroids present unique challenges for analysis due to their low biological concentrations and, when analyzed my mass spectrometry (MS), relatively low ionization efficiencies [12]. Additionally, steroids have a high number of isomers and isobars with drastically different biological functions [1]. Therefore, rapid, sensitive, and isomer-resolving analytical methods are of significant interest in multiple fields of chemistry.
A number of analytical techniques have been used to analyze steroids including immunoassays, gas chromatography (GC), liquid chromatography (LC), and MS [12–14]. Immunoassays are expensive and indirectly measure the concentrations of specific steroids, which can introduce confounding variables [12]. GC has limitations due to the need of derivatization of steroid analytes, causing difficulties with reproducibility. LC-MS and LC-MS/MS have also been applied to steroid hormone analysis with promising results. Boggs et al. reported an LC-MS/MS method to analyze multiple classes of steroids, successfully demonstrating the separation and quantification of four steroid hormones classes at biologically-relevant concentrations [12]. Advantageously, LC-MS and LC-MS/MS allow the simultaneous quantitative analysis of multiple analytes without derivatization [15, 16].
Ion mobility spectrometry (IMS) coupled with MS has also been recently applied to steroid analysis [17]. As a separation method that uses an electric field and a drift gas to separate ions based on size, shape, and charge, IMS is essentially a gas-phase analogue of electrophoresis [18–21]. While there are multiple types of IMS instruments, the all separate ions either temporally or spatially. Drift tube ion mobility spectrometry (DTIMS) and traveling wave ion mobility spectrometry (TWIMS) are temporal methods and have been used to analyze steroids coupled to mass spectrometry [22–26]. Additionally, differential ion mobility spectrometry methods (DMS, FAIMS) separate ions through space and can be used to select ions with specific mobilities and only allow those to reach a coupled MS analyzer. DMS has been shown to increase the signal to noise ratio and identification confidence of targeted steroids [27, 28]. Because IMS separations are typically accomplished on a 10−3 - 10−2 s timescale, they are well-suited for incorporation between LC (102 – 103 s timescale) and time-of-flight (TOF) MS (10−5 10−4 s timescale). This provides three orthogonal dimensions of separation: polarity, size, and mass.
Previous studies of steroids by IMS have accomplished isomer separation through derivatization and metal ion adduction. Ahonen et al. successfully separated steroid isomers through derivatization using TWIMS in both standard solutions and a mixture [22]. Using DTIMS, Chouinard et al. used sodium adducted multimers to improve the separation of six multimeric steroid isomers as individual standards [23]. This work was furthered by Rister et al., who analyzed steroid isomer separation by TWIMS using group I metal adduction to monomers and multimers in individual standard solutions [29] and in mixtures [30]. In the latter case, it was found that the use of multimeric adducts to separate certain isomers was complicated by the formation of heterodimers, where two steroid isomers adduct to the same metal ion. Overall, while IMS can successfully separate isomers under certain circumstances, some limitations remain for steroid isomer separation in complex mixtures.
In the current study, we evaluated four sets of isomeric steroid species through LC-IMS-MS with metal ion adduction. The results presented here demonstrate that with the coupling of these analytical dimensions, steroid isomers can be separated more rapidly than with LC-MS alone and more completely than with IMS-MS alone. Finally, CCSs obtained from the IMS measurements were found to be comparable to previously reported literature values and can be used as an additional and complementary parameter, along with retention time and mass-to-charge ratio (m/z), to aid in analyte identification.
2. Experimental
2.1. Solution Preparation
Testosterone, androsterone, epiandrosterone, cortisone, corticosterone, hydrocortisone, 11-deoxycortisol, corticosterone-d4, lithium acetate, sodium acetate, potassium acetate, and water were purchased from Sigma-Aldrich (St. Louis, MO, USA). Aldosterone, dehydroepiandrosterone (DHEA), and methanol were purchased from Fisher Scientific (Pittsburg, PA, USA). The steroid mixture was made containing all steroids at a concentration of 500 nM in 50% HPLC grade water/methanol (the names, molecular weights, and structures of the steroids studied here are provided in Table S1 of the Supplementary Material). For analysis, 2.5 μL were injected into the LC (approximating 375 pg of each component on column). Water and methanol with lithium acetate added at a concentration of 150 μM (to form lithium ion adducts) were used as the mobile phase.
2.2. LC, IMS, and MS Conditions
LC was performed on a nanoAcquity system (Waters; Milford, MA, USA). The column was an Acquity UPLC M-Class BEH C18 column, 300 μm x 150 mm (Waters). A gradient elution was performed using methanol and water with the conditions shown in Table S2–S4 in the Supplementary Material. The LC was coupled to a Waters Synapt G2-S HDMS Q-TWIMS-TOF-MS through an electrospray ionization (ESI) source with the capillary voltage maintained at 3.1 kV. The TWIMS parameters for wave height, wave velocity, and nitrogen drift gas flow were set at 40 V, 600 m/s, and 60 mL/min, respectively, as optimized in previous studies [29–31]. Data was analyzed using DriftScope 2.4 (Waters), MassLynx 4.1 (Waters), and ORIGAMI [32]. Further visualization was conducted Using Igor Pro 7.0 (WaveMetrics; Lake Oswego, OR, USA) and SigmaPlot 13.1 (Systat, Chicago, IL, USA).
2.3. Resolution and Collision Cross Section Determination
For the resolution calculations, LC and IMS peaks were fit with a Gaussian curve in Igor Pro 7.0. This fitting of the curve produced the centroid LC retention time or IMS drift time (t) and the width of the peak at the half maximum (wFWHM). With this data, the peak-to-peak resolution (Rs) was calculated in a manner often used in separation science according to Equation 1, where wFWHM,avg represents the average of the half-height widths of the two peaks.
| Equation 1 |
Additionally, the centroid time of the IMS peaks (t) were converted to CCSs using polyalanine ions with known DTIMS CCS values published by Bush et al. [33]. Procedures for calibrating TWIMS drift times to CCS have been well-documented elsewhere [34–37]. The CCS values reported here resulted from the average of four measurements acquired on different days.
3. Results and Discussion
3.1. LC-MS Analysis
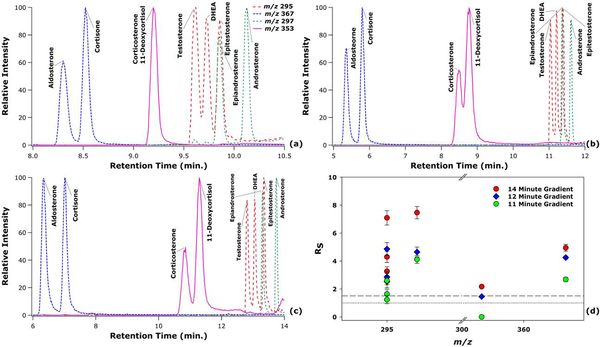
The extracted ion chromatograms for the lithium adducted monomeric steroid isomer species are shown in Figure 1. Three different gradients were established that achieved varying levels of resolution for the different steroid isomers, as depicted in Figure 1(d). As expected, the three gradients improve the resolution of isomers as the time to elute all analytes increases. While most steroids are resolved in all the gradients, the most difficult isomers to resolve were corticosterone and 11-deoxycortisol. For these isomers, the shortest gradient (11 min; Figure 1a) could not discriminate between these two isomers. The intermediate length gradient (12 min; Figure 1b) could resolve these isomers with a resolution between 1.0 and 1.5. Finally, the longest gradient (14 min; Figure 1c) could resolve these isomers at a resolution just above 1.5. Importantly, depending on the steroids of interest, gradients could be made shorter than those presented here.
Figure 1.
LC-MS extracted ion chromatograms of the steroid isomers for an 11 min LC gradient (a), 12 min LC gradient (b), and 14 min LC gradient (c). A scatter plot of the resolution values for the isomers is also provided for the three different gradients, where the dotted line represents Rs = 1.0 and the dashed line represents Rs = 1.5 (d).
An interesting trend was noted in the order of the elution of the steroids. Isomers in general tend to elute within similar retention time windows for both constitutional isomers and stereoisomers. Furthermore, the elution of the steroids is inversely related to the mass of the steroids, where the highest mass steroids elute first, and the lower mass steroids elute later. This trend is likely due to the increase in mass being corresponding to the addition of polar groups to the non-polar steroid core. This trend is in contrast to the majority of lipid classes, where increases in mass increase retention due to the elongation and saturation of the hydrophobic tails, often seen between lysophospholipids and phospholipids [38].
3.2. IMS-MS Analysis
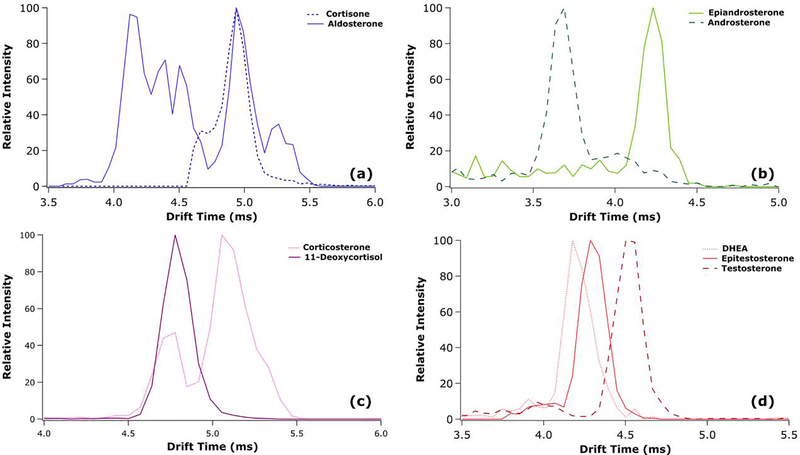
Arrival time distributions for the lithium adducted dimeric species from the 14 min gradient are presented in Figure 2, where all isomers were resolved at a value above 1.5 in the LC dimension. Additionally, CCSs are tabulated in Table S5 in the Supplementary Material and show good agreement with previously published measurements [29, 30]. Compared to previous analyses of single-component standards and mixtures of these same steroid isomers through direct infusion nanoflow ESI (nESI) analysis, the results presented here are very similar. This suggests that the ESI process (used in the present work) as compared to the nanoflow ESI ionization process (used in previous work) does not substantially impact the formation of the lithiated dimer [29, 30]. Furthermore, the resolution within the IMS dimension varies greatly between the different isomers. Due to the multiple features of aldosterone (likely arising from the presence of multiple gas-phase conformers), major features of aldosterone and cortisone are completely overlapped and unable to be analyzed by IMS alone. The major features of corticosterone and 11-deoxycortisol are separated at half height; however, the presence of a minor feature of corticosterone overlapped with 11-deoxycortisol would make quantitative analysis through IMS alone very difficult. Furthermore, testosterone is separated from epitestosterone, while DHEA and testosterone are highly overlapped. Finally, epiandrosterone and androsterone are near baseline resolved in the IMS dimension. From these results, the utility of metal adduction and IMS analysis can be shown primarily in androgen analysis; however, its limitations are also clear in the case of aldosterone and cortisone.
Figure 2.
IMS-MS extracted ion arrival time distributions for the lithiated dimeric species of cortisone and aldosterone (a), epiandrosterone and androsterone (b), corticosterone and 11-deoxycortisol (c), and DHEA, testosterone, and epitestosterone (d).
3.3. LC-IMS-MS Analysis
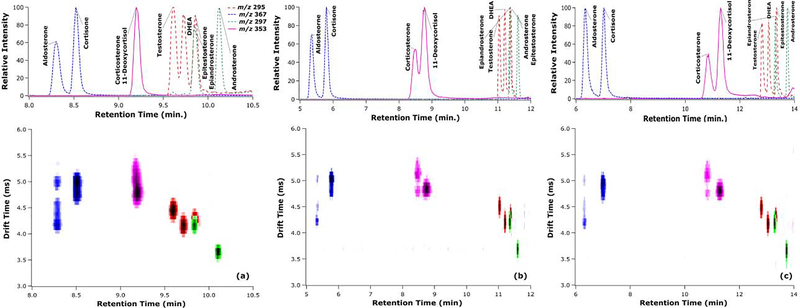
While both LC and IMS have limitations, they can complement each other well, which was demonstrated in the analysis of metal adducted dimeric steroids. Heatmaps of the LC and IMS dimensions that are color coded by mass-to-charge ratios for the three different gradients are shown in Figure 3. These heatmaps show the separation of the two major features of corticosterone and 11-deoxycortisol, allowing for qualitative analysis in the 11 min gradient, and full resolution at both the 12 min and 14 min gradients. Furthermore, other steroid isomers are shown to be separated from each other in the drift time vs. retention time space, even if they do not have a resolution above 1.5 in any one dimension. An intriguing finding emerged for those isomers with non-overlapping major features in the IMS dimension: the isomer with the lowest mobility (or longest drift time) elutes prior to any other isomers in LC. While this trend is interesting, the molecular features responsible for this trend are noteworthy and would seem to merit further study through molecular modeling. Overall, the use of LC and IMS dimensions in tandem was found to allow sufficient resolution for qualitative and quantitative analysis of steroid isomers with a shorter analysis time than could be achieved by either separation dimension alone.
Figure 3.
LC-MS chromatograms and LC-IMS-MS heatmaps for a mixture of steroid isomers separated by three different LC gradients: 11 min (a), 12 min (b), and 14 min (c). Note that as the chromatographic peak widths become narrower from (a) to (c), the heat map features may appear to become less intense; however, the peak intensities in terms of height and area are comparable throughout the series.
3.4. Corticosterone and 11-Deoxycortisol Analysis
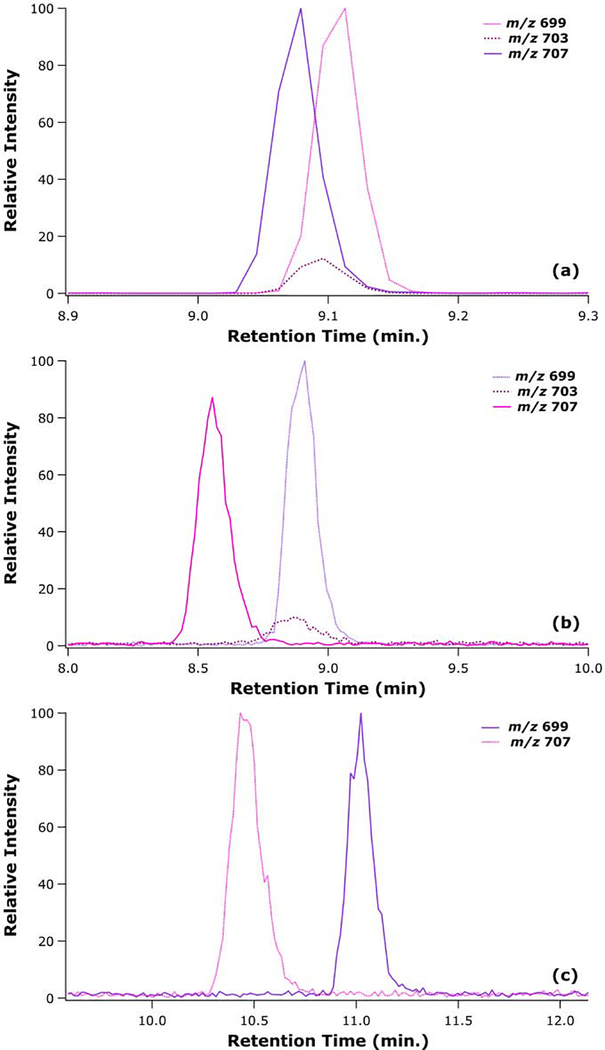
To further investigate the lithiated dimer formation of corticosterone and 11-deoxycortisol, which were the steroid isomers most difficult to separate in the LC dimension, an isotopically labeled corticosterone and 11-deoxycortisol were analyzed together in a mixture. The extracted ion chromatograms for the lithiated dimeric species of corticosterone-d4, 11-deoxycortisol, and the heterodimer for all three gradients are presented in Figure 4. In the 11 min gradient, there is a clear formation of a heterodimer that appears only during the overlapping elution of the two species. This heterodimer would significantly complicate any attempt at quantitative analysis of these analytes. On the other hand, using the 12 min gradient, a species at the m/z of a heterodimer was also observed; however, the retention time of this feature coincided only with the elution of 11-deoxycortisol. This suggests that the primary component leading to this feature was the naturally occurring M+4 isotopic species. Finally, the isomers were baseline resolved by the 14 min gradient, and there was no detectable species at the m/z of a heterodimer. The analysis of isotopically labeled isomers allow for further understanding of the complexities introduced by the formation of heterodimers, which seems to be dependent upon the LC gradient used.
Figure 4.
LC-MS extracted ion chromatograms for 11-deoxycortisol, isotopically labelled corticosterone, and the mass for the heterodimer of the above species for at three LC gradients of 11 min (a), 12 min (b), and 14 min (c).
4. Conclusions
Steroids are of increasing analytical importance due to their use as biomarkers, their abuse as performance enhancing drugs, and their status as emerging environmental contaminants. However, steroids are difficult to analyze due to the low biological concentrations and the presence of multiple steroid isomers. While previous research has discussed the use of IMS-MS and metal ion adduction for the analysis of steroid isomers, the current study combines the analytical approaches of LC, IMS, and MS to the analysis of metal adducted steroid isomers. Here, the benefits of LC-IMS-MS are demonstrated through the rapid analysis of steroids that achieves resolution value above 1.5 for all groups of isomers analyzed. We also observed that LC allows for the separation to reduce the formation of heterodimers discussed here and elsewhere in the literature. Furthermore, the IMS dimension can further separate ions that are difficult to separate through LC alone. We note that this untargeted LC-IMS-MS approach is highly complementary to targeted approaches such as LC-MS/MS using selected reaction monitoring (SRM). Moreover, because steroid isomers tend to yield fragments with the same mass-to-charge values, SRM scans may fail to discriminate between isomers or to detect a mixture thereof. Thus, the parallel analysis of complex mixtures incorporating the LC, IMS, and MS dimensions can provide orthogonal information on isomeric composition and analyte identity when applied in tandem with existing quantitative measurements by SRM. Overall, the work presented here is the first combined LC-IMS-MS analysis of metalated steroid isomers and suggests that this mode of analysis has the potential to bring significant analytical advantages to the determination of these compounds.
Supplementary Material
HIGHLIGHTS.
Steroid isomers were analyzed by LC-IMS-MS for enhanced separation.
Three gradients were established with varying levels of LC separation.
Lithium adduction allowed for enhanced IMS separation of steroid isomers.
The IMS dimension allows for separation in faster gradients than LC-MS alone.
CCS were measured for steroids with good agreement to past measurements.
Acknowledgements
This work was supported in part by funding from the National Science Foundation, Division of Chemistry, through the Chemical Measurement and Imaging Program (award number 1507989). Funding from the National Institutes of Health, National Institute of General Medical Sciences, was also received through a fellowship to A.L.R. from the Molecular Mechanisms of Disease Predoctoral Training Program (award number T32GM107001) and through the use of core facilities sponsored in part by the Nebraska Center for Integrated Biomolecular Communication (award number P20GM113126). The authors thank Dr. Jennifer Wood (University of Nebraska Lincoln, Department of Animal Science) for the kind gift of corticosterone-d4. Finally, the authors thank Ms. Jessica L. Minnick for constructive comments on a draft of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hu J, Zhang Z, Shen WJ, Azhar S, Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones, Nutr. Metab. 7 (2010) 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alda MJ, Barcelo D, Review of analytical methods for the determination of estrogens and progestogens in waste waters, Anal. Chem. 371 (2001) 437–447. [DOI] [PubMed] [Google Scholar]
- [3].Arlt W, Biehl M, Taylor AE, Hahner S, Libe R, Hughes BA, Schneider P, Smith DJ, Stiekema H, Krone N, Porfiri E, Opocher G, Bertherat J, Mantero F, Allolio B, Terzolo M, Nightingale P, Shackleton CH, Bertagna X, Fassnacht M, Stewart PM, Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors, J. Clin. Endocrinol. Metab. 96 (2011) 3775–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gouveia MJ, Brindley PJ, Santos LL, Correia da Costa JM, Gomes P, Vale N, Mass spectrometry techniques in the survey of steroid metabolites as potential disease biomarkers: a review, Metabolism 62 (2013) 1206–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guddat S, Thevis M, Kapron J, Thomas A, Schanzer W, Application of FAIMS to anabolic androgenic steroids in sport drug testing, Drug Test Anal. 1 (2009) 545–553. [DOI] [PubMed] [Google Scholar]
- [6].Morrow L, Porcu P, Neuroactive steroid biomarkers of alcohol sensitivity and alcoholism risk, in: Ritsner M (Ed.) The Handbook of Neuropsychiatric Biomarkers, Endophenotypes and Genes, Springer Science, 2009. [Google Scholar]
- [7].Severi G, Morris HA, MacInnis RJ, English DR, Tilley W, Hopper JL, Boyle P, Giles GG, Circulating steroid hormones and the risk of prostate cancer, Cancer Epidemiol. Biomarkers Prev. 15 (2006) 86–91. [DOI] [PubMed] [Google Scholar]
- [8].Lewis J, Steroid analysis in saliva: an overview, Clin. Biochem. Rev. 27 (2006) 139–146. [PMC free article] [PubMed] [Google Scholar]
- [9].Liu R, Zhou JL, Wilding A, Simultaneous determination of endocrine disrupting phenolic compounds and steroids in water by solid-phase extractiongas chromatography-mass spectrometry, J. Chromatogr. A 1022 (2004) 179–189. [DOI] [PubMed] [Google Scholar]
- [10].Nozaki O, Steroid analysis for medical diagnosis, J. Chromatogr. A 935 (2001) 267–278. [DOI] [PubMed] [Google Scholar]
- [11].Van Renterghem P, Van Eenoo P, Sottas PE, Saugy M, Delbeke F, A pilot study on subject-based comprehensive steroid profiling: novel biomarkers to detect testosterone misuse in sports, Clin. Endocrinol. 75 (2011) 134–140. [DOI] [PubMed] [Google Scholar]
- [12].Boggs AS, Bowden JA, Galligan TM, Guillette LJ Jr., Kucklick JR, Development of a multi-class steroid hormone screening method using Liquid Chromatography/Tandem Mass Spectrometry (LC-MS/MS), Anal. Bioanal. Chem. 408 (2016) 4179–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kasal A, Budesinsky M, Griffiths WJ, Spectroscopic Methods of Steroid Analysis, in: Steroid Analysis, 2010, pp. 27–161. [Google Scholar]
- [14].Giese RW, Measurement of endogenous estrogens: analytical challenges and recent advances, J. Chromatogr. A 1000 (2003) 401–412. [DOI] [PubMed] [Google Scholar]
- [15].Pitarch-Motellon J, Sancho JV, Ibanez M, Pozo O, Roig-Navarro AF, Determination of selected endogenous anabolic androgenic steroids and ratios in urine by ultra high performance liquid chromatography tandem mass spectrometry and isotope pattern deconvolution, J. Chromatogr. A 1515 (2017) 172–178. [DOI] [PubMed] [Google Scholar]
- [16].Settlage J, Oglesby T, Rajasekaran A, Williard C, Scott G, The importance of chromatographic resolution when analyzing steroid biomarkers, Steroids 99 (2015) 45–48. [DOI] [PubMed] [Google Scholar]
- [17].Rister AL, Dodds ED, Steroid analysis by ion mobility spectrometry, Steroids 153 (2020) 108531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kanu AB, Dwivedi P, Tam M, Matz L, Hill HH Jr., Ion mobility-mass spectrometry, J. Mass Spectrom. 43 (2008) 1–22. [DOI] [PubMed] [Google Scholar]
- [19].Campuzano I, Bush MF, Robinson CV, Beaumont C, Richardson K, Kim H, Kim HI, Structural characterization of drug-like compounds by ion mobility mass spectrometry: comparison of theoretical and experimentally derived nitrogen collision cross sections, Anal. Chem. 84 (2012) 1026–1033. [DOI] [PubMed] [Google Scholar]
- [20].Cumeras R, Figueras E, Davis CE, Baumbach JI, Gracia I, Review on ion mobility spectrometry. Part 1: current instrumentation, Analyst 140 (2015) 1376–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Giles K, Williams JP, Campuzano I, Enhancements in travelling wave ion mobility resolution, Rapid Commun. Mass Spectrom. 25 (2011) 1559–1566. [DOI] [PubMed] [Google Scholar]
- [22].Ahonen L, Fasciotti M, Gennas GB, Kotiaho T, Daroda RJ, Eberlin M, Kostiainen R, Separation of steroid isomers by ion mobility mass spectrometry, J. Chromatogr. A 1310 (2013) 133–137. [DOI] [PubMed] [Google Scholar]
- [23].Chouinard CD, Beekman CR, Kemperman RHJ, King HM, Yost RA, Ion mobility-mass spectrometry separation of steroid structural isomers and epimers, Int. J. Ion Mobil. Spec. 20 (2017) 31–39. [Google Scholar]
- [24].Chouinard CD, Cruzeiro VW, Roitberg AE, Yost RA, Experimental and theoretical investigation of sodiated multimers of steroid epimers with ion mobility-mass spectrometry, J. Am. Soc. Mass Spectrom. 28 (2017) 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kaur-Atwal G, Reynolds JC, Mussell C, Champarnaud E, Knapman TW, Ashcroft AE, O’Connor G, Christie SD, Creaser CS, Determination of testosterone and epitestosterone glucuronides in urine by ultra performance liquid chromatographyion mobility-mass spectrometry, Analyst 136 (2011) 3911–3916. [DOI] [PubMed] [Google Scholar]
- [26].Shvartsburg AA, Smith RD, Fundamentals of traveling wave ion mobility spectrometry, Anal. Chem. 80 (2008) 9689–9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ray JA, Kushnir MM, Yost RA, Rockwood AL, Wayne Meikle A, Performance enhancement in the measurement of 5 endogenous steroids by LC-MS/MS combined with differential ion mobility spectrometry, Clin. Chim. Acta 438 (2015) 330–336. [DOI] [PubMed] [Google Scholar]
- [28].Kolakowski BM, Mester Z, Review of applications of high-field asymmetric waveform ion mobility spectrometry (FAIMS) and differential mobility spectrometry (DMS), Analyst 132 (2007) 842–864. [DOI] [PubMed] [Google Scholar]
- [29].Rister AL, Martin TL, Dodds ED, Application of group I metal adduction to the separation of steroids by traveling wave ion mobility spectrometry, J. Am. Soc. Mass Spectrom. 30 (2019) 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rister AL, Martin TL, Dodds ED, Formation of multimeric steroid metal adducts and implications for isomer mixture separation by traveling wave ion mobility spectrometry, J. Mass Spectrom. 54 (2019) 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rister AL, Dodds ED, Ion mobility spectrometry and tandem mass spectrometry analysis of estradiol glucuronide isomers, J. Am. Soc. Mass Spectrom. 30 (2019) 2037–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Migas LG, France AP, Bellina B, Barran PE, ORIGAMI: A software suite for activated ion mobility mass spectrometry (aIM-MS) applied to multimeric protein assemblies, Int. J. Mass Spectrom. 427 (2018) 20–28. [Google Scholar]
- [33].Bush MF, Campuzano ID, Robinson CV, Ion mobility mass spectrometry of peptide ions: effects of drift gas and calibration strategies, Anal. Chem. 84 (2012) 7124–7130. [DOI] [PubMed] [Google Scholar]
- [34].Gelb AS, Jarratt RE, Huang Y, Dodds ED, A study of calibrant selection in measurement of carbohydrate and peptide ion-neutral collision cross sections by traveling wave ion mobility spectrometry, Anal. Chem. 86 (2014) 11396–11402. [DOI] [PubMed] [Google Scholar]
- [35].Ruotolo BT, Benesch JLP, Sandercock AM, Hyung SJ, Robinson CV, Ion mobility-mass spectrometry analysis of large protein complexes, Nat. Protoc. 3 (2008) 1139–1152. [DOI] [PubMed] [Google Scholar]
- [36].Smith DP, Knapman TW, Campuzano I, Malham RW, Berryman JT, Radford SE, Ashcroft AE, Deciphering drift time measurements from travelling wave ion mobility spectrometry-mass spectrometry studies, Eur. J. Mass Spectrom. 15 (2008) 113–130. [DOI] [PubMed] [Google Scholar]
- [37].Thalassinos K, Grabenauer M, Slade SE, Hilton GR, Bowers MT, Scrivens JH, Characterization of phosphorylated peptides using traveling wave-based and drift cell ion mobility mass spectrometry, Anal. Chem. 81 (2008) 248–254. [DOI] [PubMed] [Google Scholar]
- [38].Cajka T, Fiehn O, Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry, Trends Analyt. Chem 61 (2014) 192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.