Abstract
Cell-mediated immunity may play an important role in lung carcinogenesis. We investigated the associations for circulating levels of tryptophan, kynurenine, kynurenine:tryptophan ratio (KTR), kynurenine metabolite—quinolinic acid (QA), and neopterin as markers of interferon-gamma-induced cellular immune activation with lung cancer risk in 5,364 cases and 5,364 individually matched control subjects from 20 prospective cohorts included the international Lung Cancer Cohort Consortium (LC3). Tryptophan, kynurenine, QA, and neopterin were quantified by mass spectrometry-based methods in serum/plasma samples collected on average 6 years before lung cancer diagnosis. Odds ratios (ORs) and 95% confidence intervals (CIs) for lung cancer associated with different levels of these metabolites and KTR were calculated using conditional logistic regression with adjustment for matched smoking variables and circulating cotinine. Overall, the highest quintiles of circulating kynurenine, KTR, QA and neopterin were associated with a 20–30% higher risk of lung cancer, and tryptophan with a 15% lower risk compared with the lowest quintile (all Ptrend <0.05). The strongest associations were seen for current smokers, where the adjusted ORs (95% CIs) of lung cancer for the highest quintile of KTR, QA and neopterin were 1.42 (1.15–1.75), 1.42 (1.14–1.76) and 1.45 (1.13–1.86), respectively, compared with the lowest quintile. The associations with KTR and QA were strongest for lung squamous cell carcinoma followed by adenocarcinoma and for lung cancer diagnosed within the first 2 years after blood draw. Strongest associations among current smokers suggest a key role for cell-mediated immunity in smoking-related lung carcinogenesis.
Keywords: lung cancer, kynurenine, tryptophan, neopterin, quinolinic acid
INTRODUCTION
Lung cancer is one of the most common cancers accounting for 2.09 million incident cases and 1.76 million deaths worldwide in 2018 1. The 5-year survival rate for lung cancer cases is only 17.7% in the United States (US) 2, and is even worse globally 3. This underscores the importance of improving prevention and treatment to reduce lung cancer morbidity and mortality. Whilst the role of the immune system in the development of lung cancer has been increasingly recognized, the mechanisms by which immune mediators influence risk are only partially understood 4, 5.
Previous epidemiological studies that focused on the associations between circulating cytokines and risk of lung cancer have provided inconsistent results. For example, interleukin-6 and interleukin-8 were associated with increased risk of lung cancer in two prospective studies in the US 6 and Europe 7, but these same markers were not associated with lung cancer risk in a second US cohort 8 that evaluated the associations between 77 inflammatory markers and lung cancer risk, perhaps due to low statistical power. Furthermore, these previous studies were not well powered to study risk in important sub-groups, such as never smokers. In addition, concentrations of cytokines are generally low in the circulation of healthy individuals who have no active infection or malignancy 9. Thus investigations of alternative biomarkers associated with inflammation, immune response, and lung cancer risk is warranted.
Interferon gamma (IFN-gamma) is a cytokine produced predominantly by cells involved in cell-mediated immunity such as natural killer cells, natural killer T cells, and CD4 and CD8 cytotoxic T cells 10. Animal studies have shown that IFN-gamma-induced cellular immunity is important for the inhibition of lung cancer development 11, 12. However, the association between IFN-gamma and lung cancer risk in human epidemiology is understudied. The half-life of IFN-gamma in the circulation is short 13 and it is therefore not readily measurable in large-scale epidemiological studies. IFN-gamma activates indoleamine 2,3-dioxygenase (IDO), and upregulates metabolism through the kynurenine pathway of tryptophan metabolism 14. As such, the kynurenine:tryptophan ratio (KTR) can be used as a surrogate of IDO activity and IFN-gamma-mediated cellular immune activation 15. IFN-gamma also stimulates the production of neopterin, a metabolite of guanosine triphosphate, by macrophages 16. One previous epidemiological study observed an association between KTR and higher risk of lung cancer 17. A second prospective study showed associations between KTR or neopterin and risk of cancer overall, but no association was observed for risk of lung cancer specifically, possibly due to lack of statistical power 18. Thus replication studies are needed to confirm these earlier findings. In addition, the downstream metabolites of kynurenine pathway (Figure 1) may also play a role in the initiation and progression of lung cancer. For example, quinolinic acid (QA), a downstream metabolite of kynurenine, has immuno-regulatory effects 19, 20 and may be important in lung cancer risk, but has not been studied in large epidemiological studies.
Figure 1:
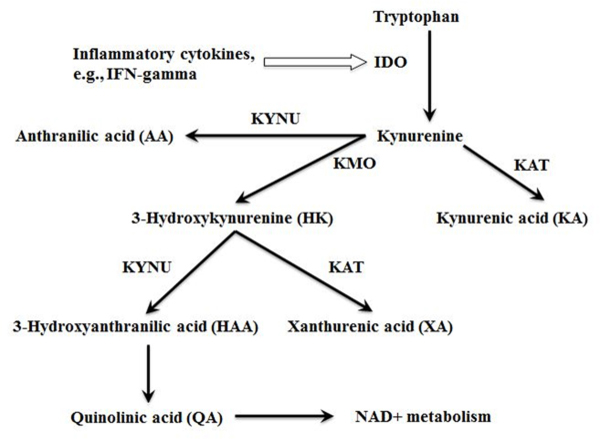
The kynurenine pathway of tryptophan metabolism
The purpose of the current study conducted among 20 prospective cohorts from Asia, Australia, Europe and the US is to comprehensively investigate the associations between circulating concentrations of KTR, QA and neopterin as markers of IFN-gamma-induced cellular immune activation and lung cancer risk. Our large sample size (5364 case-control pairs) allows us to further investigate these associations by smoking status, histology, and time from blood draw to diagnosis.
MATERIALS AND METHODS
Study population
The design of the Lung Cancer Cohort Consortium (LC3) including cohort design and follow-up procedures has been reported previously 21. The current investigation included case-control studies of incident lung cancer cases and individually matched controls nested within 20 prospective cohorts from the US, Europe, Australia, and Asia. At recruitment into each cohort, participants signed informed consent forms, completed questionnaires, had blood sample drawn and anthropometric measurements taken. The LC3 was approved by the Institutional Review Board of each contributing cohort.
Selection of cases and controls
Lung cancer cases were defined on the basis of the International Classification of Diseases for Oncology, Second Edition (ICD-O-2), and included all invasive cancers coded as C34.0 to C34.9. Altogether, 11,399 incident lung cancer cases with pre-diagnostic serum or plasma samples among members of the US National Cancer Institute Cohort Consortium in 2009 were eligible for participation. From this, the LC3 selected a total of 5,545 lung cancer cases, and to optimize the statistical power in smoking stratified analyses, never and former smoking cases were oversampled. For each case, one control was randomly selected within the same cohort among all eligible participants who were alive and free of cancer (except non-melanoma skin cancer) at the same length of time from enrollment as was the index case at diagnosis. Matching criteria were race (US only), sex, date of blood collection (± 1 month, relaxed to ± 3 months for sets without available controls), and date of birth (± 1 year, relaxed to ± 3 years), as well as smoking status in 5 categories (never smokers, short and long-term quitters among former smokers with <10 years or ≥10 years since quitting, and light and heavy current smokers (<15 or ≥15 cigarettes per day). After excluding cases who were not able to be correctly matched on smoking status in 5 categories defined above (n=126 cases), had insufficient serum/plasma samples (n=42), or had a revised date of diagnosis prior to blood draw (n=13), a total of 5364 lung cancer case-control pairs remained eligible for the current analysis.
Biochemical analyses
Serum or plasma samples from all LC3 study participants were sent on dry ice to the Bevital A/S laboratory (http://www.bevital.no) in Bergen, Norway, and were kept at −80°C until later analysis. Concentrations of tryptophan, kynurenine 22, quinolinic acid (QA), neopterin and cotinine 23 – a biomarker of recent tobacco exposure were determined by mass spectrometry based methods (LC-MS/MS, GC-MS/MS). Biochemical analysis was performed in 96-well plates, each containing 86 study samples, 6 calibration samples, 3 quality control samples, and 1 blank sample. Samples of the index case and the matched control subjects were put next to each other in a random order and always analyzed together in the same batch. The laboratory personnel were blind to the case/control status of the test samples.
Statistical Analysis
The KTR ratio was calculated as kynurenine concentration divided by tryptophan multiplied by 1000. We logarithmically transformed (base e) original values of all biomarker concentrations and KTR to normalize their skewed distributions. The pair-wise correlations between biomarkers were assessed using Spearman correlation coefficients. The relationships between circulating concentrations of biomarkers and socio-demographic, lifestyle and clinical factors, including age at blood draw, body mass index (BMI), estimated glomerular filtration rate (eGFR; a measurement of kidney function that influences the circulating levels of kynurenine and its metabolites), and geographical region were evaluated using Analysis of Covariance (ANCOVA). The eGFR was calculated based on participant’s age, gender, and creatinine concentration in plasma or serum according to the previously published method 24.
Study participants were divided into quintiles based on the distributions of biomarker concentrations among controls within a specific cohort. Odds ratios (ORs) of lung cancer for quintiles of biomarker concentrations were calculated relative to the first quintile using conditional logistic regression 25. Ordinal values (e.g., 1, 2, 3, 4, and 5) for individual biomarkers were used for testing linear trends across quintiles in the biomarker-lung cancer risk associations.
In addition to matching on cohort, race (US only), sex, date of blood draw, date of birth, and the combination of smoking status with years of quitting and number (for former smokers) and number of cigarettes per day (for current smokers), the multivariable conditional logistic regression models included the following reported risk factors for lung cancer and determinants of kynurenine metabolites as potential confounders: cotinine concentration (continuous, a biomarker of recent nicotine intake) 26, educational attainment (six categories), body mass index (BMI) in kg/m2 (<18.5, 18.5– <25, 25– <30, ≥30), and eGFR.
Fully adjusted regression models were used in analyses stratified by smoking status (current, former, never smokers), histological subtypes of lung cancer (adenocarcinoma, large-cell carcinoma, small-cell carcinoma, and squamous cell carcinoma), time between blood draw and lung cancer diagnosis (<2, 2–<5, and ≥5 years), and geographical region (US, Europe/Australia, Asia). Potential effect modification of associations between biomarkers and lung cancer risk by demographic, lifestyle, or other factors were examined by including their product term in the multivariate regression models.
Statistical analyses were carried out using SAS software version 9.3 (SAS Institute, Cary, NC). All P values reported are two-sided, and those that were <0.05 were considered to be statistically significant.
RESULTS
Baseline characteristics of cases and controls
The current study sample included 5,364 incident lung cancer cases and 5,364 individually matched controls (Table 1). Overall, slightly more participants were male (54.2%). Participants from Europe/Australia (EU/AU) and Asia were also predominantly male (57.9% and 69.2%, respectively) whereas participants from the US were predominantly female (58.7%). Current smokers accounted for nearly half the overall study participants (47%, 2,519 case-control pairs), with former (28.3%, 1,518 case-control pairs) and never smokers (24.7%, 1,327 case-control pairs) contributing approximately one-quarter each. Cases and controls had, on average, similar characteristics including BMI and age at recruitment (60 years).
Table 1.
Baseline and clinical characteristics of study participants overall and by continent, the Lung Cancer Cohort Consortium (LC3) Study
| Baseline and Clinical Characteristics |
US cohorts | EU/AU cohorts | Asian cohorts | Overall | ||||
|---|---|---|---|---|---|---|---|---|
| No.(%) of participants in group | No.(%) of participants in group | No.(%) of participants in group | No.(%) of participants in group | |||||
| Cases (n=2400) |
Matched controls (n=2400) |
Cases (n=1189) |
Matched controls (n=1189) |
Cases (n=1775) |
Matched controls (n=1775) |
Cases (n=5364) |
Matched controls (n=5364) |
|
| Sex | ||||||||
| Men | 991 (41.3%) | 991 (41.3%) | 688 (57.9%) | 688 (57.9%) | 1229 (69.2%) | 1229 (69.2%) | 2908 (54.2%) | 2908 (54.2%) |
| Women | 1409 (58.7%) | 1409 (58.7%) | 501 (42.1%) | 501 (42.1%) | 546 (30.8%) | 546 (30.8%) | 2456 (45.8%) | 2456 (45.8%) |
| Smoking status | ||||||||
| Never | 569 (23.7%) | 569 (23.7%) | 156 (13.1%) | 156 (13.1%) | 602 (33.9%) | 602 (33.9%) | 1327 (24.7%) | 1327 (24.7%) |
| Former | 1007 (42%) | 1007 (42%) | 335 (28.2%) | 335 (28.2%) | 176 (9.9%) | 176 (9.9%) | 1518 (28.3%) | 1518 (28.3%) |
| Current | 824 (34.3%) | 824 (34.3%) | 698 (58.7%) | 698 (58.7%) | 997 (56.2%) | 997 (56.2%) | 2519 (47%) | 2519 (47%) |
| Education | ||||||||
| less than high school | 237 (9.9%) | 215 (9%) | 662(55.6%) | 598(50.2%) | 898 (50.6%) | 883 (49.7%) | 1797 (33.5%) | 1696 (31.6%) |
| completed high school | 357 (14.9%) | 374 (15.6%) | 159 (13.4%) | 180 (15.2%) | 243 (13.7%) | 230 (13%) | 759 (14.1%) | 784 (14.6%) |
| vocational school | 422 (17.6%) | 435 (18.1%) | 180 (15.2%) | 200 (16.8%) | 289 (16.3%) | 279 (15.7%) | 891 (16.6%) | 914 (17%) |
| some college | 402 (16.8%) | 393 (16.4%) | 107 (9%) | 129 (10.9%) | 171 (9.6%) | 196 (11%) | 680 (12.7%) | 718 (13.4%) |
| college graduate | 357 (14.9%) | 319 (13.3%) | 63 (5.3%) | 64 (5.4%) | 104 (5.9%) | 113 (6.4%) | 524 (9.8%) | 496 (9.2%) |
| graduate studies | 574 (23.9%) | 637 (26.5%) | 10 (0.8%) | 8 (0.7%) | 62 (3.5%) | 65 (3.7%) | 646 (12%) | 710 (13.2%) |
| Unknown | 51 (2.1%) | 27 (1.1%) | 8 (0.7%) | 10 (0.8%) | 8 (0.5%) | 9 (0.5%) | 67 (1.2%) | 46 (0.9%) |
| Body Mass indexa | ||||||||
| <18.5 | 30 (1.3%) | 31 (1.3%) | 14 (1.2%) | 10(0.8%) | 157 (8.8%) | 113 (6.4%) | 201 (3.7%) | 154 (2.9%) |
| 18.5– <25 | 1088 (45.3%) | 1020 (42.5%) | 521 (43.8%) | 435 (36.6%) | 1203 (67.8%) | 1192 (67.2%) | 2812 (52.4%) | 2647 (49.3%) |
| 25– <30 | 841 (35%) | 858 (35.8%) | 468 (39.4%) | 536 (45.1%) | 369 (20.8%) | 424 (23.9%) | 1678 (31.3%) | 1818 (33.9%) |
| ≥30 | 378 (15.8%) | 430 (17.9%) | 185 (15.6%) | 206(17.4%) | 46 (2.6%) | 46 (2.6%) | 609 (11.4%) | 682 (12.7%) |
| Unknown | 63 (2.6%) | 61 (2.5%) | 1 (0.1%) | 2 (0.2%) | 0 (0%) | 0 (0%) | 64 (1.2%) | 63 (1.2%) |
| Continuous variables, median (5th-95th percentile) | ||||||||
| Age at recruitment (years) | 60 (42–74) | 60 (42–74) | 60 (45–70) | 60 (45–70) | 60 (46–72) | 60 (46–71 | 60 (44–72) | 60 (44–72) |
| Circulating concentrations for biomakers | ||||||||
| Tryptophan, μmol/L | 63.9 (41.3–89.1) | 64.4 (43.7–90.5) | 67.8 (48.9–92.7) | 68.1 (50.1–91.1) | 67.3 (48.6–91.2) | 67.5 (49.1–90.1) | 66.0 (44.9–90.8) | 66.5 (46.2–90.7) |
| Kynurenine, μmol/L | 1.51 (1.00–2.37) | 1.53 (1.02–2.34) | 1.52 (1.06–2.19) | 1.52 (1.07–2.18) | 1.49 (1.08–2.18) | 1.48 (1.09–2.14) | 1.50 (1.04–2.25) | 1.51 (1.05–2.22) |
| Kynurinine:Tryptophan ratio (x 1000) | 22.6 (16.6–38.6) | 23.6 (16.6–37.0) | 22.3 (16.4–33.8) | 22.0 (16.5–32.1) | 21.9 (15.9–34.1) | 21.9 (16.0–32.1) | 22.6 (16.2 −36.2) | 22.6 (16.4–34.5) |
| Quinolinic acid, nmol/L | 364 (200–789) | 363 (207–741) | 341 (201–633) | 334 (202–605) | 350 (207–651) | 350 (216–605) | 354 (203–708) | 353 (208–685) |
| Neopterin, nmol/L | 12.0 (5.74–25.0) | 11.8 (5.66–25.5) | 10.2 (4.78–20.9) | 10.3 (4.38–19.5) | 10.6 (5.29–24.0) | 10.7 (5.28–24.6) | 11.1 (5.31–24.0) | 11.0 (5.14–24.6) |
| Clinical characteristics, case participants only | ||||||||
| Age at diagnosis, median (range), years | 70 (55–83) | 69 (54–80) | 69 (52–80) | 69.8 (53.6–82.0) | ||||
| Time from blood draw to diagnosis (years) | 5.2 (1–15.5) | 10.0 (1.5–16.0) | 5.8 (0.7–16.5) | 6.3 (1.0–16.0) | ||||
| Histology, No. (%) | ||||||||
| Large cell carcinoma | 112 (4.6%) | 46 (4%) | 16 (1%) | 174 (3.3%) | ||||
| Small cell carcinoma | 245 (10.4%) | 150 (12.5%) | 99 (5.5%) | 492 (9.2%) | ||||
| Squamous cell carcinoma | 291 (11.9%) | 231 (19.5%) | 319 (17.9%) | 836 (15.5%) | ||||
| Adenocarcinoma | 1034 (42.7%) | 419 (34.5%) | 615 (34.6%) | 2056 (38.4%) | ||||
| Missing / Unknown | 735 (31.4%) | 357 (29.5%) | 726 (41%) | 1806 (33.6%) | ||||
Body mass index is calculated as weight in kilograms divided by height in meters squared
Median age at lung cancer diagnosis was 69.8 (range 53.6 – 82.0) with little variation across geographic regions. The median time between blood draw and lung cancer diagnosis was 5.2 years for the US, 5.8 years for Asian, and 10 years for cohorts in Australia and Europe. Histologically, the majority of lung cancer cases were adenocarcinoma, followed by squamous cell, small cell and large cell carcinoma. Due to a larger overall sample size, the US cohorts contributed the majority of all adenocarcinoma cases (50.3%), small cell carcinoma cases (49.8%), and large cell carcinoma cases (64.4%). The proportion of squamous cell cases did not differ substantially by region, with each region contributing approximately one-third of cases.
Biomarker distribution in study population
The geometric means of biomarkers were significantly different among groups by smoking status in control subjects. The mean concentration of tryptophan was highest for current smokers and lowest for never smokers whereas the levels of kynurenine, KTR, QA, and neopterin were the highest for former smokers (Supplementary Table 1). Concentrations [median (20th −80th percentile)] of circulating biomarkers did not differ substantially across cohorts within geographic region, with few exceptions. For US cohorts, circulating tryptophan concentrations were 20 μmol/L higher in the American Cancer Society Cancer Prevention Study-II (CPS-II) Nutrition cohort compared to the Women’s Health Initiative (WHI) cohort (Supplementary Table 2). In addition, circulating neopterin concentrations were different among different cohorts within a region; the highest levels were observed in the Multiethnic Cohort (MEC) among the US cohorts and in the Singapore Chinese Health Study among Asian cohorts. Overall kynurenine, KTR, QA, and neopterin concentrations were positively correlated with each other (Spearman correlation coefficient [r] = 0.34–0.66) whereas tryptophan was positively correlated with kynurenine (r = 0.45) and QA (r = 0.13), but inversely correlated with KTR (r = −0.43) and was not correlated with neopterin (r = −0.01) (Supplementary Table 3).
Overall and stratified associations of circulating biomarkers and lung cancer risk
The highest quintiles of circulating kynurenine, KTR, QA, and neopterin were associated with a 22–31% higher risk of lung cancer as compared with the lowest quintiles after controlling for smoking status, duration and intensity, circulating levels of cotinine, and other potential confounders (Table 2). In contrast, the highest quintile of tryptophan was associated with a 15% lower risk of lung cancer compared with the lowest quintile (Table 2).
Table 2:
Odds ratios of lung cancer incidence comparing higher quintiles with the lowest quintile of circulating biomarkers
The Lung Cancer Cohort Consortium (LC3) Study (n = 5364 cases and 5364 controls)
| Odds Ratio (95% Confidence Interval) by quintiles of biomarkera | ||||||
|---|---|---|---|---|---|---|
| Biomarkers | Q1 | Q2 | Q3 | Q4 | Q5 | P trend |
| Tryptophan (μmol/L) | 1.00 | 0.86 (0.76–0.97) | 0.87 (0.77–0.98) | 0.86 (0.76–0.97) | 0.85 (0.75–0.96) | 0.019 |
| Kynurenine (μmol/L) | 1.00 | 1.05 (0.92–1.18) | 1.02 (0.89–1.16) | 1.01 (0.89–1.15) | 1.22 (1.06–1.40) | 0.033 |
| KTR b | 1.00 | 0.97 (0.86–1.10) | 0.96 (0.84–1.09) | 1.01 (0.89–1.15) | 1.31 (1.14–1.50) | <0.001 |
| Quinolinic acid (nmol/L) | 1.00 | 0.95 (0.84–1.08) | 0.94 (0.83–1.06) | 1.09 (0.96–1.25) | 1.31 (1.14–1.51) | <0.001 |
| Neopterin (nmol/L) | 1.00 | 1.12 (0.99–1.27) | 1.09 (0.96–1.24) | 1.12 (0.98–1.28) | 1.31 (1.14–1.51) | 0.001 |
All models were adjusted for educational attainment (categorical), body mass index (kg/m2) (categorical), serum cotinine concentrations (continuous), estimated glomerular filtration rate (continuous), and cohort.
KTR, kynurenine to tryptophan ratio (x 1000),
Table 3 shows the odds ratios for lung cancer associated with higher quintiles of biomarkers in current, former, and never smokers separately. Among current smokers, ORs (95% CIs) for lung cancer in the highest quintiles of KTR, QA and neopterin were 1.42 (1.15–1.75), 1.42 (1.14–1.75), and 1.45 (1.13–1.86), respectively (all Ptrend ≤ 0.005). The corresponding ORs (95% CIs) in former smokers were 1.32 (1.00–1.74), 1.20 (0.90–1.59), and 1.43 (0.97–1.86) (all Ptrend were borderline significant). There was no association between these biomarkers and lung cancer risk among never smokers (all Ptrend > 0.16). However, no interaction between any biomarker and smoking status for lung cancer risk was detected all P’s for multiplicative interaction > 0.05) (data not shown).
Table 3:
Odds ratios of lung cancer incidence comparing higher quintiles with the lowest quintile of circulating biomarkers stratified by smoking status
The Lung Cancer Cohort Consortium (LC3) Study (n = 5364 cases and 5364 controls)
| Smoking status and biomarker |
Odds Ratio (95% Confidence Interval) by quintiles of biomarkera | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P trend | |
| Current smokers (2519 cases and 2519 controls) | ||||||
| Tryptophan (μmol/L) | 1.00 | 0.80 (0.66–0.98) | 0.79 (0.65–0.96) | 0.81 (0.66–0.98) | 0.78 (0.63–0.95) | 0.046 |
| Kynurenine (μmol/L) | 1.00 | 0.86 (0.72–1.04) | 1.04 (0.86–1.26) | 1.05 (0.86–1.28) | 1.16 (0.93–1.46) | 0.057 |
| KTR b | 1.00 | 0.94 (0.79–1.11) | 0.95 (0.79–1.13) | 1.04 (0.87–1.26) | 1.42 (1.15–1.75) | 0.005 |
| Quinolinic acid (nmol/L) | 1.00 | 0.97 (0.83–1.14) | 1.04 (0.87–1.24) | 1.26 (1.04–1.53) | 1.42 (1.14–1.76) | <0.001 |
| Neopterin (nmol/L) | 1.00 | 1.10 (0.91–1.34) | 1.21 (0.98–1.48) | 1.28 (1.02–1.61) | 1.45 (1.13–1.86) | 0.003 |
| Former smokers (1518 cases and 1518 controls) | ||||||
| Tryptophan (μmol/L) | 1.00 | 0.73 (0.58–0.92) | 0.81 (0.64–1.03) | 0.82 (0.65–1.04) | 0.74 (0.58–0.94) | 0.077 |
| Kynurenine (μmol/L) | 1.00 | 1.12 (0.84–1.49) | 1.23 (0.95–1.6) | 1.02 (0.78–1.34) | 1.08 (0.82–1.41) | 0.955 |
| KTR b | 1.00 | 1.06 (0.80–1.41) | 1.18 (0.89–1.54) | 1.13 (0.86–1.49) | 1.32 (1.00–1.74) | 0.035 |
| Quinolinic acid (nmol/L) | 1.00 | 1.00 (0.74–1.33) | 0.82 (0.62–1.08) | 1.17 (0.89–1.55) | 1.20 (0.90–1.59) | 0.037 |
| Neopterin (nmol/L) | 1.00 | 1.14 (0.86–1.50) | 1.14 (0.85–1.54) | 1.01 (0.74–1.37) | 1.34 (0.97–1.86) | 0.196 |
| Never smokers (1327 cases and 1327 controls) | ||||||
| Tryptophan (μmol/L) | 1.00 | 1.00 (0.80–1.26) | 1.04 (0.81–1.32) | 1.16 (0.88–1.53) | 0.87 (0.64–1.18) | 0.911 |
| Kynurenine (μmol/L) | 1.00 | 1.06 (0.85–1.33) | 1.12 (0.88–1.43) | 1.05 (0.8–1.38) | 1.17 (0.85–1.59) | 0.406 |
| KTR b | 1.00 | 1.00 (0.79–1.27) | 0.92 (0.72–1.19) | 0.92 (0.71–1.19) | 1.17 (0.88–1.54) | 0.562 |
| Quinolinic acid (nmol/L) | 1.00 | 0.89 (0.69–1.13) | 0.70 (0.54–0.9) | 0.92 (0.71–1.2) | 1.07 (0.79–1.44) | 0.707 |
| Neopterin (nmol/L) | 1.00 | 1.09 (0.85–1.40) | 1.18 (0.89–1.55) | 1.30 (0.97–1.74) | 1.19 (0.86–1.63) | 0.168 |
All models were adjusted for educational attainment (categorical), body mass index (kg/m2) (categorical), serum cotinine concentrations (continuous), estimated glomerular filtration rate (continuous), and cohort.
KTR, kynurenine to tryptophan ratio (x 1000).
When data were analyzed by histological subtype of lung cancer, associations for KTR and QA with risk of lung squamous cell carcinoma, and for QA with adenocarcinoma were seen (Table 4). The associations for other biomarkers with risk of adenocarcinoma or squamous cell carcinoma, and for all biomarkers with large cell and small cell carcinomas did not reach statistical significance.
Table 4:
Odds ratios of lung cancer incidence by histological subtype comparing higher quintiles with the lowest quintile of circulating biomarkers
The Lung Cancer Cohort Consortium (LC3) Study (n = 3558 cases and 3558 controls)a
| Histological subtype and biomarker |
Odds Ratio (95% Confidence Interval) by quintiles of biomarkerb |
|||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P trend | |
| Large cell carcinoma (174 cases and 174 controls) | ||||||
| Tryptophan (μmol/L) | 1.00 | 1.88 (0.94–3.74) | 1.94 (0.95–3.98) | 1.97 (0.90–4.31) | 1.96 (0.80–4.81) | 0.142 |
| Kynurenine (μmol/L) | 1.00 | 1.62 (0.73–3.57) | 1.25 (0.54–2.88) | 2.05 (0.96–4.36) | 2.28 (0.92–5.68) | 0.048 |
| KTR b | 1.00 | 0.40 (0.17–0.90) | 0.74 (0.34–1.61) | 1.02 (0.47–2.18) | 0.90 (0.40–2.04) | 0.339 |
| Quinolinic acid (nmol/L) | 1.00 | 1.04 (0.49–2.19) | 0.65 (0.31–1.37) | 1.12 (0.50–2.48) | 1.68 (0.68–4.12) | 0.344 |
| Neopterin (nmol/L) | 1.00 | 1.33 (0.51–3.49) | 1.70 (0.69–4.15) | 1.11 (0.41–3.02) | 1.97 (0.66–5.87) | 0.390 |
| Small cell carcinoma (492 cases and 492 controls) | ||||||
| Tryptophan (μmol/L) | 1.00 | 0.77 (0.51–1.17) | 0.74 (0.48–1.13) | 0.57 (0.37–0.88) | 0.82 (0.53–1.25) | 0.189 |
| Kynurenine (μmol/L) | 1.00 | 0.87 (0.58–1.32) | 1.02 (0.67–1.55) | 1.01 (0.64–1.59) | 0.99 (0.62–1.57) | 0.838 |
| KTR b | 1.00 | 0.65 (0.43–1.01) | 1.08 (0.70–1.65) | 0.74 (0.47–1.16) | 1.13 (0.71–1.80) | 0.447 |
| Quinolinic acid (nmol/L) | 1.00 | 0.83 (0.54–1.28) | 0.79 (0.51–1.25) | 1.37 (0.88–2.13) | 1.32 (0.81–2.14) | 0.071 |
| Neopterin (nmol/L) | 1.00 | 1.20 (0.75–1.92) | 1.07 (0.64–1.80) | 0.89 (0.53–1.50) | 1.29 (0.71–2.36) | 0.823 |
| Squamous cell carcinoma (836 cases and 836 controls) | ||||||
| Tryptophan (μmol/L) | 1.00 | 0.67 (0.49–0.93) | 0.68 (0.48–0.96) | 0.72 (0.51–1.01) | 0.76 (0.54–1.06) | 0.304 |
| Kynurenine (μmol/L) | 1.00 | 0.71 (0.50–1.00) | 1.21 (0.86–1.69) | 1.04 (0.73–1.47) | 1.22 (0.84–1.77) | 0.066 |
| KTRc | 1.00 | 1.06 (0.77–1.46) | 0.99 (0.71–1.38) | 1.01 (0.72–1.41) | 1.68 (1.17–2.43) | 0.023 |
| Quinolinic acid (nmol/L) | 1.00 | 1.58 (1.15–2.16) | 1.38 (0.99–1.93) | 1.56 (1.11–2.20) | 1.99 (1.35–2.91) | 0.003 |
| Neopterin (nmol/L) | 1.00 | 1.61 (1.14–2.26) | 1.21 (0.83–1.75) | 1.36 (0.92–2.01) | 1.34 (0.88–2.04) | 0.468 |
| Adenocarcinoma (2056 cases and 2056 controls) | ||||||
| Tryptophan (μmol/L) | 1.00 | 0.96 (0.79–1.17) | 0.98 (0.80–1.20) | 1.26 (1.01–1.56) | 0.89 (0.70–1.12) | 0.764 |
| Kynurenine (μmol/L) | 1.00 | 1.04 (0.85–1.27) | 1.14 (0.93–1.39) | 1.00 (0.80–1.24) | 1.09 (0.86–1.39) | 0.615 |
| KTRb | 1.00 | 1.02 (0.83–1.24) | 1.00 (0.82–1.23) | 1.00 (0.81–1.24) | 1.12 (0.89–1.40) | 0.426 |
| Quinolinic acid (nmol/L) | 1.00 | 0.85 (0.68–1.05) | 0.85 (0.68–1.06) | 1.01 (0.80–1.27) | 1.36 (1.05–1.74) | 0.009 |
| Neopterin (nmol/L) | 1.00 | 1.04 (0.84–1.29) | 1.13 (0.90–1.43) | 1.19 (0.92–1.52) | 1.27 (0.97–1.66) | 0.059 |
1,806 cases were other or unknown histological types of cancer, thus they and their individually matched controls were excluded from this analysis.
All models were adjusted for educational attainment (categorical), body mass index (kg/m2) (categorical), serum cotinine concentrations (continuous), estimated glomerular filtration rate (continuous), and cohort.
KTR, kynurenine to tryptophan ratio (x 1000).
In the sensitivity analysis, the associations for circulating levels of kynurenine, KTR and QA were observed for the risk of lung cancer diagnosed within 2 years after blood draw (Table 5). Higher levels of neopterin were associated with higher risk of lung cancer diagnosed within 2 to <5 years after blood draw. The association between QA and lung cancer risk remained, albeit weakened, even 5 or more years after blood collection.
Table 5:
Odds ratios of lung cancer incidence comparing higher quintiles with the lowest quintile of circulating biomarkers stratified by time from blood draw to cancer diagnosis, The Lung Cancer Cohort Consortium (LC3) Studya
| Time from blood draw to Cancer diagnosis and biomarkersa |
Odds Ratio (95% Confidence Interval) by quintiles of biomarkerb |
|||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P trend | |
| < 2 years (552 cases and 552 controls) | ||||||
| Tryptophan (μmol/L) | 1.00 | 0.56 (0.37–0.85) | 0.65 (0.43–0.99) | 0.73 (0.47–1.14) | 0.72 (0.45–1.13) | 0.542 |
| Kynurenine (μmol/L) | 1.00 | 1.21 (0.78–1.89) | 1.22 (0.76–1.96) | 1.15 (0.71–1.87) | 1.86 (1.13–3.08) | 0.032 |
| KTRc | 1.00 | 1.14 (0.72–1.80) | 0.95 (0.60–1.52) | 0.98 (0.61–1.57) | 1.92 (1.17–3.14) | 0.024 |
| Quinolinic acid (nmol/L) | 1.00 | 1.20 (0.76–1.90) | 0.86 (0.53–1.38) | 1.62 (1.00–2.61) | 2.28 (1.38–3.77) | <0.001 |
| Neopterin (nmol/L) | 1.00 | 1.18 (0.73–1.89) | 1.22 (0.74–2.01) | 1.44 (0.82–2.51) | 1.52 (0.85–2.72) | 0.154 |
| 2–< 5 years (3093 cases and 3093 controls) | ||||||
| Tryptophan (μmol/L) | 1.00 | 1.02 (0.75–1.39) | 1.03 (0.75–1.41) | 0.97 (0.70–1.35) | 0.83 (0.59–1.17) | 0.279 |
| Kynurenine (μmol/L) | 1.00 | 1.02 (0.72–1.44) | 0.97 (0.69–1.36) | 0.95 (0.66–1.37) | 1.13 (0.76–1.66) | 0.689 |
| KTRc | 1.00 | 0.98 (0.71–1.35) | 0.93 (0.67–1.29) | 1.08 (0.78–1.51) | 1.29 (0.90–1.84) | 0.142 |
| Quinolinic acid (nmol/L) | 1.00 | 0.87 (0.62–1.21) | 0.77 (0.55–1.07) | 1.10 (0.79–1.54) | 1.24 (0.85–1.79) | 0.136 |
| Neopterin (nmol/L) | 1.00 | 0.98 (0.70–1.38) | 1.05 (0.72–1.54) | 1.76 (1.16–2.68) | 1.72 (1.10–2.67) | 0.003 |
| ≥ 5 years (467 cases and 467 controls) | ||||||
| Tryptophan (μmol/L) | 1.00 | 0.85 (0.70–1.03) | 0.84 (0.70–1.02) | 0.93 (0.77–1.13) | 0.85 (0.69–1.04) | 0.413 |
| Kynurenine (μmol/L) | 1.00 | 0.95 (0.79–1.13) | 1.13 (0.94–1.35) | 1.05 (0.87–1.28) | 1.17 (0.94–1.45) | 0.092 |
| KTRc | 1.00 | 0.99 (0.84–1.17) | 1.08 (0.91–1.29) | 1.09 (0.91–1.31) | 1.21 (0.98–1.48) | 0.058 |
| Quinolinic acid (nmol/L) | 1.00 | 0.96 (0.81–1.13) | 0.96 (0.8–1.15) | 1.21 (1.00–1.45) | 1.28 (1.03–1.59) | 0.005 |
| Neopterin (nmol/L) | 1.00 | 1.07 (0.89–1.28) | 1.09 (0.89–1.33) | 1.09 (0.87–1.35) | 1.22 (0.96–1.56) | 0.158 |
1,253 cases had missing information on the time interval from blood draw to cancer diagnosis, thus these cases and their individually matched controls were excluded from this analysis.
All models were adjusted for educational attainment (categorical), body mass index (kg/m2) (categorical), serum cotinine concentrations (continuous), estimated glomerular filtration rate (continuous), and cohort.
KTR, kynurenine to tryptophan ratio (x 1000).
DISCUSSION
Principal findings
The current study is the largest prospective epidemiological study to investigate the novel associations of KTR and neopterin, proinflammatory immune biomarkers with risk of lung cancer. The study demonstrated significant associations between increasing levels of these cellular immunity biomarkers and higher risk of lung cancer in current smokers, and to a lesser extent, in former smokers. These positive associations were strongest for lung squamous cell cancer and for lung cancer cases diagnosed within the first two years after blood draw.
Higher circulating KTR concentrations and risk of lung cancer
The observed associations between KTR and lung cancer risk in the current study have plausible biological explanations. Tryptophan is an essential amino acid for immune cell proliferation, and depletion of tryptophan results in T cell apoptosis 27. IDO catalyzes the initial step of the kynurenine pathway, the conversion of tryptophan to formylkyurenine, which is rapidly converted to kynurenine. Thus when IDO is high, the KTR would be expected to be higher. IDO can be induced by a series of inflammatory cytokines, especially IFN-gamma. In addition, IDO is over-expressed in several cancers, including lung cancer 28. Clinical studies conducted in lung cancer patients showed that mRNA expression of IDO was higher in lung cancer tissues than adjacent non-malignant lung tissues 29. IDO-expressing dendritic cells were found in tumor tissues and tumor-draining lymph nodes in patients with lung cancers 30. Serum KTR was higher in lung cancer patients than in healthy controls 31. The stronger associations between kynurenine or KTR and risk of lung cancer in individuals within <2 years of blood draw may reflect potential reverse causation. A prior epidemiological study in the European Prospective Investigation into Cancer and Nutrition (EPIC)17 reported associations for lower circulating levels of tryptophan and higher KTR with higher risk of lung cancer, similar to those found in the present study which included a much larger sample size and diverse populations.
IDO in tumor cells and infiltrating inflammatory cells, such as dendritic cells and regulatory B cells, serves as an immunosuppressive enzyme that limits T cell responses against tumors via depletion of tryptophan and by producing immuno-regulatory kynurenine metabolites 30, 32. Inhibition of IDO by 1-methyltryptophan significantly delayed the tumor outgrowth in a mouse model of Lewis Lung carcinoma 28. Kynurenine can promote the proliferation of regulatory T cells, which suppress the antitumor immune response, contributing to cancer immune escape 33. Another possible mechanism linking the kynurenine pathway and lung cancer development is through the interaction between kynurenine metabolites and aryl hydrocarbon receptor, a protein that regulates the metabolic pathways of exogenous chemicals. Kynurenine is a ligand for aryl hydrocarbon receptor, which activates the carcinogenesis pathway of polycyclic aromatic hydrocarbons, in particular benzo[a]pyrene, a strong lung carcinogen derived from tobacco smoke 34, 35. Lung squamous cell carcinoma is more strongly associated with polycyclic aromatic hydrocarbons present in tobacco smoke 36 whereas adenocarcinoma is more strongly associated with tobacco-specific nitrosamines such as 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone (NNK) in animal models 37. This may explain why our observed associations between KTR and lung cancer risk were confined to current smokers, and stronger for squamous cell carcinoma than adenocarcinoma of the lung.
Higher circulating quinolinic acid concentrations and risk of lung cancer
The current study is the first to evaluate the association between QA and lung cancer risk. We found that a higher concentration of QA in pre-diagnostic blood samples was associated with higher risk of lung cancer. QA, a downstream metabolite of kynurenine, has long been known as a neurotoxin via stimulation of the presynaptic receptor which induces oxidative stress, and enhances the production of pro-inflammatory cytokines in the brain 38. Increased levels of QA were found in cerebrospinal fluid of patients with neuro-degenerative disorders, such as Huntington’s disease, Alzheimer’s disease, and AIDS dementia complex 20. In gerbils, QA level increased following acute systemic immune stimulation via upregulation of enzymes in the kynurenine pathway 39. In the current study, circulating QA concentrations were moderately correlated with the inflammatory markers KTR (r=0.57) and neopterin (r=0.40), which is consistent with the fact that QA concentrations are correlated with IDO expression 20. Previous studies showed that during inflammation, QA synthesis occurs mainly in immune cells 20. Given that QA is a precursor of nicotinamide adenine dinucleotide, a coenzyme for redox reactions, accumulation of QA within immune cells could provide substrates for nicotinamide adenine dinucleotide synthesis to meet the enhanced requirements during an immune response 20. Taken together, the observed association between QA and increased risk of lung cancer could reflect immune response against cancer prior to its clinical presentation. In addition, recent evidence showed that QA can inhibit the proliferation of cancer-killing T and natural killer cells 40. Therefore, the higher concentrations of QA may promote tumor growth via its role in immune suppression. The association for QA with risk of lung cancer within <2 years of blood draw is stronger than those with longer time intervals, which suggests that this marker may be related to the progression of lung cancer and could be developed as biomarker for early detection of lung cancer.
Strengths and limitations
The strengths of the study include a prospective design using pre-diagnostic plasma/serum samples, and a large sample size with sufficient power to conduct analysis stratified by smoking status, and histology of lung cancer. We measured circulating metabolites of the kynurenine pathway, including the novel use of QA as an inflammatory marker. In addition to matching on smoking status, intensity and duration in the study design, the analysis for the association between biomarkers of cellular immune activation and lung cancer risk was adjusted for circulating cotinine, a biomarker of recent tobacco exposure 41, and eGFR, a renal function measurement that is a strong determinant for circulating concentrations of kynurenine and its metabolites 42. The present study measured novel biomarkers for cellular immune activation, such as KTR and neopterin with a high intra-individual correlation in our previously published work on the development of the methodology, in which the intraclass correlation coefficients (ICCs) for KTR and neopterin in 4 different sampling visits over 3.5 years were 0.74 and 0.67, respectively 43, indicating that a single time point measurement is a relatively reliable biomarker for long-term exposure, and these biomarkers may be better than traditional cytokine biomarkers such as IFN-gamma and interleukins with lower ICCs 44. As any observational study, the observed association between serum biomarkers and lung cancer risk could be confounded by other factors such as smoking. For example, smoking is an established risk factor for lung cancer. Smokers in the present study had significantly higher concentration of tryptophan and lower levels of KTR and QA, as well as neopterin. Furthermore, lung cancer risk was only significantly inversely associated with tryptophan and positively associated with KTR and QA, as well as neopterin, among current smokers. Although smoking status, density and duration were matched for cases and controls in the present study and circulating cotinine was additionally adjusted for in the statistical analysis, the residual confounding of smoking on the observed biomarker-lung cancer risk associations cannot completely be ruled out.
CONCLUSION
The current study demonstrates that markers of cellular immunity, KTR and QA, as well as neopterin, were associated with increased risk of lung cancer for current smokers. Findings from experimental studies also support a role for inflammation and immunity in the development of lung cancer. Taken together, our results suggest that biomarkers for early detection of smoking related lung cancer could be found in the kynurenine pathway. Our novel observation of a positive association between QA and lung cancer risk warrants mechanistic studies on the role of QA in lung carcinogenesis.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all participants, investigators and staff of the 20 original cohort studies that contributed data and biospecimens to the international Lung Cancer Cohort Consortium (LC3) project. For the Nurses’ Health Study and the Health Professionals Follow-up Study, we acknowledge following state cancer registries for their help to ascertain cancer cases: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. For the Campaign Against Cancer and Stroke (CLUE I) and the Campaign Against Cancer and Heart Disease (CLUE II) cohorts, we thank the Maryland Cancer Registry, Center for Cancer Surveillance and Control, Department of Health and Mental Hygiene for providing Cancer Incidence Data,
FUNDING
The Lung Cancer Cohort Consortium (LC3) was supported by National Institutes of Health/National Cancer Institute grant No. 1U01CA155340. The work of T. L. Larose presented in this paper was undertaken during a postdoctoral placement at the International Agency for Research on Cancer, within the framework of an agreement between the Research Council of Norway and the Norwegian University of Science and Technology.
We acknowledge the main funding sources to individual participating cohorts (Funding agency, grant number): The Nurses’ Health Study and the Health Professionals Follow-up Study (NIH UM1CA167552, UM1CA186107, and P01CA87969); the Shanghai Cohort Study and the Singapore Chinese Health Study (NIH R01CA1144034 and UM1CA182876); the Shanghai Men’s Health Study (NIH UM1 CA173640); The Shanghai Women’s Health Study (NIH UM1 CA182910); The Southern Community Cohort Study (NIH R01 CA092447 and U01 CA202979); The Women’s Health Initiative (NIH contracts HHSN268201100046C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, HHSN268201600004C, and HHSN271201600004C); the Women’s Health Study (NIH CA047988, CA182913, HL043851, HL080467, and HL099355). The Alpha-Tocopherol Beta-Carotene Study (HHSN261201500005C and NCI Intramural Research Program), the Prostate Lung Colorectal Ovarian Cancer Screening Trial (NIH Contracts from Division of Cancer Prevention and the Division of Cancer Epidemiology and Genetics); the Melbourne Collaborative Cohort Study (VicHealth and Cancer Council Victoria and Australian National Health and Medical Research Council grants 209057, 251553 and 1050198); The CLUE I and II (the State of Maryland, the Maryland Cigarette Restitution Fund, and the National Program of Cancer Registries); and the HUNT Study is a collaboration between NTNU (Norwegian University of Science and Technology, Faculty of Medicine and Health Sciences), Trøndelag County Council, Central Norway Health Authority, and the Norwegian Institute of Public Health.
Abbreviations:
- ANCOVA
Analysis of Covariance
- BMI
body mass index
- CI
confidence interval
- eGFR
estimated glomerular filtration rate
- IDO
Indoleamine 2,3-dioxygenase
- IFN-gamma
interferon-gamma
- KTR
kynurenine to tryptophan ratio
- LC3
Lung Cancer Cohort Consortium
- OR
odds ratio
- QA
quinolinic acid
REFERENCES
- 1.World Health Organization. Cancer Fact Sheet, vol. 2018, 2018. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, et al. SEER Cancer Statistics Review, 1975–2013, National Cancer Institute Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April., 2016. [Google Scholar]
- 3.Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32: 605–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell 2010;17: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomes M, Teixeira AL, Coelho A, Araujo A, Medeiros R. The role of inflammation in lung cancer. Adv Exp Med Biol 2014;816: 1–23. [DOI] [PubMed] [Google Scholar]
- 6.Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, Bowman ED, Engels EA, Caporaso NE, Harris CC. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst 2011;103: 1112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner DR, Fanidi A, Grankvist K, Muller DC, Brennan P, Manjer J, Byrnes G, Hodge A, Severi G, Giles GG, Johansson M, Johansson M. Inflammatory Cytokines and Lung Cancer Risk in 3 Prospective Studies. Am J Epidemiol 2017;185: 86–95. [DOI] [PubMed] [Google Scholar]
- 8.Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G, Caporaso NE, Pinto LA, Chaturvedi AK. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 2013;105: 1871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biancotto A, Wank A, Perl S, Cook W, Olnes MJ, Dagur PK, Fuchs JC, Langweiler M, Wang E, McCoy JP. Baseline levels and temporal stability of 27 multiplexed serum cytokine concentrations in healthy subjects. PLoS One 2013;8: e76091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol 2007;96: 41–101. [DOI] [PubMed] [Google Scholar]
- 11.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001;410: 1107–11. [DOI] [PubMed] [Google Scholar]
- 12.Hodge G, Barnawi J, Jurisevic C, Moffat D, Holmes M, Reynolds PN, Jersmann H, Hodge S. Lung cancer is associated with decreased expression of perforin, granzyme B and interferon (IFN)-gamma by infiltrating lung tissue T cells, natural killer (NK) T-like and NK cells. Clin Exp Immunol 2014;178: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen MH, Pedersen EK, Nordbo Y, Varhaug JE, Midttun O, Ueland PM, Nygard OK, Mellgren G, Lien EA. Vitamin B6 status and interferon-gamma-mediated immune activation in primary hyperparathyroidism. Journal of internal medicine 2012;272: 583–91. [DOI] [PubMed] [Google Scholar]
- 14.Kolodziej LR, Paleolog EM, Williams RO. Kynurenine metabolism in health and disease. Amino acids 2011;41: 1173–83. [DOI] [PubMed] [Google Scholar]
- 15.Ulvik A, Midttun O, Pedersen ER, Eussen SJ, Nygard O, Ueland PM. Evidence for increased catabolism of vitamin B-6 during systemic inflammation. Am J Clin Nutr 2014;100: 250–5. [DOI] [PubMed] [Google Scholar]
- 16.Pingle SK, Tumane RG, Jawade AA. Neopterin: Biomarker of cell-mediated immunity and potent usage as biomarker in silicosis and other occupational diseases. Indian journal of occupational and environmental medicine 2008;12: 107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuang SC, Fanidi A, Ueland PM, Relton C, Midttun O, Vollset SE, Gunter MJ, Seckl MJ, Travis RC, Wareham N, Trichopoulou A, Lagiou P, et al. Circulating biomarkers of tryptophan and the kynurenine pathway and lung cancer risk. Cancer Epidemiol Biomarkers Prev 2014;23: 461–8. [DOI] [PubMed] [Google Scholar]
- 18.Zuo H, Tell GS, Vollset SE, Ueland PM, Nygard O, Midttun O, Meyer K, Ulvik A, Eussen SJ. Interferon-gamma-induced inflammatory markers and the risk of cancer: the Hordaland Health Study. Cancer 2014;120: 3370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell death and differentiation 2002;9: 1069–77. [DOI] [PubMed] [Google Scholar]
- 20.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunology and cell biology 2003;81: 247–65. [DOI] [PubMed] [Google Scholar]
- 21.Fanidi A, Muller DC, Yuan JM, Stevens VL, Weinstein SJ, Albanes D, Prentice R, Thomsen CA, Pettinger M, Cai Q, Blot WJ, Wu J, et al. Circulating Folate, Vitamin B6, and Methionine in Relation to Lung Cancer Risk in the Lung Cancer Cohort Consortium (LC3). J Natl Cancer Inst 2018;110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueland PM, Midttun O, Windelberg A, Svardal A, Skalevik R, Hustad S. Quantitative profiling of folate and one-carbon metabolism in large-scale epidemiological studies by mass spectrometry. Clin Chem Lab Med 2007;45: 1737–45. [DOI] [PubMed] [Google Scholar]
- 23.Midttun O, Kvalheim G, Ueland PM. High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using HPLC-MS/MS. Analytical and bioanalytical chemistry 2013;405: 2009–17. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Annals of internal medicine 2009;150: 604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslow N, Day N. Statistical methods in cancer research, vol.1: The analysis of case–control studiesed. Lyon: IARC: IARC Scientific Pub No. 32, 1980. [PubMed] [Google Scholar]
- 26.Benowitz NL, Hukkanen J, Jacob P, 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handbook of experimental pharmacology 2009: 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 2004;4: 762–74. [DOI] [PubMed] [Google Scholar]
- 28.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med 2004;10: 15–8. [DOI] [PubMed] [Google Scholar]
- 29.Karanikas V, Zamanakou M, Kerenidi T, Dahabreh J, Hevas A, Nakou M, Gourgoulianis KI, Germenis AE. Indoleamine 2,3-dioxygenase (IDO) expression in lung cancer. Cancer biology & therapy 2007;6: 1258–62. [DOI] [PubMed] [Google Scholar]
- 30.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Mellor AL Jr.. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 2002;297: 1867–70. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D, Nakamura Y, Inui N, Nakamura H, Chida K. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer 2010;67: 361–5. [DOI] [PubMed] [Google Scholar]
- 32.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003;9: 1269–74. [DOI] [PubMed] [Google Scholar]
- 33.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 2010;185: 3190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prendergast GC. Cancer: Why tumours eat tryptophan. Nature 2011;478: 192–4. [DOI] [PubMed] [Google Scholar]
- 35.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011;478: 197–203. [DOI] [PubMed] [Google Scholar]
- 36.Wang GZ, Cheng X, Zhou B, Wen ZS, Huang YC, Chen HB, Li GF, Huang ZL, Zhou YC, Feng L, Wei MM, Qu LW, et al. The chemokine CXCL13 in lung cancers associated with environmental polycyclic aromatic hydrocarbons pollution. Elife 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujimoto J, Nunomura-Nakamura S, Liu Y, Lang W, McDowell T, Jakubek Y, Ezzeddine D, Kapere Ochieng J, Petersen J, Davies G, Fukuoka J, Wistuba II, et al. Development of Kras mutant lung adenocarcinoma in mice with knockout of the airway lineage-specific gene Gprc5a. Int J Cancer 2017;141: 1589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lugo-Huitron R, Ugalde Muniz P, Pineda B, Pedraza-Chaverri J, Rios C, Perez-de la Cruz V. Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid Med Cell Longev 2013;2013: 104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saito K, Crowley JS, Markey SP, Heyes MP. A mechanism for increased quinolinic acid formation following acute systemic immune stimulation. The Journal of biological chemistry 1993;268: 15496–503. [PubMed] [Google Scholar]
- 40.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. The Journal of experimental medicine 2002;196: 459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boffetta P, Clark S, Shen M, Gislefoss R, Peto R, Andersen A. Serum cotinine level as predictor of lung cancer risk. Cancer Epidemiol Biomarkers Prev 2006;15: 1184–8. [DOI] [PubMed] [Google Scholar]
- 42.Theofylaktopoulou D, Midttun O, Ulvik A, Ueland PM, Tell GS, Vollset SE, Nygard O, Eussen SJ. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: the Hordaland Health Study. Clin Exp Immunol 2013;173: 121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Midttun O, Townsend MK, Nygard O, Tworoger SS, Brennan P, Johansson M, Ueland PM. Most blood biomarkers related to vitamin status, one-carbon metabolism, and the kynurenine pathway show adequate preanalytical stability and within-person reproducibility to allow assessment of exposure or nutritional status in healthy women and cardiovascular patients. J Nutr 2014;144: 784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epstein MM, Breen EC, Magpantay L, Detels R, Lepone L, Penugonda S, Bream JH, Jacobson LP, Martinez-Maza O, Birmann BM. Temporal stability of serum concentrations of cytokines and soluble receptors measured across two years in low-risk HIV-seronegative men. Cancer Epidemiol Biomarkers Prev 2013;22: 2009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


