Abstract
Objective
The Cardiothoracic Surgical Trials Network (CTSN) reported that left ventricular reverse remodeling at 2-years did not differ between patients with moderate ischemic mitral regurgitation (MR) randomized to CABG plus mitral-valve (MV) repair (n=150) or CABG alone (n=151). To address health resource use implications, we compared costs and quality-adjusted survival.
Methods
We used individual patient data from the CTSN trial on survival, hospitalizations, quality-of-life, and U.S. hospitalization costs to estimate cumulative costs and quality-adjusted life years (QALYs). A microsimulation model was developed to extrapolate to 10-years. Bootstrap and deterministic sensitivity analyses were performed to address uncertainty.
Results
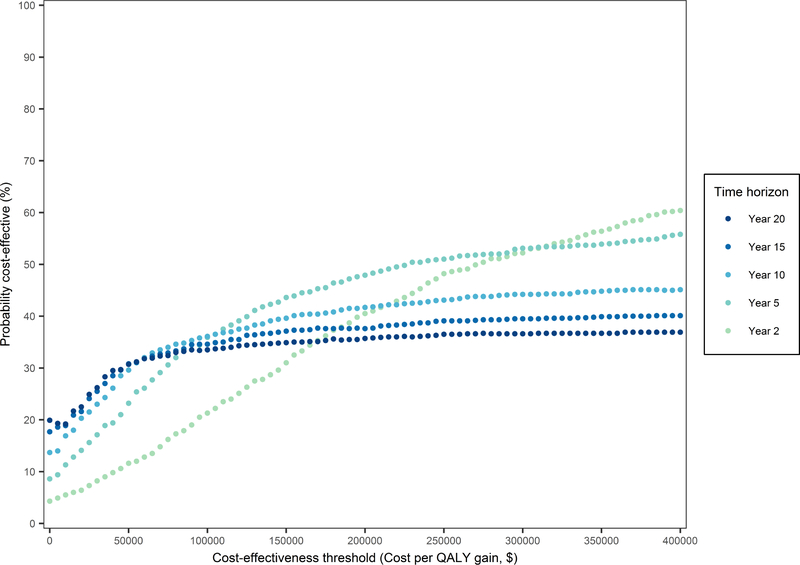
In-hospital costs were $59,745 for CABG plus MV repair vs $51,326 for CABG alone, difference $8,419 (95% uncertainty interval 2,259–18,757). Two-year costs were $81,263 vs $67,341 and QALYs were 1.35 vs 1.30, difference 0.05 (−0.04–0.14), resulting in an incremental cost-effectiveness ratio (ICER) of $308,343/QALY for CABG plus MV repair. At 10-years, its costs remained higher ($107,733 vs $88,583, difference 19,150 [−3,866–56,826]) and QALYs showed no difference (−0.92–0.87), with 5.08 vs 5.08. The likelihood that CABG plus MV repair would be considered cost-effective at 10-years based on a cost-effectiveness threshold of $100k/QALY did not exceed 37%. Only when this procedure reduces the death rate by a relative 5% will the ICER fall below $100k/QALY.
Conclusions
Addition of MV repair to CABG for patients with moderate ischemic MR is unlikely to be cost-effective. Only if late mortality benefits can be demonstrated will it meet commonly used cost-effectiveness criteria.
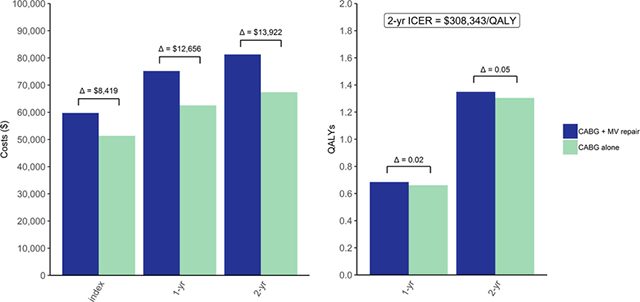
Central Picture.
Within-trial cost-effectiveness analysis results
Central Message
Adding MV repair to CABG for patients with moderate ischemic MR is unlikely to be cost-effective; if late mortality benefits can be demonstrated it may meet commonly used cost-effectiveness criteria.
Perspective Statement
The CTSN previously showed that left ventricular remodeling does not improve when MV repair is added to CABG for moderate ischemic MR. Although providing more durable correction, it is also associated with more adverse events. Based on trial data and microsimulations, costs are higher for CABG plus MV repair, and its cost-effectiveness would become only acceptable if there was late mortality benefit.
Introduction
Ischemic mitral regurgitation (MR) is present in up to 60% of patients with myocardial infarction,1–3 and is typically caused by a change in the geometry of the left ventricle following the myocardial injury, which impedes adequate coaptation of normal mitral leaflets.4 Regardless of severity, ischemic MR has been consistently associated with worse left ventricular function, increased risk of heart failure, and higher mortality rates.5 However, although broad consensus exists for the correction of severe ischemic MR at the time of coronary-artery bypass graft (CABG) surgery, for moderate ischemic MR this practice remains controversial.
To address this surgical controversy, the Cardiothoracic Surgical Trials Network (CTSN) performed a randomized trial that compared CABG plus MV repair to CABG alone in patients with chronic, moderate ischemic MR, the results of which were recently published in two separate reports.6, 7 Three hundred and one randomized patients were followed for two years and the primary endpoint was left ventricular end-systolic volume index (LVESVI). Secondary end points included residual MR, mortality, major adverse cardiovascular events, hospitalization, and quality-of-life. LVESVI improved in both groups at 1 and 2 years, but no significant differences between treatment groups were observed (P=0.71). Two-year mortality rates did not differ as well: 10.6% in the CABG alone group vs 10.0% in the CABG plus MV repair group (P=0.78). However, while the 2-year risk of moderate or severe residual MR was significantly higher among those who underwent CABG alone (32.3% vs 11.2%, P < 0.001), those who underwent the combined procedure had significantly higher rate of neurologic events (14 events vs. 4 events, P = 0.02) and supraventricular arrhythmias (24 events vs. 11 events, P = 0.04) at 2-years. The only quality-of-life metric that showed a statistically significant difference between treatment groups was the Duke Activity Status Index,8 which demonstrated higher functional capacity in those that received the combined procedure (P=0.02).
The increasing emphasis on value-based care has made economic analyses become more relevant for interpreting trial results to further inform medical decision making. With economic analyses conducted alongside trials, both the expected costs and clinical outcomes are compared across study arms. In a formal cost-effectiveness analysis (CEA), the health outcome or “effectiveness” is expressed as survival time accounting for time spent at less than full quality, i.e. quality adjusted life years (QALYs). Decision makers can then decide which intervention would meet our expectation of good value based on the extra cost spent per gain in QALYs.{Ferket/Oxman} Given the trial results, adding MV repair to CABG is expected to result in differential upfront costs and health risks that may be off-set by differential long-term improvements in morbidity, survival and health care resource use. As such, the selection of the analytic time horizon can substantially impact our conclusions on cost-effectiveness and therefore costs and effectiveness outcomes need to be extrapolated to a longer follow-up.
In order to comprehensively assess the trade-offs associated with the two surgical strategies, we performed a CEA using individual-level patient data from the trial with microsimulation to extrapolate outcomes beyond the trial follow-up duration.
Methods
Trial Design and Population
The CTSN moderate ischemic MR trial6, 7 was funded by the National Institutes of Health (NIH) and the Canadian Institutes of Health Research (CIHR) and conducted at 26 clinical centers. In summary, 301 patients with multivessel coronary artery disease and moderate ischemic MR were randomized between 2009 and 2013 to undergo either CABG alone (n=151) or CABG plus MV repair (n=150). CABG was performed on-pump, using standard techniques and all patients received guideline-directed medical therapy. In patients randomized to CABG plus MV repair, a rigid or semi-rigid complete annuloplasty ring was used. The ring was downsized by two sizes when possible to correct for annular dilatation. The specific ring type, implantation technique, and myocardial preservation method were at the surgeon’s discretion. Causes of death and adverse events were adjudicated by an independent committee of experts. An NIH-appointed data and safety monitoring board oversaw trial progress.
Cost and Quality-of-Life Data
Costing was done from a U.S. healthcare sector perspective. Costs were derived from uniform billing (UB) medical claims forms associated with index hospitalizations (N=172) and hospital readmissions (N=104) at U.S. study sites and these data were obtained from Vizient, a healthcare improvement company,9 or directly from study sites themselves. Costs were calculated per hospitalization, by converting charges using departmental cost-to-charge ratios matched to reported revenue codes. Departmental cost-to-charge ratios were derived from Centers for Medicare & Medicaid Services annual hospital cost reports. All costs were expressed into 2015 U.S. dollars using the Personal Health Care index for hospital care.10 Generic health status was converted into a utility weight using the Short-Form Six-Dimension (SF-6D) health utility index (0=death, 1=optimal quality-of-life),11 which was derived from patient-level 12-Item Short Form Health Survey (SF-12) data collected at baseline, 6, 12 and 24 months during the trial. We performed multiple imputation for missing costs and SF-6D utility scores (see Supplemental Methods). All analyses were performed based on intention-to-treat.
Within-Trial Cost-effectiveness Analysis
We initially performed a within-trial CEA. Cumulative costs were calculated by totaling hospitalization costs for each patient during the trial follow-up period. QALYs were calculated from longitudinal SF-6D utility scores using an area-under-the-curve approach with a trapezoidal rule. For the base case, when an interval death occurred, we assumed that the SF-6D utility score would follow a sudden drop to zero at the moment of death.12 Year 2 costs and QALYs were discounted using a rate of 3%.13 We then calculated the difference in average costs and QALYs between treatment groups. An incremental cost-effectiveness ratio (ICER) was calculated when the more expensive strategy would also provide more effectiveness (for details see Supplemental Methods).
Long-Term Cost-effectiveness Analysis
For predicting costs and QALYs over a 5 and 10-year time horizon we developed an individual-level state-transition (‘microsimulation’) model (Supplemental Figure I). We designed the model to make forecasts of mortality, readmissions for heart failure, other cardiovascular disease (CVD), and non-CVD related reasons, reoperations, as well as to track the expected costs and loss of quality-of-life related to these adverse events. To increase the precision of event rates, we combined the moderate ischemic MR trial data with data from the CTSN severe ischemic MR trial14, 15 and included a trial-treatment interaction term to model the MV repair effect. In the CTSN severe ischemic MR trial, 251 patients were randomized between December 2008 and April 2012 to undergo either mitral-valve repair (n=126) or replacement (n=125). The design and protocol of both trials were nearly identical, and 21 of the 22 participating study sites for the CTSN severe ischemic MR trial also participated in the moderate ischemic MR trial. Event rates were further adjusted by age and gender, and we included time-dependent covariates to allow for an increase in event rates after readmissions, which were tracked in the model with “tracker variables”. Hazard ratios (HRs) for these variables were estimated by Andersen-Gill models,16 an extension of the Cox proportional-hazards model allowing for recurrent events (Supplemental Table I). For the base case, we modeled baseline readmission rates with restricted cubic splines assuming a Weibull distribution for extrapolations beyond the trial follow-up period. We chose this more data-driven approach for smooth estimation of readmission rates in absence of literature indicating the underlying distribution of readmission rates. Competing mortality rates were assumed to follow an exponential survival distribution in concordance with long-term survival curves from cohort studies, which predominantly included ischemic moderate MR patients with rigorous long-term (>5 year) follow-up for death (Supplemental Figure II).17–23 Model validity was assessed by comparing model-based predictions with empirical 2-year event rates, cumulative costs and QALYs obtained from each MR trial separately (Supplemental Figure III–VII). SF-6D utility scores were assumed to remain stable beyond trial duration.24 Cost and quality-of-life penalties were conditioned on readmissions and estimated by prediction models that included age at admission, gender, study arm, and reason for admission. We implemented a 1-month cycle length and assumed events would occur halfway for calculating costs and QALYs. We discounted costs and QALYs with a 3% annual rate.13 For details about the model inputs used in the long-term CEA see Supplemental Methods.
We assessed parameter uncertainty by a bootstrap procedure in which we randomly sampled trial participant data with replacement, ensuring each bootstrap sample had the same size as the original trial dataset, and generated 1,000 bootstrap datasets. Analysis steps performed for both the within-trial and long-term CEA were repeated in each bootstrap dataset. Results were summarized as: 1) 95% uncertainty intervals (UIs) using a bias-corrected and accelerated method; 2) as a scatter plot of the 1,000 pairs of difference in average costs and QALYs; and 3) as cost-effectiveness acceptability curves. In the latter, the percentage of bootstraps in which CABG plus MV repair was deemed to be cost-effective was plotted against a range of time horizons and cost-effectiveness thresholds.13
Sensitivity Analyses
To evaluate robustness and heterogeneity of our findings, we conducted a number of deterministic sensitivity and scenario analyses. For the within-trial CEA, we tested the assumption that patients who died would have a gradual decline in quality-of-life from the last value measured until death. For the long-term CEA, we tested the impact of varying the annual discount rate from 0 to 5%; of applying different distribution assumptions for extrapolating readmission and mortality rates; and of extending the analytic time horizon to 15 and 20 years of follow-up. We also evaluated the impact of varying the effect of CABG plus MV repair on long-term rates beyond the 2-year time point for mortality, heart failure and other cardiovascular readmissions within 95% UI limits. In addition, we examined the effect of increasing the risk of reoperation in the CABG only group. Finally, we looked at the effect of varying hospitalization costs within 95% UI limits and varying baseline age of the entire trial cohort from 45 to 85 (see Supplemental Methods for further details).
Results
Study Population
The average age of the trial cohort was 65±11 years and the majority of patients (68%) were male. The mean baseline LVESVI was 57.2±25.4 mL/m2. The majority of patients had a prior history of myocardial infarction or revascularization and nearly half had diabetes mellitus. Three of the 150 patients assigned to CABG plus MV repair received CABG alone, and eight patients assigned to CABG alone group underwent a combined procedure. For more details on study population baseline and operative characteristics see our earlier publications6, 7 and Supplemental Table II and III.
Within-Trial Cost-Effectiveness Outcomes
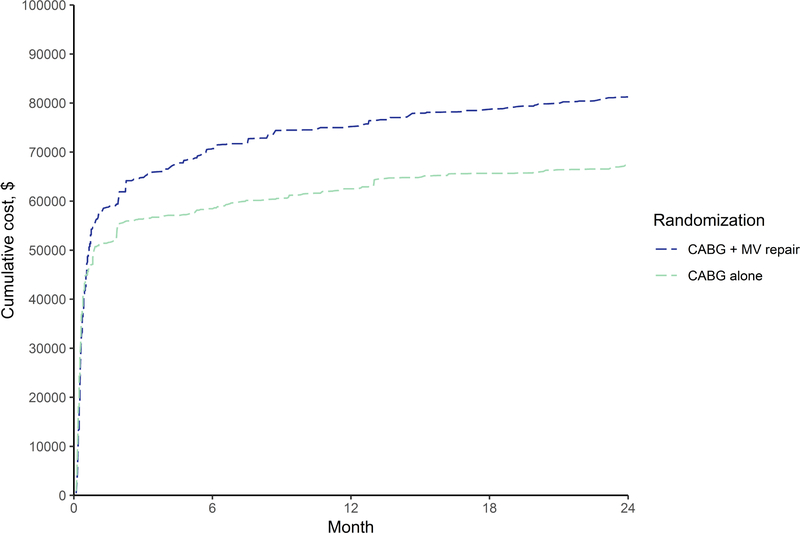
Cardiopulmonary bypass time, time to discharge and ICU stay were longer for patients randomized to CABG plus MV repair (Supplemental Table III). Index hospitalization costs were $59,745 for CABG plus MV repair vs $51,326 for CABG alone (difference $8,419, 95% UI $2,259–18,757), and also 2-year costs were higher for the combined procedure (difference $13,922, 95% UI $2,370–28,888), see Table 1 and Figure 1. There were small differences in SF-6D utility scores during the 2 years of follow-up (Supplemental Figure VIII), and by 2 years the combined procedure group accrued 0.05 more QALYs (95% UI −0.04–0.14) than the CABG only group (Table 1 and Supplemental Figure IX). The 2-year ICER of CABG plus MV repair compared to the CABG alone was $308,343/QALY.
Table 1.
Base case cost-effectiveness outcomes (95% UI)
| Outcome | CABG alone N=151 |
CABG plus mitral-valve repair N=150 |
|---|---|---|
| Costs, $ | ||
| Index hospitalization | 51,326 (47,320–59,903) | 59,745 (54,439–71,512) |
| Δ | Reference | 8,419 (2,259–18,757) |
| 1-year | 62,514 (56,491–72,971) | 75,170 (66,737–88,746) |
| Δ | Reference | 12,656 (2,152–24,662) |
| 2-year | 67,341 (59,233–76,778) | 81,263 (71,535–96,596) |
| Δ | Reference | 13,922 (2,370–28,888) |
| 5-year | 77,883 (66,660–92,099) | 94,610 (80,376–122,163) |
| Δ | Reference | 16,727 (−354–40,476) |
| 10-year | 88,583 (71,953–111,801) | 107,733 (87,978–153,433) |
| Δ | Reference | 19,150 (−3,866–56,826) |
| QALYs | ||
| 1-year | 0.66 (0.63–0.69) | 0.68 (0.66–0.71) |
| Δ | Reference | 0.02 (−0.01–0.06) |
| 2-year | 1.30 (1.23–1.36) | 1.35 (1.29–1.41) |
| Δ | Reference | 0.05 (−0.04–0.14) |
| 5-year | 2.98 (2.76–3.18) | 3.04 (2.82–3.28) |
| Δ | Reference | 0.06 (−0.27–0.37) |
| 10-year | 5.08 (4.48–5.65) | 5.08 (4.45–5.77) |
| Δ | Reference | 0.00 (−0.92–0.87) |
| ICER, $/QALY | ||
| 1-year | Reference | 530,418 |
| 2-year | Reference | 308,343 |
| 5-year | Reference | 266,983 |
| 10-year | Reference | Dominated |
Shown are average costs and QALYs (95% UIs) and their differences (95% UI) for N=151 for CABG alone and N=150 for CABG plus mitral-valve repair. ICERs are shown for CABG plus mitral-valve repair vs CABG alone when both average costs and QALYs were higher for CABG plus mitral-valve repair. The CABG plus mitral-valve repair strategy is considered dominated when its average costs are higher but its average QALYs are equal or lower. Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; UI, uncertainty interval.
Figure 1. Within-trial cumulative average cost by study arm.
Shown are cumulative costs over the trial duration of two years. Total costs incurred until each time point indicated by a hospital discharge are averaged across N=150 for CABG plus mitral-valve repair and N=151 for CABG alone. Abbreviations: MV, mitral-valve.
Long-Term Cost-Effectiveness Outcomes
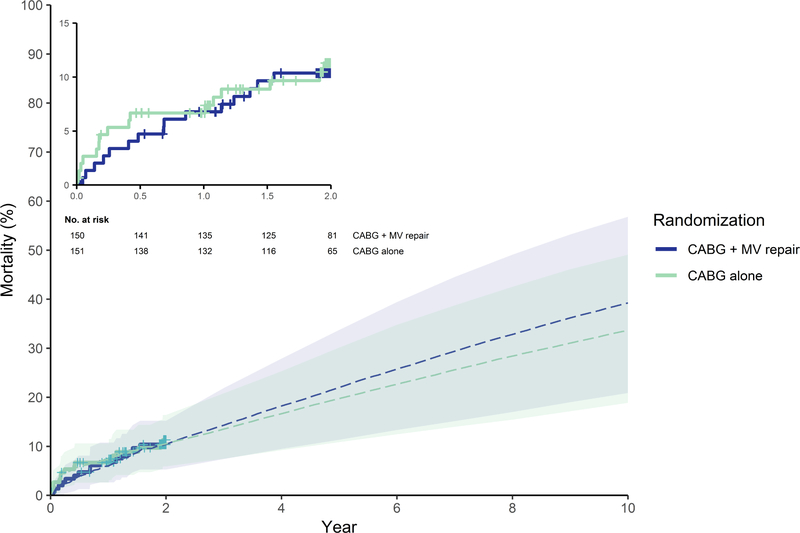
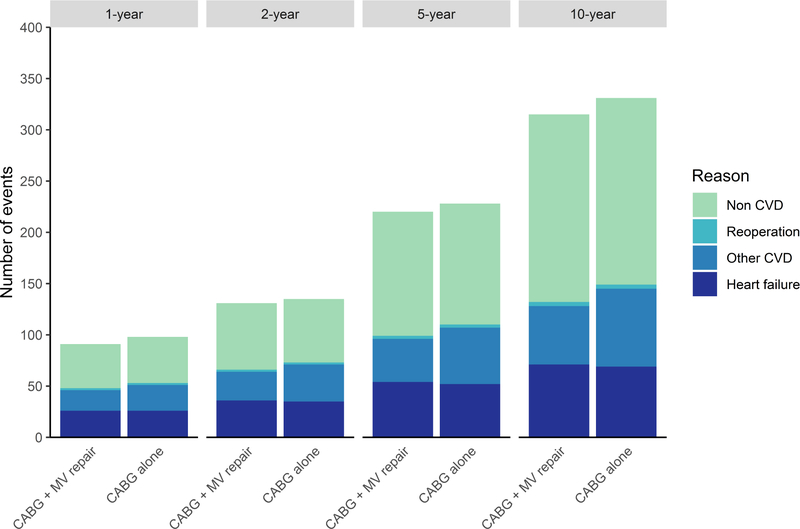
Mortality rates between 90 days and two years post-randomization were on average higher for CABG plus MV repair than CABG only, but this difference was not statistically significant (adjusted HR of 1.46 [95% UI 0.56–3.33]). However, when extrapolating beyond two years, the average mortality benefit with CABG alone becomes more pronounced (Figure 2). Adjusted HRs for readmissions were not statistically significant (Supplemental Table I) and the difference in long-term readmission rates for the two surgical strategies was small on average (Figure 3). Higher average costs for the combined procedure persisted beyond the 2-year trial duration. At 10 years, the difference in costs was $19,150 (95% UI $-3,866–56,826), but in QALYs it was 0.00 (95% UI −0.92–0.87) (Table 1). Using cost-effectiveness thresholds of $50k and $100k/QALY, the likelihood that CABG plus MV repair would be cost-effective at 10 years was 30% and 37%, respectively (i.e. < 300 and 370 of 1,000 bootstrap replicates). Even using a cost-effectiveness threshold of $200K/QALY this probability did not exceed 50% (Figure 4).
Figure 2. Observed and simulated all-cause mortality estimates by study arm.
Shown are all-cause mortality (%) estimates based on Kaplan-Meier curves of N=150 for CABG plus mitral-valve repair and N=151 for CABG alone with censoring at 2-year (solid lines) and simulated mortality estimates used for extrapolation in the base case long-term cost-effectiveness analysis (dashed lines). Shown are numbers at risk with accounting for censored observations for the first 2 years of follow-up, beyond the 2 years simulated mortality applies. Abbreviations: MV, mitral-valve.
Figure 3. Readmissions by type during 10-year follow-up.
Number of readmissions is shown by type at 1- and 2-year based on the empirical trial data. The counts shown at 5- and 10-year are based on adding simulated outcomes occurring within 2–5 and 2–10 year time intervals as predicted by the base case long-term cost-effectiveness analysis. Abbreviations: MV, mitral-valve.
Figure 4. Cost-effectiveness acceptability curves for CABG plus mitral-valve repair vs CABG alone according to time horizon.
These curves indicate the probability (%) of CABG plus mitral-valve repair being cost-effective as compared with CABG alone using different time horizons. Each curve equals the obtained percentage of bootstrap iterations (1,000 in total) in which the repeated cost-effectiveness analysis showed that CABG plus mitral-valve repair was dominant (less costly and QALYs ≥ CABG alone) or had a favourable incremental cost-effectiveness ratio (ICER) with a cost($) per QALY gained value lower than the selected cost-effectiveness threshold on the X-axis. The probability of CABG alone being cost-effective equals 100% minus the depicted probability of CABG plus mitral-valve repair being cost-effective.
Sensitivity Analyses
Results of the within-trial CEA did not change when quality-of-life was assumed to decline gradually prior to death (Supplemental Figure X). Using different discount rates and distributions for extrapolation of readmission rates did not impact conclusions about the incremental cost-effectiveness of the combined procedure. Increasing the long-term reoperation rate following CABG alone did not have a significant impact on the cost and incremental cost-effectiveness of the combined procedure. Neither did increasing the cost of readmissions to the upper limit of the 95% UI have a significant impact: CABG plus MV repair continued to have a cost that was $14,000 higher (Table 2).
Table 2.
Sensitivity analyses using a 10-year time horizon
| Model parameter | Costs CABG alone, $ | Costs CABG plus MV repair, $ | Δ costs CABG plus MV repair vs CABG alone, $ | QALYs CABG alone | QALYs CABG plus MV repair | Δ QALYs CABG plus MV repair vs CABG alone | ICER, $/QALY CAGB plus MV repair vs CABG alone |
|---|---|---|---|---|---|---|---|
| Discount rate | |||||||
| 0% | 92,383 | 112,418 | 20,035 | 5.79 | 5.78 | −0.02 | Dominated |
| 5% | 86,453 | 105,104 | 18,651 | 4.69 | 4.69 | 0.01 | 2,908,611 |
| Assumed distribution for modeling baseline mortality rates | |||||||
| Spline-Weibull | 91,013 | 111,618 | 20,605 | 5.41 | 5.49 | 0.08 | 264,312 |
| Weibull from | 90,253 | 111,230 | 20,977 | 5.36 | 5.44 | 0.08 | 276,035 |
| Log-logistic | 90,322 | 111,067 | 20,745 | 5.37 | 5.44 | 0.07 | 311,249 |
| Assumed distribution for modeling baseline readmission rates | |||||||
| Weibull | 87,646 | 105,667 | 18,021 | 5.08 | 5.09 | 0.01 | 1,878,783 |
| Log-logistic | 85,718 | 103,377 | 17,659 | 5.09 | 5.09 | 0.01 | 2,830,045 |
| HR for mortality comparing CABG plus MV repair vs CABG alone | |||||||
| Lower limit 95% UI | 88,583 | 111,476 | 22,894 | 5.08 | 5.47 | 0.39 | 58,592 |
| Upper limit 95% UI | 88,583 | 101,771 | 13,189 | 5.08 | 4.45 | −0.63 | Dominated |
| HR for heart failure readmissions comparing CABG plus MV repair vs CABG alone | |||||||
| Lower limit 95% UI | 88,583 | 105,323 | 16,740 | 5.08 | 5.10 | 0.02 | 960,585 |
| Upper limit 95% UI | 88,583 | 111,871 | 23,289 | 5.08 | 5.07 | −0.01 | Dominated |
| HR for other cardiovascular readmissions comparing CABG plus MV repair vs CABG alone | |||||||
| Lower limit 95% UI | 88,583 | 105,541 | 16,959 | 5.08 | 5.10 | 0.02 | 1,115,282 |
| Upper limit 95% UI | 88,583 | 112,551 | 23,969 | 5.08 | 5.07 | −0.01 | Dominated |
| HR for heart failure and other cardiovascular readmissions comparing CABG plus MV repair vs CABG alone | |||||||
| Lower limit 95% UI | 88,583 | 103,046 | 14,463 | 5.08 | 5.10 | 0.01 | 994,711 |
| Upper limit 95% UI | 88,583 | 116,980 | 28,398 | 5.08 | 5.07 | −0.01 | Dominated |
| Assumed increase in reoperation risk in CABG alone | |||||||
| 100% higher risk (2× risk of base case analysis) | 89,092 | 107,733 | 18,641 | 5.08 | 5.08 | 0.00 | Dominated |
| 300% higher risk (4× risk of base case analysis) | 89,945 | 107,733 | 17,788 | 5.08 | 5.08 | 0.00 | Dominated |
| Costs heart failure readmissions | |||||||
| Lower limit 95% UI | 89,195 | 102,829 | 13,634 | 5.08 | 5.08 | 0.00 | Dominated |
| Upper limit 95% UI | 96,184 | 110,221 | 14,037 | 5.08 | 5.08 | 0.00 | Dominated |
| Costs other cardiovascular readmissions | |||||||
| Lower limit 95% UI | 85,720 | 103,356 | 17,636 | 5.08 | 5.08 | 0.00 | Dominated |
| Upper limit 95% UI | 96,608 | 111,477 | 14,870 | 5.08 | 5.08 | 0.00 | Dominated |
| Costs non-cardiovascular readmissions | |||||||
| Lower limit 95% UI | 85,031 | 99,177 | 14,147 | 5.08 | 5.08 | 0.00 | Dominated |
| Upper limit 95% UI | 97,289 | 111,557 | 14,267 | 5.08 | 5.08 | 0.00 | Dominated |
| Age of entire cohort | |||||||
| Age 45 | 81,547 | 98,784 | 17,237 | 5.19 | 5.26 | 0.07 | 250,737 |
| Age 50 | 83,132 | 100,952 | 17,820 | 5.15 | 5.21 | 0.06 | 295,911 |
| Age 55 | 84,788 | 103,245 | 18,457 | 5.08 | 5.13 | 0.05 | 378,967 |
| Age 60 | 86,457 | 105,495 | 19,038 | 5.02 | 5.01 | 0.00 | Dominated |
| Age 65 | 88,805 | 108,512 | 19,707 | 4.90 | 4.85 | −0.05 | Dominated |
| Age 70 | 91,033 | 111,341 | 20,309 | 4.75 | 4.67 | −0.08 | Dominated |
| Age 75 | 92,753 | 113,985 | 21,231 | 4.54 | 4.42 | −0.12 | Dominated |
| Age 80 | 95,757 | 116,502 | 20,745 | 4.54 | 4.16 | −0.38 | Dominated |
| Age 85 | 97,581 | 118,520 | 20,939 | 4.00 | 3.84 | −0.15 | Dominated |
Shown are average costs and QALYs for N=151 for CABG alone and N=150 for CABG plus mitral-valve repair as calculated in each sensitivity analysis. ICERs are shown for CABG plus mitral-valve repair when both average costs and QALYs are higher than in the CABG alone strategy. The combined procedure strategy was dominated when its average costs were higher but its average QALYs were equal or lower. Abbreviations: HR, hazard ratio; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; UI, uncertainty interval.
However, the ICER of the combined procedure falls to less than $100k/QALY with a post-trial mortality HR of 0.95 and reached $58,592/QALY when the HR is at the lower 95% UI limit (0.558 see Supplemental Table I). On the other hand, assuming the lower 95% UI extremes for HRs of CABG plus MV repair for heart failure and cardiovascular readmission rates, the incremental cost-effectiveness of CABG plus MV repair only minimally improved. Also with lower mortality rates beyond the trial duration, i.e. assuming that extrapolated rates are not constant over time, but are leveling off or assuming a younger baseline age, the incremental cost-effectiveness of the combined-procedure improves, but continues to exceed $200K/QALY (Table 2).
Discussion
We found that costs are higher with CABG plus MV repair compared with CABG alone and this difference already materialized early on. Our finding of higher upfront costs with MV repair is consistent with trial observations that cardiopulmonary bypass time, time to discharge, and length of stay in the ICU during the index hospitalization were longer. In addition, the combined procedure led to a higher number of serious adverse events from baseline to one year follow-up, whereas from one year to two year follow-up the number was nearly equal.6, 7 Consequently, when restricting the time horizon to the trial duration of two years, the small downstream health benefit (0.05 QALY on average) with the combined procedure unlikely justifies the increase in costs (> $13,000 on average at 2-years and > $19,000 at 10-years). Also microsimulations of readmission, reoperation, and mortality rates beyond the trial follow-up period did not project a marked improvement of the incremental effectiveness of the combined procedure over time, whereas its costs continued to be higher, resulting in unfavorable long-term cost-effectiveness. Only when assuming significantly lower late mortality rates than forecasted in the base case analysis does the incremental cost-effectiveness of the combined procedure fall in the range of levels that are commonly used for healthcare interventions with good value, i.e. ICERs that fall below $50,000/QALY (high value) or within $50,000–150,000/QALY (intermediate value).25
This raises the question of whether the combined procedure will actually yield a late mortality benefit. In the CTSN trial, the rate of significant MR two years after the operation was two times higher among those randomized to CABG alone, which presumably will lead to higher morbidity and mortality rates later on. Although such an effect was observed in patients with degenerative MR,26 for moderate ischemic MR this is highly uncertain. It is also possible that the potential future benefits of lower post-surgical MR rates following a combined procedure might be offset by increases in mortality due to more frequent postoperative complications, including neurologic events and supraventricular arrhythmias. In fact, the small difference in average QALYs favoring CABG plus MV repair observed at 2-years decreased over time due to a small, but uncertain increment in late-phase mortality with the combined procedure.
Comparison with Previous Studies
Yet, recently published meta-analyses summarizing estimates from observational studies and small randomized trials that compared CABG plus MV repair to CABG alone for ischemic MR prior to the CTSN trial failed to show any statistically significant differences in short and long-term mortality rates.27, 28 Also no statistically significant differences were found for readmission or reoperation rates,27 although only four studies, including the CTSN moderate ischemic MR trial, reported on these outcomes.7, 20, 29, 30 Unfortunately, an unequivocal interpretation of the findings from these meta-analyses on late-phase mortality rates is hampered by wide variation in the estimated rates of perioperative and early-phase mortality in the included studies, which themselves varied substantially in their study design and patient population. However, one reasonably large observational study that included only patients with moderate ischemic MR (N=251) and used multivariable adjustment found a non-significant trend towards an increased hazard with CABG plus MV repair: HR 1.41 (95% confidence interval [CI] 0.93 to 2.12) from 1 to 10 years post-surgery.20 In addition, two larger observational studies,21, 23 which predominantly included patients with moderate ischemic MR, showed similar trends to worsened survival with CABG plus MV repair in the late post-surgical phase: after multivariable adjustment, resulting HRs were 1.23 (95% CI not reported) and 1.54 (95% CI 0.79 to 2.99), respectively. Thus, the late-phase mortality benefits required for outweighing additional costs with MV repair have not been demonstrated in the current literature and may not be achievable.
Study Limitations
Some limitations of our analysis deserve to be mentioned. First, simulations of event rates beyond the trial follow-up period utilized data from a second randomized clinical trial (the CTSN severe ischemic MR trial) in addition to the individual patient-level trial data from the CTSN moderate ischemic MR trial.14, 15 Because death rates were particularly low in the moderate ischemic MR trial, this approach enabled a more precise extrapolation of survival beyond the trial follow-up period. However, the CTSN study centers and surgeons participating in both ischemic MR trials were nearly identical and in addition, predictions from our microsimulation model validated well against observed data from each trial separately (Supplemental Figures III–VI). Moreover, our long-term survival estimates (5 and 10 year) were similar to those reported in previous studies,17–23 and in line with current practice. Another potential limitation in our study is the wide between-individual variation in observed trial costs that led to uncertainty around the average cost estimates over time. Yet, when a choice between alternative interventions cannot be deferred, the decision maker should base health policy decisions on point estimates, and further research should be guided by the impact of uncertainty on which intervention is best.31 In this context, given the impact of uncertain death rates on our conclusions, further research should particularly focus on collecting complete long-term survival data accounting for potential confounding factors. Last, our cost-effectiveness estimates were based on trial data from 26 experienced surgical centers, and therefore may not be fully extrapolated to clinical centers nationwide. Although large-scale claims and registry data may yield more generalizable data, their usefulness is limited by their lack of detailed clinical information,32 which impedes the appropriate adjustment for known confounders.
Influential guideline and expert consensus reports on the management of MR have been recently updated after publication of the findings from the CTSN moderate ischemic MR trial, emphasizing that the net benefit of performing MV repair at the time of CABG is uncertain for the overall patient population.33–35 These guidelines, therefore, advocate an individualized approach that takes into account the trade-off between short-term surgical risks and potential long-term benefits following MV repair on a patient-by-patient basis. Such individualized decision-making would entail reserving MV repair for patients for whom the net clinical benefit is expected to be high, i.e. those with a low risk of neurologic events and supraventricular arrhythmias and a sufficient long life expectancy to benefit from MV repair. Our cost-effectiveness analysis shows that only when we can demonstrate substantial late mortality benefits the value of a combined procedure may become acceptable. Thus, further research should focus on collecting data for individualized prediction of long-term survival outcomes for both procedures in order to better inform surgical decisions.
Conclusions
Our cost-effectiveness analysis suggests that the addition of MV repair to CABG for patients with moderate ischemic MR is unlikely to provide additional quality-adjusted survival at a cost that would meet commonly used cost-effectiveness criteria, and only if late mortality benefits can be demonstrated, would adding MV repair to CABG become economically attractive. Further research should focus on individualized prediction of long-term survival to better inform surgical decisions.
Supplementary Material
Acknowledgements
Study Concept and Design: Ferket, Chang, Bagiella, Gelijns, Moskowitz. Acquisition, Analysis, or Interpretation of Data: All authors. Critical Revision of the Manuscript for Important Intellectual Content: All authors. Statistical Analysis: Ferket and Chang. Obtained Funding: Gelijns and Ferket. Administrative, Technical, or Material Support: Cardiothoracic Surgical Trials Network (CTSN).
Sources of Funding:
This work was supported by a cooperative agreement (U01 HL088942) funded by the National Heart, Lung, and Blood Institute and the National Institute of Neurological Disorders and Stroke of the NIH and the Canadian Institutes of Health Research. Dr. Ferket was supported by American Heart Association Grant #16MCPRP31030016 (Ferket). The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health; or the United States Department of Health and Human Services.
Glossary of Abbreviations
- CABG
coronary artery bypass graft
- CEA
cost-effectiveness analysis
- ICER
incremental cost-effectiveness ratio
- MR
mitral regurgitation
- MV
mitral-valve
- QALY
quality-adjusted life year
- U.S.
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
B.S. Ferket: None. V.H. Thourani: None. P. Voisine: None. S.F. Hohmann: None. H.L. Chang: None. P.K. Smith: None. R.E. Michler: None. G. Ailawadi: None. L.P. Perrault: None. M.A. Miller: None. K. O’Sullivan: None. S.L. Mick: None. E. Bagiella: None. M.A. Acker: None. E. Moquete: None. J.W. Hung: None. J.R. Overbey: None. A. Lala: None. M. lraola: None. J.S. Gammie: None. A.C. Gelijns: None. P.T. O’Gara: None. A.J. Moskowitz: None.
Trial Registration: ClinicalTrials.gov Identifier: NCT00806988.
IRB approval: The institutional review board at each center approved the protocol, and all patients provided written informed consent.
References
- 1.Hillis GS, Moller JE, Pellikka PA, Bell MR, Casaclang-Verzosa GC, Oh JK. Prognostic significance of echocardiographically defined mitral regurgitation early after acute myocardial infarction. Am Heart J. 2005;150:1268–1275. [DOI] [PubMed] [Google Scholar]
- 2.Lamas GA, Mitchell GF, Flaker GC, Smith SC Jr., Gersh BJ, Basta L, et al. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and ventricular enlargement investigators. Circulation. 1997;96:827–833. [DOI] [PubMed] [Google Scholar]
- 3.Tcheng JE, Jackman JD Jr., Nelson CL, Gardner LH, Smith LR, Rankin JS, et al. Outcome of patients sustaining acute ischemic mitral regurgitation during myocardial infarction. Ann Intern Med. 1992;117:18–24. [DOI] [PubMed] [Google Scholar]
- 4.Pierard LA, Carabello BA. Ischaemic mitral regurgitation: Pathophysiology, outcomes and the conundrum of treatment. Eur Heart J. 2010;31:2996–3005. [DOI] [PubMed] [Google Scholar]
- 5.Sannino A, Smith RL 2nd, Schiattarella GG, Trimarco B, Esposito G, Grayburn PA. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: A systematic review and meta-analysis. JAMA Cardiol. 2017;2:1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith PK, Puskas JD, Ascheim DD, Voisine P, Gelijns AC, Moskowitz AJ, et al. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2014;371:2178–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michler RE, Smith PK, Parides MK, Ailawadi G, Thourani V, Moskowitz AJ, et al. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2016;374:1932–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, et al. A brief selfadministered questionnaire to determine functional capacity (the duke activity status index). Am J Cardiol. 1989;64:651–654. [DOI] [PubMed] [Google Scholar]
- 9.Vizient. 2018. https://www.vizientinc.com/. Accessed January 24, 2019.
- 10.Dunn A, Grosse SD, Zuvekas SH. Adjusting health expenditures for inflation: A review of measures for health services research in the united states. Health Serv Res. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the sf-12. Med Care. 2004;42:851–859. [DOI] [PubMed] [Google Scholar]
- 12.Glasziou PP, Cole BF, Gelber RD, Hilden J, Simes RJ. Quality adjusted survival analysis with repeated quality of life measures. Stat Med. 1998;17:1215–1229. [DOI] [PubMed] [Google Scholar]
- 13.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–1103. [DOI] [PubMed] [Google Scholar]
- 14.Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein D, Moskowitz AJ, Gelijns AC, Ailawadi G, Parides MK, Perrault LP, et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen PK, Gill RD. Cox’s regression model for counting processes: A large sample study. Ann Statist. 1982;10:1100–1120. [Google Scholar]
- 17.Harris KM, Sundt TM 3rd, Aeppli D, Sharma R, Barzilai B. Can late survival of patients with moderate ischemic mitral regurgitation be impacted by intervention on the valve? Ann Thorac Surg. 2002;74:1468–1475. [DOI] [PubMed] [Google Scholar]
- 18.Trichon BH, Glower DD, Shaw LK, Cabell CH, Anstrom KJ, Felker GM, et al. Survival after coronary revascularization, with and without mitral valve surgery, in patients with ischemic mitral regurgitation. Circulation. 2003;108 Suppl 1:II103–110. [DOI] [PubMed] [Google Scholar]
- 19.Diodato MD, Moon MR, Pasque MK, Barner HB, Moazami N, Lawton JS, et al. Repair of ischemic mitral regurgitation does not increase mortality or improve long-term survival in patients undergoing coronary artery revascularization: A propensity analysis. Ann Thorac Surg. 2004;78:794–799; discussion 794–799. [DOI] [PubMed] [Google Scholar]
- 20.Wong DR, Agnihotri AK, Hung JW, Vlahakes GJ, Akins CW, Hilgenberg AD, et al. Long-term survival after surgical revascularization for moderate ischemic mitral regurgitation. Ann Thorac Surg. 2005;80:570–577. [DOI] [PubMed] [Google Scholar]
- 21.Mihaljevic T, Lam BK, Rajeswaran J, Takagaki M, Lauer MS, Gillinov AM, et al. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol. 2007;49:2191–2201. [DOI] [PubMed] [Google Scholar]
- 22.Deja MA, Grayburn PA, Sun B, Rao V, She L, Krejca M, et al. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation. 2012;125:2639–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castleberry AW, Williams JB, Daneshmand MA, Honeycutt E, Shaw LK, Samad Z, et al. Surgical revascularization is associated with maximal survival in patients with ischemic mitral regurgitation: A 20-year experience. Circulation. 2014;129:2547–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdallah MS, Wang K, Magnuson EA, Spertus JA, Farkouh ME, Fuster V, et al. Quality of life after pci vs cabg among patients with diabetes and multivessel coronary artery disease: A randomized clinical trial. JAMA. 2013;310:1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, et al. Acc/aha statement on cost/value methodology in clinical practice guidelines and performance measures: A report of the american college of cardiology/american heart association task force on performance measures and task force on practice guidelines. J Am Coll Cardiol. 2014;63:2304–2322. [DOI] [PubMed] [Google Scholar]
- 26.Suri RM, Clavel MA, Schaff HV, Michelena HI, Huebner M, Nishimura RA, et al. Effect of recurrent mitral regurgitation following degenerative mitral valve repair: Long-term analysis of competing outcomes. J Am Coll Cardiol. 2016;67:488–498. [DOI] [PubMed] [Google Scholar]
- 27.Anantha Narayanan M, Aggarwal S, Reddy YNV, Alla VM, Baskaran J, Kanmanthareddy A, et al. Surgical repair of moderate ischemic mitral regurgitation-a systematic review and meta-analysis. Thorac Cardiovasc Surg. 2017;65:447–456. [DOI] [PubMed] [Google Scholar]
- 28.Altarabsheh SE, Deo SV, Dunlay SM, Erwin PJ, Obeidat YM, Navale S, et al. Meta-analysis of usefulness of concomitant mitral valve repair or replacement for moderate ischemic mitral regurgitation with coronary artery bypass grafting. Am J Cardiol. 2017;119:734–741. [DOI] [PubMed] [Google Scholar]
- 29.Bouchard D, Jensen H, Carrier M, Demers P, Pellerin M, Perrault LP, et al. Effect of systematic downsizing rigid ring annuloplasty in patients with moderate ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2014;147:1471–1477. [DOI] [PubMed] [Google Scholar]
- 30.Prifti E, Bonacchi M, Frati G, Giunti IG, Leacche M, Proietti P, et al. Should mild-to-moderate and moderate ischemic mitral regurgitation be corrected in patients with impaired left ventricular function undergoing simultaneous coronary revascularization? J Card Surg. 2001;16:473–483. [DOI] [PubMed] [Google Scholar]
- 31.Claxton K. Exploring uncertainty in cost-effectiveness analysis. Pharmacoeconomics. 2008;26:781–798. [DOI] [PubMed] [Google Scholar]
- 32.Mack MJ, Herbert M, Prince S, Dewey TM, Magee MJ, Edgerton JR. Does reporting of coronary artery bypass grafting from administrative databases accurately reflect actual clinical outcomes? J Thorac Cardiovasc Surg. 2005;129:1309–1317. [DOI] [PubMed] [Google Scholar]
- 33.O’Gara PT, Grayburn PA, Badhwar V, Afonso LC, Carroll JD, Elmariah S, et al. 2017 acc expert consensus decision pathway on the management of mitral regurgitation: A report of the american college of cardiology task force on expert consensus decision pathways. J Am Coll Cardiol. 2017;70:2421–2449. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, et al. 2017 aha/acc focused update of the 2014 aha/acc guideline for the management of patients with valvular heart disease: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2017. [DOI] [PubMed] [Google Scholar]
- 35.American Association For Thoracic Surgery Ischemic Mitral Regurgitation Consensus Guidelines Writing C, Kron IL, LaPar DJ, Acker MA, Adams DH, Ailawadi G, et al. 2016 update to the american association for thoracic surgery consensus guidelines: Ischemic mitral valve regurgitation. J Thorac Cardiovasc Surg. 2017;153:1076–1079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.