Abstract
Anatomically interconnected, the ventromedial prefrontal cortex (vmPFC) and amygdala interact in emotion processing. However, no meta-analyses have focused on studies that reported concurrent vmPFC and amygdala activities. With activation likelihood estimation (ALE) we examined 100 experiments that reported concurrent vmPFC and amygdala activities, and distinguished responses to positive vs. negative emotions and to passive exposure to vs. active regulation of emotions. We also investigated whole-brain experiments for other regional activities. ALE and contrast analyses identified convergent anterior and posterior vmPFC response to passive positive and negative emotions, respectively, and a subregion in between to mixed emotions. A smaller area in the posterior ventral vmPFC is specifically involved in regulation of negative emotion. Whereas bilateral amygdala was involved during emotional exposure, only the left amygdala showed convergent activities during active regulation of negative emotions. Whole brain analysis showed convergent activity in left ventral striatum for passive exposure to positive emotions and down-regulation of negative emotions, and in the posterior cingulate cortex and ventral precuneus for passive exposure to negative emotions. These findings highlight contrasting, valence-specific subregional vmPFC as well as other regional responses during passive exposure to emotions. The findings also suggest that hyperactivation of the vmPFC is associated with diminished right amygdala activities during regulation of negative emotions. Together, the findings extend the literature by specifying the roles of subregional vmPFC and amygdala activities in emotion processing.
Keywords: meta-analysis, vmPFC, amygdala, fMRI, emotion processing, emotion regulation
1. Introduction
Emotion is a mental state that comprises thoughts or feelings of personal well-being that are often contingent on environmental influences (Cardinal et al., 2002; Damasio, 1998). Emotion regulation refers to our ability to generate, maintain, and control emotion (Langner et al., 2018; Park et al., 2019), and emotion dysregulation is implicated in a wide range of psychological conditions (Cheetham et al., 2010; Kret and Ploeger, 2015; Langner et al., 2018; Phillips et al. 2003a; Robinson and Berridge, 2003). Sustained bias toward negative emotions and anhedonia are among the hallmarks of major depression (Donofry et al., 2016; Ma, 2015), whereas fluctuating mood and affect characterizes bipolar disorder (Green et al., 2007). Behavioral or pharmacological interventions have aimed to ameliorate negative emotions and maladaptive coping strategies for individuals with these clinical conditions (Sloan et al., 2017). Thus, understanding the neural bases of emotion processing and regulation has been of central importance in cognitive and clinical neuroscience research.
Previous work has highlighted responses of many cortical and subcortical regions to emotion perception and regulation (Kober et al., 2008; Lieberman et al., 2019; Lindquist et al., 2012; Phillips et al., 2003b). Among these regions, the amygdala and ventromedial prefrontal cortex (vmPFC) are known to play a central role in emotion processing (Costafreda et al., 2008; Di et al., 2017; Lindquist et al., 2012). Lesion studies in humans and animals have suggested the amygdala as a hub for threat detection and negative emotional experience (Adolphs et al., 2005; Anderson and Phelps, 1998; Davis et al., 2008). Electrical stimulation of the amygdala elicited defensive behavior (al Maskati and Zbrozyna, 1989) and intensified startle responses to acoustic stimuli in rats (Rosen and Davis, 1988). Further, autonomic responses accompanied amygdala activation to negative emotions (al Maskati and Zbrozyna, 1989; Brierley et al., 2004; LeDoux et al., 1990). These studies together suggest an outsized role of the amygdala in negative emotion processing. On the other hand, neuroimaging studies showed amygdala activation during exposure to happy facial expressions (Ebner et al., 2012; Hurlemann et al., 2008), and meta-analyses have suggested that the amygdala partakes more broadly in response to salient, including both positive and negative, emotional stimuli (Lindquist et al., 2012; Di et al., 2017).
Commonly described as a bridge between cognitive and emotional processes, the vmPFC is involved in emotion and reward processing, value-based decision making, as well as social cognition and self-referential activities (Bartra et al., 2013; Hiser and Koenigs, 2018; Lieberman et al., 2019). Patients with focal vmPFC lesions showed blunted emotional and physiological response to aversive stimuli (Barrash et al., 2000; Damasio et al., 1990). Importantly, the vmPFC seems particularly critical for regulation of negative emotions (Hiser and Koenigs, 2018).
Indeed, both rodent and non-human primate studies have characterized anatomical projections from the vmPFC to inhibitory interneurons in the amygdala (McDonald et al., 1996; Ghashghaei and Barbas, 2002). Subdivisions of the prefrontal cortex (PFC) that project to the amygdala also receive inputs from amygdala. These bidirectional connections are not uniformly or symmetrically distributed across the PFC subregions: cytoarchitecturally agranular regions have the densest connections with the amygdala in primates (Ray and Zald, 2012; Stefanacci and Amaral, 2002). Subgenual cingulate cortex or Broadmann area (BA) 25 and dorsal anterior cingulate cortex (BA 24) project most heavily to the amygdala, and the perigenual and rostral cingulate cortex or BA 32 also provide significant inputs to the amygdala in monkeys (Ghashghaei et al., 2007; Price, 2007). Thus, in sharp contrast with dorsolateral PFC (dlPFC), the vmPFC has more direct and heavier anatomical connectivity with the amygdala (Ray and Zald, 2012). Between-species anatomical homologies likely vary across brain regions. In rodents the medial PFC can be divided into infralimbic (IL) and prelimbic (PrL) subregions, homologous to BA25 and BA32 of primates, including humans, respectively (Gabbott et al., 2003; Myers-Schulz and Koenigs, 2012; Quirk and Beer, 2006). Electrical stimulation of the IL suppressed conditioned fear by decreasing amygdala activity (Milad and Quick, 2002; Quirk et al., 2003). To note, the anatomical and functional homology of BA32 between monkeys and humans remains an active focus of research. Considering the cytoarchitecture, Ongur et al. suggested that subregion BA32pl in humans is homologous to monkey BA32, whereas BA32ac in humans is homologous to monkey BA24 (Ongur et al., 2003).
Many studies support functional interaction between the vmPFC and amygdala. For instance, patients with vmPFC lesions demonstrated elevated amygdala responses to aversive images relative to control subjects, suggesting a role of the vmPFC in regulating amygdala activity and emotional experience (Motzkin et al., 2015). Numerous imaging studies have highlighted the roles of the amygdala in fear conditioning and of the vmPFC in down-regulating amygdala activity during fear extinction (Icenhour et al., 2015; Kalisch et al., 2006; Milad et al., 2009; Phelps et al, 2004). Likewise, studies have characterized concurrent vmPFC and amygdala activities in response to positive or negative emotions, suggesting a broader realm of functional interactions (Britton et al., 2006; Heller et al., 2014; Mobbs et al., 2007). A number of meta-analyses of emotion processing and regulation have described amygdala and vmPFC connectivity during behavioral challenges, in support of their interacting roles in emotion and saliency processing (Hiser and Koenigs, 2018; Di et al., 2017; Roy et al., 2012; Lindquist et al., 2012; Wager et al., 2008a). Another meta-analysis focusing on studies of psychophysiological interaction of the right amygdala also reported connectivity targets in the vmPFC during emotional exposures (Smith et al., 2016).
However, it remains less clear how the vmPFC and amygdala respond to negative versus positive emotions or to passive exposure to emotional stimuli as compared to active regulation (e.g., reappraisal) of emotions. Summarizing fMRI and PET studies of emotion induction and experience, Kober and colleagues reported that the vmPFC and amygdala respond to both positive and negative emotions (Kober et al., 2008). Another overview of PET and fMRI studies reported that pleasant and unpleasant emotional experience was associated with vmPFC and amygdala activity, respectively (Wager et al., 2008a). A review of human and animal data suggested that posterior vmPFC/subgenual anterior cingulate cortex (sgACC) and anterior vmPFC/pregenual anterior cingulate cortex (pgACC) are associated with processing of negative and positive emotions, respectively (Myers-Schulz and Koenigs, 2012). In a recent review using Neurosynth fMRI data, the same group showed that the anterior vmPFC was associated with value processing, decision making and social cognition, whereas the posterior vmPFC and amygdala were more related to emotion processing (Hiser and Koenigs, 2018). On the other hand, the meta-analysis has not substantiated the differential association of anterior/posterior vmPFC with positive/negative emotions (Hiser and Koenigs, 2018). Other meta-analysis endorsed amygdala response during passive exposure to negative emotions (Garcia-Garcia et al., 2016; Lindquist et al., 2012) and sgACC response to both negative and positive emotions, but suggested that the amygdala or vmPFC was not critically involved in emotion regulation (Langner et al. 2018).
Together, a few conclusions can be drawn from the extant studies. First, the vmPFC and amygdala are anatomically and functionally connected. Second, the vmPFC and amygdala may respond concurrently to emotional processing; whereas the amygdala may respond to both positive and negative emotions, the anterior and posterior vmPFC appears to respond differentially to positive and negative emotions, respectively, although the latter has not been confirmed in meta-analyses. Third, evidence appears to support a role of the vmPFC in regulating emotions; however, whether the amygdala responds to emotion regulation or how it responds in link with vmPFC activity remains unclear. Thus, a few important questions are outstanding. First, when the amygdala is engaged concurrently, are different subregions of the vmPFC involved in processing positive, negative and both positive and negative (mixed) emotions? Second, do the amygdala and vmPFC respond in the same or opposite direction (activation vs. “deactivation”) to emotional processing? Do the patterns of concurrent vmPFC and amygdala response depend on the valence of emotions? Third, do different subregions of the vmPFC and amygdala respond to passive exposure to emotions and active regulation of emotions?
We performed a meta-analysis using activation likelihood estimation (ALE) algorithm to address three aims. First, we examined whether the anterior and posterior vmPFC is each involved in positive and negative emotion processing, as suggested in earlier work (Myers-Schulz and Koenigs, 2012) but yet to be substantiated in meta-analysis. We also evaluated if the vmPFC and amygdala respond in the same or opposite direction to passive exposure to emotions, whether this directional relationship depends on the valence of emotions, and whether subregions of the vmPFC may respond differently in relation to the direction of amygdalar response. As previous meta-analyses have largely focused on experiments reporting “activation” but not “deactivation,” it would require a thorough consideration of the opposing patterns of activities to understand the roles of the vmPFC and amygdala in emotion processing. Second, we examined whether passive exposure to and regulation of negative emotions involved distinct vmPFC subregional and amygdala activities. Third, we performed ALE analysis of whole-brain experiments to examine if other brain regions showed convergence of activation concurrently with vmPFC and amygdala each during passive exposure to and active regulation of emotions.
2. Methods
2.1. Literature search
Literature search was conducted in PubMed and Web of Science (from the inception until February 26th, 2019), supplemented by screening of references in relevant reviews. Unlike the amygdala, the vmPFC does not have well-defined anatomical boundaries. The imaging literature has referred to a wide swath of cortical regions as the vmPFC and sometimes used the term medial orbitofrontal cortex (mOFC) to refer to the vmPFC. We thus searched with different combinations of terms, including ‘amygdala’ AND ‘vmPFC’ OR ‘ventromedial prefrontal cortex’ OR ‘ventral medial prefrontal cortex’ OR ‘mOFC’ OR ‘medial OFC’ OR ‘medial orbital frontal cortex’ OR ‘perigenual’ OR ‘subgenual’ AND ‘fMRI’ OR ‘functional MRI’ OR ‘functional magnetic’ for ‘All Fields’ in PubMed and ‘Title/Keywords/Abstract’ in Web of Science. We included not only studies of emotions but also those of other cognitive tasks that evoke emotional responses. For instance, a contrast of wins vs.no-wins in a reward-based decision-making task would be considered as an experiment of positive emotions. A complete list of the studies is available in the Supplementary Tables.
Papers were included based on following criteria: (1) published in English; (2) healthy human subjects. If a study involved both patient and healthy subjects, it was included in the analyses only if the coordinates of regional activities were reported for healthy controls; (3) original reports of fMRI study of emotion and related paradigms. Consequently, resting-state experiments and studies utilizing other imaging modalities such as PET were excluded; (4) reports showing findings that clearly reflect within-subject differences in valenced emotional response or regulation of vs. passive exposure to emotions. Thus, experiments showing regional activations to emotions of the same valence (e.g., fearful vs. angry face) were excluded. Studies or findings of fear extinction were not included since the extinction phase involves rather complex processes that are not amenable to classification as positive or negative in valence. Studies of individual differences (e.g., age, sex, genotypes) or other inter-subject variables (e.g., drug, sleep, treatment) were not included; (5) vmPFC and amygdala showed activation or deactivation within the same contrast; (6) regional activations reported in standard stereotactic coordinates (Talairach [TAL] or Montreal Neurological Institute [MNI]); (7) studies that did not involve duplicate subjects; (8) studies reporting vmPFC activity but with coordinates actually located in the dorsomedial PFC or lateral OFC were excluded. We used a rather broad vmPFC mask to encompass all potential vmPFC coordinates from the literature (Suppl. Figure 1).
2.2. Search results and sorting of all experiments for ALE analyses
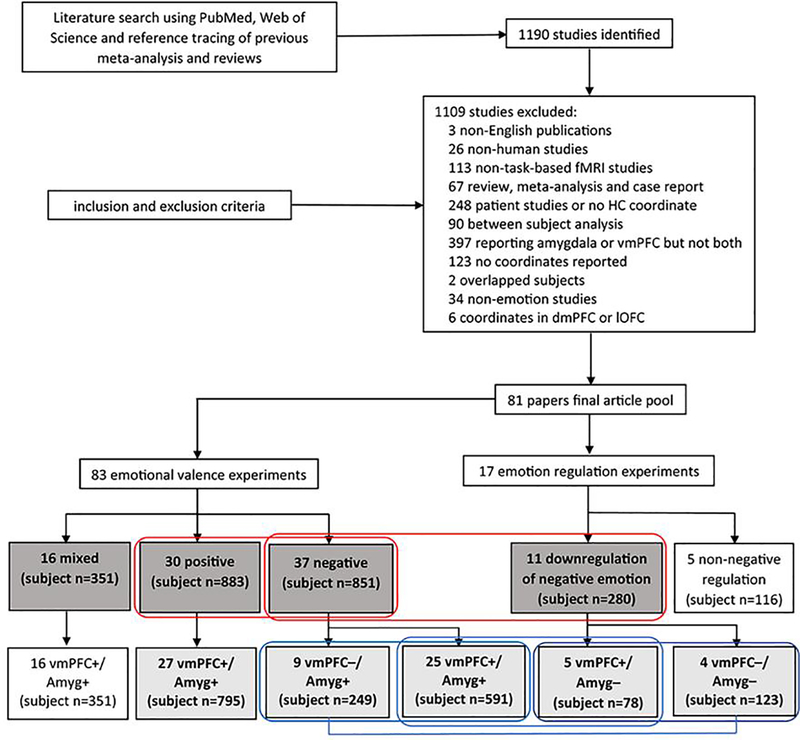
We identified 1190 non-duplicate papers (studies), and 81 papers containing 100 experiments were included in the final literature pool (Fig. 1). The 100 experiments were first divided into two exclusive pools: those reporting findings on passive exposure to emotions (n=83) and those on emotion regulation vs. passive exposure (n=17). For the former we further distinguished experiments on positive emotion (n=30; Suppl. Table S1), negative emotion (n=37; Suppl. Table S2), and mixed (positive and negative) emotions (n=16; Suppl. Table S3). For experiments containing multiple contrasts, we selected the one involving a comparison with the baseline; that is, for studies reporting findings from both ‘positive vs. neutral’ and ‘positive vs. negative’ contrasts, we employed ‘positive vs. neutral’ in ALE analyses. Thus, in experiments of passive exposure to emotional stimuli, the contrasts of interest comprised “positive > neutral” (positive emotion), “negative > neutral” (negative emotion), and “mixed (or both positive and negative) > neutral.” For experiments on regulation, the contrast of interest comprised “regulation > passive exposure.” In the below and in the figures, we refer to response to “emotion > neutral” as “activation” or “+” and to “neutral > emotion” as “deactivation” or “−”. The 17 experiments on regulation vs. passive exposure concerned mainly down-regulation of negative emotions (n=11, Suppl. Table S4; see also below). Of the other 6, one involved up-regulation of negative emotions and 5 involved regulation (1 up- and 4 down- regulation) of positive or mixed emotions. Thus, we first conducted a total of 4 primary ALE analyses, each on passive exposure to positive (n=30), negative (n=37), and mixed (n=16) emotions and on down-regulation of vs. passive exposure of negative emotion (n=11). The experimental stimuli employed in the experiments are summarized in the Supplement – Exp. Stimuli.
Figure 1.
Flow chart of literature search, study selection and experiment grouping according to the specific aims. +: activation; −: deactivation; dmPFC: dorsomedial prefrontal cortex; lOFC: lateral orbitofrontal cortex; Amyg: Amygdala. The boxes in dark and light gray each indicates experiments included in primary and subgroup ALE analyses. The two red rectangles indicate the experiments included in primary contrast analyses. For instance, we contrasted the 30 and 37 experiments each on passive exposure to positive and negative emotions to identify distinct responses to valenced emotions. The three blue rectangles covering and the line connecting two light gray boxes indicate the experiments included in subgroup contrast analyses.
The second set of ALE analyses – subgroup analyses – concerned experiments showing different directions of vmPFC and amygdala activations (Fig. 1). In experiments on passive exposure to positive emotions (n=30), 3 each reporting vmPFC− /amygdala− (Xu et al., 2011), vmPFC+/amygdala− (Kim et al., 2004), and vmPFC− /amygdala+ (Kohn et al., 2014) were excluded from the subgroup analyses. The remaining 27 experiments all involved co-activation of the vmPFC and amygdala (vmPFC+/Amyg+). In experiments on passive exposure to negative emotions, 1 experiment reporting vmPFC+/amygdala− (Rubio et al., 2015) and 2 reporting both vmPFC− /amygdala− (Herde et al., 2007; Mobbs et al., 2007) were excluded. The remaining 34 experiments comprised 25 showing vmPFC and amygdala co-activation (vmPFC+/Amyg+) and 9 showing vmPFC deactivation and amygdala activation (vmPFC−/Amyg+), and were so separated in subgroup analyses. Of the 17 emotion regulation experiments, we excluded 3 on regulation of positive emotion (Koush et al., 2019; Kober et al., 2010; Winecoff et al., 2011), 2 on down-regulation of mixed emotions (Kanske et al., 2011), and 1 on up-regulation of negative emotion (Eippert et al., 2007). Of the remaining 11 experiments that involved down-regulation of negative emotion, 2 reporting vmPFC+/amygdala+ were also removed (Dougherty et al., 2015; Stephanou et al., 2017). Thus, the subgroup analyses of negative emotion down-regulation were performed on experiments demonstrating vmPFC activation and amygdala deactivation (vmPFC+/Amyg−, n=5) and vmPFC and amygdala co-deactivation (vmPFC−/Amyg−, n=4). The vmPFC coordinates of the experiments removed from subgroup ALE analyses were shown in Suppl. Figure S2 (see Results).
2.2. ALE analyses of “whole-brain” experiments
The afore-described samples for ALE analysis examined whether or which subregions of the vmPFC may respond concurrently with the amygdala during passive exposure to positive emotion, to negative emotion, and to emotion irrespective of valence, as well as during down-regulation of negative emotion. Thus, we included both whole-brain and ROI studies in the meta-analysis. Here we examined the activities of brain regions in addition to the amygdala and vmPFC, and thus included only those experiments reporting whole-brain findings. Studies presenting vmPFC activity in whole brain analysis but amygdala activity in ROI analysis were also included as the amygdala is commonly examined as an ROI.
2.3. Activation likelihood estimation (ALE)
We used GingerALE (version 3.0) from BrainMap Project to perform ALE meta-analysis (Eickhoff et al., 2009; Eickhoff et al., 2012; Turkeltaub et al., 2012). ALE algorithm finds agreement across experiments and spatial convergence of activation foci with the data modeled in common stereotactic space. The ALE results were examined at a threshold of cluster-forming voxel-level p < 0.001, uncorrected and then cluster p < 0.05, FWE-corrected, on the basis of 5,000 p-value permutations. We identified convergence of activities across whole brain and ROI studies. In a separate analysis that focused only on whole-brain studies, we located the convergent patterns of activity in other brain regions (Eickhoff et al., 2011; Laird et al., 2005). The analysis was performed in MNI space, with coordinates reported in Talairach (TAL) space transformed into MNI space using Lancaster transform function, as implemented in GingerALE. For published coordinates transformed using “Brett”, we proceeded with Brett transformation from TAL to MNI in GingerALE to “un-Brett”.
For all contrast analyses, voxel-wise differences between ALE datasets were compared. All contributing foci datasets were pooled and randomly divided into two equal-sized sets of contrasted experiments. ALE scores for each voxel within the randomly assembled groups were subtracted from one another and compared with the true data. We performed 5,000 such p value permutations using GingerALE and examined the results at p<0.01 with a minimum cluster volume of 200mm3 (Eickhoff et al., 2011). ALE values were transformed into Z scores as computed from p value images in GingerALE to facilitate the interpretation of the contrast results.
ALE clusters from single dataset meta-analysis and Z score maps for contrast analysis were overlaid onto a MNI template using Mango Image Viewer (www.ric.uthscsa.edu/mango).
2.3.1. ALE: primary analysis
We computed convergence of activities during exposure to emotional stimuli (positive, negative, and mixed) and during down-regulation of negative emotion using single dataset ALE analysis. We conducted contrast analysis to examine the differences between the ALE maps of said groups of experiments. We contrasted pairwise ALE files incorporating the coordinates of the vmPFC and amygdala. The contrasts of interest comprised passive exposure to positive vs. negative emotions and passive exposure to vs. regulation of negative emotions (Fig. 1).
Both experiments on exposure to negative emotions and those on regulation of negative emotions comprised subsets of a number of experiments with different directions of vmPFC and/or amygdala activities. Although the contrast analyses did not show significant convergent differences between these subsets of experiments (sections 2.3.2 and 3.2), the results of the primary ALE analyses should be considered with this issue in mind. That is, both primary single dataset ALE and contrast analyses were conducted without distinguishing experiments with different directions of vmPFC and/or amygdala response.
2.3.2. ALE: Subgroup analysis
We also performed single-dataset ALE analysis for subgroups of experiments showing vmPFC+/Amyg+ to negative emotions, vmPFC−/Amyg+ to negative emotions; experiments showing vmPFC−/Amyg− and vmPFC+/Amyg− during down-regulation of negative emotion; and experiments showing vmPFC+/Amyg+ to positive emotions. In contrast analyses, the contrasts of interest comprised [vmPFC+/Amyg+ (passive)] vs. [vmPFC−/Amyg+ (passive)] to examine whether, with amygdala activation to negative emotions, distinct subregions of the vmPFC showed activation and deactivation; [vmPFC+/Amyg+ (passive)] vs. [vmPFC+/Amyg− (regulation)] as well as [vmPFC−/Amyg+ (passive)] vs. [vmPFC−/Amyg− (regulation)] to examine whether subregions of the vmPFC were specifically engaged that would influence amygdala response during down-regulation of, as compared with passive exposure to, negative emotions; and [vmPFC−/Amyg− (regulation)] vs. [vmPFC+/Amyg− (regulation)] to examine whether distinct subregions of the vmPFC exhibited activation vs. deactivation in diminishing amygdala response during down-regulation of negative emotions (Fig. 1).
2.3.3. ALE: Whole-brain analysis
We further performed ALE analysis for whole-brain experiments showing vmPFC+/Amyg+ to passive exposure of positive (n=16), negative (n=15), and mixed (n=9) emotions, respectively; and vmPFC−/Amyg− to down-regulation of negative emotions (n=4).
3. Results
3.1. Overview and meta-analysis of emotion exposure and emotion regulation experiments
We identified 67 papers containing 83 experiments involving passive exposure to emotions, and 15 papers containing 17 experiments involving regulation of emotions, respectively, with one study including experiments on both passive exposure to and down-regulation of mixed emotions (Kanske et al., 2011) (Fig. 1). The experiments on exposure were divided into those involving positive emotions (n=30; 883 subjects), negative emotions (n=37; 851 subjects), and mixed emotions (n=16; 351 subjects). The emotion regulation experiments included 11 involving down-regulation of negative emotions (280 subjects). Among the 83 passive exposure experiments with concurrent vmPFC and amygdala activity, a similar number of experiments reported on positive and negative emotions. Most experiments on positive emotions (27 out of 30) and all 16 experiments on mixed emotions reported vmPFC+/Amyg+, whereas 25 and 9 experiments reported vmPFC+/Amyg+ and vmPFC−/Amyg+, respectively, for exposure to negative emotions. Of the 17 experiments with concurrent vmPFC and amygdala activity during emotion regulation, 11 concerned down-regulation of negative emotions and 5 and 4 of the 11 reported vmPFC+/Amyg− and vmPFC−/Amyg−, respectively.
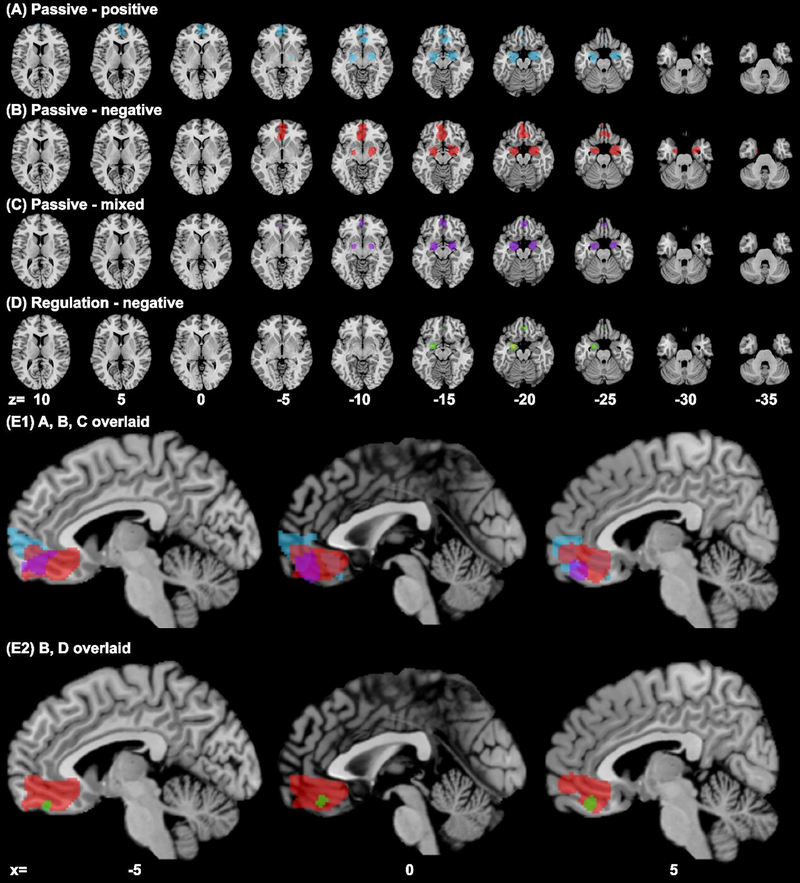
ALE analysis revealed convergence of activity for all emotion exposure experiments in bilateral amygdala and vmPFC, whereas down-regulation of negative emotion showed convergence of activity in the left amygdala and vmPFC (Table 1, Fig. 2). Passive exposure to positive emotions involved the vmPFC including the frontopolar cortex and the frontopolar part of the OFC, bilateral amygdala and the hippocampus and entorhinal cortex that surround the amygdala. Passive exposure to negative emotions involved the relatively posterior and ventral vmPFC and likewise involved the hippocampus and entorhinal cortex in addition to the amygdala. Passive exposure to mixed emotions involved similar structures as those to negative emotions but in more limited extent. Regulation of negative emotions involved a small locus in the medial OFC, as well as the left but not right amygdala.
Table 1.
ALE of responses to passive exposure to emotions and to regulation of negative emotions
| Brain Region | Peak MNI | Cluster Size (mm3) | Weighted (MNI) | ALE value (× 103) | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||
| A. Passive - positive | ||||||||
| vmPFC (FPC; BA10) | 0 | 58 | −2 | 6952 | −1 | 56 | −4 | 45.0 |
| Amygdala | 26 | −4 | −20 | 6536 | 24 | −5 | −18 | 42.4 |
| Amygdala | −24 | −8 | −16 | 6216 | −23 | −7 | −18 | 44.6 |
| Hippocampus | −28 | −20 | −10 | 10.3 | ||||
| vmPFC (pgACC; BA32) | 6 | 34 | −12 | 2128 | 7 | 33 | −1 3 | 18.3 |
| vmPFC (sgACC; BA25) | 2 | 26 | −24 | 10.2 | ||||
| B. Passive - negative | ||||||||
| vmPFC (BA32/11) | 0 | 38 | −18 | 12368 | −1 | 40 | −13 | 30.3 |
| vmPFC (BA32/11) | −8 | 34 | −14 | 26.9 | ||||
| vmPFC (FPC, BA10) | 0 | 54 | −6 | 25.5 | ||||
| vmPFC (FPC, BA10) | 4 | 52 | −6 | 24.7 | ||||
| vmPFC (BA11) | 10 | 34 | −22 | 18.7 | ||||
| Amygdala | 26 | −4 | −18 | 8952 | 25 | −3 | −2 0 | 53.0 |
| Amygdala | 28 | −2 | −22 | 52.4 | ||||
| Amygdala | −20 | −6 | −16 | 5032 | −21 | −4 | −1 9 | 39.3 |
| C. Passive - mixed | ||||||||
| Amygdala | −20 | −6 | −18 | 4064 | −21 | −5 | −18 | 41.9 |
| Amygdala | 22 | −4 | −18 | 3792 | 22 | −3 | −18 | 36.8 |
| vmPFC (BA11) | 2 | 46 | −18 | 2352 | 1 | 49 | −17 | 18.1 |
| vmPFC (BA10) | −6 | 40 | −6 | 8.4 | ||||
| D. Regulation - negative | ||||||||
| Amygdala | −24 | −4 | −20 | 2016 | −23 | −3 | −20 | 22.4 |
| vmPFC (BA11) | 4 | 38 | −20 | 520 | 2 | 40 | −20 | 10.0 |
| vmPFC (BA11) | −6 | 44 | −22 | 8.1 | ||||
Note: BA: Brodmann Area; FPC: frontopolar cortex; pgACC: perigenual anterior cingulate cortex; sgACC: subgenual anterior cingulate cortex
Figure 2.
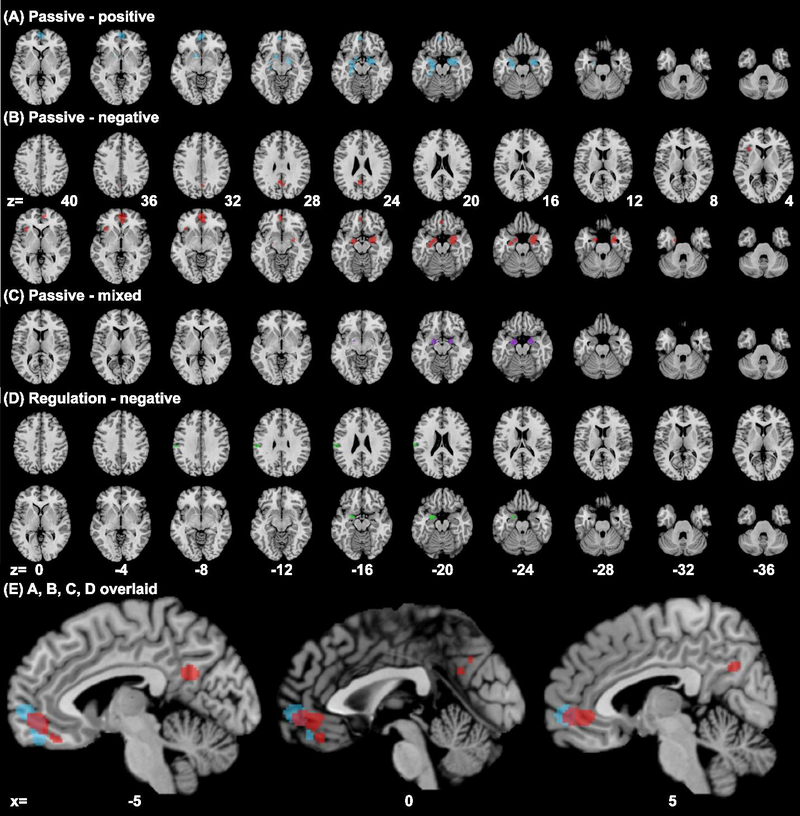
Results of ALE meta-analysis (cluster level p < 0.05, FWE corrected, with a cluster-forming threshold of voxel p < 0.001) of experiments on passive exposure to (A) positive emotion (blue), (B) negative emotion (red), (C) mixed emotion (purple), and (D) down-regulation of negative emotions (green); clusters overlaid on axial sections from z=+10 to −35, 5mm part. Panel (E1) shows A, B, C clusters overlaid, and (E2) shows B, D clusters overlaid on parasagittal sections at x=−5, 0, and 5. Passive exposure to positive and negative emotions each engaged anterior and posterior regions of the vmPFC with the area in between engaged during exposure to mixed emotions. Regulation of negative emotions involved a smaller ventral posterior region within the vmPFC cluster identified for passive exposure to negative emotions.
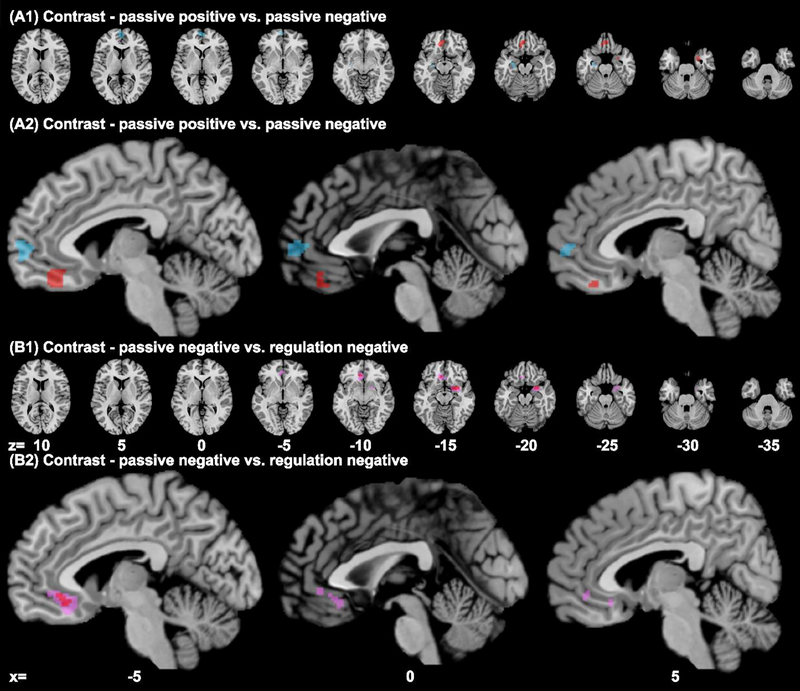
The contrast of [positive > negative (passive)] showed convergence of activity in the anterior vmPFC (cluster size: 1120 voxels; peak voxel MNI coordinates: −2, 58, 1) and left hippocampus (560 voxels, peak: −28, −14, −20). [Negative > positive (passive)] showed convergence in posterior vmPFC (1392 voxels, peak: −6, 37, −19); right entorhinal cortex and amygdala (344 voxels, peak: 28, 1, −30) (Suppl. Table S5, Fig. 3A1–A2). Thus, passive exposure to positive and negative emotions involved the anterior and posterior vmPFC, respectively.
Figure 3.
Results of contrast analysis (images thresholded at p<0.01 with a minimum cluster volume of 200mm3) of experiments on passive exposure to positive vs. negative emotions (A1, A2): positive > negative (blue); negative > positive (red); and of experiments on passive exposure to vs. regulation of negative emotions (B1, B2) passive exposure > down-regulation (red); For the latter we also used a p < 0.05 (pink; 2000 voxels, peak: −7, 31, −12); no clusters for down-regulation > passive exposure.
In additional analyses, we identified “pure emotion” experiments, including 17 that involved exposure to positive facial emotions, pleasant pictures, positive social feedbacks or erotic stimuli, and 36 that involved exposure to negative facial emotions, unpleasant pictures, videos or scripts, negative social feedbacks, or electric shocks. The results of ALE analyses likewise revealed convergence in the anterior and posterior vmPFC each for positive and negative emotions (Supp. Figure S3, Suppl. Table S6).
The contrast of [passive exposure > down-regulation] of negative emotion showed convergence of activity in posterior vmPFC (296 voxels, peak: −9, 28, −15) and a small cluster in the right entorhinal cortex and amygdala (616 voxels, peak: 26, 4, −18), whereas [down-regulation – passive exposure] contrast yielded no significant clusters (Suppl. Table S7, Fig. 3B1–B2). That is, whereas passive exposure to negative emotions involved a wide swath of the posterior vmPFC, a more limited subregion in the ventral posterior vmPFC showed convergence specifically for experiments involving down-regulation of negative emotions. Further, passive exposure to but not regulation of negative emotions involved the right amygdala.
3.2. Overview and meta-analysis of experiments with different patterns of vmPFC and amygdala activity
In our experiment pool, vmPFC hyperactivity (vmPFC+) was broadly reported for passive processing of positive and mixed emotions (96%) as well as negative emotions (70%), and 7 out of 11 (64%) experiments on down-regulation of negative emotions. Of the 37 experiments on passive exposure of negative emotion, 25 showed vmPFC+/Amyg+, and only one reported vmPFC+/Amyg−, whereas out of the 11 experiments on down-regulation of negative emotion, 2 reported vmPFC+/Amyg+ and 5 reported vmPFC+/Amyg− (OR = 47.29, p = 0.0005; two-sided Fisher exact test), suggesting that increases in vmPFC activity may directly or indirectly decrease amygdala activity during emotion regulation. In experiments reporting vmPFC hypoactivity (vmPFC−), 9 out of the 37 on passive exposure to negative emotion reported vmPFC−/Amyg+ and 2 reported vmPFC−/Amyg−, whereas 4 out of the 11 experiments on down-regulation of negative emotion reported vmPFC−/Amyg− and none reported vmPFC−/Amyg+ (OR = inf, p = 0.0110). The latter finding suggested that, the amygdala tended to become hypoactive during emotion regulation even in experiments showing vmPFC deactivation.
We identified subgroups of experiments according to the direction of vmPFC and amygdala response, including vmPFC+/Amyg+ to passive exposure of positive emotion (n=27; 795 subjects); vmPFC+/Amyg+ (n=25; 591 subjects) and vmPFC−/Amyg+ (n=9; 249 subjects) to passive exposure of negative emotion; vmPFC−/Amyg− (n=4; 123 subjects), and vmPFC+/Amyg− (n=5; 78 subjects) to down-regulation of negative emotion (Fig. 1) for ALE. All vmPFC coordinates of experiments excluded from subgroup analyses were labeled in Suppl. Figure S2.
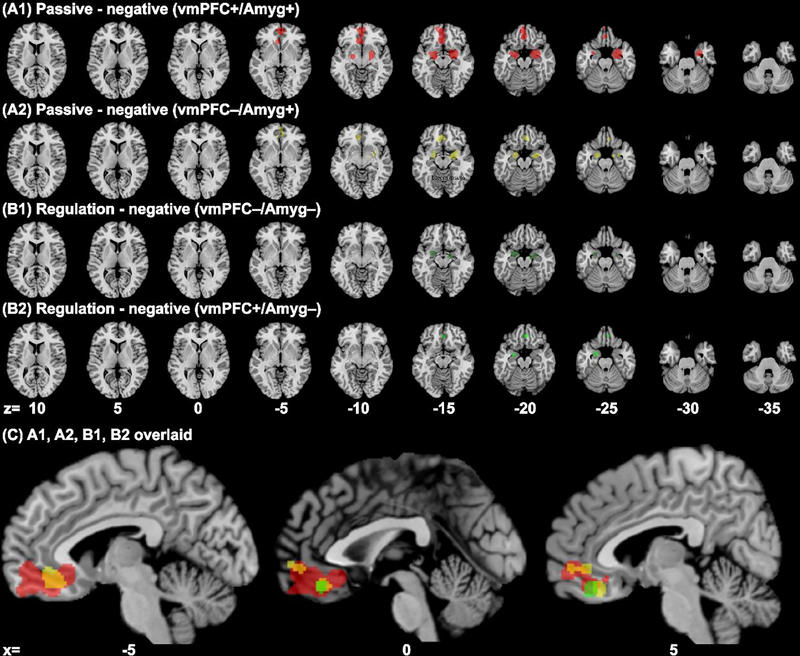
The experiments showing vmPFC+/Amyg+ or vmPFC−/Amyg+ to passive negative emotions both showed convergence of response in relatively posterior vmPFC and bilateral amygdala in ALE analysis (Table 2A1–A2, Fig. 4A1–A2). Experiments of down-regulation of negative emotion showing vmPFC−/Amyg− revealed convergence of deactivation in bilateral amygdala and right entorhinal cortex (Table 2B1, Fig. 4B1), whereas those showing vmPFC+/Amyg− converged in the left amygdala and posterior vmPFC (Table 2B2, Fig. 4B2–3C). Experiments with vmPFC+/Amyg+ to positive emotions showed a convergence of activation in a large cluster in the anterior vmPFC, a smaller cluster in the posterior vmPFC, and bilateral amygdala (Table 2C).
Table 2.
Subgroup ALE of responses to passive exposure to emotions and to regulation of negative emotions
| Brain Region | MINI | Cluster Size (mm3) | Weighted (MNI) | ALE value (× 103) | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||
| A1. Passive - negative (vmPFC+/Amyg+) | ||||||||
| vmPFC (BA32/11) | 0 | 40 | −18 | 8120 | −2 | 42 | −13 | 21.5 |
| vmPFC (BA32) | −6 | 26 | −10 | 21.4 | ||||
| vmPFC (FPC, BA10) | −2 | 54 | −8 | 20.1 | ||||
| vmPFC (FPC, BA10) | 6 | 52 | −6 | 17.3 | ||||
| Amygdala | 28 | −2 | −24 | 6992 | 26 | −2 | −2 0 | 43.8 |
| Amygdala | −20 | −6 | −16 | 3096 | −22 | −5 | −17 | 30.4 |
| Amygdala | −30 | 0 | −20 | 15.9 | ||||
| A2. Passive - negative (vmPFC−/Amyg+) | ||||||||
| Amygdala | 26 | −6 | −16 | 2360 | 27 | −4 | −17 | 19.8 |
| Entorhinal cortex (BA34) | 18 | −6 | −24 | 8.7 | ||||
| Globus Pallidus | 16 | −8 | −12 | 8.3 | ||||
| vmPFC (BA32) | −8 | 36 | −12 | 1896 | −3 | 35 | −1 5 | 12.7 |
| vmPFC (BA32) | 4 | 34 | −18 | 10.1 | ||||
| Amygdala | −20 | −4 | −22 | 1392 | −2 0 | −4 | −2 1 | 14.8 |
| vmPFC (BA32/24) | 6 | 42 | −6 | 520 | 4 | 48 | −6 | 10.8 |
| vmPFC (FPC, BA10) | 2 | 52 | −6 | 9.1 | ||||
| B1. Regulation - negative (vmPFC−/Amyg−) | ||||||||
| Amygdala | −24 | −4 | −20 | 1216 | −24 | −3 | −19 | 14.0 |
| Entorhinal cortex (BA28) | 16 | −10 | −16 | 960 | 19 | −9 | −18 | 11.4 |
| Amygdala | 26 | −6 | −22 | 8.9 | ||||
| B2. Regulation - negative (vmPFC+/Amyg−) | ||||||||
| vmPFC (BA11) | 4 | 38 | −20 | 536 | 4 | 39 | −20 | 9.8 |
| Amygdala | −24 | −4 | −22 | 512 | −23 | −3 | −23 | 9 |
| C. Passive - positive (vmPFC+/Amyg+) | ||||||||
| vmPFC (FPC, BA10) | 0 | 58 | −2 | 6216 | −1 | 56 | −3 | 45.0 |
| Amygdala | 26 | −4 | −20 | 6136 | 24 | −5 | −18 | 36.3 |
| Amygdala | 24 | −8 | −14 | 34.4 | ||||
| Amygdala | −24 | −8 | −16 | 6120 | −2 3 | −7 | −1 8 | 44.6 |
| vmPFC (BA32) | 8 | 34 | −12 | 1120 | 6 | 33 | −15 | 16.3 |
| vmPFC (sgACC, BA25) | 2 | 26 | −24 | 10.2 | ||||
Note: BA: Brodmann Area; FPC: frontopolar cortex; pgACC: perigenual anterior cingulate cortex; sgACC: subgenual anterior cingulate cortex
Figure 4.
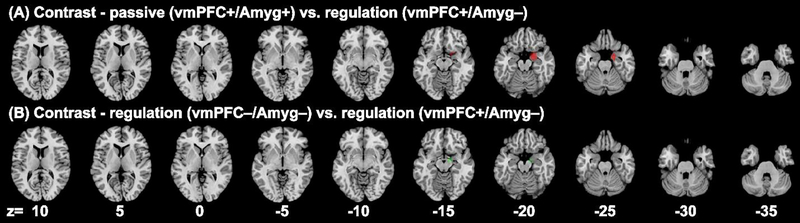
Results of subgroup ALE analysis of experiments involving negative emotions and showing different patterns of vmPFC and amygdala activities (cluster level p < 0.05, FWE corrected, with a cluster-forming threshold of voxel p < 0.001): (A1) [vmPFC+/Amyg+ (passive)] (red), (A2) [vmPFC−/Amyg+ (passive)] (yellow); (B1) [vmPFC−/Amyg− (regulation)] (dark green), (B2) [vmPFC+/Amyg− (regulation)] (green). Panel (C) shows A1, A2, B1 and B2 clusters overlaid on parasagittal sections. Voxels that overlapped between A1 and A2 appeared orange.
In contrast analyses we examined whether experiments showing distinct patterns of vmPFC/amygdala activities may engage different subregions of the vmPFC. We focused on four contrasts: [vmPFC+/Amyg+ (passive)] vs. [vmPFC−/Amyg+ (passive)]; [vmPFC+/Amyg+ (passive)] vs. [vmPFC+/Amyg− (regulation)]; [vmPFC−/Amyg+ (passive)] vs. [vmPFC−/Amyg− (regulation)]; and [vmPFC−/Amyg− (regulation)] vs. [vmPFC+/Amyg− (regulation)]. The results showed that none of these contrasts yielded convergence of differences in the vmPFC, but only in the amygdala or adjacent regions. The contrast of [vmPFC+/Amyg+ (passive)] > [vmPFC+/Amyg− (regulation)] reported convergence in right entorhinal cortex and amygdala (1336 voxels, peak: 21, −2, −21) (Fig. 5A). The contrast [vmPFC−/Amyg− (regulation)] > [vmPFC+/Amyg− (regulation)] reported convergence in the right entorhinal cortex (216 voxels, peak: 14, −8, −17) (Suppl. Table S8, Fig. 5B).
Figure 5.
Results of subgroup contrast analyses (images thresholded at p<0.01 with a minimum cluster volume of 200mm3): (A) [vmPFC+/Amyg+ (passive)] > [vmPFC+/Amyg− (regulation)] (red), (B) [vmPFC−/Amyg− (regulation)] > [vmPFC+/Amyg− (regulation)] (green).
3.3. Other brain regions: meta-analysis of “whole-brain” experiments
We performed whole-brain ALE analyses for experiments that reported whole-brain results. In total, four sets of whole brain analyses were performed for passive exposure to positive emotion (vmPFC+/Amyg+; n=16, 407 subjects), to negative emotion (vmPFC+/Amyg+; n=15, 295 subjects), mixed emotions (vmPFC+/Amyg+; n=9, 170 subjects); and for down-regulation of negative emotion showing (vmPFC−/Amyg−; n=4, 123 subjects).
Besides vmPFC, amygdala and the adjacent entorhinal and hippocampal areas, whole brain subgroup analysis identified convergence of activation in left ventral striatum (880 voxels, peak: −13, 6, −12) from experiments on passive exposure to positive emotions (vmPFC+/Amyg+; Table 3A, Fig. 6A). The analyses identified the ventral precuneus and posterior cingulate cortex (864 voxels, peak: −2, −59, 28) from experiments on passive exposure to negative emotions (vmPFC+/Amyg+; Table 3B, Fig. 6B). No additional clusters were identified for experiments on mixed emotions (vmPFC+/Amyg+; Table 3C, Fig. 6C). For experiments on down-regulation of negative emotions (vmPFC−/Amyg−), the results showed convergence of deactivation in left ventral striatum (472 voxels, peak: −3, 5, −7). Further, unlike the other whole-brain analyses, which identified bilateral amygdala, the left but not right amygdala was reported in this subgroup of experiments on down-regulation of negative emotions (Table 3D, Fig. 6D–E).
Table 3.
ALE of whole brain experiments on responses to passive exposure to emotions and to regulation of negative emotions
| Brain Region | MINI | Cluster Size (mm3) | Weighted center (MNI) | ALE value (× 103) | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||
| A. Passive - positive (vmPFC+/Amyg+) | ||||||||
| Amygdala | 24 | −4 | −20 | 3464 | 23 | −4 | −19 | 27.9 |
| Entorhinal cortex | 16 | −2 | −16 | 26.9 | ||||
| vmPFC (FPC, BA10) | 2 | 56 | −4 | 2832 | −1 | 54 | −7 | 29.0 |
| vmPFC (FPC, BA10) | −6 | 50 | −10 | 18.9 | ||||
| vmPFC (FPC, BA10) | −6 | 50 | −20 | 16.1 | ||||
| Entorhinal cortex | −22 | −16 | −20 | 2792 | −2 5 | −1 1 | −2 0 | 26.5 |
| Amygdala | −30 | −4 | −20 | 17.8 | ||||
| Ventral striatum | −14 | 10 | −10 | 880 | −1 3 | 6 | −1 2 | 18.5 |
| Entorhinal cortex | −14 | 2 | −16 | 17.2 | ||||
| B. Passive - negative (vmPFC+/Amyg+) | ||||||||
| Amygdala | 26 | −2 | −22 | 3160 | 26 | −3 | −21 | 34.9 |
| vmPFC (BA10/32) | −2 | 52 | −8 | 2496 | 1 | 50 | −7 | 24.6 |
| vmPFC (BA10/32) | 6 | 50 | −4 | 23.0 | ||||
| Amygdala | −20 | −8 | −16 | 1384 | −2 4 | −1 0 | −1 9 | 19.0 |
| Hippocampus | −28 | −14 | −22 | 17.4 | ||||
| PCC(BA31) | −4 | −58 | 26 | 864 | −2 | −5 9 | 28 | 18.0 |
| PCC(BA31) | 4 | −62 | 30 | 14.9 | ||||
| C. Passive - mixed (vmPFC+/Amyg+) | ||||||||
| Amygdala | −20 | −6 | −18 | 2064 | −20 | −5 | −18 | 26.8 |
| Amygdala | −30 | −4 | −22 | 10.7 | ||||
| Entorhinal cortex | 20 | −4 | −18 | 1640 | 20 | −3 | −1 9 | 28.5 |
| D. Regulation - negative (vmPFC−/Amyg−) | ||||||||
| Amygdala | −24 | −4 | −20 | 648 | −24 | −3 | −19 | 14.0 |
| Ventral striatum | −4 | 6 | −6 | 472 | −3 | 5 | −7 | 16.8 |
Note: BA: Brodmann Area; FPC: frontopolar cortex; PCC: posterior cingulate cortex
Figure 6.
Whole brain results showing significant clusters from subgroup ALE meta-analysis (cluster level p < 0.05, FWE corrected, with a cluster-forming threshold of voxel p < 0.001) for experiments involving (A) [vmPFC+/Amyg+ (passive)] of positive emotion (blue), (B) [vmPFC+/Amyg+ (passive)] of negative emotion (red), (C) [vmPFC+/Amyg+ (passive)] of mixed emotions (purple), and (D) [vmPFC−/Amyg− (down-regulation)] of negative emotion (green). Panel (E) shows A-D clusters overlaid.
4. Discussion
On the bases of fMRI studies that engaged emotional responses and reported vmPFC and amygdala activation or deactivation, we sought to determine convergence and contrast of activity in subareas of vmPFC and amygdala in healthy subjects. We conducted primary group ALE analyses to identify regional differences in response to passive exposure to positive, negative and mixed emotions and to regulation of negative emotions. In subgroup analyses we focused on experiments with distinct and consistent patterns of vmPFC and amygdala activities and examined whether these experiments engaged different vmPFC and amygdala subregions. Finally, we conducted ALE analysis on experiments that reported findings for the whole-brain to examine convergence of other concurrent regional activities. We highlighted the major findings in the following.
4.1. Passive processing of positive vs. negative emotions
As demonstrated by contrast analysis, passive exposure to positive emotions engaged the anterior vmPFC, whereas passive exposure to negative emotions engaged the posterior vmPFC, substantiating the hypotheses raised in earlier studies (Myers-Schulz and Koenigs, 2012; Hiser and Koenigs, 2018). Further, passive exposure to mixed emotions engaged a subregion in between.
In the vmPFC, from the olfactory core anteriorly to the ventral frontal pole, as well as laterally to the ventrolateral regions, the cortical architecture changes from the evolutionally oldest agranular cortex with less neuronal density and myelination to dysgranular and granular cortex in primates (Barbas, 1988; Ray and Zald, 2012; Yeterian and Pandya, 1991). Electrophysiological studies have also provided evidence of vmPFC subregions in processing valenced outcomes. For instance, multi-electrode recording and cortical stimulation showed that the pregenual anterior cingulate cortex (pACC) responded to the delivery of both food and air puffs, with responses in ventral bank of pACC biased toward negative stimuli in macaques (Amemori and Graybiel, 2012). Compared to other pACC subregions, the ventral pACC is anatomically unique with denser projections to the caudate and amygdala in rhesus monkeys (Eblen and Graybiel, 1995; Ghashghaei et al., 2007). Thus, although many experiments have shown amygdala and vmPFC responses to both positive and negative emotions, the anterior and posterior vmPFC can be anatomically and functionally differentiated. Along with earlier studies, the current findings suggest that the anterior and posterior vmPFC is each distinctly involved in the processing of positive and negative emotions.
Integrating human PET and fMRI studies using Multilevel Peak Kernel Density (MKDA) meta-analysis, Hayes et al. demonstrated that vmPFC subregions, amygdala, hippocampus, and thalamus, among others, were central to processing both appetitive and aversive stimuli, with the very anterior vmPFC showing selectivity for appetitive processes (Hayes et al. 2014). Another MKDA meta-analysis of fMRI and PET studies reported both vmPFC and amygdala as valence-general regions by intersecting positive > neutral and negative > neutral contrasts (Lindquist et al., 2016). The latter study also highlighted a preference of the amygdala for negativity by studying negative > positive paradigms. It is worth noting that these meta-analyses included both fMRI and PET experiments and identified vmPFC and amygdala activities independently, whereas the current work included only fMRI studies that reported concurrent vmPFC and amygdala responses. Thus, the current findings need to be considered in the context of concurrent vmPFC and amygdala activities.
4.2. Role of vmPFC and amygdala in emotion regulation
ALE showed that down-regulation of negative emotions similarly involved the posterior vmPFC, though in a smaller locus, as compared with passive exposure to negative emotions. Contrast analysis showed convergence of activity for “passive exposure > down-regulation” in the posterior vmPFC, right amygdala, entorhinal cortex, and putamen, which are all anatomically connected. It should be noted that a smaller number of the experiments were identified that involved down-regulation of negative emotions, which might have undermined the statistical power of the ALE of these responses.
Although both vmPFC and amygdala are known to be involved in emotion processing, previous studies have not consistently implicated both structures in the regulation of emotions. The posterior vmPFC cluster identified here (peak coordinates: 2, 40, −20) coincides well with the vmPFC cluster (peak: 6, 40, −22) reported from a previous fMRI ALE meta-analysis of down-regulation of negative emotion (Diekhof et al., 2011). Another earlier meta-analysis of experiments across a wide range of paradigms showed that bilateral amygdala increased in activity during exaggeration of experienced emotion, and decreased during down-regulation of emotions (Frank et al., 2014). In contrast, reviewing both fMRI and PET imaging studies involving reinterpretation, distancing or suppression of emotions, Langner et al. reported no significant convergence of activation in the vmPFC or amygdala (Langner et al., 2018). A later study also noted that the amygdala and vmPFC were not involved in emotion regulation with distancing strategies (Powers and LeBar, 2019). Instead, the dorsomedial prefrontal cortex (PFC), including the dorsal ACC and supplementary motor area, dorsolateral PFC (dlPFC), ventrolateral PFC, and lateral orbitofrontal cortex (OFC) were more commonly involved in emotion reappraisal (Chen et al., 2018; Langner et al., 2018; Wager et al., 2008b). It was suggested that the dlPFC, which did not appear to project directly to the amygdala may partake in emotion reappraisal and exert its effects on the amygdala via connections with the subgenual or dorsal ACC (Ray and Zald, 2012). That is, the medial PFC may play a role in emotion regulation indirectly by actuating the influences from these “higher-order” cognitive regions. Another consideration is that, as part of the default mode network, the vmPFC is involved in processing self-relevant information (Hiser and Koenigs, 2018; Northoff et al., 2006). Thus, the vmPFC may participate in down-regulation of emotion by detachment or distancing (i.e., decreasing self-relevance) but not as much by strategies that involve reappraisal, modification or selection. New studies are warranted to test this hypothesis.
We also showed that the left but not the right amygdala demonstrated convergence in experiments with active regulation of vs. passive exposure to emotions. That is, active regulation suppresses the response of the right amygdala to emotional stimuli. These findings are consistent with earlier meta-analyses showing that the left amygdala was more frequently reported than the right amygdala during emotion processing and regulation (Baas et al., 2004; Sergerie et al., 2008; Wager et al., 2003). Wager et al. demonstrated a left-hemispheric predominance of amygdala activity in emotion processing and a bias toward negative emotions (Wager et al., 2003). Combining fMRI and PET studies, Baas et al. also showed left lateralization of amygdala activity during emotion processing, irrespective of stimulus type or task instructions (Baas et al., 2004). By investigating visual emotional perception studies that reported amygdala activation, Sergerie et al. showed bilateral amygdala response to both positive and negative stimuli, with more studies (particularly those with a block design) reporting left amygdala activity, likely reflecting a more sustained response in the left amygdala (Sergerie et al., 2008). Indeed, the amygdala is known to demonstrate hemispheric functional asymmetry, with the right amygdala responding to the onset of emotional stimuli and habituating more quickly than the left amygdala and the left amygdala responding more to sustained engagement in emotions (Baas et al., 2004; Kilpatrick and Cahill, 2003).
Together, these findings suggest that, when the vmPFC and amygdala are both involved, a region in the ventral posterior vmPFC and the left but not right amygdala are engaged in down-regulation of negative emotions. It is important to note that by restricting the meta-analyses to experiments reporting activities of both the vmPFC and amygdala, leaving out those showing only vmPFC or amygdala activity, the current meta-analyses do not address the issue whether these or other cerebral structures are involved in emotion processing across all experiments. The current findings thus need to be considered within the context of concurrent vmPFC and amygdala activities.
4.3. Directions of vmPFC and amygdala activities during emotion processing
Nearly all passive exposure experiments (94%) reported amygdala activation, whereas most (82%) experiments of down-regulation of negative emotions reported amygdala deactivation. This finding is consistent with previous reports that the amygdala responded to salient stimuli and that amygdala activity was inhibited during down-regulation of negative emotion (Myers-Schulz and Koenigs, 2012).
Previous studies have suggested a role of the vmPFC in value-based decision making, self-referential processing, and emotion regulation likely through interaction with the amygdala (Icenhour et al., 2015; Myers-Schulz and Koenigs, 2012). In our experiment pool, vmPFC hyperactivity was broadly reported for passive processing of positive and mixed emotions (96%) as well as negative emotions (70%), and most (64%) experiments on down-regulation of negative emotions. More experiments on passive exposure of negative emotion showed vmPFC+/Amyg+, whereas more on down-regulation of negative emotion reported vmPFC+/Amyg−, suggesting vmPFC modulation of amygdala activity during emotion regulation. In experiments reporting vmPFC deactivation, more on passive exposure to negative emotion reported vmPFC−/Amyg+, and no experiments on down-regulation of negative emotion reported vmPFC−/Amyg+. One is tempted to speculate that decreases in amygdala and vmPFC activity may indicate successful and unsuccessful down-regulation of negative emotions, respectively. Together, the current findings add to an earlier work (Diekhof et al., 2011) by showing that a posterior subregion (peak: 4, 39, −20) of the vmPFC engages in down-regulation of negative emotions by decreasing amygdala activity.
In subgroup analyses of experiments showing different directions of vmPFC and amygdala responses, a comparison between [vmPFC−/Amyg+ (passive, negative)] and [vmPFC−/Amyg− (regulation, negative)], and between [vmPFC+/Amyg+ (passive)] and [vmPFC−/Amyg+ (passive)] did not yield significant differences. The contrast of [vmPFC+/Amyg+ (passive)] > [vmPFC+/Amyg− (regulation)], and [vmPFC−/Amyg− (regulation)] > [vmPFC+/Amyg− (regulation)] both reported differences in the right amygdala or nearby regions. To note, [vmPFC+/Amyg− (regulation)] experiments reported convergence of activity only in left amygdala and vmPFC, consistent with the findings of subgroup contrast analysis. However, the sample sizes of these subgroups of experiments were very small and these findings should be considered as tentative.
The two subgroups of experiments vmPFC+/Amyg− and vmPFC−/Amyg− did not appear to differ systematically in the experiment design or regulation strategies. Three of the 4 vmPFC−/Amyg−experiments reported dlPFC and dmPFC activation (Ochsner et al., 2002; Schardt et al., 2010; Winecoff et al., 2011), with the remaining study contrasting mainly down- and up-regulation (van Reekum et al., 2007). Of the 5 (4 whole-brain) studies showing vmPFC+/Amyg−, one study showed concurrent dlPFC activation with vmPFC (Delgado et al., 2008); one showed dlPFC deactivaton (Pitskel et al., 2011); two didn’t report dlPFC or dmPFC clusters (Koenigsberg et al., 2010; Kompus et al., 2009); and the remaining (ROI) study demonstrated vmPFC modulation of the interaction between dorsal frontopolar cortex (BA10) and amygdala (Urry et al., 2006). It thus appears that, although the small sample did not converge to show dlPFC or dmPFC response in whole-brain analysis of the vmPFC−/Amyg− subgroup, these prefrontal structures may be involved in down-regulating amygdala activity and negative emotions, when the vmPFC is deactivated. Notably, ALE of vmPFC+/Amyg+ or vmPFC−/Amyg+ whole-brain experiments on passive exposure to negative emotions did not reveal convergence in the dlPFC or dmPFC. Nonetheless, studies are warranted to identify under which conditions the vmPFC, dlPFC, dmPFC or other regions may be commonly or differentially engaged in the regulation of emotions.
4.4. Other regional activities during emotion processing
Whole brain ALE analysis identified convergence of activation in the ventral precuneus, posterior cingulate cortex (PCC), and left hippocampus besides the vmPFC and bilateral amygdala activities in passive exposure to negative emotion. As key components of the default mode network, both PCC and ventral precuneus are pivotal for integration of a wide variety of brain functions (Buckner et al., 2008, Zhang and Li, 2012a). Functionally connected with the vmPFC, both the PCC and ventral precuneus support self-referential processing (Cavanna and Trimble, 2006; Leech and Sharp, 2014; Zhang and Li, 2012b) that are central to emotional experience. The hippocampus, with its reciprocal anatomical connections with the amygdala, has been broadly implicated in fear conditioning and extinction (Chaaya et al., 2018; Yang and Wang, 2017, Vianna et al., 2004).
The analyses of whole brain experiments identified convergent activation in left ventral striatum (VS) and entorhinal cortex for passive exposure to positive emotion, and right entorhinal cortex for mixed emotions. Anatomically connected with the amygdala and hippocampus, the entorhinal cortex (BA28, BA34) is central to the formation, maintenance, and retrieval of episodic, including emotional, memory (Kohler et al., 1998; McDonald et al., 2000; Owen et al., 1996). The VS is a hub of the dopaminergic motivation and reward circuit (Frank et al., 2019, Valjent et al., 2019). A meta-analysis across 40 studies demonstrated that the vmPFC, left VS and thalamus responded in positive correlation with subjective pleasantness (Kuhn and Gallinat, 2012). Focusing on fMRI studies of reward prediction error (PE), a recent work reported a valence network including the VS and vmPFC in response to positive > negative PE (Fouragnan et al., 2018). It has also been suggested that the vmPFC plays a critical role in reward or value-based decision making through its interaction with the VS and amygdala (Hiser and Koenigs, 2018). We also observed convergent hypoactivation of the VS in the subgroup (vmPFC−/Amyg−) of experiments on down-regulation of negative emotion. The VS responds to salient stimuli of both positive and negative valence (Jensen et al., 2003); thus, the latter result suggested that the VS may show diminished response as negative emotion is dampened and become less salient. An alternative explanation is that the convergent deactivation of VS of vmPFC−/Amyg− experiments reflected a failure in down-regulation of negative emotions. The number of the vmPFC−/Amyg− experiments was small and more studies are required to address this issue.
4.5. Limitations, other considerations, and conclusions
Several limitations need to be considered for the study. First, as with other meta-analyses, we used the ALE algorithm to examine spatial consistency of brain activities. Convergence of activity was determined on the basis of peak activation coordinates of experiments and subject numbers but not activity intensity. Second, a smaller number of studies showing differential vmPFC and amygdala responses were identified for subgroup analyses. As meta-analyses with an experiment number less than 17 may not be statistically powered to detect subtle effects (Eickhoff, et al., 2011; Muller et al., 2018), these results should be interpreted with caution and confirmed in future work. Third, we included studies with behavioral paradigms that did not intend to address emotion processing; thus, the current results should be considered in a broader behavioral context of emotional experience. One also could not rule out the possibility that some of the findings may be specific to the emotional stimuli. For instance, 7 of the passive positive but only 1 passive negative experiments involved monetary stimuli; in contrast, 7 passive negative but only 1 passive positive experiments involved electric stimuli. The experimental details are provided in the Supplement. Fourth, new studies are needed to investigate how the dlPFC and dmPFC interact with the vmPFC and amygdala in regulating emotions. Fifth, the “vmPFC” has been referred to with many different terms across different areas such as neuroeconomics and clinical research (Delgado et al., 2016). We searched the literature with “ventral paracingulate” and did not identify any relevant studies. However, we did not search with “medial prefrontal cortex”. Thus, we cannot rule out the possibility that the current work did not include all pertinent studies. Sixth, BOLD signal drop-out and spatial warping is prominent in parts of vmPFC and one would consider this issue in evaluating the current findings. Finally, the method of meta-analytic connectivity modeling (MACM) can be used to identify regional co-activations (Robinson et al., 2010). With a seed, one obtains co-activations of other brain regions across all task-based fMRI or PET studies from the databases. Therefore, the findings of MACM do not distinguish between tasks/experiments or, with respect to the current study, positive vs. negative emotions or passive exposure to vs. active regulation of negative emotions. Further, these databases usually include a more restricted number of studies, in contrast to the literature located by manual search of PUBMED and Web of Science etc. Another consideration is that the Sleuth software is used to perform MACM analysis. Sleuth only allows a seed region less than 10,000 mm3, and thus for the current work the vmPFC would not be allowable as an ROI.
In sum, the anterior and posterior vmPFC activity was associated with positive and negative emotion processing, respectively. Down-regulation of, as compared to passive exposure to, negative emotions engaged a smaller area of the posterior vmPFC and the left but not right amygdala. Concurrent with vmPFC, amygdala and adjacent regional responses, activity also converged in the ventral precuneus and PCC during passive exposure to negative emotion, and in the left VS during passive exposure to positive emotion and down-regulation of negative emotion.
Supplementary Material
Acknowledgments
5.4 Funding
This study was supported by NIH grants DA023248, AA021449, MH113134 and DA044749. The NIH is otherwise not involved in the conceptualization of the study, data collection and analysis, or in the decision to publish the current results
Footnotes
5. Compliance with Ethical Standards
5.1 Disclosure of potential conflicts of interest
All authors declare no conflicts of interest in the current work
5.2 Research involving Human Participants and/or Animals
This study employed published data in the public domain for meta-analyses and did not involve human participants or animals.
5.3 Informed consent
This study employed published data in the public domain for meta-analyses and did not involve human participants.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR, 2005. A mechanism for impaired fear recognition after amygdala damage. Nature 433, 68–72. [DOI] [PubMed] [Google Scholar]
- al Maskati HA, Zbrozyna AW, 1989. Cardiovascular and motor components of the defence reaction elicited in rats by electrical and chemical stimulation in amygdala. J Auton Nerv Syst 28, 127–131. [DOI] [PubMed] [Google Scholar]
- Amemori K, Graybiel AM, 2012. Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nat Neurosci 15, 776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA, 1998. Intact recognition of vocal expressions of fear following bilateral lesions of the human amygdala. Neuroreport 9, 3607–3613. [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS, 2004. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Brain Res Rev 45, 96–103. [DOI] [PubMed] [Google Scholar]
- Barbas H, 1988. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol 276, 313–342. [DOI] [PubMed] [Google Scholar]
- Barrash J, Tranel D, Anderson SW, 2000. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol 18, 355–381. [DOI] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW, 2013. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 76, 412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley B, Medford N, Shaw P, David AS, 2004. Emotional memory and perception in temporal lobectomy patients with amygdala damage. J Neurol Neurosurg Psychiatry 75, 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Taylor SF, Sudheimer KD, Liberzon I, 2006. Facial expressions and complex IAPS pictures: common and differential networks. Neuroimage 31, 906–919. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL, 2008. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ, 2002. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26, 321–352. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR, 2006. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583. [DOI] [PubMed] [Google Scholar]
- Chaaya N, Battle AR, Johnson LR, 2018. An update on contextual fear memory mechanisms: Transition between Amygdala and Hippocampus. Neurosci Biobehav Rev 92, 43–54. [DOI] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Yucel M, Lubman DI, 2010. The role of affective dysregulation in drug addiction. Clin Psychol Rev 30, 621–634. [DOI] [PubMed] [Google Scholar]
- Chen T, Becker B, Camilleri J, Wang L, Yu S, Eickhoff SB, Feng C, 2018. A domain-general brain network underlying emotional and cognitive interference processing: evidence from coordinate-based and functional connectivity meta-analyses. Brain Struct Funct 223, 3813–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH, 2008. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev 58, 57–70. [DOI] [PubMed] [Google Scholar]
- Damasio AR, 1998. Emotion in the perspective of an integrated nervous system. Brain Res Brain Res Rev 26, 83–86. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H, 1990. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res 41, 81–94. [DOI] [PubMed] [Google Scholar]
- Davis M, Antoniadis EA, Amaral DG, Winslow JT, 2008. Acoustic startle reflex in rhesus monkeys: a review. Rev Neurosci 19, 171–185. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA, 2008. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 59, 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Beer JS, Fellows LK, Huettel SA, Platt ML, Quirk GJ, Schiller D, 2016. Viewpoints: Dialogues on the functional role of the ventromedial prefrontal cortex. Nat Neurosci 19, 1545–1552. [DOI] [PubMed] [Google Scholar]
- Di X, Huang J, Biswal BB, 2017. Task modulated brain connectivity of the amygdala: a meta-analysis of psychophysiological interactions. Brain Struct Funct 222, 619–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O, 2011. Fear is only as deep as the mind allows A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage 58, 275–285. [DOI] [PubMed] [Google Scholar]
- Donofry SD, Roecklein KA, Wildes JE, Miller MA, Erickson KI, 2016. Alterations in emotion generation and regulation neurocircuitry in depression and eating disorders: A comparative review of structural and functional neuroimaging studies. Neurosci Biobehav Rev 68, 911–927. [DOI] [PubMed] [Google Scholar]
- Dougherty LR, Blankenship SL, Spechler PA, Padmala S, Pessoa L, 2015. An fMRI Pilot Study of Cognitive Reappraisal in Children: Divergent Effects on Brain and Behavior. J Psychopathol Behav Assess 37, 634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eblen F, Graybiel AM, 1995. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J Neurosci 15, 5999–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Johnson MK, Fischer H, 2012. Neural mechanisms of reading facial emotions in young and older adults. Front Psychol 3, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT, 2012. Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT, 2011. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57, 938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT, 2009. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30, 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S, 2007. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp 28, 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouragnan E, Retzler C, Philiastides MG, 2018. Separate neural representations of prediction error valence and surprise: Evidence from an fMRI meta-analysis. Hum Brain Mapp 39, 2887–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Hussein AA, Smart LM, Sabatinelli D, 2014. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev 45, 202–211. [DOI] [PubMed] [Google Scholar]
- Frank LE, Preston AR, Zeithamova D, 2019. Functional connectivity between memory and reward centers across task and rest track memory sensitivity to reward. Cogn Affect Behav Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Bacon SJ, 2003. Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res 993, 59–71. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia I, Kube J, Gaebler M, Horstmann A, Villringer A, Neumann J, 2016. Neural processing of negative emotional stimuli and the influence of age, sex and task-related characteristics. Neurosci Biobehav Rev 68, 773–793. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H, Hilgetag CC, Barbas H, 2007. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34, 905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H, 2002. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 115, 1261–1279. [DOI] [PubMed] [Google Scholar]
- Green MJ, Cahill CM, Malhi GS, 2007. The cognitive and neurophysiological basis of emotion dysregulation in bipolar disorder. J Affect Disord 103, 29–42. [DOI] [PubMed] [Google Scholar]
- Hayes DJ, Duncan NW, Xu J, Northoff G, 2014. A comparison of neural responses to appetitive and aversive stimuli in humans and other mammals. Neurosci Biobehav Rev 45, 350–368. [DOI] [PubMed] [Google Scholar]
- Heller AS, Lapate RC, Mayer KE, Davidson RJ, 2014. The face of negative affect: trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. J Cogn Neurosci 26, 2102–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herde L, Forster C, Strupf M, Handwerker HO, 2007. Itch induced by a novel method leads to limbic deactivations a functional MRI study. J Neurophysiol 98, 2347–2356. [DOI] [PubMed] [Google Scholar]
- Hiser J, Koenigs M, 2018. The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol Psychiatry 83, 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Rehme AK, Diessel M, Kukolja J, Maier W, Walter H, Cohen MX, 2008. Segregating intra-amygdalar responses to dynamic facial emotion with cytoarchitectonic maximum probability maps. J Neurosci Methods 172, 13–20. [DOI] [PubMed] [Google Scholar]
- Icenhour A, Kattoor J, Benson S, Boekstegers A, Schlamann M, Merz CJ, Forsting M, Elsenbruch S, 2015. Neural circuitry underlying effects of context on human pain-related fear extinction in a renewal paradigm. Hum Brain Mapp 36, 3179–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S, 2003. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron 40, 1251–1257. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ, 2006. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci 26, 9503–9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M, 2011. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex 21, 1379–1388. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L, Cahill L, 2003. Modulation of memory consolidation for olfactory learning by reversible inactivation of the basolateral amygdala. Behav Neurosci 117, 184–188. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ, 2004. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci 16, 1730–1745. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD, 2008. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage 42, 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN, 2010. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A 107, 14811–14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise K, Pizzarello S, Dorantes C, Tecuta L, Guerreri S, Goodman M, New A, Flory J, Siever LJ, 2010. Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia 48, 1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, McIntosh AR, Moscovitch M, Winocur G, 1998. Functional interactions between the medial temporal lobes and posterior neocortex related to episodic memory retrieval. Cerebral Cortex 8, 451–461. [DOI] [PubMed] [Google Scholar]
- Kohn N, Falkenberg I, Kellermann T, Eickhoff SB, Gur RC, Habel U, 2014. Neural correlates of effective and ineffective mood induction. Soc Cogn Affect Neurosci 9, 864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompus K, Hugdahl K, Ohman A, Marklund P, Nyberg L, 2009. Distinct control networks for cognition and emotion in the prefrontal cortex. Neurosci Lett 467, 76–80. [DOI] [PubMed] [Google Scholar]
- Koush Y, Pichon S, Eickhoff SB, Van De Ville D, Vuilleumier P, Scharnowski F, 2019. Brain networks for engaging oneself in positive-social emotion regulation. Neuroimage 189, 106–115. [DOI] [PubMed] [Google Scholar]
- Kret ME, Ploeger A, 2015. Emotion processing deficits: a liability spectrum providing insight into comorbidity of mental disorders. Neurosci Biobehav Rev 52, 153–171. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J, 2012. The neural correlates of subjective pleasantness. Neuroimage 61, 289–294. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT, 2005. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Leiberg S, Hoffstaedter F, Eickhoff SB, 2018. Towards a human self-regulation system: Common and distinct neural signatures of emotional and behavioural control. Neurosci Biobehav Rev 90, 400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM, 1990. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci 10, 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ, 2014. The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Straccia MA, Meyer ML, Du M, Tan KM, 2019. Social, self, (situational), and affective processes in medial prefrontal cortex (MPFC): Causal, multivariate, and reverse inference evidence. Neurosci Biobehav Rev 99, 311–328. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Wager TD, Weber J, Barrett LF, 2016. The Brain Basis of Positive and Negative Affect: Evidence from a Meta-Analysis of the Human Neuroimaging Literature. Cereb Cortex 26, 1910–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF, 2012. The brain basis of emotion: a meta-analytic review. Behav Brain Sci 35, 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, 2015. Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol Psychiatry 20, 311–319. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L, 1996. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71, 55–75. [DOI] [PubMed] [Google Scholar]
- McDonald B, Highley JR, Walker MA, Herron BM, Cooper SJ, Esiri MM, Crow TJ, 2000. Anomalous asymmetry of fusiform and parahippocampal gyrus gray matter in schizophrenia: A postmortem study. Am J Psychiatry 157, 40–47. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL, 2009. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66, 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, 2002. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420, 70–74. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD, 2007. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science 317, 1079–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M, 2015. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry 77, 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller VI, Cieslik EC, Laird AR, Fox PT, Radua J, Mataix-Cols D, Tench CR, Yarkoni T, Nichols TE, Turkeltaub PE, Wager TD, Eickhoff SB, 2018. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev 84, 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M, 2012. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry 17, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J, 2006. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage 31, 440–457. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD, 2002. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14, 1215–1229. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL, 2003. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 460, 425–449. [DOI] [PubMed] [Google Scholar]
- Owen AM, Milner B, Petrides M, Evans AC, 1996. A specific role for the right parahippocampal gyrus in the retrieval of object-location: a positron emission tomography study. J Cogn Neurosci 8, 588–602. [DOI] [PubMed] [Google Scholar]
- Park C, Rosenblat JD, Lee Y, Pan Z, Cao B, Iacobucci M, McIntyre RS, 2019. The Neural Systems of Emotion Regulation and Abnormalities in Major Depressive Disorder. Behav Brain Res [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE, 2004. Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43, 897–905. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R, 2003a. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry 54, 515–528. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R, 2003b. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry 54, 504–514. [DOI] [PubMed] [Google Scholar]
- Pitskel NB, Bolling DZ, Kaiser MD, Crowley MJ, Pelphrey KA, 2011. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Dev Cogn Neurosci 1, 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers JP, LaBar KS, 2019. Regulating emotion through distancing: A taxonomy, neurocognitive model, and supporting meta-analysis. Neurosci Biobehav Rev 96, 155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, 2007. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann Ny Acad Sci 1121, 54–71. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS, 2006. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol 16, 723–727. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D, 2003. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23, 8800–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RD, Zald DH, 2012. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci Biobehav Rev 36, 479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, 2003. Addiction. Annu Rev Psychol 54, 25–53. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT, 2010. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp 31, 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Davis M, 1988. Enhancement of acoustic startle by electrical stimulation of the amygdala. Behav Neurosci 102, 195–202, 324. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD, 2012. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci 16, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio A, Van Oudenhove L, Pellissier S, Ly HG, Dupont P, Lafaye de Micheaux H, Tack J, Dantzer C, Delon-Martin C, Bonaz B, 2015. Uncertainty in anticipation of uncomfortable rectal distension is modulated by the autonomic nervous system - A fMRI study in healthy volunteers. Neuroimage 107, 10–22. [DOI] [PubMed] [Google Scholar]
- Schardt DM, Erk S, Nusser C, Nothen MM, Cichon S, Rietschel M, Treutlein J, Goschke T, Walter H, 2010. Volition diminishes genetically mediated amygdala hyperreactivity. Neuroimage 53, 943–951. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL, 2008. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 32, 811–830. [DOI] [PubMed] [Google Scholar]
- Sloan E, Hall K, Moulding R, Bryce S, Mildred H, Staiger PK, 2017. Emotion regulation as a transdiagnostic treatment construct across anxiety, depression, substance, eating and borderline personality disorders: A systematic review. Clin Psychol Rev 57, 141–163. [DOI] [PubMed] [Google Scholar]
- Smith DV, Gseir M, Speer ME, Delgado MR, 2016. Toward a cumulative science of functional integration: A meta-analysis of psychophysiological interactions. Hum Brain Mapp 37, 2904–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG, 2002. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol 451, 301–323. [DOI] [PubMed] [Google Scholar]
- Stephanou K, Davey CG, Kerestes R, Whittle S, Harrison BJ, 2017. Hard to look on the bright side: neural correlates of impaired emotion regulation in depressed youth. Soc Cogn Affect Neurosci 12, 1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P, 2012. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp 33, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ, 2006. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci 26, 4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Biever A, Gangarossa G, Puighermanal E, 2019. Dopamine signaling in the striatum. Adv Protein Chem Struct Biol 116, 375–396. [DOI] [PubMed] [Google Scholar]
- van Reekum CM, Urry HL, Johnstone T, Thurow ME, Frye CJ, Jackson CA, Schaefer HS, Alexander AL, Davidson RJ, 2007. Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. J Cogn Neurosci 19, 237–248. [DOI] [PubMed] [Google Scholar]
- Vianna MR, Coitinho AS, Izquierdo I, 2004. Role of the hippocampus and amygdala in the extinction of fear-motivated learning. Curr Neurovasc Res 1, 55–60. [DOI] [PubMed] [Google Scholar]
- Wager TD, Barrett LF, Bliss-Moreau E, Lindquist K, Duncan S, Kober H, Joseph J, Davidson M, Mize J, 2008a. The Neuroimaging of Emotion. The handbook of emotion, 249–271. [Google Scholar]
- Wager T, 2008b. The roles of medial prefrontal cortex in emotion: Neuroimaging evidence for functional subdivisions and cortical-subcortical pathways. Biol Psychiat 63, 151s–151s. [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF, 2003. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage 19, 513–531. [DOI] [PubMed] [Google Scholar]
- Winecoff A, Labar KS, Madden DJ, Cabeza R, Huettel SA, 2011. Cognitive and neural contributors to emotion regulation in aging. Soc Cogn Affect Neurosci 6, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Aron A, Brown L, Cao G, Feng T, Weng X, 2011. Reward and motivation systems: a brain mapping study of early-stage intense romantic love in Chinese participants. Hum Brain Mapp 32, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang JZ, 2017. From Structure to Behavior in Basolateral Amygdala-Hippocampus Circuits. Front Neural Circuits 11, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN, 1991. Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. J Comp Neurol 312, 43–67. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li CS, 2012a. Functional networks for cognitive control in a stop signal task: independent component analysis. Hum Brain Mapp 33, 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CS, 2012b. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage 59, 3548–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.