Abstract
Survival differences by racial and ethnic group have been reported in children and adolescents with germ cell tumors (GCTs), but whether these differences depend on stage of disease is unclear. Using the SEER 18 registries (2000-2015), we examined GCT survival differences by race/ethnicity (non-Hispanic white [NHW], Black, Asian/Pacific Islander [API], Hispanic) separately for males and females aged 0-19 years at diagnosis. We used Kaplan Meier survival curves (Log-Rank p-values) to characterize survival differences. Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (95% CI) for the association between race/ethnicity and death. Using an inverse odds weighting mediation analysis, we estimated the association between race/ethnicity and death treating stage of disease as the mediator. There were no significant racial/ethnic survival differences among females. Male survival differed by race/ethnicity (p<0.0001) with NHW males having the best survival. Compared to NHW, API and Hispanic males had significantly higher risks of death (API HR: 2.18; 95% CI: 1.32-3.56; Hispanic HR: 1.98; 95% CI: 1.42-2.78) (model adjusted for age and year at diagnosis, tumor histology and location, stage). This association was mediated by stage of disease only among Hispanic males with gonadal tumors (indirectHR: 1.18; 95% CI: 1.03-1.35).
The increased risk of death after a testicular GCT diagnosis observed among Hispanic males was mediated by stage of disease. For API males and Hispanic males with extragonadal tumors, other unidentified factors including differences in exposures, tumor biology or treatment received may impact the observed racial/ethnic survival disparities.
Keywords: pediatric germ cell tumors, survival, racial disparities
Introduction
Studies of testicular cancer in adolescent and adult populations have reported racial/ethnic differences in the risk of death, with non-Hispanic white (NHW) males having better outcomes.1,2 Survival differences by race/ethnicity among females diagnosed with ovarian GCTs have also been reported.3 A recent study shows that among children and adolescents diagnosed with a GCT, Hispanic children have higher all-cause mortality than non-Hispanic black or white children.4 Less is known about racial/ethnic differences in survival after a GCT diagnosis when males and females are considered separately, which is important as there are established differences in GCT survival by sex.5,6
GCTs are heterogeneous with regard to both tumor location and histology, which vary by sex, age at diagnosis, and race/ethnicity and also impact survival.2,6-13 For example, tumors of mixed histology display a higher rate of death than other histologic subtypes in females.13 In males diagnosed with testicular cancer at ages less than 40 years, nonseminoma histology is associated with a higher risk of death than seminoma.2 Gonadal tumors are associated with better survival than extragonadal tumors.6,14,15 Thus, observed racial/ethnic differences in tumor location and histology may contribute to racial differences in survival.6
In general, racial/ethnic disparities in childhood cancer survival are postulated to arise from differences in socioeconomic status (SES);16 however, a recent analysis considering SES as a potential mediator of the association between race and the risk of death after a GCT diagnosis did not find that SES mediated the association between Hispanic ethnicity, compared to NHW race, and the risk of death.4 The racial/ethnic differences in GCT survival may depend on differences in clinical characteristics, delay in diagnosis, treatment received, pharmacogenetics, tumor biology, or other factors.
Using the Surveillance, Epidemiology, and End Results (SEER) Program 18 registries (2000-2015), we estimated survival differences by race/ethnicity (NHW, non-Hispanic black, Asian/Pacific Islander [API], Hispanic) for males and females aged 0-19 years with a GCT diagnosis, as this association has been more extensively studied in the adult population as reported elsehwere.1-3 We estimated the association between race/ethnicity and death for each sex separately. Then, to explore the role of stage of disease in the association between race/ethnicity and death after a GCT diagnosis, we used an inverse odds weighted (IOW) mediation analysis approach to estimate the association between race/ethnicity and death after a GCT diagnosis while treating stage of disease as a mediator.4,17-19
Materials and Methods
Study population.
Cases aged 0-19 years (n=4,172) were identified using the publicly available Surveillance, Epidemiology, and End Results (SEER) Program 18 registries20 (2000-2015), which includes Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles, San Jose-Monterey, Rural Georgia, the Alaska Native Tumor Registry, greater California, greater Georgia, Kentucky, Louisiana, and New Jersey. Included individuals had a first primary germ cell tumor (GCT) diagnosis defined by the International Classification of Childhood Cancer 3rd edition21 as either Xa Intracranial and intraspinal GCT, Xb Extracranial and extragonadal GCT, or Xc Malignant gonadal GCT. Included cases also had information available on race/ethnicity according to the SEER definition: non-Hispanic white (NHW), non-Hispanic black (black), Asian/Pacific Islander (API), and Hispanic (all races). Finally, the main analysis was restricted to tumors that were classified as germinoma, yolk sac tumor, mixed histology, and other histologic types including choriocarcinoma, embryonal carcinoma and tumors labeled as ‘other’ by SEER (n=3,314) as defined using the International Classification of Diseases for Oncology 3 (ICD-O-3) SEER codes and the classification scheme used by Poynter et al. 2010.6 Teratomas (n=858) were excluded as they are generally not malignant, are highly curable with surgery alone, and are thought to be a different disease than other types of GCTs.22
This analysis used existing data with no personal identifiers; therefore, the study was exempt from review by the University of Minnesota Institutional Review Board.
Variables of interest.
From SEER, we obtained the age at diagnosis (<1, 1-4, 5-9, 10-14, 15-19 years), year of diagnosis (2000-2015), race/ethnicity (defined above), tumor histology (defined above), tumor size (<2cm, 2-<5cm, ≥5cm), stage of disease (local, regional, distant), mediastinal tumor location (yes/no; obtained from ICD-O topography codes C38.1-C38.3 in SEER), surgery (yes, no/unknown), chemotherapy (yes, no/unknown), radiation (yes, no/unknown), months of survival (continuous), and vital status. The distribution of treatment received by GCT histology and location is shown in Supplemental Table 1.
Statistical analysis.
Chi-squared tests were conducted to detect differences in the distribution of patient and tumor characteristics by race/ethnicity for males and females separately except for treatment received as this data is not reliably captured in SEER.23 Five-year overall survival was calculated for males and females of all races combined and stratified by race/ethnicity. We also used Kaplan-Meier survival curves and Log-Rank p-values to compare survival differences by sex and by racial/ethnic group for GCTs overall and stratified by tumor location. Cox proportional hazards models were used to compute sex-specific hazard ratios (HR) and the corresponding 95% confidence intervals (95% CI) for the association between race/ethnicity and the risk death with NHW serving as the referent group. There was no violation of the proportional hazards assumption when entering an interaction term between race/ethnicity and time into the model.
We conducted a mediation analysis in racial/ethnic groups where we observed a significant association between race/ethnicity and the risk of death, treating stage of disease as the mediator. We used an inverse odds weighting (IOW) method, described by Nguyen et al. (2014)17 and used elsewhere.4,18,19 Briefly, the use of the weighted Cox proportional hazards model allows for estimation of the association between race/ethnicity and the risk of death, independent of stage of disease. The weight for race/ethnicity was estimated from a logistic regression model for stage of disease in association with race/ethnicity where NHW served as the referent category and was assigned a weight of 1. Black, API, and Hispanic males were assigned a value of the inverse odds of the aforementioned logistic models conducted separately for each racial/ethnic group. The indirect effect of race/ethnicity on the risk of death operating through stage of disease was calculated by subtracting the direct effect beta from the total effect beta (βindirect=βtotal-βdirect), which was estimated from the Cox proportional hazards model without the IOW specification. For the total, direct, and indirect effects, the resulting HRs were estimated and bootstrapped standard errors (1000 replications) were used to estimate the 95% CI. A significant indirect effect was interpreted as evidence of mediation by stage of disease in the race/ethnicity-risk of death association. Cox regression analyses were conducted in SAS v9.4 (SAS Institute, Cary, NC), mediation analyses were conducted in Stata v15.0 (College Station, Texas), and figures were generated in GraphPad Prism v8.0.0 (GraphPad Software, La Jolla, CA). Statistical significance was determined using two-sided hypothesis test (alpha of 0.05).
Data availability.
SEER 18 data is publicly available20 (2000-2015) and can be accessed on the SEER website (https://seer.cancer.gov).
Results
Demographic and clinical characteristics by race/ethnicity in males
There were 2,432 males with germ cell tumors (GCTs) who had race/ethnicity and survival information available for this analysis (Table 1). NHW and Hispanic boys made up the majority of male GCT cases (50% and 37%, respectively). The average age at diagnosis ranged from 13.9 years (API) to 15.8 years (NHW). We found significant differences in the distributions of tumor location and histology by race/ethnicity (both chi-squared p<0.0001). Over 75% of tumors diagnosed in NHW and Hispanic males were gonadal while under half of the tumors among black and API were gonadal (chi-squared p<0.0001). Black and API males had higher percentages of intracranial/intraspinal tumors than NHW or Hispanic males. NHW and Hispanic males were more likely to have tumors of mixed histology and germinoma when compared with black and API males.
Table 1.
Population demographic and tumor characteristics for children with germ cell tumors by race/ethnicity and sex, SEER 18 2000-2015
| Males | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non- Hispanic White |
Black | Asian/ Pacific Islander |
Hispanic | Non- Hispanic White |
Black | Asian/ Pacific Islander |
Hispanic | |||
| N (%) 1241 (51.0) |
N (%) 77 (3.2) |
N (%) 208 (8.6) |
N (%) 906 (37.3) |
N (%) 395 (44.8) |
N (%) 137 (15.5) |
N (%) 97 (11.0) |
N (%) 253 (28.7) |
|||
| n (%) | n (%) | n (%) | n (%) | chi-squared p-value |
n (%) | n (%) | n (%) | n (%) | chi-squared p-value |
|
| Age at diagnosis (years) | ||||||||||
| Average (Standard Deviation) | 15.8 (4.5) | 14.6 (5.5) | 13.9 (5.7) | 15.4 (4.8) | 11.8 (6.0) | 12.0 (6.1) | 12.1 (5.6) | 12.9 (5.0) | ||
| <1 | 22 (1.8) | 3 (3.9) | 8 (3.8) | 21 (2.3) | 0.003 | 18 (4.6) | 11 (8.0) | 5 (5.2) | 6 (2.4) | 0.08 |
| 1-4 | 54 (4.4) | 6 (7.8) | 19 (9.1) | 48 (5.3) | 50 (12.7) | 12 (8.8) | 9 (9.3) | 16 (6.3) | ||
| 5-9 | 33 (2.7) | 1 (1.3) | 8 (3.8) | 27 (3.0) | 53 (13.4) | 12 (8.8) | 10 (10.3) | 30 (11.9) | ||
| 10-14 | 138 (11.1) | 12 (15.6) | 38 (18.3) | 100 (11.0) | 106 (26.8) | 37 (27.0) | 34 (35.1) | 81 (32.0) | ||
| 15-19 | 994 (80.1) | 55 (71.4) | 135 (64.9) | 710 (78.4) | 168 (42.5) | 65 (47.4) | 39 (40.2) | 120 (47.4) | ||
| Tumor Location | ||||||||||
| Intracranial/Intraspinal | 237 (19.1) | 31 (40.3) | 89 (42.8) | 137 (15.1) | <0.0001 | 61 (15.4) | 18 (13.1) | 34 (35.1) | 34 (13.4) | <0.0001 |
| Extracranial/Extragonadal | 58 (4.7) | 8 (10.4) | 31 (14.9) | 66 (7.3) | 83 (21.0) | 46 (33.6) | 17 (17.5) | 44 (17.4) | ||
| Gonadal | 946 (76.2) | 38 (49.4) | 88 (42.3) | 703 (77.6) | 251 (63.5) | 73 (53.3) | 46 (47.4) | 175 (69.2) | ||
| Tumor Histology | ||||||||||
| Germinoma | 361 (29.1) | 37 (48.1) | 105 (50.5) | 248 (27.4) | <0.0001 | 187 (47.3) | 38 (27.7) | 51 (52.6) | 117 (46.2) | 0.0002 |
| Yolk sac tumor | 80 (6.4) | 11 (14.3) | 37 (17.8) | 89 (9.8) | 92 (23.3) | 41 (29.9) | 26 (26.8) | 48 (19.0) | ||
| Mixed | 613 (49.4) | 20 (26.0) | 51 (24.5) | 447 (49.3) | 91 (23.0) | 36 (26.3) | 13 (13.4) | 64 (25.3) | ||
| Other | 187 (15.1) | 9 (11.7) | 15 (7.2) | 122 (13.5) | 25 (6.3) | 22 (16.1) | 7 (7.2) | 24 (9.5) | ||
| Tumor size (cm) | ||||||||||
| ≤2 | 202 (23.6) | 10 (21.7) | 22 (15.9) | 103 (15.4) | <0.0001 | 21 (8.8) | 7 (8.5) | 4 (6.6) | 10 (6.4) | 0.06 |
| >2-≤5 | 434 (50.7) | 29 (63.0) | 76 (55.1) | 303 (45.2) | 40 (16.7) | 8 (9.8) | 19 (31.1) | 27 (17.2) | ||
| >5 | 220 (25.7) | 7 (15.2) | 40 (29.0) | 265 (39.5) | 179 (74.6) | 67 (81.7) | 38 (62.3) | 120 (76.4) | ||
| missing | 385 | 31 | 70 | 235 | 155 | 55 | 36 | 96 | ||
| Stage of disease | ||||||||||
| Local | 784 (64.6) | 43 (58.9) | 129 (64.2) | 497 (56.3) | 0.002 | 167 (44.9) | 52 (40.3) | 55 (57.3) | 105 (43.0) | 0.02 |
| Regional | 245 (20.2) | 16 (21.9) | 43 (21.4) | 194 (22.0) | 127 (34.1) | 36 (27.9) | 20 (20.8) | 70 (28.7) | ||
| Distant | 185 (15.2) | 14 (19.2) | 29 (14.4) | 192 (21.7) | 78 (21.0) | 41 (31.8) | 21 (21.9) | 69 (28.3) | ||
| missing | 27 | 4 | 7 | 23 | 23 | 8 | 1 | 9 | ||
| Surgery (yes) | 1073 (86.5) | 62 (80.5) | 139 (66.8) | 792 (87.4) | 334 (84.6) | 115 (83.9) | 71 (73.2) | 214 (84.6) | ||
| Radiation (yes) | 246 (19.8) | 32 (41.6) | 78 (37.5) | 144 (15.9) | 51 (12.9) | 14 (10.2) | 27 (27.8) | 30 (11.9) | ||
| Chemotherapy (yes) | 667 (53.7) | 44 (57.1) | 133 (63.9) | 526 (58.1) | 277 (70.1) | 96 (70.1) | 74 (76.3) | 182 (71.9) | ||
| Vital status | ||||||||||
| Alive | 1172 (94.4) | 70 (90.9) | 181 (87.0) | 810 (89.4) | <0.0001 | 369 (93.4) | 128 (93.4) | 89 (91.8) | 241 (95.3) | 0.6 |
| Dead | 69 (5.6) | 7 (9.1) | 27 (13.0) | 96 (10.6) | 26 (6.6) | 9 (6.6) | 8 (8.2) | 12 (4.7) | ||
| Year diagnosed | ||||||||||
| 2000:2004 | 394 (31.7) | 30 (39.0) | 59 (28.4) | 200 (22.1) | <0.0001 | 136 (34.4) | 41 (29.9) | 26 (26.8) | 75 (29.6) | 0.5 |
| 2005:2009 | 419 (33.8) | 23 (29.9) | 58 (27.9) | 283 (31.2) | 116 (29.4) | 40 (29.2) | 34 (35.1) | 71 (28.1) | ||
| 2010:2015 | 428 (34.5) | 24 (31.2) | 91 (43.8) | 423 (46.7) | 143 (36.2) | 56 (40.9) | 37 (38.1) | 107 (42.3) | ||
Among male cases with tumor size available, nearly half of the tumors diagnosed in each group were intermediate in size (>2-≤5cm); however, a large number of cases were missing tumor size data so these results should be cautiously interpreted. Approximately 60% of male cases were diagnosed with local stage of disease, but there were differences by race/ethnicity (p=0.002). Black and Hispanic males had the highest proportions of cases diagnosed with distant disease.
Demographic and clinical characteristics by race/ethnicity in females
For females diagnosed with a GCT from 2000-2015 (N=882) that had race/ethnicity and survival information available, the race/ethnicity distribution differed slightly from that observed for males (Table 1). GCTs were most commonly diagnosed in NHW and Hispanic females as observed in males; however, nearly 2/3 of GCTs in black children were diagnosed among females, which was the only racial/ethnic group to have a female predominance. The age at diagnosis for females was younger than observed for males at 12-13 years of age in each group. Overall, females were more frequently diagnosed with extracranial/extragonadal tumors than males and extracranial/extragonadal tumors tended to be larger in size than tumors of other locations (84% >5cm compared to 8% >5cm for intracranial/intraspinal and 45% >5cm for gonadal; chi-squared p<0.0001). While all groups were most frequently diagnosed with gonadal tumors, API females had the highest proportion of intracranial/intraspinal tumors and black females had the highest proportion of extracranial/extragonadal tumors (chi-squared p<0.0001). Tumor histology also differed by race/ethnicity (chi-squared p=0.0002). Females in all groups tended to have larger tumors when compared with males, with nearly three-quarters measuring over 5cm in size, though due to the large number of cases missing data these results should be interpreted with caution.
Survival differences by sex and race/ethnicity
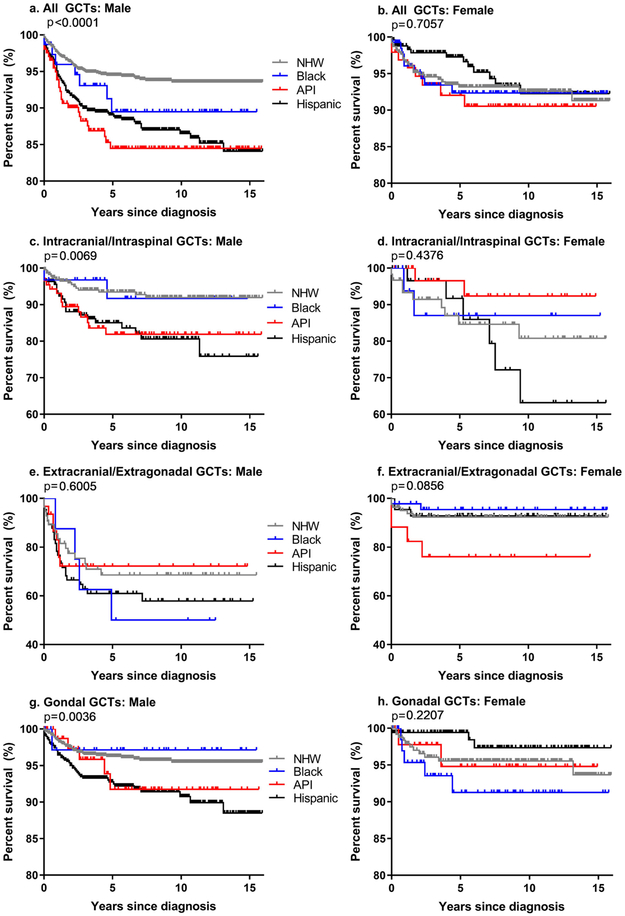
Overall, males of all races combined had slightly lower survival than females (five-year survival: males 92%, females 94%; overall survival p=0.05). The five-year survival estimates for all GCTs combined among females did not differ significantly by race/ethnicity. Conversely, overall survival differed significantly by race/ethnicity among males (log-rank p <0.0001; Figure 1). API males had the lowest five-year survival (85%) and NHW males had the highest (94%).
Figure 1.
Overall survival and log-rank p-values for males and females diagnosed with germ cell tumors by race/ethnicity for all GCTs combined (a-b), and by tumor location (c-h) SEER 18, 2000-2015
Survival differences by tumor location
Kaplan-Meier survival curves for males and females with GCTs overall and stratified by location are shown in Figure 1. For intracranial and intraspinal GCTs, males and females both had five-year survival of 89% (log-rank p=0.5). There were differences in overall survival between racial/ethnic groups among males, but not females, diagnosed with intracranial/intraspinal GCTs (male log-rank p=0.007). Extracranial/extragonadal tumors among all females combined resulted in 27% higher five-year survival than observed for males (five-year survival: males 64%, females 91%; log-rank p<0.0001). In sex-stratified analyses for extracranial/extragonadal tumors, there were no significant differences in overall survival between racial/ethnic groups for males or females (males 95%, females 96%; log-rank p=0.2). Among gonadal tumors, there was no significant difference in overall survival observed between racial/ethnic groups in females, but there was a significant difference found for males between racial/ethnic groups (male log-rank p=0.004).
Differences in the risk of death by sex and race/ethnicity
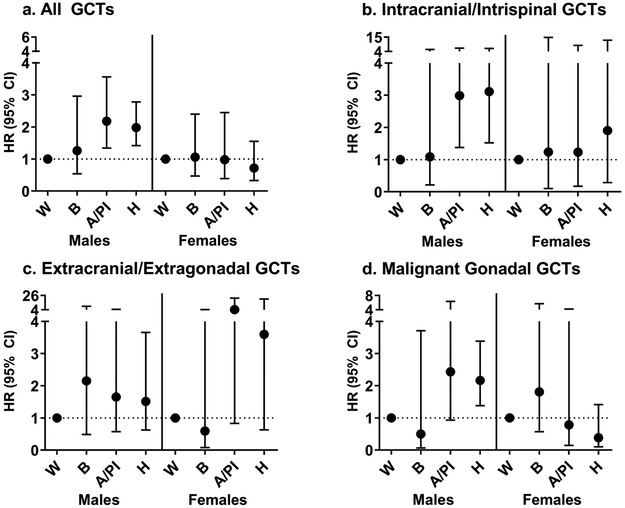
Compared to females, males had a significantly higher risk of death (hazard ratio [HR] 1.68; 95% confidence interval [95% CI]: 1.20-2.36) after adjustment for race/ethnicity, age at diagnosis, tumor histology and location, and year of diagnosis. Estimates were similar when adjusting for stage of disease as well. We observed significant differences in survival for Hispanic and API males compared to NHW males (Figure 2, Supplemental Table 2). In the adjusted model, which accounted for stage of disease and other demographic and tumor characteristics, there was a significantly increased risk of death observed among API males (HR: 2.18; 95% CI: 1.34-3.56) and Hispanic males (HR: 1.98; 95% CI:1.42-2.78) (NHW referent). There was no significant effect observed for black males, although sample size was limited. These estimates were similar in direction and magnitude to those from the crude models.
Figure 2.
Hazard ratios (HR) and 95% confidence intervals (95% CI) for the association between race/ethnicity and the risk of death after a germ cell tumor diagnosis among males and females (non-Hispanic, white [NHW] as referent) for a) all GCTs combined, b) intracranial/intraspinal GCTs, c) extracranial/extragonadal GCTs and d) malignant gonadal GCTs, SEER 18, 2000-2015. Model adjusted for tumor location (all GCTs only), tumor histology, age at diagnosis, year of diagnosis, stage of disease, and mediastinal tumor location (extracranial/extragonadal tumors only).
Differences in the risk of death by sex, race/ethnicity, and tumor location
There were no significant differences in the risk of death between racial/ethnic groups among females for any tumor location. Among males, when stratifying by tumor location, there was significant risk of death observed among API males, compared to NHW males, for intracranial/intraspinal tumors. Hispanic males had a significant risk of death, relative to NHW males, for both intracranial/intraspinal and gonadal tumors. Among extracranial/extragonadal tumors, adjustment for mediastinal tumor location (yes, n=120), which is associated with poor prognosis9, did not alter effect estimates relative to the adjusted model estimates without considering mediastinal tumor location, which were non-significant for all racial/ethnic groups.
Evidence of mediation by stage of disease
Because stage of disease lies on the temporal pathway between race/ethnicity and the risk of death, we conducted a mediation analysis between race/ethnicity and the risk of death treating stage of disease as the mediator. These analyses were restricted to Hispanic, API and NHW males as the referent because these were the only groups where we observed significant survival differences. In the first-leg results for the inverse odds weighting (IOW) mediation analysis including API and Hispanic males, who displayed a significantly higher risk of death than NHW males, there were differences in the association between race/ethnicity and stage of disease. Hispanic males were more likely to have regional and distant stage of disease compared to NWH males (Supplemental Table 3). When we evaluated GCTs in all locations, there was no evidence of mediation by stage of disease as evidenced by the non-significant indirect effects and the similar direct and total effect estimates observed for each race/ethnicity group relative to NHW males (Table 2). In analyses stratified by tumor location, we observed a mediating effect of stage of disease for the association between race/ethnicity and death in Hispanic males with gonadal tumors (indirectHR: 1.18, 95% CI: 1.03-1.35).
Table 2.
Hazard ratios (HR) and 95% confidence intervals (95% CI) from the inverse odds weighting mediation analysis for the association between race/ethnicity and the risk of death among Asian/Pacific Islander and Hispanic males compared to non-Hispanic, white males diagnosed with germ cell tumors mediated by stage of disease for the model adjusted for age and year of diagnosis and tumor location and histology, SEER 18 registries (2000-2015). Only race/ethnicity groups with significant differences in the risk of death as compared to NHW males for all GCTs combined and by tumor location are included.
| Indirect | Direct | Total | % Change from total to direct effecta |
||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| All GCTs | |||||||
| API vs NHW | 0.85 (0.45-1.59) | 0.6 | 2.71 (1.15-6.38) | 0.02 | 2.30 (1.29-4.09) | <0.01 | −20 |
| Hispanic vs NHW | 1.10 (0.98-1.23) | 0.1 | 2.21 (1.55-3.16) | <0.01 | 2.42 (1.71-3.43) | <0.01 | 10 |
| Intracranial/Intraspinal GCTs | |||||||
| API vs NHW | 0.83 (0.4-1.73) | 0.6 | 3.93 (1.29-11.96) | 0.02 | 3.25 (1.30-8.14) | 0.01 | −16 |
| Hispanic vs NHW | 1.10 (0.74-1.66) | 0.6 | 3.14 (1.26-7.82) | 0.01 | 3.47 (1.52-7.94) | <0.01 | 8 |
| Gonadal GCTs | |||||||
| Hispanic vs NHW | 1.18 (1.03-1.35) | 0.02 | 2.38 (1.46-3.88) | <0.01 | 2.80 (1.72-4.56) | <0.01 | 16 |
Percent change from total to direct effect (βtotal-βdirect)/βtotal)*100
Discussion
We used population-based data from the SEER 18 registries to identify survival differences by racial/ethnic group in male and female children and adolescents diagnosed with a GCT. We did not observe significant differences in survival by racial/ethnic group in females, which differs from a previous report on ovarian GCTs from females of all ages where NHW females had better outcomes than black females.3 Among males, we observed racial/ethnic differences in survival such that NHW males experienced better outcomes than other groups, in agreement with adult testicular cancer studies.1,2 In our study, API and Hispanic males had twice the risk of death observed for NHW males, which is in line with effect estimates from an adult testicular cancer study among nonwhite males with nonseminomas2 and among Hawaiian males, who would be included in our API category.1 Our study differs from these previous studies, which focused on adult malignancies with less information on race and ethnicity, as we are using recent data with better-defined race/ethnicity and we only included children aged 0-19 years. These differences may contribute to the lack of survival disparities among females in our study; however, our findings and the previous studies for male GCTs consistently suggests that NHW males experience better outcomes than other racial/ethnic groups.
Overall, males had worse survival and a significantly higher risk of death than females despite the fact that females had larger tumors and were more frequently diagnosed at later stage of disease (Table 1). We hypothesized that differences in tumor location and histology may explain this finding as females were more likely to have tumors of yolk sac histology, which typically have better outcomes when compared with other histologic subtypes.24 In addition, extragonadal/extracranial tumors are more common in females,25 and these tumors are typically larger in size and have better outcomes compared with tumors in other locations, particularly among females (Figure 1). However, we found that even after adjustment for tumor location and histology, males displayed an increased risk of death relative to females. These findings suggest that factors other than tumor histology or location, such as tumor biology or response to therapy, may underlie the observed sex differences in GCT survival.
In our study, we observed a slight mediating effect for stage of disease for the association between race/ethnicity and the risk of death among Hispanic males diagnosed with gonadal tumors. We estimated that approximately 16% of the association between Hispanic ethnicity and death after a gonadal GCT diagnosis may operate through stage of disease. The presence of significant direct effects for Hispanic and API males for all GCTs combined and for intracranial/intraspinal and Hispanic males for gonadal GCTs suggests that factors other than stage of disease including tumor biology, diagnosis delay, treatment received, response to therapy may underlie the observed racial/ethnic differences in survival.
GCT biology may vary by race/ethnicity and may impact cancer outcomes, as observed among adults in various tumor types.26-28 Racial/ethnic differences in pediatric GCT tumor biology remain to be identified. However, if such differences in tumor biology exist, they may impact therapeutic efficacy29 and may explain some of the observed racial/ethnic survival disparities among males with GCTs. GCTs display differing patterns of methylation and gene expression30 by histologic subtype.31,32 Consideration of racial/ethnic differences in GCT gene expression and methylation may be important factors for identifying biologic mechanisms underlying survival disparities and may prove useful in the development of targeted therapies.
Racial/ethnic differences in treatment received and adverse events may explain some of the observed survival differences among children and adolescents with GCTs. In a recent study of children with acute lymphoblastic leukemia, Hispanic children had twice the risk of neurotoxicity following methotrexate treatments than NHW children.33 Children who experienced neurotoxic events often received 2.25 fewer doses of chemotherapy33, which could affect survival. The investigation of racial and ethnic differences in treatment received and adverse events among children with GCTs will be an important component in our understanding of the observed racial/ethnic disparities in GCT survival.
Pharmacogenomics may play an integral role in the observed racial/ethnic survival differences in children with GCTs.34,35 Germline genetic differences between racial/ethnic groups can lead to differences in drug metabolism36 impacting drug efficacy and survival from cancer. Overall, the inclusion of racial/ethnic minorities in clinical trials and drug development studies has been minimal.37 Even though 70% of children diagnosed with cancer in the United States are enrolled in clinical trials, young Hispanic children and older white children are underrepresented in therapy studies for solid tumors.38 Increasing the inclusion of children with diverse racial/ethnic backgrounds remains important for developing effective therapies for solid tumors such as GCTs, where the racial/ethnic differences in survival among males are largely unexplained.
Although we present a large, population-based study characterizing survival differences by race/ethnicity among children aged 0-19 years diagnosed with GCTs, our findings should be interpreted in light of the following limitations. To categorize race/ethnicity, we relied upon the SEER categorization, which arises from medical records, physician report, or death certificates. While this been found to be fairly reliable when compared to patient self-report39, we cannot disentangle the social construct of race/ethnicity from that of genetics, which may shed light on the biologic mechanisms contributing to the observed survival disparities among males. While SEER provides a wealth of data to investigate racial/ethnic disparities in cancer survival, it does not present detailed information for important clinical characteristics such treatment received. 23 Therefore, we viewed stage of disease as a surrogate for treatment received, which presumably does not depend on race/ethnicity, but this warrants validation in studies with detailed treatment data. In SEER, stage of disease has been harmonized over the included years (2000-2015) and does not represent the exact staging system used in the clinic for GCTs, which is described in detail elsewhere22; however, stage is presumed to be fairly reliably captured in SEER as it is reported by cancer registries.40
In conclusion, using data from over 3,300 children and adolescents diagnosed with a GCT between 2000-2015, we observed significant racial/ethnic differences in survival among males but not females. Importantly, this association was not mediated by stage of disease, except for gonadal tumors in Hispanic males. These findings, in addition to a lack of mediation by SES as reported elsewhere4, suggest that the observed racial/ethnic disparities in survival for males with GCTs may be biologic or may depend on the efficacy of treatment received. Future studies with detailed treatment data are necessary to infer the role of treatment on the observed disparities. Future molecular studies should seek to characterize racial/ethnic differences in pharmacogenomics and tumor biology among males diagnosed with GCTs, which could inform drug development for race/ethnicity-specific therapies and be a step toward decreasing the racial/ethnic survival disparities among male children and adolescents diagnosed with GCTs.
Supplementary Material
Novelty and Impact.
Among children with germ cell tumors (GCTs), survival differences by race/ethnicity have been reported for GCTs overall in children and for testicular GCTs in adult males. We characterized racial/ethnic survival differences by sex among children and adolescents diagnosed with GCTs. There were no racial/ethnic survival differences among females, but Hispanic and Asian/Pacific Islander males had worse survival than whites, independent of stage of disease at diagnosis, suggesting other factors may be driving the observed disparity.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (grant number T32 CA099936 to LAW) and the Children’s Cancer Research Fund, Minneapolis, MN.
Abbreviations:
- (GCT)
germ cell tumor
- (NHW)
non-Hispanic white
- (API)
Asian/Pacific Islander
- (HR)
hazard ratio
- (95% CI)
95% confidence interval
- (SES)
socioeconomic status
- (SEER)
Surveillance, Epidemiology, and End Results
- (ICD-O-3)
International Classification of Diseases for Oncology 3
- (IOW)
inverse odds weighting
Footnotes
Conflict of Interest
JNP and LAW have no financial conflicts of interest to disclose. ALF serves on the Clinical Advisory Board for Decibel Therapeutics for work unrelated to the research presented herein.
References
- 1.Biggs M Lou, Schwartz SM. Differences in testis cancer survival by race and ethnicity: A population-based study, 1973-1999 (United States). Cancer Causes Control 2004;15:437–44. [DOI] [PubMed] [Google Scholar]
- 2.Fossa SD, Cvancarova M, Chen L, Allan AL, Oldenburg J, Peterson DR, Travis LB. Adverse prognostic factors for testicular cancer-specific survival: A population-based study of 27,948 patients. J Clin Oncol 2011;29:963–70. [DOI] [PubMed] [Google Scholar]
- 3.Bryant CS, Kumar S, Shah JP, Mahdi H, Ali-Fehmi R, Munkarah AR, Deppe G, Morris RT. Racial disparities in survival among patients with germ cell tumors of the ovary-United States. Gynecol Oncol [Internet] 2009;114:437–41. Available from: 10.1016/j.ygyno.2009.05.039 [DOI] [PubMed] [Google Scholar]
- 4.Kehm RD, Spector LG, Poynter JN, Vock DM, Altekruse SF, Osypuk TL. Does socioeconomic status account for racial and ethnic disparities in childhood cancer survival? Cancer 2018;August 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horner M, Ries L, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse S, Feuer E, Huang L, Mariotto A, Miller B, Lewis D, et al. SEER Cancer Statistics Review, 1975-2006 [Internet]. 2009. [cited 2011 Aug 20]. Available from: https://seer.cancer.gov/csr/1975_2006/
- 6.Poynter JN, Amatruda JF, Ross JA. Trends in incidence and survival of pediatric and adolescent patients with germ cell tumors in the United States, 1975 to 2006. Cancer [Internet] 2010;116:612–25. Available from: http://onlinelibrary.wiley.com/doi/10.1002/cncr.25454/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson TA, Schneider DT, Perlman EJ. Principles and Practice of Pediatric Oncology. Sixth Philadelphia, PA: Lippincott Williams & Wilkins, 2011. 1045–1067p [Google Scholar]
- 8.Rakheja D, Teot LA. Pediatric Germ Cell Tumors: Pathology of Germ Cell Tumors. 2014. 37–58p [Google Scholar]
- 9.Olson TA, Schneider DT, Perlman EJ. Germ Cell Tumors: Principles and Practice in Pediatric Oncology. Sixth Philadelphia, PA: Lippincott Williams & Wilkins, 2011. 1045–1067p [Google Scholar]
- 10.Poynter JN. Pediatric Germ Cell Tumors: Epidemiology of Germ Cell Tumors. First Heidelberg New York Dordrecht London: Springer-Verlag, 2014. 17–36p [Google Scholar]
- 11.Cusack M, Scotting P. DNA methylation in germ cell tumour aetiology: Current understanding and outstanding questions. Reproduction 2013;146:R49–60. [DOI] [PubMed] [Google Scholar]
- 12.Fustino N, Rakheja D, Ateek CS, Neumann JC, Amatruda JF. BMP signaling activity distinguishes histologic subsets of pediatric germ cell tumors. Int J Androl 2011;34:e218–e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith H, Berwick M, Verschraegen C, Wiggins C, Lansing L, Muller C, Qualls C. Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol 2006;107:1075–85. [DOI] [PubMed] [Google Scholar]
- 14.De Backer A, Madern CC, Pieters R, Haentjens P, Hakvoort-Cammel FCAJ, Oosterhuis JW, Hazebroek FWJ. Influence of tumor site and histology on long-term survival in 193 children with extracranial germ cell tumors. Eur J Pediatr Surg 2008;18:1–6. [DOI] [PubMed] [Google Scholar]
- 15.Mortazavi N, Mahzooni P, Taheri D, Jalilian M, Novin K. Germ Cell Tumor’s Survival Rate in Young Patients. Iran J Cancer Prev 2015;8:0–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colton MD, Hawkins M, Goulding D, Cockburn M, Green AL. Socioeconomics, Race, and Ethnicity in Childhood Cancer Survival: Accessing and Addressing Root Causes of Disparities. Cancer [Internet] 2018;1–4. Available from: http://doi.wiley.com/10.1002/cncr.31558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen QC, Osypuk TL, Schmidt NM, Glymour MM, Tchetgen EJT. Practical guidance for conducting mediation analysis with multiple mediators using inverse odds ratio weighting. Am J Epidemiol 2015;181:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tchetgen Tchetgen EJ. Inverse odds ratio-weighted estimation for causal mediation analysis. Stat Med 2013;32:4567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams LA, Richardson M, Kehm RD, McLaughlin CC, Mueller BA, Chow EJ, Spector LG. The association between sex and most childhood cancers is not mediated by birthweight. Cancer Epidemiol 2018;57:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data, Nov 2017 Sub (2000-2015) [Internet]. Available from: http://www.seer.cancer.gov
- 21.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer, third edition. Cancer 2005;103:1457–67. [DOI] [PubMed] [Google Scholar]
- 22.Frazier AL, Amatruda F J. Pediatric Germ Cell Tumors [Internet]. Springer; Heidelberg New Yor Dordrecht London, 2009. 911–961pAvailable from: http://www.crossref.org/deleted_DOI.html [Google Scholar]
- 23.Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, Warren JL. Comparison of SEER Treatment Data With Medicare Claims. Med Care 2016;54:e55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frazier AL, Hale JP, Rodriguez-Galindo C, Dang H, Olson T, Murray MJ, Amatruda JF, Thornton C, Arul GS, Billmire D, Shaikh F, Pashankar F, et al. Revised Risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J Clin Oncol 2015;33:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams LA, Richardson M, Marcotte EL, Poynter JN, Spector LG. Sex ratio among childhood cancers by single year of age. Pediatr Blood Cancer 2019;66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien KM, Cole SR, Tse C-K, Perou CM, Carey LA, Foulkes WD, Dressler LG, Geradts J, Millikan RC. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res [Internet] 2010. [cited 2013 Aug 27];16:6100–10. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3029098&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enokida H, Shiina H, Urakami S, Igawa M, Ogishima T, Pookot D, Li LC, Tabatabai ZL, Kawahara M, Nakagawa M, Kane CJ, Carroll PR, et al. Ethnic group-related differences in CpG hypermethylation of the GSTP1 gene promoter among African-American, Caucasian and Asian patients with prostate cancer. Int J Cancer 2005;116:174–81. [DOI] [PubMed] [Google Scholar]
- 28.Williams LA, Butler EN, Sun X, Allott EH, Cohen SM, Fuller AM, Hoadley KA, Perou CM, Geradts J, Olshan AF, Troester MA. TP53 protein levels, RNA-based pathway assessment, and race among invasive breast cancer cases. npj Breast Cancer [Internet] 2018;June 25:4:13 Available from: 10.1038/s41523-018-0067-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, et al. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science (80- ) 2004;304:1497–501. [DOI] [PubMed] [Google Scholar]
- 30.Palmer RD, Barbosa-Morais NL, Gooding EL, Muralidhar B, Thornton CM, Pett MR, Roberts I, Schneider DT, Thorne N, Tavaré S, Nicholson JC, Coleman N. Pediatric malignant germ cell tumors show characteristic transcriptome profiles. Cancer Res 2008;68:4239–47. [DOI] [PubMed] [Google Scholar]
- 31.Williams LA, Mills L, Hooten AJ, Langer E, Roesler M, Frazier AL, Krailo M, Nelson HH, Bestrashniy J, Amatruda JF, Poynter JN. Differences in DNA methylation profiles by histologic subtype of pediatric germ cell tumors: A report from the Children’s Oncology Group. Br J Cancer 2018;119:864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amatruda JF, Ross JA, Christensen B, Fustino NJ, Chen KS, Hooten AJ, Nelson H, Kuriger JK, Rakheja D, Frazier AL, Poynter JN. DNA methylation analysis reveals distinct methylation signatures in pediatric germ cell tumors. BMC Cancer [Internet] 2013;13:313 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3701494&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor OA, Brown AL, Brackett J, Dreyer ZE, Moore IK, Mitby P, Hooke MC, Hockenberry MJ, Lupo PJ, Scheurer ME. Disparities in Neurotoxicity Risk and Outcomes among Pediatric Acute Lymphoblastic Leukemia Patients. Clin Cancer Res 2018;24:5012–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortega VE, Meyers DA. Pharmacogenetics: Implications of race and ethnicity on defining genetic profiles for personalized medicine. J Allergy Clin Immunol 2014;133:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urban TJ. Race, Ethnicity, Ancestry and Pharamacogenetics. Mt Sanai J Med 2010;77:133–9. [DOI] [PubMed] [Google Scholar]
- 36.Xie H, Kim R, Wood A, Stein C. Molecular Basis of Ethnic Differences in Drug Disposition and Response. Annu Rev Pharmacol Toxicol 2001;41:815–50. [DOI] [PubMed] [Google Scholar]
- 37.Kwiatkowski K, Coe K, Bailar JC, Swanson GM. Inclusion of minorities and women in cancer clinical trials, a decade later: Have we improved? Cancer 2013;119:2956–63. [DOI] [PubMed] [Google Scholar]
- 38.Lund MJ, Eliason MT, Haight AE, Ward KC, Young JL, Pentz RD. Racial/ethnic diversity in children’s oncology clinical trials: Ten years later. Cancer 2009;115:3808–16. [DOI] [PubMed] [Google Scholar]
- 39.Clegg LX, Reichman ME, Hankey BF, Miller BA, Lin YD, Johnson NJ, Schwartz SM, Bernstein L, Chen VW, Goodman MT, Gomez SL, Graff JJ, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: Implications for health disparity studies. Cancer Causes Control 2007;18:177–87. [DOI] [PubMed] [Google Scholar]
- 40.Cronin KA, Ries LAG, Edwards BK. Collaborative Staging and Its Impact on Cancer Registry Data: Information for Data Users on Analysis and Interpretation of Registry Data. Cancer 2014;120:3755–7.25412387 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
SEER 18 data is publicly available20 (2000-2015) and can be accessed on the SEER website (https://seer.cancer.gov).