Abstract
Background
IgG4-related disease (IgG4-RD) is a fibro-inflammatory condition marked by rapid clinical improvement after selective depletion of B lymphocytes with rituximab. This feature suggests that B cells might participate in fibrogenesis and wound healing.
Objective
In the present work we aimed to demonstrate that B lymphocytes contribute directly to tissue fibrosis in IgG4-RD.
Methods
Total circulating CD19+ B-lymphocytes, naïve B cells, memory B cells, or plasmablasts from IgG4-RD patients were cultivated with human fibroblasts. Pro-fibrotic soluble factors and collagen production in the co-cultures were assessed by ELISA and Luminex assays. RNA-sequencing and quantitative RT-PCR were used to assess fibroblast activation in the presence of B cells, as well as the induction of pro-fibrotic pathways in B cell subsets. Relevant pro-fibrotic and inflammatory molecules were confirmed in vitro by functional experiments and on IgG4-RD tissue sections by multi-color immunofluorescence studies.
Results
B cells from IgG4-RD patients (i) produced the pro-fibrotic molecule PDGF-B and stimulated collagen production by fibroblasts; (ii) expressed enzymes implicated in extracellular matrix remodeling such as LOXL2; (iii) produced the chemotactic factors CCL-4, CCL-5, and CCL-11; and (iv) induced the production of these same chemokines by activated fibroblasts. Plasmablasts expressed sets of genes implicated in fibroblast activation and proliferation, and therefore represent cells with intrinsic pro-fibrotic properties
Conclusion
We have demonstrated that B cells, contribute directly to tissue fibrosis in IgG4-RD. These unanticipated pro-fibrotic properties of B lymphocytes, particularly of plasmablasts, might be relevant for fibrogenesis in other fibro-inflammatory disorders and for wound healing processes in physiological conditions.
Keywords: IgG4, IgG4-related disease, B-cells, plasmablasts, fibrosis, fibroblasts, rituximab, LOXL2, PDGF
CAPSULE SUMMARY
The present work unveils unanticipated fibrogenic properties of B lymphocytes in humans and demonstrates their direct contribution to tissue fibrosis in the pathogenesis of IgG4-related disease.
INTRODUCTION
Fibrosis is a reversible physiological phenomenon of tissue scarring occurring during wound healing and the resolution of inflammation. When dysregulated, fibrosis can become irreversible and lead to tissue distortion and organ damage, thereby representing a major negative prognostic determinant across a host of different chronic inflammatory disorders [1]. In general, tissue fibrosis arises from the deposition of collagenous proteins synthesized by activated fibroblasts, but the complex network of pro-fibrotic signals that sustains fibroblast activation remains only partially elucidated. In particular, although a variety of cell types and molecular pathways have been causally associated with fibroblast activation, these events appear tightly integrated, interdependent and, probably, redundant [2]. All these aspects complicate the identification of their relative contribution to fibrogenesis. This is, indeed, the main reason why there are few - if any - treatment strategies available to reverse the course of most fibrotic disorders [3].
Unlike most other fibrotic diseases, IgG4-related disease (IgG4-RD), a fibroinflammatory condition characterized by mass-forming lesions and lymphoplasmacytic infiltrates with an abundance of IgG4-positive plasma cells, promptly responds to B-cell depletion with rituximab [4]. This disorder provides the ideal clinical condition to explore the contribution of B cells to fibrosis since B-cell depletion in this disease leads to rapid shrinkage of the typical fibrous lesions and provides durable clinical improvement in most patients [5]. In addition, rituximab attenuates serological biomarkers of fibrosis and reverses myofibroblast activation in affected organs, suggesting that fibrogenesis in IgG4-RD might be halted by selectively targeting the B-cell compartment [6]. Despite this indirect evidence supporting the possible involvement of B lymphocytes in tissue fibrosis [7–13], most pathogenic studies on IgG4-RD conducted so far have focused on putative fibrogenic T lymphocytes - namely CD4+ cytotoxic T lymphocytes (CD4+CTLs), Th2 cells, and macrophages. While B cells have been assumed to contribute to this disease indirectly, either by antigen presentation to potentially pathogenic helper T cells or by virtue of auto-antibody generation, no direct pro-fibrotic role for B cells in this condition has been hitherto postulated [14–19].
In the present work, we hypothesize a direct contribution of B lymphocytes to fibrogenesis in IgG4-RD that extends beyond their established roles as antibody-producing and antigen-presenting cells. By co-cultivating circulating B cells from IgG4-RD patients with human fibroblasts, we demonstrate that B lymphocytes are capable of orchestrating tissue fibrosis through both direct and indirect mechanisms, namely: (i) the secretion of soluble profibrotic factors that induce collagen production by activated fibroblasts; (ii) the secretion of chemotactic signals for pro-fibrotic populations of T lymphocytes and macrophages; (iii) the production of collagenous proteins; and (iv) the organization of the extracellular matrix. In addition, by profiling the transcriptome of circulating B-cell subpopulations in IgG4-RD patients, we have identified previously unknown pro-fibrotic properties of circulating plasmablasts that might contribute to the pathogenesis of this condition as well as that of other chronic fibrosing disorders.
MATERIALS AND METHODS
Patients
This study was approved by the institutional review board of San Raffaele Scientific Institute (Milan, Italy) and of Massachusetts General Hospital (Boston, USA). Informed written consent was obtained from all patients with IgG4-RD referred to the rheumatology clinics of both Hospitals and enrolled in the study. Samples from 20 patients with IgG4-RD AIP were used (organ involvement and patients’ demographics are listed in Table E1 in this article’s Online Repository). Patients with IgG4-RD were compared with 5 healthy control subjects. Tissue sections from 5 patients with IgG4-related sialadenitis, 5 patients with IgG4-RD AIP, and 5 patients with Sjögren’s syndrome obtained from the Massachusetts General Hospital and the Kyushu University Hospital Department of Oral and Maxillofacial Surgery, Fukuoka, Japan, were also studied.
Flow cytometry and microscopy
A detailed description of the methods used for PBMC isolation, flow cytometry, cell sorting, immunohistochemistry, and immunofluorescence is provided in the Methods section of this article’s Online Repository.
Co-culture experiments
Co-culture experiments were carried out with B cells and B-cell subsets from patients with IgG4-RD AIP, and primary human pancreatic or skin fibroblasts. Polycarbonate semipermeable membranes (Nunc Dominique Dutscher, Brumath, France) were used to identify soluble factors or contact mediated pro-fibrotic mechanisms. Soluble collagen concentration in the supernatant of fibroblasts/CD19+ B cells co-cultures was evaluated using Sircol collagen assay (Biocolor Ltd. Interchim, Montluçon, France). Soluble collagen concentration in the supernatant of fibroblasts/B-cells subsets co-cultures was evaluated using the Pro-collagen Iα1 ELISA kit (R&D Systems Inc., Minneapolis, MN, USA). Cytokines and chemokines present in the supernatant of fibroblasts/CD19+ B cells co-cultures were measured using the Bio-Plex Pro Human Cytokine Grp I Panel 27-plex assay (Bio-Rad, Hercules, CA, USA). A detailed description of the methods used for the establishment of primary human pancreatic fibroblasts cell lines and co-cultures with total B cells and B-cell subsets is provided in the Methods section of this article’s Online Repository.
Gene Expression Analysis
For Quantitative real-time Polymerase Chain Reaction Analysis total RNA was extracted from cultured fibroblasts using ReliaPrep™ RNA Cell Miniprep System (Promega) and reverse transcribed with random hexameric primers. Real-time quantitative PCR for human GAPDH, COL1A1, COLA1A2, hCOL3A1, hACTA2, and hCD19 genes was performed with the KAPA SYBR® FAST qPCR Master Mix (2X) Kit (Bio-Rad, Hercules, CA, USA) using a Roche LightCycler® (Roche Molecular Diagnostic, Pleasanton, CA, USA). For RNA sequencing analysis on B cells and fibroblasts, cells were directly harvested in RLT buffer from Qiagen containing 1% 2-Mercaptoethanol (Pierce/ThermoScientific, Waltham, Massachusetts) and RNA was isolated with the RNeasy Plus Micro kit (Qiagen). Libraries were prepared using Superscript II Reverse Transcriptase (Thermo Fisher Scientific) and sequenced using the Illumina NextSeq sequencer (San Diego, California, USA). Differential gene expression analysis was performed using DESeq2 Analyzer. Detailed methodology is included in the Methods section in this article’s Online Repository.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software 6.0 (La Jolla, CA, USA). Normal distribution of continuous variables was assessed with the D’Agostino & Pearson omnibus normality test. Non-normally distributed variables were compared using the Mann-Whitney U-test. Unpaired t-test were used to perform comparisons between two groups. A p-value < 0.05 was considered significant. Continuous variables are expressed as mean ± standard deviation (SD), unless otherwise specified.
RESULTS
B lymphocytes from IgG4-RD patients release soluble signals that increase collagen production by human fibroblasts
In order to assess a direct role of B lymphocytes in fibroblast activation and collagen secretion, we set up co-cultures of primary human fibroblasts with CD19+ B cells isolated from the peripheral blood of 5 patients with IgG4-RD and performed transcriptomic analyses on RNA extracted from cultured fibroblasts. We used primary human pancreatic fibroblasts and B cells from IgG4-RD patients with pancreatic involvement to reproduce in vitro some of the possible interactions occurring between these two cell types in IgG4-related autoimmune pancreatitis (AIP), the most well-characterized manifestation of IgG4-RD. Clinical features of the patient cohort are reported in the Table E1. B cells purified from the peripheral blood of 5 sex and age-matched healthy donors were used as controls. As shown in Figure 1.A, B cells from IgG4-RD AIP patients induced the activation of pro-fibrotic pathways in co-cultured fibroblasts with a significant up-regulation of genes involved in epithelial-to-mesenchymal transition, myogenesis, and formation of apical junctions compared to control fibroblasts, as assessed by transcriptomic analysis on fibroblast RNA (see also Figure E1 and Table E2).
Figure 1. B lymphocytes drive pro-fibrotic genes expression and collagen production by human pancreatic fibroblasts by means of soluble factors.
(A) Top results of GSEA performed on log2 fold changes between gene expression of fibroblasts co-cultured with B cells from patients with IgG4-RD AIP in the absence of semipermeable membranes and of control fibroblasts at 72hrs (see Online Supplementary Material for details). The network graph shows top scoring enriched gene-sets (FDR q-value <0.01) and their associated leading edge genes (those responsible of the core enrichment of the gene-set). Nodes representing genes are coloured according to their log2 fold change and genes considered of particular interest are labelled. (B) Quantitative PCR showing fold change expression of ACTA2, COL1A1, COL1A2, and COL3A1 genes in pancreatic fibroblasts after 3 days co-culture with B lymphocytes from patients with IgG4-RD AIP (n=5) and from healthy donors (n=5). (C) Collagen concentration in the supernatant of different experimental conditions. Human pancreatic fibroblasts (FBL); FBL treated with TGFβ1 20ng/ml (TGFβ); FBL co-cultured with B cells from healthy controls (blue bars); FBL co-cultured with B cells from patients with IgG4-RD (red bars) in the presence (Transwell) or absence (Contact) of semipermeable membranes. * = p < 0.05 for the comparison with fibroblasts alone; ° = p < 0.05 for the comparison with co-cultures in the presence of B cells from healthy controls. Results are expressed as mean ± SD of 5 experiments.
We confirmed activation of pro-fibrotic pathways by performing quantitative PCR for α-Smooth Muscle Actin (ACTA2), COL1A1, COL1A2, and COL3A1 collagen genes (Figure 1.B). The expression of ACTA2, COL1A1, and COL1A2 genes, but not of COL3A1, were also significantly increased in fibroblasts co-cultured with B cells from healthy donors but to a significantly lower level than in fibroblasts co-cultured with B cells from IgG4-RD patients (Figure 1.B). Accordingly, collagen production in the co-cultures of fibroblasts with B cells from IgG4-RD patients was significantly increased compared to both fibroblasts cultured alone and fibroblasts cultured with B cells from healthy donors (Figure 1.C).
In order to evaluate whether the increased collagen secretion observed in the presence of B cells was due to soluble or contact-mediated factors, we performed co-cultures with and without semipermeable membranes (transwells) to respectively avoid or allow physical interactions between B cells and fibroblasts. As shown in Figure 1.B–C, pro-fibrotic gene expression and collagen production by pancreatic fibroblasts did not differ between co-cultures performed in the presence or absence of transwells, both being significantly increased compared to control fibroblasts regardless of any physical interaction. Similar results were obtained using human skin fibroblasts (Figure 2.D).
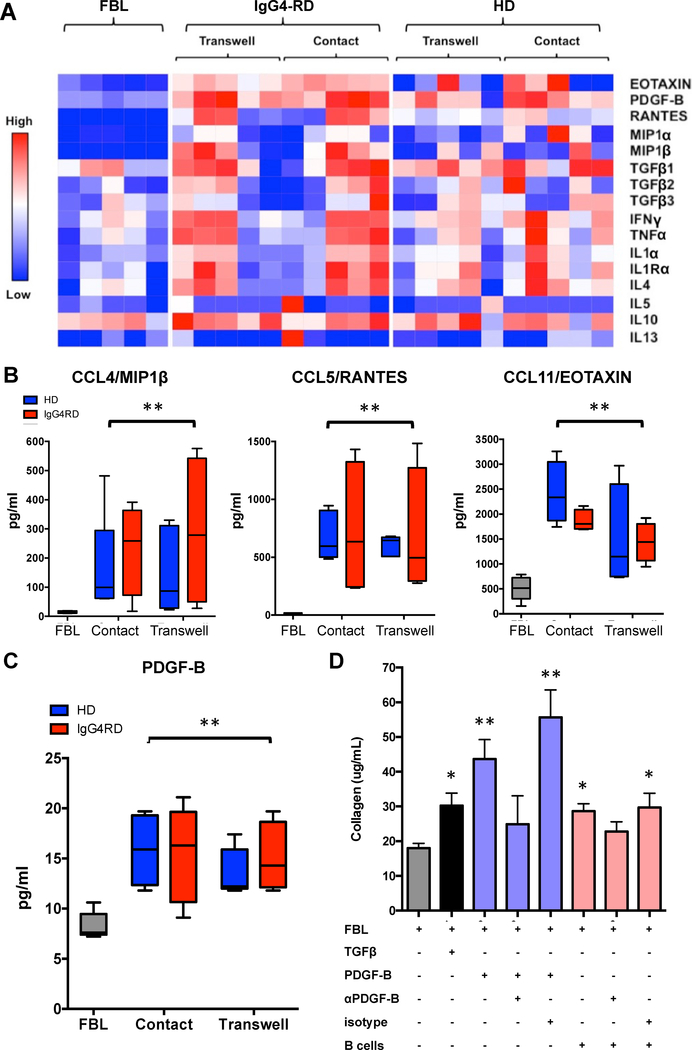
Figure 2. PDGF-B is increased in the co-cultures of fibroblasts with B lymphocytes together with CCL-4, CCL-5, and CCL-11, and mediates collagen production.
(A) Quantification of different cytokines and chemokines by Luminex analysis after 3 days of culture with specific focus on those significantly increased in the supernatant of the co-cultures compared to control fibroblasts: (B) CCL-4, CCL-5, CCL-11, and (C) PDGF-B. Abbreviations: primary human pancreatic fibroblast (FBL); FBL stimulated with 20 ng/ml of recombinant human TGF-β1 (TGFβ); FBL co-cultured with B cells from healthy controls (n=5) (blue bars); FBL co-cultured with B cells from patients with IgG4-RD (n=5) (red bars), in the presence (Transwell) or absence (Contact) of semipermeable membranes. Results are expressed as mean ± SD. (D) PDGF-B inhibition decreases B-cell induced collagen production by primary human skin fibroblasts. Abbreviations: primary human skin fibroblasts (FBL); FBL stimulated with 20 ng/ml of recombinant human TGF-β1 (TGFβ); FBL stimulated with 10 ng/ml of recombinant human PDGF-B (PDGF-B); FBL incubated with 0.5 μg/ml of anti-PDGF-B antibody two hours prior to stimulation with 10 ng/ml of recombinant human PDGF-B (αPDGF-B); FBL incubated with 0.5 μg/ml of IgG isotype antibody two hours prior to stimulation with 10 ng/ml of recombinant human PDGF-B (Isotype); co-cultures with B cells from patients with IgG4-RD (B cells); * = p < 0.05. Results are expressed as mean ± SD of 5 experiments.
These findings demonstrate that CD19+ B lymphocytes from patients with IgG4-RD and, to lesser extent, from healthy individuals are capable of driving myofibroblast differentiation and collagen production through the secretion of soluble factors rather than physical interactions.
The pro-fibrotic factor PDGF-B, and the inflammatory chemokines CCL-4, CCL-5, CCL-11 are increased in the co-cultures of human fibroblasts with B lymphocytes
In order to identify soluble pro-fibrotic molecules secreted by B lymphocytes we collected the supernatants of the co-cultures of B cells from either healthy controls or IgG4-RD patients and pancreatic fibroblasts. We then quantitated cytokines and chemokines known to be implicated in fibrosis and wound healing using a Luminex based approach. As shown in Figure 2.A, the presence of B cells from IgG4-RD patients and from healthy subjects both led to significantly increased concentrations of platelet derived growth factor-B (PDGF-B), CCL-4 (MIP1β), CCL-5 (RANTES), and CCL-11 (EOTAXIN) compared to fibroblasts alone. In particular, CCL-4 and CCL-5 production was induced by the presence of B lymphocytes, while low levels of PDGF-B and CCL-11 were constitutively secreted by untreated fibroblasts. No significant difference was observed in the concentrations of PDGF-B, CCL-4, CCL-5, and CCL-11 between co-cultures of fibroblasts with B cells from IgG4-RD patients and from healthy donors, or between co-cultures with and without semipermeable membranes (Figure 2.A–C). The levels of other pro-fibrotic molecules such as IL-1α, IL-4, IL-5, IL-10, IL-13, IFNγ, TNFα, and TGFβ did not differ between fibroblasts alone and fibroblasts co-cultured with B cells from either IgG4-RD patients or healthy donors.
These results indicate that B lymphocytes either secrete the pro-fibrotic molecule PDGF-B or stimulate activated fibroblasts to produce it, or do both. These results also demonstrate that fibroblasts stimulated by B cells or perhaps B cells themselves, secrete chemokines that might contribute to the recruitment of other inflammatory cells types in vivo.
PDGF-B mediates B-lymphocyte dependent collagen production by human fibroblasts
PDGF-B is known to contribute to tissue fibrosis by stimulating fibroblast activation in several ways, including cell proliferation and stimulation of collagen production [20–21]. In order to assess the biological relevance of PDGF-B in our model, we performed co-cultures of skin fibroblasts and B cells from IgG4-RD patients, and used blocking anti-PDGF-B antibody or isotype control to interfere with collagen production. As shown in Figure 2–D, PDGF-B blockade attenuated the fibrotic effect induced by B cells from IgG4-RD patients while nonimmune IgG of the same isotype did not inhibit the collagen production induced by the same B lymphocytes. These results support the notion that B lymphocytes might contribute to the activation of fibroblasts into a pro-fibrotic state as observed in B cell/fibroblast co-cultures, either through the production of PDGF-B or by inducing the secretion of PDGF-B by activated fibroblasts. It is also possible that both mechanisms occur together, thus perpetuating autocrine and paracrine pro-fibrotic loops. Of note, despite showing similar levels of PDGF-B in the supernatant, co-cultures of fibroblasts with B cells from IgG4-RD patients yielded significantly higher amounts of collagen compared with co-cultures of fibroblasts with B cells from healthy individuals (Figure 1.B–C). This suggests that factors other than PDGF-B might also contribute to the increased fibrotic effect of B cells from IgG4-RD patients.
B lymphocytes and fibroblasts infiltrating IgG4-RD affected organs express PDGF-B, CCL-4, CCL-5, and CCL-11
To localize the cellular source of PDGF-B, CCL-4, CCL-5, and CCL-11 in our co-culture system - whether B cells or pancreatic fibroblasts – and to confirm the relevance of these molecules to the pathogenesis of IgG4-RD, we performed multicolour immunofluorescence and quantitative analysis on tissue sections of patients with IgG4-RD AIP. Healthy pancreatic sections were used as controls. As shown in Figure 3.A the CD19+ B cell infiltrate in IgG4-RD AIP was localized in the proximity of pancreatic ducts and its distribution corresponded to areas of dense fibrosis observed in sequential tissue slides (Figure 3.B). Tertiary lymphoid structures rich in CD19+ cells were also observed. Few scattered CD19+ B cells were detected in healthy pancreatic tissue, with no organized lymphoid structures (Figure E2).
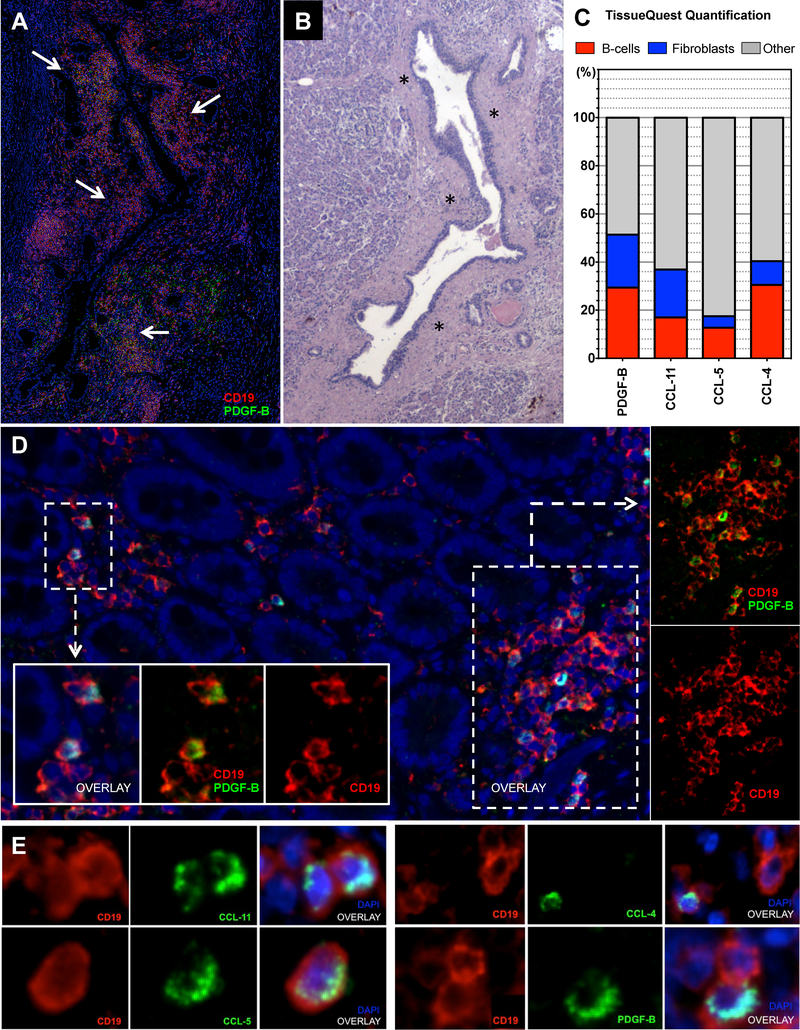
Figure 3. B-lymphocytes and fibroblasts infiltrating IgG4-RD autoimmune pancreatitis express PDGF-B, CCL-4, CCL-5, and CCL-11.
(A) Immunofluorescence staining of an IgG4-RD AIP tissue, showing PDGF-B expressing B cells (arrows) surrounding pancreatic ducts (CD19 (red), PDGF-B (green), and DAPI (blue)). (B) Sequential matched Hematoxylin/Eosin stain showing a dense fibrosis (asterisks) likely generated as a consequence of the peri-ductal inflammatory infiltrate described in A. (C) Quantification of double-positive cells for CD19+ (red bars) or αSMA+ (blue bars) and CCL-4, CCL-5, CCL-11, or PDGF-B in IgG4-RD AIP expressed in percentage of total CCL-4, CCL-5, CCL-11, or PDGF-B positive cells (i.e. CD19+PDGF-B+ cells/PDGF-B+ cells). Bar length represents the average quantification of 5 tissue slides. (D) PDGF-B expressing B lymphocytes infiltrating the sub-epithelial layer of the duodenum in a case of IgG4-RD AIP of the pancreatic head (low magnification); inserts (high magnification). (E) Immunofluorescence staining of CD19 (red), CCL-4, CCL-5, CCL-11, and PDGF-B (all green), and DAPI (blue) positive cells in representative tissue samples from a patient with IgG4-RD AIP (high magnification).
CD19+ B lymphocytes expressing either PDGF-B, CCL-4, CCL-5, or CCL-11 represented on average 29.2% (range 23.1%−35.2%), 30.9% (range 25.3%−39.1%), 12.7% (range 10.2%−15.4%), and 16.9% (range 15.1%−20.4%), of total PDGF-B, CCL-4, CCL-5, CCL-11 expressing cells, respectively (Figure 3.C–E). α-SMA+ fibroblasts expressing either PDGF-B, CCL-4, CCL-5, or CCL-11 represented on average 22.2% (range 21.3%−25.2%), 8.3% (range 5.3%−12.1%), 4.5% (range 1.2%−5.4%), and 19.6% (range 14.1%−21.2%) of total PDGF-B, CCL-4, CCL-5, and CCL-11 expressing cells, respectively (Figure 3.C and Figure E3). α-SMA+ myofibroblasts and CD19+ B lymphocytes were observed in close proximity in IgG-RD AIP tissues (Figure E3), suggesting that interactions between these two types of cells are likely to occur at disease sites. These findings indicate that the pro-fibrotic molecule PDGF-B and the inflammatory chemokines CCL-4, CCL-5, and CCL-11 are produced by both B lymphocytes and myofibroblasts infiltrating IgG4-RD lesions in vivo. Of note, only a fraction of total CD19+ cells and α-SMA+ cells expressed PDGF-B, CCL-4, CCL-5, or CCL-11, suggesting the existence of pathogenic subsets of B lymphocytes and myofibroblasts capable of driving inflammation within affected tissues (Figure E4.A). Our results also indicate that other cell types known to abundantly infiltrate IgG4-RD tissues likely contribute to the production of these mediators such as macrophages, eosinophils, and T cells. Finally, these results support the notion that both B lymphocytes and pancreatic fibroblasts represent a source of PDGF-B, CCL-4, CCL-5, and CCL-11 in the co-culture studies described above.
Plasmablasts from IgG4-RD patients induce collagen secretion by human fibroblasts, produce collagenous proteins, and express extracellular-matrix remodelling enzymes
A major finding of our previous experiments was the increased collagen production observed in co-cultures of fibroblasts with B lymphocytes from healthy subjects compared to control fibroblasts (Figure 1.B–C). Yet, despite similar levels of PDGF-B in the supernatants of the two culture conditions, the collagen production induced by B cells from healthy donors was significantly lower compared to that induced by B cells from patients with IgG4-RD AIP (Figure 1.B–C). This result was further confirmed by the significant up-regulation of pro-fibrotic pathways in pancreatic fibroblasts cultured with B cells from IgG4-RD patients compared to similar fibroblasts cultured with B cells from healthy individuals, suggesting the existence of fibrogenic B-lymphocytes in the peripheral blood of individuals with active untreated IgG4-RD (Figure 4.A, Figure E1 and Table E3). Indeed, we know from our previous studies that, in contrast to healthy individuals, active IgG4-RD is characterized by an oligoclonal expansion of circulating plasmablasts, and that circulating plasmablasts dramatically decrease during disease remission [22,23]. Plasmablast expansions in IgG4-RD patients also correlate with serological biomarkers of collagen deposition, indicating that these activated B cells might contribute to disease pathogenesis and tissue fibrosis [6,24].
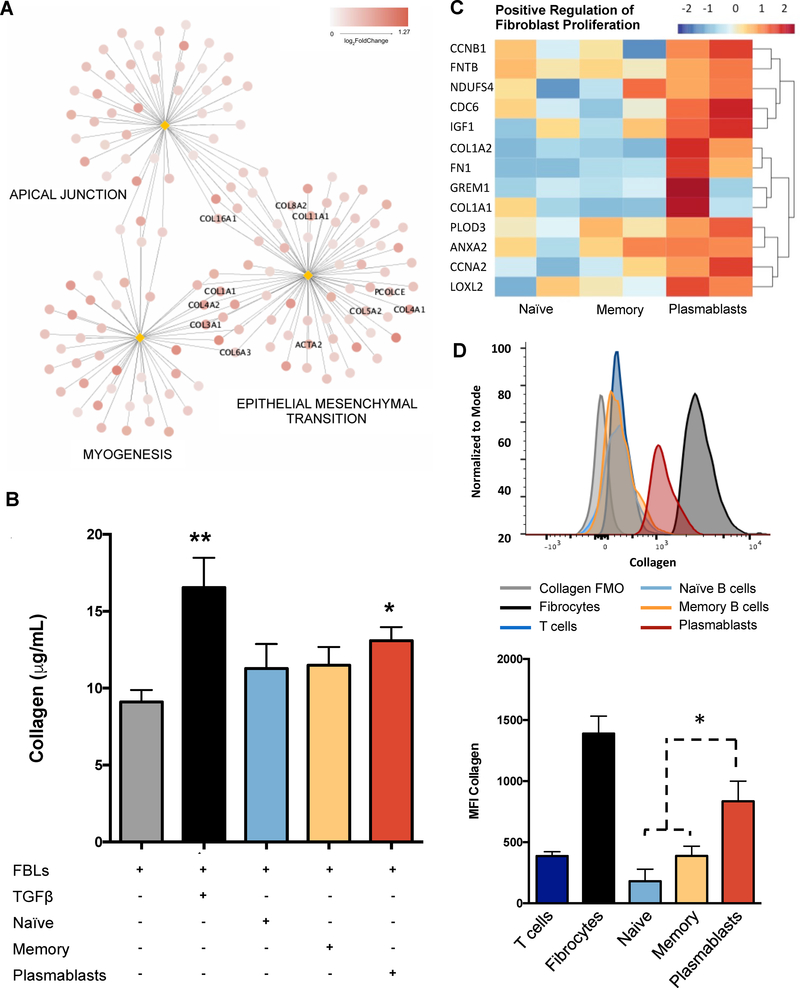
Figure 4. Plasmablasts from IgG4-RD patients induce collagen secretion by human fibroblasts, produce collagenous proteins, and express extracellular-matrix remodelling enzymes.
(A) Top results of GSEA performed on log2 fold changes of gene expression in fibroblasts co-cultured with B cells from patients with IgG4-RD AIP and that in fibroblasts co-cultured with B cells from healthy controls at 72hrs in the presence of semipermeable membranes (see Online Supplementary Material for details). The network has been realized with the same logic described for Figure 1.A (B) Collagen production is increased in co-cultures with plasmablasts compared to control fibroblasts. Primary human skin fibroblasts (FBL); FBL stimulated with 20 ng/ml of recombinant human TGF-β1 (TGFβ); FBL co-cultured with naïve B cells, memory B cells, or circulating plasmablasts. Results are presented as mean ± SD of 5 independent experiments. * = p < 0.05. (C) Heatmap showing up-regulation in circulating plasmablasts of genes significantly mapping to the Gene Ontology “GO:0048146: positive regulation of fibroblasts proliferation”. (D) Flow cytometry showing increased collagen expression in circulating plasmablasts compared to naïve B cells, and memory B cells. Circulating fibrocytes and CD3+ T cells are used as positive and negative controls, respectively. Overlaid histograms represent collagen expression in the different cell types in a representative patient. Histograms represent Mean Fluorescence Intensity (MFI) of intracellular collagen in different cell types. * = p < 0.05. Results are expressed as mean ± SD of 5 experiments.
In order to address the pro-fibrotic properties of B cell subsets in patients with IgG4-RD, we set up co-cultures of skin fibroblasts with flow-cytometrically sorted naïve B cells, memory B cells, and circulating plasmablasts from 5 patients with active untreated IgG4-RD AIP. As shown in Figure 4.B, fibroblasts cultured with circulating plasmablasts produced significantly more collagen compared to control fibroblasts. Naïve or memory B cells were significantly less proficient at eliciting collagen production (Figure 4.B).
In order to better characterize the pro-fibrotic properties of circulating plasmablasts more thoroughly, we performed whole transcriptome analyses of the 3 different B cell subsets. Compared to naïve and memory B cells, circulating plasmablasts showed significant upregulation of genes implicated in positive regulation of fibroblast proliferation, such as the collagen genes COL1A1 and COL1A2, the insulin-like growth factor-1 (IGF-1) gene, and the lysyl oxidase homolog 2 (LOXL2), and gremlin-1 (GREM-1) genes (Figure 4.C). Collagen expression by circulating plasmablasts was significantly higher than that of naïve and memory B cells, as assessed by flow-cytometry (Figure 4.D). Whole transcriptome analysis of fibroblasts cultured with naïve B cells, memory B cells, or plasmablasts, also revealed that CCL-11 and PDGF-B expression was significantly increased by all three B-lymphocyte subpopulations compared to control fibroblasts. Conversely, fibroblast expression of CCL-4 and CCL-5 was induced by naïve B cells and circulating plasmablasts but not by memory B cells (Figure E4.B). Taken together these results indicate that plasmablasts possess pro-fibrotic properties that enable them to activate fibroblasts and to contribute to the organization of the extracellular matrix. Further, plasmablasts directly express collagenous proteins and stimulate fibroblasts to produce inflammatory chemokines.
LOXL2 is abundantly expressed by plasma cells infiltrating IgG4-related disease tissues
LOXL2 is a member of a group of enzymes known as the lysyl oxidase family, that control tissue stiffness and elasticity by catalysing the covalent crosslinking of both collagen and elastin fibres [3,27]. Different members of the LOX family are up-regulated in human fibrotic disorders, and the overexpression of LOXL2 has been implicated in the progression of experimental fibrosis [1–3,28–30]. Indeed, stiffening of the extracellular matrix is emerging as a major driver of full myofibroblast differentiation, and mechanoceptors are now considered as important as soluble factors in promoting fibrogenesis [1–3,32].
To confirm LOXL2 expression by plasmablasts infiltrating IgG4-RD, we performed multicolour immunofluorescence and quantitative analyses on salivary glands from patients with IgG4-RD. As shown in Figure 5, up to 30% of LOXL2 positive cells expressed CD19 and did not express CD20, representing plasmablasts or plasma cells (Figure 5.A–B). LOXL2 expression by B cells infiltrating IgG4-RD lesions was observed in a proportion of IgG4+ and IgG1+ plasmablasts, suggesting that, at least in IgG4-RD patients, class-switched B cells bear the capability of modulating extra-cellular matrix stiffness (Figure 5.B–C). CD19 and IgG4 double-positive cells infiltrating IgG4-RD tissues also expressed the activation-induced cytidine deaminase (AID), a master regulator of immunoglobulin gene recombination, further supporting on-going processes of class-switch and somatic hypermutation [33].
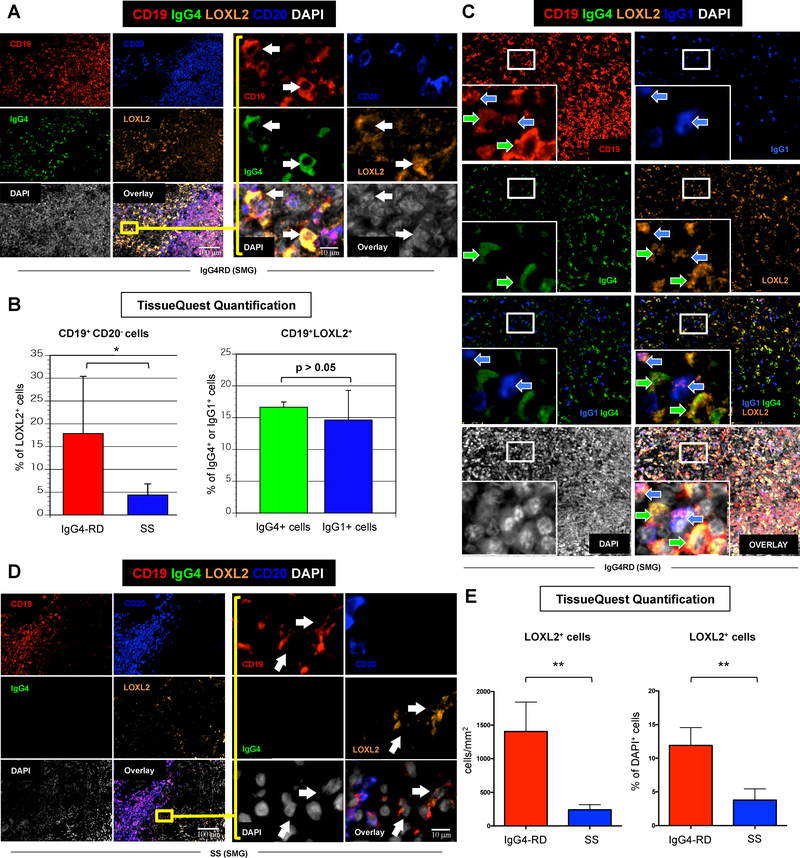
Figure 5. LOXL2 is highly expressed by plasmablasts/plasma cells in IgG4-related sialoadenitis.
(A) Immunofluorescence staining of CD19 (red), IgG4 (green), LOXL-2 (orange), CD20 (blue), and DAPI (white) in salivary glands from a representative patient with IgG4-RD showing abundant infiltrate of LOXL2 expressing IgG4+ plasmablasts (arrows) (inserts: high magnification). (B) Quantification of LOXL2+ plasmablasts (CD19+ CD20− B cells) in salivary glands from patients with IgG4-RD (red bar) and Sjögren’s syndrome (SS) (blue bar) using TissueQuest software. * = p < 0.05 (mean ± SD of n=5 experiments). Quantification of LOXL2+ cells within the total pool IgG4+ (red bar) or IgG1+ (blue bar) plasmablasts in salivary glands from patients with IgG4-RD using TissueQuest software (mean ± SD of n=5 experiments). (C) Immunofluorescence staining of CD19 (red), IgG4 (green), LOXL-2 (orange), IgG1 (blue), DAPI (white) in the salivary gland of a representative patient with IgG4-RD showing LOXL2 expression by both IgG4 (green arrows) and IgG1 (blue arrows) producing B cells (inserts: high magnification). (D) Immunofluorescence staining of CD19 (red), IgG4 (green), LOXL-2 (orange), CD20 (blue), and DAPI (white) in salivary glands from a representative patient with SS showing few LOXL2 expressing IgG4+ B lymphocytes (arrows) (inserts: high magnification). (E) Quantification of LOXL2+ cells in salivary glands from patients with IgG4-RD (red bar) and SS (blue bar) showing a significantly lower number of LOXL2 expressing cells in SS compared to IgG4-RD. ** = p < 0.01 (mean ± SD of n=5 experiments).
To assess the relevance of LOXL2 to the pathogenesis of IgG4-RD, we performed multicolour immunofluorescence and quantitative analyses on salivary glands from patients with Sjögren’s syndrome (SS). Sjögren’s syndrome was used as a control condition because of the chronic lymphoplasmacytic inflammation in the absence of significant tissue fibrosis when compared to IgG4-RD. LOXL2 was expressed at significantly higher frequency in IgG4-RD sialoadenitis than in SS, suggesting an important role of this enzyme in the pathophysiology of IgG4-RD (Figure 5.D–E). In particular, the absolute number of LOXL2 positive cells in IgG4-RD and in SS was 1406 cells/mm2 (± 436 cells/mm2) and 240 cells/mm2 (± 43 cells/mm2) respectively, representing on average the 12.1% (± 2.6%) and the 3.7% (± 0.9%) of total cells in the tissue (p < 0.05 for both comparisons) (Figure 5.E). CD19+ and IgG4+ cells were also increased in IgG4-RD sialoadenitis compared to SS, with IgG4+ B cells accounting for 48.4% (± 7.7%) of total CD19+ B lymphocytes in IgG4-RD and for 20.1% (± 9.1%) in SS (p < 0.05) (Figure E5.A). Likewise, LOXL2+CD19+CD20− plasmablasts were significantly more abundant in IgG4-RD sialoadenitis than in SS, accounting for 17.8% (± 12.5%), and 4.3% (± 2.4%) of total tissue infiltrating LOXL2+ cells, respectively (Figure 5.B–D and Figure E5.A). These results demonstrate that plasmablasts represent a sizable proportion of LOXL2 expressing cells in IgG4-RD lesions, thus reinforcing the pathogenic relevance of B lymphocytes for the development of tissue fibrosis in this condition.
Finally, in order to assess whether LOXL2 expression by plasmablasts/plasma cells represents a feature unique to IgG4-RD or is common to physiological and pathological plasmablasts, we performed immunohistochemistry and immunofluorescence studies on human tonsils from otherwise healthy individuals, on bone marrow smears from patients with multiple myeloma (MM), and on lymph nodes from patients with Castleman disease (CD). In analogy with IgG4-RD, in fact, MM and CD represent haematological disorders characterized by expansions of IgG secreting B lymphocytes. As shown in Figure E5.B–D, LOXL2 was expressed by a proportion of plasma cells residing in tonsils with reactive follicular hyperplasia, in the bone marrow of patients with MM, and in lymph nodes of patients with CD, indicating that plasmablats/plasma cells may display a previously overlooked pro-fibrotic machinery both in physiological and pathological conditions. Of note, LOXL2+CD19+CD20− plasmablasts were more abundant in IgG4-RD sialoadenitis than in SS, and CD, accounting for 17.9% (± 12.5%), 4.4% (± 2.4%), and 8.9% (± 10.6%) of total tissue infiltrating LOXL2 positive cells, respectively (Figure E5.D).
DISCUSSION
The pathogenicity of B lymphocytes in immune-mediated disorders has been traditionally attributed to the production of auto-antibodies and to the activation of auto-reactive T cells via antigen-presentation. Accordingly, the B-cell depleting agent rituximab has found successful application both in conditions caused by pathogenic auto-antibodies, such as autoimmune thrombotic thrombocytopenic purpura and hemolytic anemia, as well as in diseases presumably driven by auto-reactive T lymphocytes, such as progressive multiple sclerosis and rheumatoid arthritis [34–38].
Rituximab is also highly effective in IgG4-RD, a primarily fibrotic disorder in which the relevance of autoantibodies and T cells to fibrosis is still being explored [5,39]. Our recent studies have implicated antibodies to galectin-3 in a subset of IgG4-RD patients, and others have identified auto-antibodies to a recombinant truncated form of laminin 511, but definitive proof of a pathogenic role of these antibodies has not yet been demonstrated [40,41]. Similarly, our studies have revealed expansions of CD4+CTLs in IgG4-RD as well as the secretion of profibrotic cytokines and the potential induction of apoptosis by these cells, but conclusive evidence that they drive fibrosis have not yet been obtained [14–16]. Indeed, the fact that B-cell depletion has been shown to correlate with rapid improvement of tissue fibrosis in IgG4-RD affected organs raises the hypothesis that B-lymphocytes might be involved in fibrogenesis through mechanisms unrelated to either the production of auto-antibodies or the activation of fibrogenic T cells [6]. Despite the prominent plasma cell infiltrate found in IgG4-RD lesions, however, the possibility that B cells could directly drive collagen deposition has not been investigated formally. The precise mechanisms through which rituximab leads to prompt clinical effects remain elusive.
In the present work we describe for the first time different mechanisms by which B lymphocytes may actively contribute to tissue fibrosis in IgG4-RD. In particular, we demonstrate that B cells from IgG4-RD patients (i) stimulate collagen production by activated fibroblasts through soluble pro-fibrotic signals such as PDGF-B; (ii) organize the extracellular matrix through specialized enzymes such as lysyl oxidases; and (iii) directly produce collagenous proteins. In addition, we demonstrate that B cells secrete the chemotactic factors CCL-4, CCL-5, and CCL-11, and induce the production of these same chemokines by activated fibroblasts. Finally, we demonstrate that, among different B-cell subsets, plasmablasts display intrinsic fibrogenic properties because they express sets of genes implicated in fibroblast activation and proliferation.
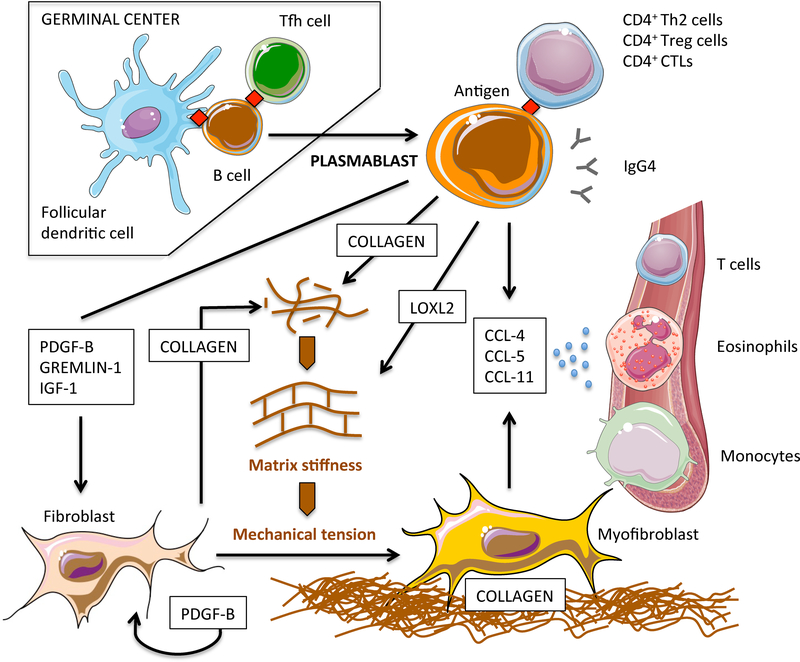
Taken together, these novel findings add important pieces to the complex pathogenesis of IgG4-RD and suggest a possible central role for B-cells in the orchestration of fibrosis in this condition. In particular, our results suggest a revised pathogenetic model of IgG4-RD whereby B lymphocytes exploit five major functions in addition to antibody secretion and to the presumed activation of pathogenic T lymphocytes, namely: (1) they induce epithelial-to-mesenchymal transition of myofibroblast precursors and stimulate collagen production by activated fibroblasts; (2) they directly contribute to tissue fibrosis by secreting collagenous proteins; (3) they regulate extracellular matrix stiffness; (4) they secrete chemotactic factors for putative fibrogenic CD4+CTLs and M2 macrophages; and, (5) they stimulate fibroblasts to amplify the recruitment of inflammatory cells (Figure 6). The depletion of B cells through strategies that target CD20 therefore might interfere with all these processes, thereby rapidly halting fibroblast activation and collagen deposition [6].
Figure 6. Revised pathogenetic model of IgG4-RD pathogenesis: B-cell contribution to tissue fibrosis.
After germinal centre reaction, antigen experienced naïve B cells differentiate into class-switched plasmablasts and migrate into inflamed sites where they contribute to tissue fibrosis in different ways. Plasmablasts initiate fibroblast differentiation into myofibroblasts through soluble factors that activate transcriptional programs implicated in mesenchymal transition. Plasmablasts also stimulate fibroblasts to secrete collagen and PDGF-B, thus amplifying fibroblast activation in an autocrine/paracrine loop. Plasmablasts directly contribute to tissue fibrosis by secreting collagenous proteins. Plasmablasts regulate extracellular matrix stiffness by secreting enzymes responsible for the crosslinking of collagen molecules such as LOXL2. Increased extracellular matrix stiffness leads to the activation of mechanoceptors on fibroblasts, thus fostering their full differentiation into myofibroblasts. Plasmablasts attract inflammatory cells with additional fibrotic potential such as CD4+ CTLs, eosinophils, and M2 macrophages, by secreting CCL-4, CCL-5, and CCL-11, and by stimulating fibroblasts to produce these same chemokines. The pathogenic relevance of IgG4 antibodies and of T-cell populations presumably sustained by cognate antigen specific plasmablasts remains to be defined.
Another major finding of our study was the increased collagen production observed in co-cultures of fibroblasts with B lymphocytes from healthy subjects compared to control fibroblasts. Collagen production induced by B cells from healthy donors was, however, significantly lower compared to that induced by B cells from patients with IgG4-RD AIP, even though we found similar levels of PDGF-B in the supernatants of both co-cultures. On the one hand, these results indicate that pro-fibrotic mediators other than PDGF-B contribute to the increased collagen production observed in the presence of B-lymphocytes from IgG4-RD patients. On the other hand, these data also suggest that normal B lymphocytes in the absence of disease possess some potential pro-fibrotic properties that may possibly contribute to normal wound healing, but activated B cells seen in a disease context are far more capable of contributing to the process of fibrosis
Indeed, we know from our previous studies that a population of circulating plasmablasts is disproportionately expanded in active IgG4-RD patients compared to healthy individuals and to other inflammatory conditions, but they have not previously been causally linked to fibrotic phenomena [22–23]. Here we provide the first evidence that plasmablasts represent a B-cell subset with intrinsic fibrogenic potential because in contrast to both naïve and memory B cells, they express higher levels of genes implicated in fibroblast activation as well as in the pathogenesis of various human fibrotic disorders. IGF-1, for instance, synergizes with PDGF-B to promote mesenchymal cell proliferation and has been linked to liver fibrosis and to stromal reaction in Grave’s orbitopathy [42–44]. Gremlin1 is known for antagonizing bone morphogenetic protein and for up-regulating fibroblast growth factors in different connective tissue and developmental disorders [45–46]. LOXL2 has been causally associated with liver and pulmonary fibrosis, and clinical trials with LOXL2 inhibitors are ongoing for the treatment of various types of fibrotic diseases [27–32,47]. In addition, LOXL2 and other members of the LOX family have been implicated in epithelial-to-mesenchymal transition in cancer thus contributing to the acquisition of metastatic properties by neoplastic cells [48–49].
These pro-fibrotic properties of expanded plasmablasts did not seem to depend on IgG class-switch nor to be unique to IgG4-RD. LOXL2, in fact, was expressed by IgG4+ and IgG1+ B cells in IgG4-RD lesions, as well as by plasmablasts from patients with SS, CD, and MM, suggesting that a certain ability to regulate extracellular-matrix stiffness represents a constitutive feature of some terminally differentiated antibody producing cells. In this sense, it is tempting to speculate that the abundant infiltrate of plasmablasts/plasma cells that characterizes IgG4-RD and MM lesions is more likely to drive a fibrotic outcome compared to SS, where the number of LOXL2+ B cells is far less prominent. Indeed, in contrast to SS, tissue fibrosis represents an established negative prognostic factor for end-stage organ damage in IgG4-RD and for overall survival in patients with MM [4,50].
Of note, although plasmablasts induced a significant increase of collagen secretion in the co-cultures compared to naïve and memory B cells, the former were no more stimulatory than naive B cells in terms of PDGF-B, CCL-4, CCL-5, and CCL-11 expression by fibroblasts, suggesting that plasmablasts stimulate fibroblasts activation through mechanisms that differ from other B cell subsets. The surprising observation that plasmablasts from IgG4-RD patients expressed type I collagen genes, for instance, raises the possibility that the increased collagen production observed in fibroblasts co-cultured with plasmablasts (and B cells, in general) was due at least in part to the direct secretion of collagenous proteins by B lymphocytes. Altogether, these results unveil unanticipated direct pro-fibrotic properties of plasmablasts/plasma cells, and indicate that the acquisition of these properties likely represents an intrinsic feature of plasmablasts or plasma cells that was not previously appreciated. This ability to secrete pro-fibrotic molecules is likely acquired at a discrete stage of terminal antigen-driven B cell differentiation and this subset of LOXL2 secreting plasmablasts/plasma cells is enriched in the tissues of certain fibrotic diseases.
Finally, we have demonstrated both in vitro and in affected tissues that B cells could potentially contribute to orchestrating the inflammatory infiltrate in IgG4-RD by directly secreting the chemotactic factors CCL-4, CCL-5, and CCL-11 for cell types with fibrogenic properties typically found in IgG4-RD lesions, such as T lymphocytes, macrophages, and eosinophils. B cells also stimulate fibroblasts secretion of these chemokines, thus amplifying the recruitment of inflammatory cells at sites of disease. In particular, CCL-4 is a chemokine involved in monocyte recruitment and activation by engaging the chemokine receptor CCR5 [51]; CCL-5 attracts T cells, eosinophils, and basophils, by interacting with CCR1, CCR3, and CCR5 [52]; and CCL-11 selectively recruits eosinophils through CCR2, CCR3, and CCR5 [53]. Interestingly, CCL-4, CCL-5, and CCL-11 secreted by both B lymphocytes and fibroblasts might also recruit CD4+CTLs, a population of CD4+ T lymphocytes with cytotoxic properties that is increasingly considered central to IgG4-RD pathogenesis [15–16]. CD4+CTLs, in fact, express high levels of CCR5 and their decline observed following rituximab treatment might be due to the depletion of B cells responsible for their recruitment at disease sites [16].
Our study is not the first addressing a potential role of B cells in disease-related fibrogenesis. B regulatory cells, in fact, are known to produce the pro-fibrotic cytokines TGFβ and IL-10, but their contribution to tissue fibrosis has never been assessed in specific disease settings [7]. Putative auto-reactive B cell clones are presumed to produce anti-PDGF-R and anti-matrix metalloproteinase-1 auto-antibodies in patients with systemic sclerosis, but the relevance in vivo of these antibodies is yet to be confirmed [8,13]. More recently, two different studies on chronic lymphocytic leukemia (CLL) and systemic sclerosis have specifically investigated the role of B lymphocytes in activating stromal cells. Ding and colleagues demonstrated that B cells from CLL patients produce PDGF and that conditioned medium obtained from these cells activates marrow stromal cells through PDGF receptor activation [11]. Francois et al showed that B cells from healthy individuals induce collagen production by skin fibroblasts from scleroderma patients through contact mediated mechanisms that depend on TGFβ production [12]. In this sense, our work not only confirms the production of PDGF by activated B lymphocytes as well as the pro-fibrotic properties of B cells from healthy subjects, but also unveils and extensively characterizes a fully functional pro-fibrotic machinery in B lymphocyte that makes them an unexpected professional key player in wound healing processes and fibrogenesis. Whether the prominent plasma cell infiltrate that is typically observed in IgG4-RD lesions is the major cell type responsible for the characteristic tissue fibrosis seen remains difficult to conclude in the absence of animal models. Yet, the results of our study and the prompt response of IgG4-RD lesions to B-cell depletion with rituximab strongly support this possibility.
Our approaches have both potential strengths and weaknesses. Major potential strengths are (i) the adoption of two different human mesenchymal stromal cells (pancreatic and dermal fibroblasts) for co-culture experiments; (ii) the use of B lymphocytes from both healthy donors and patients with active untreated IgG4-RD; and (iii) the unbiased approach adopted to identify relevant pro-fibrotic and immunomodulatory molecules in our system. Pancreatic fibroblasts, may have in fact, allowed us to recapitulate some of the possible interactions occurring between fibroblasts and B cells in AIP, the prototypical clinical-pathological scenario of IgG4-RD. On the other hand, whole transcriptome analysis represented the most powerful instrument to broadly detect changes in fibroblast gene expression induced by B lymphocytes as well as pro-fibrotic pathways in B-cell subsets before focusing on specific molecules of interest. In addition, it is noteworthy that all the reported experiments were performed on human samples from three referral centres for IgG4-RD, thus assuring accurate patient selection, definitive diagnosis, and robust confirmation of our results on different study cohorts. The major limitation of our work is that we only provide correlative in vitro and histological data because reliable animal models of IgG4-RD suitable for confirmatory pathogenic in vivo studies have not yet been developed. IgG4-RD represents, indeed, a rare condition and immune-pathological pathways amenable to mouse modelling remain unknown. Also, being our work focused on IgG4-RD, we limited our tissue analysis on IgG4 class-switched B lymphocytes, but we cannot exclude that pro-fibrotic properties of B cells are shared by class-switched plasmablasts in general.
In conclusion, our work provides novel insights into the pathophysiology of IgG4-RD and unveils unexpected pro-fibrotic properties of B lymphocytes in humans, over and beyond the prevalent view of B cells functioning primarily as antigen presenting cells and providers of surviving signals to putative pathogenic T cells. Because it is likely that the mechanisms described here are not unique to IgG4-RD, selective targeting of pro-fibrotic B-lymphocyte subpopulations and/or pro-fibrotic mediators such as PDGF-B and LOXL2 may offer alternative therapeutic approaches for IgG4-RD and for other fibrosing conditions characterized by B-cell activation and lymphoplasmacytic infiltration.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Dr. Filippo Canducci, Roberto Ferrarese and Cristina Scielzo (IRCCS San Raffaele Scientific Institute, Milan, Italy) for helpful discussion, to Prof. Lorenzo Piemonti (IRCCS San Raffaele Scientific Institute, Milan, Italy) for providing primary human pancreatic fibroblasts cell lines, to Dr. Giovanni Tonon and Paolo Provero (Center for Translational Genomics and BioInformatics (CTGB), San Raffaele Scientific Institute, Milan, Italy) for insightful discussion about RNA sequencing and bioinformatics analysis, and to Stefano Grassi and Francesca Inverizzi from Pathology Unit for performing immunohistochemical stainings.
FUNDING SOURCE - This study was funded by a “Giovani Ricercatori 2018 – Research Grant” award to EDT from “Cariplo Foundation”, by a “TRIDEO 2014 – Research Grant” award to EDT from the “Italian Association for Cancer Research (AIRC)/Cariplo Foundation”, by an AIRC IG 2017–20351 to AAM, by the “5×1000” Italian Ministry of Health, and by NIH U19 AI110495 to SP. EDT received support from the “Collegio Ghislieri” (Pavia, Italy), CP was supported by NIH T32 AR007258, VSM was supported by AI 113163 from the NIH, and TM was supported by a Japanese Society for the Promotion of Science Postdoctoral Fellowship for Studies Abroad.
ABBREVIATIONS
- IgG4-RD
IgG4-related disease
- CD4+CTLs
CD4+ cytotoxic T lymphocytes
- AIP
Autoimmune pancreatitis
- ACTA2
α-Smooth Muscle Actin
- PDGF-B
Platelet derived growth factor-B
- IGF-1
Insulin-like growth factor-1
- LOXL2
Lysyl oxidase homolog 2
- CLL
Chronic lymphocytic leukemia
- PBMC
Peripheral blood mononuclear cells
Footnotes
CONFLICT of INTEREST - Nothing to report.
DISCLOSURE - The authors have not received any financial support or other benefits from commercial sources for the work reported in the manuscript, or any other financial interests that could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease.Nat Med. 2012;18:1028–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockey DC, Bell PD, Hill JA. Fibrosis-a Common Pathway to Organ Injury and Failure. NEJM. 2015;372:1138–49. [DOI] [PubMed] [Google Scholar]
- 3.Ho YY, Lagares D, Tager AM, Kapoor M. Fibrosis-a lethal component of systemic sclerosis. Nat Rev Rheumatol. 2014;10:390–402. [DOI] [PubMed] [Google Scholar]
- 4.Della-Torre E, Lanzillotta M, Doglioni C. Immunology of IgG4-related disease. Clin Exp Immunol 2015;181:191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carruthers MN, Topazian MD, Khosroshahi A, Witzig TE, Wallace ZS, Hart PA, et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis. 2015;74:1171–7. [DOI] [PubMed] [Google Scholar]
- 6.Della-Torre E, Feeney E, Deshpande V, Mattoo H, Mahajan V, Kulikova M, et al. B-cell depletion attenuates serological biomarkers of fibrosis and myofibroblast activation in IgG4-related disease. Ann Rheum Dis 2015;74:2236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cell Mol Immunol. 2013;10:122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato S, Hayakawa I, Hasegawa M, Fujimoto M, Takehara K. Function blocking autoantibodies against matrix metalloproteinase-1 in patients with systemic sclerosis. J Invest Dermatol. 2003;120:542–547. [DOI] [PubMed] [Google Scholar]
- 9.Jordan S, Distler JH, Maurer B, Huscher D, van Laar JM, Allanore Y, et al. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis. 2015;74:1188–94. [DOI] [PubMed] [Google Scholar]
- 10.Keir GJ, Maher TM, Ming D, Abdullah R, de Lauretis A, Wickremasinghe M, et al. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology. 2014;19:353–9. [DOI] [PubMed] [Google Scholar]
- 11.Ding W, Knox TR, Tschumper RC, Wu W, Schwager SM, Boysen JC, et al. Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood. 2010;116:2984–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.François A, Chatelus E, Wachsmann D, Sibilia J, Bahram S, Alsaleh G, et al. B lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis Res Ther. 2013;15:R168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354:2667–76. [DOI] [PubMed] [Google Scholar]
- 14.Maehara T, Mattoo H, Ohta M, Mahajan VS, Moriyama M, Yamauchi M, et al. Lesional CD4+ IFN-γ+ cytotoxic T lymphocytes in IgG4-related dacryoadenitis and sialoadenitis. Ann Rheum Dis. 2017;76:377–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattoo H, Mahajan VS, Maehara T, Deshpande V, Della-Torre E, Wallace ZS, et al. Clonal expansion of CD4+ cytotoxic T lymphocytes in patients with IgG4-related disease. J Allergy Clin Immunol. 2016;138:825–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Della-Torre E, Bozzalla-Cassione E, Sciorati C, Ruggiero E, Lanzillotta M, Bonfiglio S, et al. A CD8α- Subset of CD4+ SLAMF7+ Cytotoxic T Cells is Expanded in Patients with IgG4-Related Disease and Decreases following Glucocorticoid Treatment. Arthritis Rheumatol. 2018;70:1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn WA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furukawa S, Moriyama M, Tanaka A, Maehara T, Tsuboi H, Iizuka M, et al. Preferential M2 macrophages contribute to fibrosis in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz’s disease. Clin. Immunol. 2016;156:9–18. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe K Yamashita, S. Fujikawa, Sakurai T, Kudo M, Shiokawa M, et al. Involvement of activation of toll-like receptors and nucleotide-binding oligomerization domain-like receptors in enhanced IgG4 responses in autoimmune pancreatitis. Arthritis Rheum. 2012;64: 914–24. [DOI] [PubMed] [Google Scholar]
- 20.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demoulin JB, Essaghir A. PDGF receptor signaling networks in normal and cancer cells. Cytokine Growth Factor Rev. 2014;25:273–83. [DOI] [PubMed] [Google Scholar]
- 22.Wallace ZS, Mattoo H, Carruthers M, Mahajan VS, Della Torre E, Lee H, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis 2015;74:190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattoo H, Mahajan VS, Della-Torre E, Sekigami Y, Carruthers M, Wallace ZS, et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol 2014;134:679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanzillotta M, Della-Torre E, Stone JH. Roles of Plasmablasts and B Cells in IgG4-Related Disease: Implications for Therapy and Early Treatment Outcomes. Curr Top Microbiol Immunol. 2017;401:85–92. [DOI] [PubMed] [Google Scholar]
- 25.Lanzillotta M, Della-Torre E, Milani R, Bozzolo E, Bozzalla-Cassione E, Rovati L, et al. Effects of glucocorticoids on B-cell subpopulations in patients with IgG4-related disease. Clin Exp Rheumatol. 2019. January 11. [PubMed] [Google Scholar]
- 26.Lanzillotta M, Della-Torre E, Milani R, Bozzolo E, Bozzalla-Cassione E, Rovati L, et al. Increase of circulating memory B cells after glucocorticoid-induced remission identifies patients at risk of IgG4-related disease relapse. Arthritis Res Ther. 20183;20:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kagan HM, Li W. Lysyl oxidase: properties. specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. [DOI] [PubMed] [Google Scholar]
- 28.Rimar D, Rosner I, Nov Y, Slobodin G, Rozenbaum M, Halasz K, et al. Brief report: lysyl oxidase is a potential biomarker of fibrosis in systemic sclerosis. Arthritis Rheumatol. 2014;66:726–30. [DOI] [PubMed] [Google Scholar]
- 29.Meyringer R, Neumann E, Judex M, Landthaler M, Kullmann F, Scholmerich J, et al. Analysis of gene expression patterns in systemic sclerosis fibroblasts using RNA arbitrarily primed-polymerase chain reaction for differential display. J Rheumatol. 2007;34:747–753. [PubMed] [Google Scholar]
- 30.Cheng T, Liu Q, Zhang R, Zhang Y, Chen J, Yu R, et al. Lysyl oxidase promotes bleomycin-induced lung fibrosis through modulating inflammation. J Mol Cell Biol. 2014;6:506–15. [DOI] [PubMed] [Google Scholar]
- 31.Ikenaga N, Peng ZW, Vaid KA, Liu SB, Yoshida S, Sverdlov DY, et al. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut. 2017;66:1697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos A, Lagares D. Matrix Stiffness: the Conductor of Organ Fibrosis. Curr Rheumatol Rep. 2018;20:2. [DOI] [PubMed] [Google Scholar]
- 33.Maehara T, Mattoo H, Mahajan VS, Murphy SJH, Yuen GJ, Ishiguro N, et al. The expansion in lymphoid organs of IL-41 BATF1 T follicular helper cells is linked to IgG4 class switching in vivo. Life Science Alliance. 2018;1;doi: 10.26508/lsa.201800050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benhamou Y, Paintaud G, Azoulay E, Poullin P, Galicier L, Desvignes C, et al. Efficacy of a rituximab regimen based on B cell depletion in thrombotic thrombocytopenic purpura with suboptimal response to standard treatment: Results of a phase II, multicenter noncomparative study. Am J Hematol. 2016;91:1246–1251. [DOI] [PubMed] [Google Scholar]
- 35.Michel M, Terriou L, Roudot-Thoraval F, Hamidou M, Ebbo M, Le Guenno G, et al. A randomized and double-blind controlled trial evaluating the safety and efficacy of rituximab for warm auto-immune hemolytic anemia in adults (the RAIHA study). Am J Hematol. 2017;92:23–27. [DOI] [PubMed] [Google Scholar]
- 36.Durozard P, Maarouf A, Boutiere C, Ruet A, Brochet B, Vukusic S, et al. Efficacy of rituximab in refractory RRMS. Mult Scler. 2018;1:1352458518772748. [DOI] [PubMed] [Google Scholar]
- 37.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81. [DOI] [PubMed] [Google Scholar]
- 38.Pillai S, Mattoo H, Cariappa A. The role of B cells in autoimmunity. Current Opinion in Immunology, 2011, 23, 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Della-Torre E, Campochiaro C, Cassione EB, Albano L, Gerevini S, Bianchi-Marzoli S, et al. Intrathecal rituximab for IgG4-related hypertrophic pachymeningitis. J Neurol Neurosurg Psychiatry. 2018;89:441–444. [DOI] [PubMed] [Google Scholar]
- 40.Perugino CA, AlSalem SB, Mattoo H, Della-Torre E, Mahajan V, Ganesh G, et al. Identification of galectin-3 as an autoantigen in patients with IgG4-related disease. J Allergy Clin Immunol. 2018. May 29 pii: S0091–6749(18)30768–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiokawa M, Kodama Y, Sekiguchi K, Kuwada T, Tomono T, Kuriyama K, et al. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci Transl Med. 2018;10(453). [DOI] [PubMed] [Google Scholar]
- 42.Guo XH, Liu LX, Zhang HY, Zhang QQ, Li Y, Tian XX, et al. Insulin-like growth factor binding protein-related protein 1 contributes to hepatic fibrogenesis. J Dig Dis. 2014;15:202–10. [DOI] [PubMed] [Google Scholar]
- 43.Giannella-Neto D, Kamyar A, Sharifi B, Pirola CJ, Kupfer J, Rosenfeld RG, et al. Platelet-derived growth factor isoforms decrease insulin-like growth factor I gene expression in rat vascular smooth muscle cells and selectively stimulate the biosynthesis of insulin-like growth factor binding protein 4. Circ Res. 1992;71:646–56. [DOI] [PubMed] [Google Scholar]
- 44.Della-Torre E, Mattoo H, Mahajan VS, Deshpande V, Krause D, Song P, et al. IgG4-related midline destructive lesion. Ann Rheum Dis. 2014;73:1434–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bénazet JD, Bischofberger M, Tiecke E, Gonçalves A, Martin JF, Zuniga A, et al. A self-regulatory system of interlinked signaling feedback loops controls mouse limb patterning. Science. 2009;323: 1050–3. [DOI] [PubMed] [Google Scholar]
- 46.Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat. Genet. 2003;34:303–7. [DOI] [PubMed] [Google Scholar]
- 47.Aumiller V, Strobel B, Romeike M, Schuler M, Stierstorfer BE, Kreuz S. Comparative analysis of lysyl oxidase (like) family members in pulmonary fibrosis. Sci Rep. 2017;10;7:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millanes-Romero A, Herranz N, Perrera V, Iturbide A, Loubat-Casanovas J, Gil J, et al. Regulation of heterochromatin transcription by Snail1/LOXL2 during epithelial-to-mesenchymal transition. Mol Cell. 2013;52:746–57. [DOI] [PubMed] [Google Scholar]
- 49.Cuevas EP, Eraso P, Mazón MJ, Santos V, Moreno-Bueno G, Cano A, et al. LOXL2 drives epithelial-mesenchymal transition via activation of IRE1-XBP1 signalling pathway. Sci Rep. 2017;23:7:44988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Babarović E, Valković T, Štifter S, Budisavljević I, Seili-Bekafigo I, Duletić-Načinović A, et al. Assessment of bone marrow fibrosis and angiogenesis in monitoring patients with multiple myeloma. Am J Clin Pathol. 2012;137:870–8. [DOI] [PubMed] [Google Scholar]
- 51.Sherry B, Tekamp-Olson P, Gallegos C, Bauer D, Davatelis G, Wolpe SD, et al. Resolution of the two components of macrophage inflammatory protein 1, and cloning and characterization of one of those components, macrophage inflammatory protein 1 beta. J Exp Med. 1988;168:2251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–5. [DOI] [PubMed] [Google Scholar]
- 53.Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, et al. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med. 1994;179:881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.