Abstract
Objective
To compare the frequency of and trends in hospitalizations after heart failure (HF) diagnosis in patients with and without rheumatoid arthritis (RA) during 1987–2015.
Methods
The study included a retrospectively identified population-based cohort of patients with incident HF and prior RA (age≥18 years, 1987 ACR criteria) and a cohort of incident HF patients without RA matched 3:1 on age, sex, and year of HF diagnosis. Hospitalizations at the time of HF diagnosis were excluded. All subjects were followed until death, migration, or 12/31/2015.
Results
The study included 212 patients with RA (mean age at HF diagnosis 78.3 years; 68% female) and 636 non-RA patients (mean age at HF diagnosis 78.6 years; 68% female). The hospitalization rate after HF diagnosis was higher in RA vs non-RA (rate ratio [RR] 1.17; 95%CI 1.08–1.26). Hospitalization rates in both groups have been declining since 2005 and the difference between patients with and without RA may be decreasing after 2010. The magnitude of the increase was similar in both sexes and across all ages. Patients with RA were more likely to be hospitalized for noncardiovascular causes (RR 1.26; 95%CI 1.14–1.39), but not for HF or other cardiovascular causes compared to non-RA patients.
Conclusions
The hospitalization rate following HF diagnosis was higher in RA versus non-RA patients regardless of sex and age. Increased hospitalization risk in patients with RA was driven by increased rates of non-cardiovascular hospitalization.
Keywords: Rheumatoid arthritis, heart failure, hospitalizations
INTRODUCTION
Comorbidity burden in patients with rheumatoid arthritis (RA) is substantial and is associated with increased healthcare utilization [1]. About 80% of patients with RA have one additional chronic condition, and studies have reported a 21% increase in the prevalence of comorbidity (primarily cardiovascular conditions) in RA over the past 20 years [2, 3]. Heart failure (HF) is one of the most common cardiovascular comorbidities in patients with RA. Our prior studies showed a 2-fold increased risk of HF in RA compared to the general population, and suggested a significant contribution of HF to the excess mortality in RA [4, 5]. A recent nationwide cohort study from Denmark reported 30%-increased risk of hospitalization with incident HF in RA [6].
Population-based studies of patients with HF both from the USA and Europe have reported frequent hospitalizations following HF diagnosis, increased readmission rates [7] and high out-of-hospital mortality rates, resulting in substantial clinical and economic burden associated with HF diagnosis [8]. More recent data suggest that from 2001 to 2014 there has been a decline in hospitalization rates for HF in the United States [9].
In view of the increased occurrence of HF in patients with RA, understanding the trends in hospitalization rates following HF diagnosis in RA as compared to the general population is important for healthcare planning, resource utilization and optimization of management. However, studies reporting hospitalization rates after the diagnosis of HF in patients with RA as compared to the general population are lacking.
To address this gap, we aimed to compare the frequency of and trends in hospitalizations for any cause after HF diagnosis in patients with and without RA during 1987–2015 and to assess risk factors for hospitalizations following HF in RA.
PATIENTS AND METHODS
A retrospectively identified population-based cohort of patients with incident HF in 1987–2012 and prior RA (age≥18 years, 1987 ACR criteria) was assembled using the resources of the Rochester Epidemiology Project (REP). The REP is a unique population-based medical records linkage system that ensures ready access to the complete (in-patient and out-patient) medical records from all community medical providers in Olmsted County, MN [10]. For each patient, the earliest date of fulfillment of ≥4 1987 ACR criteria for RA was considered the RA incidence date. A comparison cohort of incident HF patients without RA matched 3:1 on age, sex, and year of HF diagnosis was assembled from the same underlying population.
HF was defined using the Framingham criteria [11, 12]. HF diagnosis requires ≥ 2 of the major criteria [i.e., paroxysmal nocturnal dyspnea or orthopnea, neck vein distention, rales, radiographic cardiomegaly (i.e., increasing heart size on chest radiograph), acute pulmonary edema, S3 gallop, increased central venous pressure ≥ 16 cm of water at the right atrium, circulation time ≥ 25 seconds, hepatojugular reflux, weight loss > 4.5 kg in 5 days in response to treatment of congestive HF)], or the presence of 1 major criterion and ≥ 2 minor criteria (i.e., bilateral ankle edema, nocturnal cough, dyspnea on ordinary exertion, hepatomegaly, pleural effusion, decrease in vital capacity by 33% from maximal value recorded, and tachycardia rate ≥ 120 beats/min). Minor criteria were counted only if they could not be attributed to another medical condition. Data on hospitalizations (i.e., admission dates, discharge dates, and admission and discharge diagnoses by International Classification of Diseases Ninth Edition [ICD-9] coding) were retrieved electronically from billing data of Olmsted County medical providers including Mayo Clinic and Olmsted Medical Center and their affiliated hospitals.
All subjects were followed until death, migration, or December 31, 2015. Hospitalizations at the time of HF diagnosis were excluded from analyses. Readmission was defined as a hospital admission date within 30 days of a prior discharge date. Data on primary discharge diagnosis were available since 1995. For analyses using discharge diagnosis, additional cases and comparators who died or emigrated from Olmsted County prior to 1995 were excluded, and follow-up began with the latter of the HF diagnosis date or January 1, 1995.
Information on the following risk factors for hospitalization for any cause following HF diagnosis was collected by retrospective medical records review in all patients with RA: smoking status (current or former smoker), body mass index, hypertension, diabetes mellitus, myocardial infarction (MI), Charlson comorbidity index, and RA disease characteristics (i.e., RA disease duration, rheumatoid factor [RF] and/ or anti-citrullinated protein antibody [ACPA] positivity, and severe extra-articular manifestations [ExRA]). Hypertension was defined according to the criteria of the Joint National Committee on Detection, Evaluation and treatment of High Blood Pressure as ≥ 2 ambulatory blood pressure readings ≥ 140 mm Hg systolic and/or ≥ 90 mm Hg diastolic obtained during a 1-year period, physician diagnosis or documented use of antihypertensive medications. Diabetes mellitus was defined as at least 2 measurements of fasting plasma glucose ≥ 126 mg/dl or a 2-hour plasma glucose ≥ 200 mg/dl, physician diagnosis or documented use of insulin and/or oral hypoglycemic agents. MI was defined according to standardized criteria [13]. Rheumatologic disease was removed from the calculation of the Charlson Comorbidity Index. Severe ExRA was defined as the presence of pericarditis, pleuritis, Felty’s syndrome, glomerulonephritis, cutaneous vasculitis, peripheral neuropathy, scleritis, episcleritis or retinal vasculitis [14]. The institutional review boards of the Mayo Clinic and the Olmsted Medical Center approved this study. This manuscript follows the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) reporting guidelines for observational studies [15].
Statistical analysis
Descriptive statistics (mean, percentages, etc.) were used to summarize the data. Rank sum and chi-square tests were used to compare characteristics between the HF patients with and without RA. Data were analyzed using person-year methods. Comparisons of person-year rates were performed using Poisson methods. Poisson regression models with smoothing splines were used to examine trends over time while allowing for non-linear effects. Comparisons of length of stay for HF patients with and without RA were performed using generalized linear models adjusted for age, sex, and calendar year with random effects to account for multiple hospitalizations in the same patient. Readmission rates were calculated as the number of readmissions divided by the number of subsequent hospitalizations excluding the first hospitalization for each patient, as this could not be a readmission by definition. Conditional frailty models were used to analyze risk factors for hospitalizations for any cause following HF diagnosis adjusting for age, sex and calendar year of HF diagnosis [16]. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria.
RESULTS
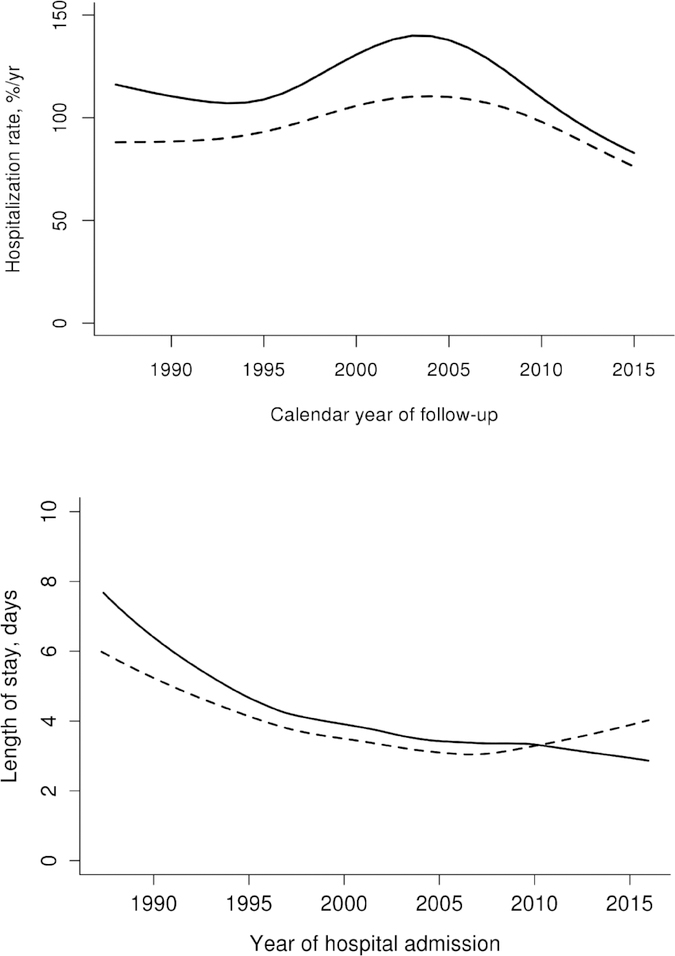
The study included 212 patients with RA and 636 patients without RA who were similar in age, sex, Charlson comorbidity index and Framingham criteria fulfillment for HF (Table 1). Table 2 shows hospitalization rates after HF diagnosis overall and subdivided according to age, sex, calendar year, HF disease duration and categories of primary discharge diagnosis among patients with and without RA. The hospitalization rate after HF diagnosis was higher in RA at 100.2 hospitalizations/100 person years, with 857 hospitalizations over 854.9 person years of follow up as compared to non-RA at 86.0 hospitalizations/100 person years, with 3047 hospitalizations over 3541.5 person years of follow up, rate ratio [RR] 1.17; 95% confidence interval [CI] 1.08–1.26; Table 2). Hospitalization rates in both groups have been declining since 2005 and the difference between patients with and without RA may be decreasing after 2010 (Figure 1, Panel A). The magnitude of the increased risk was similar in both sexes, across all ages, and throughout the duration of HF. Following HF diagnosis, patients with RA experienced a higher rate of hospitalizations for non-cardiovascular causes (RR 1.26; 95%CI 1.14–1.39), but not for HF (RR 0.96; 95%CI 0.75–1.20) or other cardiovascular causes (RR 0.99; 95%CI 0.81–1.20) compared to the patients without RA, using data on primary discharge diagnosis which were available starting 1995. The leading non-CV cause of hospitalization was respiratory disease in both cohorts.
Table 1:
Characteristics of patients with heart failure with and without rheumatoid arthritis
| Characteristic | RA (n=212) | Non-RA (n=636) |
|---|---|---|
| Age at HF diagnosis | 78.3 (±9.8) | 78.6 (±9.7) |
| Sex, female | 144 (68%) | 432 (68%) |
| Follow up, years | 4.0 (±4.2) | 5.6 (±4.8) |
| Charlson Comorbidity index | 3.9 (±2.9) | 3.8 (±3.2) |
| Framingham criteria fulfillment for HF | ||
| ≥ 2 Major | 182 (86%) | 545 (86%) |
| 1 Major and ≥ 2 Minor | 23 (11%) | 83 (13%) |
Abbreviations: RA= rheumatoid arthritis; HF= heart failure
Values in the table are mean (±standard deviation) or N (%), as indicated.
Table 2.
Hospitalization rates following diagnosis of heart failure in patients with and without rheumatoid arthritis
| RA | Non-RA | Rate Ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Group | Number of hospitalizat ions | Number of person-years | Rate (per 100 person-years) | Number of hospitaliz ations | Number of person-years | Rate (per 100 person-years) | |
| Overall | 857 | 854.9 | 100.2 | 3047 | 3541.5 | 86.0 | 1.17 (1.08–1.26) |
| Age group: | |||||||
| - 40–64 years | 70 | 98.2 | 71.3 | 174 | 280.9 | 62.0 | 1.16 (0.87–1.52) |
| - 65–79 years | 386 | 382.3 | 101.0 | 1238 | 1374.9 | 90.0 | 1.12 (1.00–1.25) |
| - ≥ 80 years | 401 | 374.4 | 107.1 | 1635 | 1885.7 | 86.7 | 1.24 (1.11–1.38) |
| Sex: | |||||||
| - Female | 577 | 623.4 | 92.6 | 1976 | 2464.0 | 80.2 | 1.15 (1.05–1.26) |
| - Male | 280 | 231.6 | 120.9 | 1071 | 1077.6 | 99.4 | 1.22 (1.07–1.39) |
| Calendar year of hospital admission | |||||||
| - 1987–1990 | 36 | 36.0 | 100.0 | 108 | 124.6 | 86.7 | 1.16 (0.78–1.66) |
| - 1991–1995 | 126 | 134.0 | 94.0 | 401 | 502.4 | 79.8 | 1.18 (0.96–1.43) |
| - 1996–2000 | 180 | 168.8 | 106.6 | 692 | 751.2 | 92.1 | 1.16 (0.98–1.36) |
| - 2001–2005 | 249 | 207.1 | 120.2 | 810 | 846.5 | 95.7 | 1.26 (1.09–1.45) |
| - 2006–2010 | 161 | 160.5 | 100.3 | 672 | 767.5 | 87.6 | 1.15 (0.96–1.36) |
| - 2011–2015 | 105 | 148.5 | 70.7 | 364 | 549.3 | 66.3 | 1.07 (0.86–1.32) |
| HF Disease duration: | |||||||
| - 0–2 years | 496 | 401.7 | 123.5 | 1517 | 1497.8 | 101.3 | 1.22 (1.10–1.35) |
| - 3–4 years | 144 | 167.8 | 85.8 | 501 | 682.3 | 73.4 | 1.17 (0.97–1.40) |
| - 5–9 years | 141 | 213.6 | 66.0 | 724 | 918.9 | 78.8 | 0.84 (0.70–1.00) |
| - ≥ 10 years | 76 | 71.9 | 105.7 | 305 | 442.5 | 68.9 | 1.54 (1.19–1.96) |
| Primary discharge diagnosis** | |||||||
| Heart failure | 87 | 714.2 | 12.2 | 387 | 3047.1 | 12.7 | 0.96 (0.75–1.20) |
| Other CV | 122 | 714.2 | 17.1 | 525 | 3047.1 | 17.2 | 0.99 (0.81–1.20) |
| Non-CV | 506 | 714.2 | 70.9 | 1714 | 3047.1 | 56.3 | 1.26 (1.14–1.39) |
Abbreviations: RA= rheumatoid arthritis; CV=cardiovascular
Available for hospitalization in 1995-present.
Figure 1.
Calendar year trends in hospitalization rates following heart failure diagnosis (Panel A) and trends in hospital length of stay (Panel B) in patients with rheumatoid arthritis (solid line) and non-RA subjects (dashed line)
Readmission rates were similar among the patients with RA (206 readmissions; 30% of 693 subsequent hospitalizations) compared to the patients without RA (667 readmissions; 27% of 2488 subsequent hospitalizations; p=0.13). There was no overall difference in hospital length of stay in patients with and without RA (mean of 5.6 vs 5.3 days, respectively, p=0.31). Length of stay was higher in earlier years in RA than non- RA and declined faster in RA than non-RA over time, with similar length of stay in both groups in recent years (interaction p=0.019, Figure 1, Panel B).
Among patients with RA, 112 (53%) were positive for RF/ACPA antibody and 19 (9%) had severe ExRA prior to HF diagnosis. Mean RA disease duration at the time of HF diagnosis was 13.6 (10.5) years. Smoking (current or former) (Hazard Ratio [HR] 1.33, 95%CI 1.06–1.68), prior MI (HR 1.37, 95%CI 1.03–1.82) and higher Charlson comorbidity index values (HR 1.10, 95%CI 1.06–1.14), but not other traditional cardiovascular risk factors and RA disease characteristics, were associated with increased risk for hospitalization (Table 3).
Table 3.
Risk factors for hospitalization for any cause following heart failure diagnosis in patients with rheumatoid arthritis
| Characteristics at heart failure diagnosis** | Value* | Hazard Ratio (95% Confidence Interval) |
|---|---|---|
| Prior myocardial infarction | 35 (17) | 1.37 (1.03–1.82) |
| Ever smoker (current or former) | 121 (58) | 1.33 (1.06–1.68) |
| Hypertension | 137 (65) | 1.23 (0.97–1.55) |
| Sex, male | 68 (32) | 1.21 (0.95–1.53) |
| Diabetes mellitus | 36 (17) | 1.15 (0.87–1.52) |
| Charlson comorbidity index | 3.9 (2.9) | 1.10 (1.06–1.14) |
| RF and/or ACPA positive | 112 (53) | 1.05 (0.84–1.31) |
| Age (per 10 years) | 78.3 (9.8) | 1.04 (0.93, 1.17) |
| RA disease duration | 13.6 (10.5) | 1.01 (1.00–1.02) |
| Calendar year | 1999 (7) | 1.00 (0.99–1.02) |
| Body mass index ≥30 kg/m2 | 63 (30) | 0.88 (0.70–1.11) |
| Severe extra-articular manifestations of RA | 19 (9) | 0.82 (0.54–1.24) |
Abbreviations: RA=rheumatoid arthritis; RF= rheumatoid factor; ACPA=anti-citrul inated protein antibodies
Values in the table are mean (±SD) for continuous characteristics and N (%) for discrete characteristics
Data on risk factors were available on all patients except 2 patients without RF/ACPA testing and 4 with unknown smoking status.
DISCUSSION
Healthcare utilization in patients with chronic conditions is one of the drivers of the healthcare crisis in the US [17, 18]. Hospitalizations of patients with HF account for a large part of healthcare costs related to HF thus presenting a major public health problem. This large population-based study is one of the first to report trends in hospitalizations following HF diagnosis in patients with RA compared to the general population. A recent large nation-wide cohort study by Khalid et al reported a 30%-increased risk of hospitalization for incident HF in RA vs the general population [6]. Our study extends these findings by showing increased rates of hospitalization subsequent to HF diagnosis in patients with versus without RA, suggesting increased healthcare use and associated economic costs in patients with RA who have HF as compared to the general population. There is a potential improving trend in HF hospitalizations in RA after 2010, which mirrors the decline in HF hospitalizations in the general population that has occurred since implementation of the 2005 ACC/AHA HF management guidelines [9].
Similar to the findings from the earlier studies of HF hospitalizations in the general population of the Olmsted County, non-cardiovascular causes were the most common reasons for hospital admission in the our RA cohort [7, 19]. Respiratory disease was one of the leading causes for hospitalization in this RA cohort, which is consistent with earlier reports of the major effect of pulmonary disease on healthcare use and mortality in RA [1].
Smoking, prior MI and higher Charlson comorbidity index values were associated with increased risk of hospitalization. This finding is concordant with the results from a prospectively followed cohort of the German register RABBIT where smoking and older age were among the risk factors for the outcome event (hospitalization or death) in patients with RA and HF [20]. A high number of comorbid conditions (> 6 comorbidities) in patients with HF who developed the outcome event was also noted in this study. Taken together, these findings suggest that common risk factors and comorbidity burden have a major effect on healthcare use and hospitalizations in RA for which an improved approach for comorbidity management should be sought.
Strengths of this study include its population-based design, availability of information on all hospitalizations in the community, long and complete follow up and the availability of a comparison cohort from the same underlying population.
Potential limitations include lack of information on anti-rheumatic medications. Lack of increase in HF hospitalizations in users of tumor necrosis factor antagonists vs users of non-biologic disease-modifying anti-rheumatic drugs has been previously reported in a large nation-wide study [21]. Due to the observational nature of this study and the inherent likelihood of confounding by indication, it is unlikely that the availability of information on anti-rheumatic medications would aid in understanding the trends in hospitalizations following HF diagnosis.
Data on primary discharge diagnosis were not available prior to 1995. As such, we cannot estimate what conditions accounted for increased risk of hospitalization following HF in earlier years and whether hospitalizations due to cardiovascular causes were more prominent contributor than after 1995. Data on hospitalizations outside the community were not available; however, these are likely rare due to the relatively isolated location of Olmsted County. Olmsted County, MN population is predominantly white, thus the findings of this study may not be readily generalizable to more ethnically diverse populations.
In summary, hospitalization rate following HF diagnosis was higher in RA than in non-RA patients regardless of age and sex. This difference was particularly apparent between 1990 and 2010. The increased hospitalization risk in patients with RA was due to an increased rate of non-cardiovascular hospitalization. Smoking, prior MI and Charlson comorbidity index were associated with increased risk of hospitalization, suggesting that increased management complexity among patients with comorbid RA may play a role in more frequent hospitalizations in patients with RA and HF.
Acknowledgments
Funding: This work was supported by a grant from the National Institutes of Health, NIAMS (R01 AR46849) and NHLBI (HL120859). Research reported in this publication was supported by the National Institute of Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: Authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Han GM, Han XF. Comorbid conditions are associated with healthcare utilization, medical charges and mortality of patients with rheumatoid arthritis. Clin Rheumatol 2016;35:1483–92. 10.1007/s10067-016-3277-y. [DOI] [PubMed] [Google Scholar]
- [2].Nikiphorou E, Norton S, Carpenter L, Dixey J, Andrew Walsh D, Kiely P, et al. Secular Changes in Clinical Features at Presentation of Rheumatoid Arthritis: Increase in Comorbidity But Improved Inflammatory States. Arthritis Care Res (Hoboken) 2017;69:21–7. 10.1002/acr.23014. [DOI] [PubMed] [Google Scholar]
- [3].Parodi M, Bensi L, Maio T, Mela GS, Cimmino MA. Comorbidities in rheumatoid arthritis: analysis of hospital discharge records. Reumatismo 2005;57:154–60. [DOI] [PubMed] [Google Scholar]
- [4].Nicola PJ, Crowson CS, Maradit-Kremers H, Ballman KV, Roger VL, Jacobsen SJ, et al. Contribution of congestive heart failure and ischemic heart disease to excess mortality in rheumatoid arthritis. Arthritis Rheum 2006;54:60–7. [DOI] [PubMed] [Google Scholar]
- [5].Davis JM 3rd, Roger VL, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum 2008;58:2603–11. 10.1002/art.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Khalid U, Egeberg A, Ahlehoff O, Lane D, Gislason GH, Lip GYH, et al. Incident Heart Failure in Patients With Rheumatoid Arthritis: A Nationwide Cohort Study. J Am Heart Assoc 2018;7(2): e007227 10.1161/JAHA.117.007227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol 2009;54:1695–702. 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Corrao G, Ghirardi A, Ibrahim B, Merlino L, Maggioni AP. Burden of new hospitalization for heart failure: a population-based investigation from Italy. Eur J Heart Fail 2014;16:729–36. 10.1002/ejhf.105. [DOI] [PubMed] [Google Scholar]
- [9].Akintoye E, Briasoulis A, Egbe A, Dunlay SM, Kushwaha S, Levine D, et al. National Trends in Admission and In-Hospital Mortality of Patients With Heart Failure in the United States (2001 −2014). J Am Heart Assoc. 2017;6(12). 10.1161/JAHA.117.006955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am 2004;30:819–34, vii. [DOI] [PubMed] [Google Scholar]
- [11].Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 1993;22(4 Suppl A):6A–13A. [DOI] [PubMed] [Google Scholar]
- [12].Mosterd A, Deckers JW, Hoes AW, Nederpel A, Smeets A, Linker DT, et al. Classification of heart failure in population based research: an assessment of six heart failure scores Eur J Epidemiol. 1997;13:491–502. [DOI] [PubMed] [Google Scholar]
- [13].Gillum RF, Fortmann SP, Prineas RJ, Kottke TE. International diagnostic criteria for acute myocardial infarction and acute stroke. Am Heart J 1984;108:150–8. [DOI] [PubMed] [Google Scholar]
- [14].Turesson C, Jacobsson L, Bergstrom U. Extra-articular rheumatoid arthritis: prevalence and mortality. Rheumatology (Oxford) 1999;38:668–74. [DOI] [PubMed] [Google Scholar]
- [15].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- [16].Box-Steffensmeier JM, De Boef S. Repeated events survival models: the conditional frailty model. Stat Med 2006;25:3518–33. [DOI] [PubMed] [Google Scholar]
- [17].Payne RA, Abel GA, Guthrie B, Mercer SW. The effect of physical multimorbidity, mental health conditions and socioeconomic deprivation on unplanned admissions to hospital: a retrospective cohort study. CMAJ 2013;185:E221–8. 10.1503/cmaj.121349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Palladino R, Tayu Lee J, Ashworth M, Triassi M, Millett C. Associations between multimorbidity, healthcare utilisation and health status: evidence from 16 European countries. Age Ageing 2016;45:431–5. 10.1093/ageing/afw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chamberlain AM, Dunlay SM, Gerber Y, Manemann SM, Jiang R, Weston SA, et al. Burden and Timing of Hospitalizations in Heart Failure: A Community Study. Mayo Clin Proc 2017;92:184–92. 10.1016/j.mayocp.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Meissner Y, Schafer M, Manger B, Zanker M, Ochs W, Listing J, et al. THU0142 The prognosis of heart failure in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases 2018;77(Suppl 2):291–2. [Google Scholar]
- [21].Solomon DH, Rassen JA, Kuriya B, Chen L, Harrold LR, Graham DJ, et al. Heart failure risk among patients with rheumatoid arthritis starting a TNF antagonist. Annals of the Rheumatic Diseases 2013;72:1813–8. 10.1136/annrheumdis-2012-202136. [DOI] [PubMed] [Google Scholar]