Abstract
2′,4′-dihydroxy-6′-methoxychalcone (cardamonin) is a natural compound with anti-proliferative effects on several cancer types including nasopharyngeal carcinoma. The effects of cardamonin on melanoma cells are unknown. The present study investigated the anti-proliferative effect of cardamonin on human melanoma cell lines (M14 and A375), and the underlying apoptosis inducing mechanisms. MTS assay showed that cardamonin inhibited M14 cells viability, and a reduction of the M14 cell density was also observed. Flow cytometry showed that cardamonin induced M14 cells apoptosis in a dose-dependent manner. Western blot analysis showed protein expression in M14 and A375; the pro-apoptotic protein BAX was upregulated, while the anti-apoptotic protein B-cell lymphoma-2 was downregulated. The protein expression of cleaved caspase-8, −9 and cleaved poly (ADP-ribose) polymerase was increased, whereas P65 was decreased. Furthermore, cardamonin inhibited M14 cell migration. These findings suggest that cardamonin may be a novel anticancer treatment for human melanoma.
Keywords: cardamonin, melanoma, apoptosis, extrinsic pathway, extrinsic pathway
Introduction
Human melanoma is a highly malignant tumor derived from abnormal melanocytes. It is responsible for >75% of skin cancer deaths (1). Melanomas metastasize in the lymph nodes through blood vessels and the prognosis is poor. Melanoma treatment by known drugs and therapies is limited and outcome is poor. The incidence of a five-year survival rate ranges between 5–15% (2). Although the effectiveness of dacarbazine as a single-agent therapy is only 4.5%, it is commonly used for the treatment of advanced melanoma as a chemotherapeutic agent (3). Moreover the majority of patients using a mono- or combination therapy comprising single-agent BRAF and/or dual specificity mitogen-activated protein kinase kinase (MEK) kinase inhibitors only exhibited a clinical improvement for a limited time (4). Therefore, the discovery of new treatments is important for the short-term prognosis of patients with melanoma.
Natural products are a potential source for new therapies. More than 75% of new anti-tumor drugs have originated from natural products, for example, paclitaxel and colchicine (5). The Food and Drug Administration (FDA) has approved 25–48% of plant derived medicines (6).
2′,4′-dihydroxy-6′-methoxychalcone (cardamonin) is a natural chalcone compound that is extracted from members of the Zingiberaceae family (7). It may increase cell apoptosis in several tumors, including nasopharyngeal carcinoma, prostate cancer and glioblastoma (8–10). Cardamomin may be effective via multiple signaling pathways, including nuclear factor (NF)-κB, signal transducer and activator of transcription 3 and mammalian target of rapamycin pathways (8,10,11). However, the underlying effects and mechanisms of cardamonin on melanoma cells remain unclear. In the present study, several assays were used to determine the anticancer activity and effects of cardamonin on human melanoma cells.
Materials and methods
Cell viability assay
An MTS assay was used to determine the effect of cardamonin on melanoma cell viability. Cells were cultured in RPMI-1640 Medium (Corning, cat. no. 10-040-CV) with 10% FBS (Shanghai ExCell Biology, Inc.; cat. no. FND500) in an incubator (37°C, 50% CO2) Human melanoma M14 cells, supplied by the Chinese Academy of Medical Sciences, (2–5 ×103/100 µl/well) were seeded in a 96-well plate and incubated for 24 h pre-cardamonin treatment at different concentrations (0, 30, 60 and 90 µM) for 24 h. Thereafter, 20 µl MTS solution (2 mg/ml) was added to each well followed by a 2 h incubation period. Absorbance was measured with a microplate reader (Infinite F50; Tecan Schweiz AG) at a wavelength of 495 nm.
Flow cytometry
Cell apoptosis caused by cardamonin was measured with a fluorescein isothiocyanate (FITC) Annexin V apoptosis detection kit (BD Biosciences; cat. no. 556547). M14 cells were seeded in a 6-well plate and treated with varying cardamonin concentrations (0, 30, 60 and 90 µM) for 24 h. Cells were collected and washed with ice-cold PBS and incubated with Annexin V-FITC and propidium iodide (PI) in the dark for 20 min. The cells were then resuspended with binding buffer and measured using a Navios flow cytometer B47903 and Navios Platform System Software v2.0 (both Beckman Coulter, Inc.).
Western blot analysis
M14 and A375 cell lines (Chinese Academy of Medical Science) were seeded in 6-well plates (2×105 per well) and treated with varying cardamonin concentrations (0, 30, 60 and 90 µM) for 24 h. Cell proteins were collected with RIPA buffer (Beijing Solarbio Science & Technology Co., Ltd.; cat. no. R0020-100 ml) supplemented with PMSF. Protein concentrations were measured using a bicinchoninic acid protein assay kit (MultiSciences). The proteins (30 µg) were separated via SDS-PAGE (15% gel) electrophoresis and transferred to PVDF membranes. The membranes were then blocked in 5% non-fat milk in TBST (0.05%Tween20) for 1 h at room temperature and incubated with homologous primary antibodies (all 1:1,000) overnight at 4°C [β-actin, cat. no. AC026; P65, cat. no. A2547; BCL2, cat. no. A2212; BAX, cat. no. A7626 (all ABclonal Biotech Co., Ltd.); Cleaved Caspase-8, cat. no. 9496; Cleaved Caspase-9, cat. no. 20750; Cleaved PARP, cat. no. 5625 (all Cell Signaling Technology, Inc.)]. The membranes were incubated with horse radish peroxidase conjugated secondary antibodies (Goat Anti-Rabbit IgG horseradish-peroxidase conjugated; cat. no. ab205718; 1:10,000) at room temperature for 2 h. After washing the membranes, enhanced chemiluminescence reagents (Beijing Solarbio Science & Technology Co., Ltd.; cat. no. PE0010) were applied to them before being scanned by a ChemiDoc XRS+ system (Bio-Rad Laboratories, Inc.).
Wound healing assay
A wound healing assay was used to test the effect of cardamonin on cell migration. M14 cells were seeded into 6-well plates (5×105 per well) (Guangzhou Jet Bio-Filtration Co., Ltd.; cat. no. TCP010006) and cultured to 80% confluence. They were then scratched through the cell monolayer using a 10 µl pipette tip before being washed with serum-free RPMI-1640 medium (Corning, Inc., cat. no. 15-040-CV) to remove floating cells. Subsequently, cells were treated with varying cardamonin concentrations (0, 30, 60 and 90 µM) for 24 h and images were captured using the ChemiDoc™ XRS+ System light miscroscope with Image Lab™ Software (Bio-Rad Laboratories, Inc., cat. no. 1708265).
Data analysis
The Gray value of three repeats was measured using ImageJ (1.50i; National Institutes of Health). Data are presented as the mean ± standard deviation. The significance difference between distinct groups were determined using one-way ANOVA (SPSS 21.0; IBM Corp.) with post-hoc test (Fisher's Least Significant Difference). P<0.05 was considered to indicate a statistically significant difference.
Results
Cardamonin inhibits cell viability
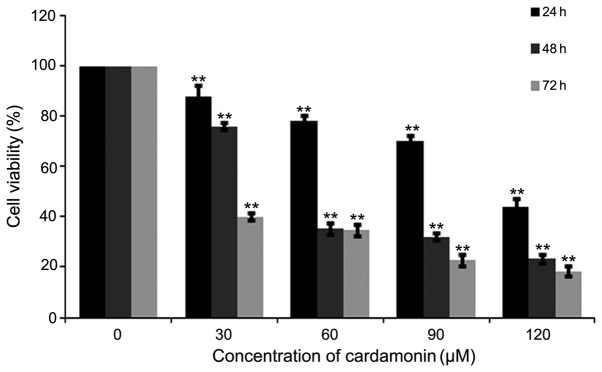
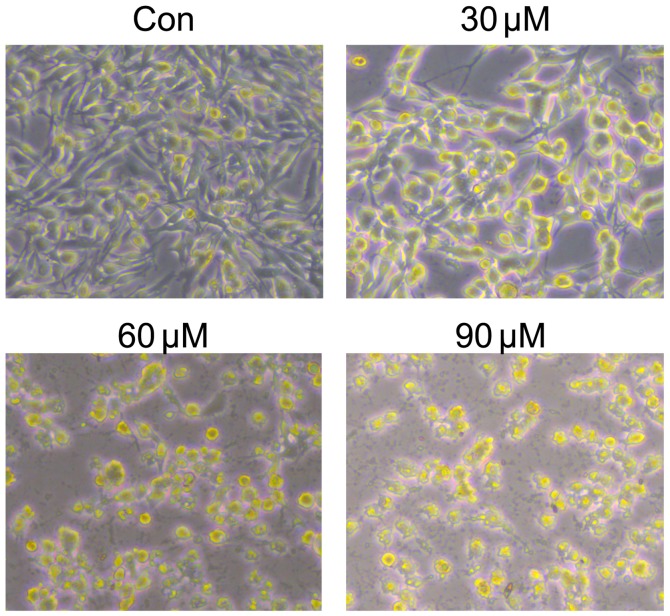
M14 cells were treated with varying cardamonin concentrations (0, 30, 60, 90 and 120 µM; n=4) for 24, 48 and 72 h. The MTS assay showed that cardamonin significantly decreased M14 cell viability (P<0.01; Fig. 1). Cell density was also decreased after cardamonin treatment for 24 h (Fig. 2). The results indicated that cardamonin could inhibit M14 cell growth.
Figure 1.
Effects of cardimonin on M14 cells growth. M14 cells were treated with various concentrations of cardamonin (0, 30, 60, 90 and 120 µM) for 24, 48 and 72 h. Cell viability was measured using an MTS assay. Graphs represent average percentages of cell proliferation normalized to M14 cells in the control group. Significance levels between distinct groups were determined using one-way analysis of variance. **P≤0.01 vs. control.
Figure 2.
Effects of cardamonin on M14 cell density. M14 cells were treated with various concentration of cardamonin (0, 30, 60 and 90 µM) for 48 h. Images are representative of three independent experiments. Con, control.
Cardamonin induces apoptosis in M14 cells
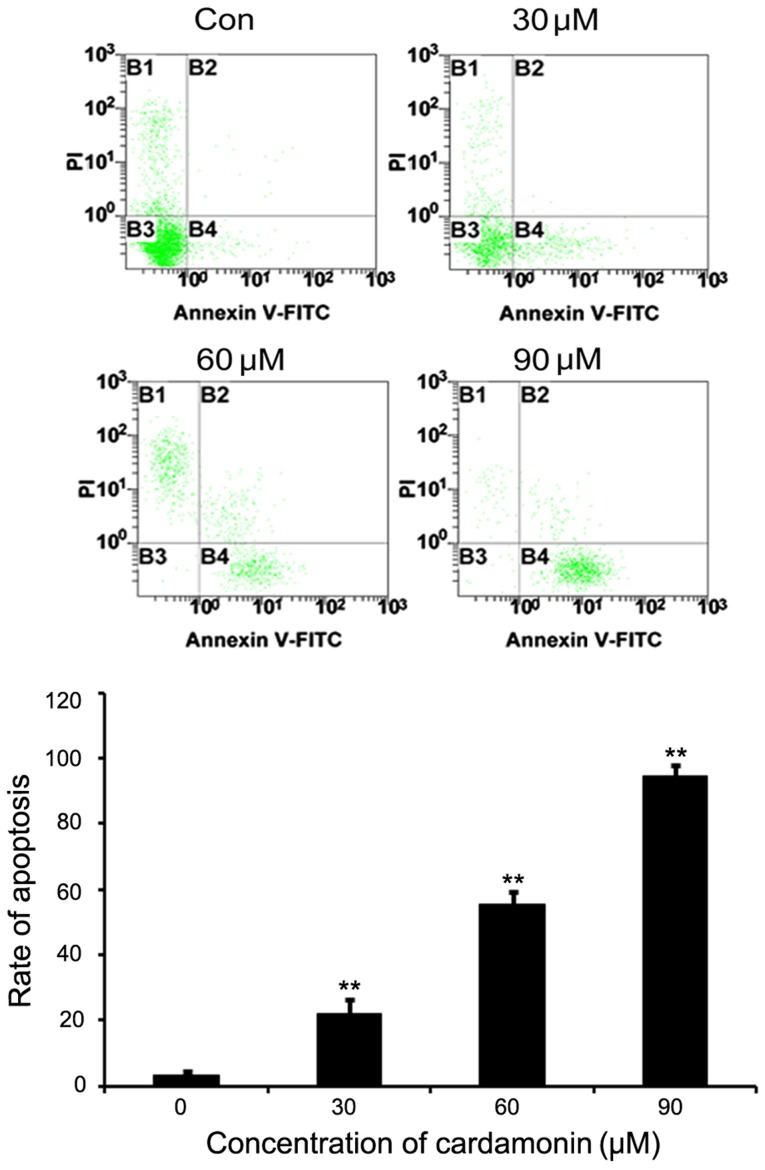
M14 cells were cultured in varying cardamonin concentrations (0, 30, 60 and 90 µM, n=3) for 24 h and then stained with Annexin V-FITC and PI for flow cytometry analysis. Results indicated that the untreated M14 cells were not stained by Annexin V and PI, whereas a significant dose-dependent increase was noted in the Annexin V and PI positive cells following cardamonin treatment (P<0.01; Fig. 3).
Figure 3.
Effects of cardamonin on M14 cell apoptosis. M14 cells were treated with different concentrations of cardamonin (0, 30, 60 and 90 µM) for 24 h. FITC, fluorescein isothiocyanate; PI, propidium iodide; Con, control. **P≤0.01 vs. control.
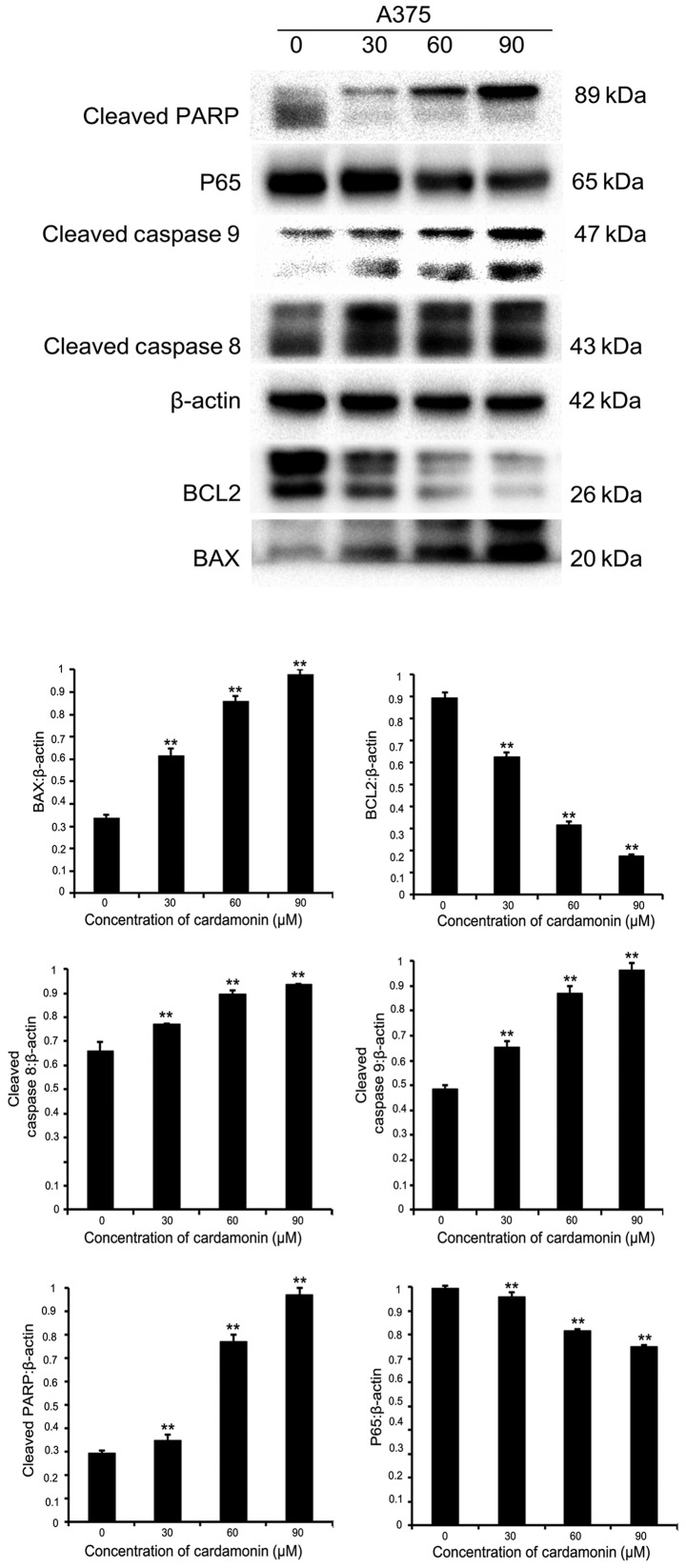
Cardamonin alters apoptosis-associated proteins in M14 cells and A375 cells
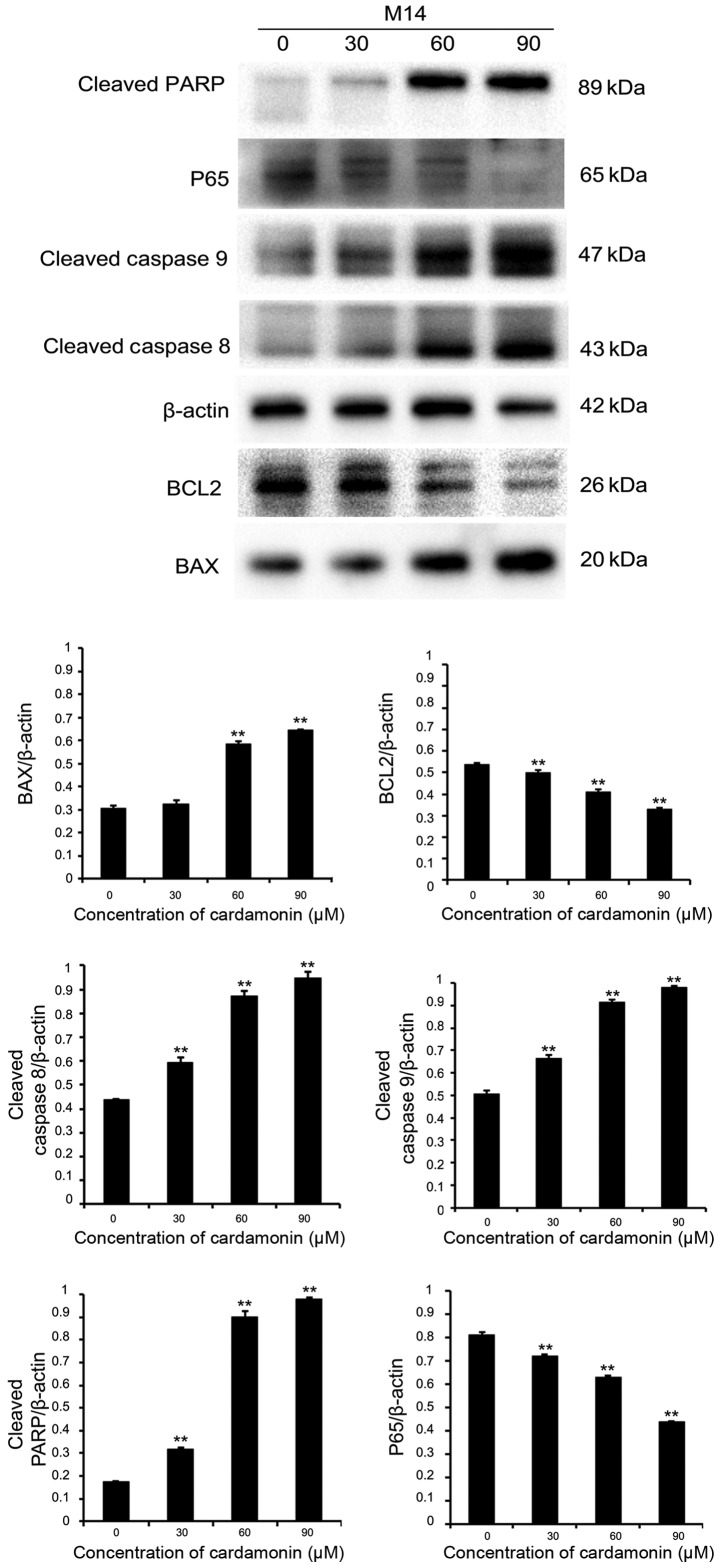
In order to investigate whether cardamonin induced melanoma cell apoptosis via the effects of apoptosis-associated proteins, immunoblotting was performed. Protein lysates were collected from two different cell lines (M14 and A375). They were cultured in varying cardamonin concentrations (0, 30, 60 and 90 µM; n=3) for 24 h. It was found that the pro-apoptotic protein BAX was upregulated, while the anti-apoptotic protein B-cell lymphoma-2 (BCL2) was downregulated in both cell lines. Intrinsic or extrinsic pathways may be involved in apoptosis, therefore the expression of cleaved caspase-8, −9 and cleaved PARP were investigated (12). These apoptotic signaling proteins increased significantly after 24-h treatment with varying cardamonin concentrations (P<0.01). P65 expression significantly decreased, thus indicating that cardamonin is a major anti-apoptotic regulator (P<0.01; Figs. 4 and 5). Results showed that cardamonin regulates the expression of important pro-apoptotic and anti-apoptotic proteins.
Figure 4.
Protein expression of Bax, BCL2, cleaved caspase-8, cleaved caspase-9, cleaved PARP, nuclear factor-κB p65 in M14 cell line detected by western blot analysis. β-actin was used as an internal control. The levels of different proteins were quantified and normalized to β-actin and are shown in a histogram. Significance levels between distinct groups were determined using one-way analysis of variance. **P≤0.01 vs. the control. PARP, poly (ADP-ribose) polymerase; BCL2, B-cell lymphoma-2.
Figure 5.
Protein expression of Bax, BCL2, cleaved caspase-8, cleaved caspase-9, cleaved PARP, NF-κB p65 in A375 cell line is detected by western blot analysis. β-actin was used as an internal control. Significance levels between distinct groups were determined using one-way analysis of variance. **P≤0.01 vs. the control. PARP, poly (ADP-ribose) polymerase; BCL2, B-cell lymphoma-2.
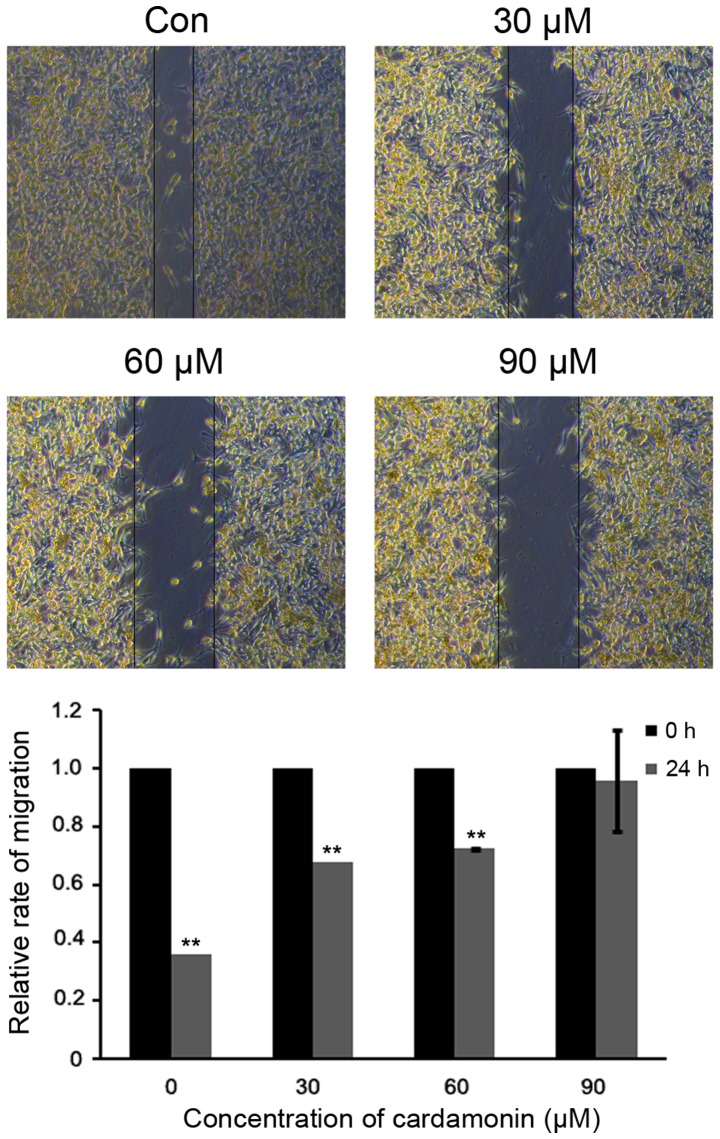
Cardamonin suppresses M14 cell migration
To assess whether cardmonin affects cell migration, a scratch-wounding assay was performed on M14 cells. Under varying cardamonin concentrations (0, 30, 60 and 90 µM; n=3), the open wound areas were significantly increased in comparison to the untreated cells (P<0.01). These data indicated that cardamonin suppressed M14 cell migration in a dose-dependent manner (Fig. 6).
Figure 6.
M14 cells are treated with different concentrations of cardamonin (0, 30, 60 and 90 µM) for 24 h, and migration is inhibited. The levels of different groups were quantified and are shown in a histogram. Significance levels between distinct groups were measured and analyzed using one-way analysis of variance. **P≤0.01 vs. control.
Discussion
Cutaneous melanoma is responsible for 1.6% of new cancer cases and 0.7% of cancer-related deaths worldwide (13). Traditional chemotherapy is ineffective in melanoma treatment and the mortality rate remains high. Dakabazine (DTIC) is the only FDA-approved anti-melanoma chemotherapy drug in use to date. In comparison to supportive therapies (5–11 months), the response rate to DTIC ranges from between 5–25% and does not increase overall survival. The development of targeted therapy has improved the response and overall survival rate in melanoma patients. Vemurafenib as an orally active BRAF inhibitor, exhibits partial and complete responses in BRAF mutated melanoma patients. Melanoma cells may easily develop drug resistance (14). Dabrafenib as another BRAF inhibitor that is similar to vemurafenib in terms of progression-free survival (PFS) and reaction rate (15). Approved immunotherapeutics include ipilimumab and nivolumab, the former being a monoclonal antibody that blocks the cytotoxic T-lymphocyte protein-4 receptor. Previous studies have shown that the combination of nivolumab (a PD-1 inhibitor) may achieve better results than the single use of ipilimumab (16–18). Nivolumab monotherapy has a higher objective response rate, a longer PFS and overall survival rate compared to chemotherapy treatments. Despite the positive effects of immunotherapy drugs, a large number of patients continue to succumb to melanoma metastasis. The ubiquitous resistance to molecular targeted therapy remains a challenge for the treatment of melanoma.
Therefore, the effective treatment of melanoma depends on the development of novel medicines. Furthermore, researchers are committed to finding drugs that are effective on cancer cells while having minimal cytotoxic effects on normal cells.
Alpinia katsumadai hayata is a traditional Chinese herbal medicine. It induces a warming of the stomach and is used for relieving gastric discomfort and a distended abdomen (19). Cardamonin is extracted from the seed of cardamom spices and it is an active ingredient of Alpinia katsumadai hayata, which has antinociceptive effects (15,20). Cardamonin has been tested as an anticancer treatment in several tumors, except melanoma. Cardamonin's proven safety and effects in tumor treatment make it a promising anti-cancer reagent. An MTS assay was performed to verify its anti-proliferative effect on M14 melanoma cells.
Apoptosis is an important physiological process whereby cells commit suicide (21). There are two major pathways of apoptosis, namely the intrinsic and the extrinsic pathway. The intrinsic pathway is controlled by the BCL2 family of proteins, which may be classified as pro-apoptotic proteins like BAX and anti-apoptotic proteins such as BCL2. BAX as a factor in the BCL2 family, plays an important role in cell apoptosis and mitochondrial function (22). BCL2 may induce apoptosis by binding to anti-apoptotic proteins, thereby replacing activators that may activate BAX (23). BCL2 is a fundamental anti-apoptotic gene that that is recognized in cancer development (24). Overexpression of BCL2 promotes tumorigenesis and tumor progression and is associated with poor patient prognosis in numerous types of cancer, for example, breast cancer, prostate cancer and melanoma (25). In order to achieve more reliable results, both M14 and A375 cell were treated with cardamonin and analyzed by western blotting. It was noted that treatment with cardamonin resulted in the downregulation of BCL2 and upregulation of BAX protein levels in M14 and A375 cells. Both proteins play a pivotal role in apoptotic pathway regulation.
Caspases are also vital pro-apoptotic proteins. Caspase-8 occurs in extrinsic apoptotic pathways while caspase-9 is involved in intrinsic (mitochondrial) pathways. Caspase-8 can process classical apoptotic caspases including caspase-9, which may lead to apoptotic initiation (26). Proenzymes synthesized by caspases, are activated by cleaving the pro-domain at a specific aspartic acid cleaving site. Activation of caspase-8 or −9 may eventually lead to the cleavage of poly (ADP-ribose) polymerase (PARP) which results in DNA fragmentation and apoptosis (27). In a previous study, upregulation of cleavage of caspase-8, caspase-9 and PARP was investigated in the treatment of prostate cancer cells with cardamonin (9). Western blot analysis results showed apoptotic induction by cardamonin via the cleavage of caspase-8, −9 and PARP. These results reflected those of a previous study that performed a Caspase-Glo 3/7, 8 and 9 assay and western blot analysis in A549 and HK1 cells (28). Ma et al (29) discovered that Deoxyarbutin inhibited Bcl-2, activated Bax and voltage-dependent anion-selective channel protein, which in turn sequentially activated caspase-9, PARP, caspase-3 and finally led to mitochondria associated apoptosis. While in a study by Bush et al (30) it was revealed that another drug curcumin induced melanoma cell death by activating caspases-3 and caspases-8 but not caspase-9. Therefore, the role of caspase-9 in melanoma cells needs to be further investigated.
NF-κB (P65) which combines with fixed nucleotide sequences of various gene promoters, is an anti-apoptotic factor. P65 has an important influence on cell proliferation and differentiation, tumor formation, invasion and metastasis. P65 directly mediates vital tumor-promoting mechanisms. NF-κB is known to activate cell proliferation, prevent apoptosis, promote tumor angiogenesis, epithelial-to-mesenchymal transition, invasiveness and metastasis (31). P65 phosphorylation is associated to the expression of the pro-apoptotic BCL2 family, which includes Bcl-2 (32). A study by Wang and Liu (33) confirmed that ingenol-3-angelate suppressed the growth of melanoma cells, in which P65 activity was downregulated. Furthermore, the effects of cardamonin on cancer cell apoptosis via the P65 signaling pathway have been proven. P65 expression was tested in M14 and A375 cell lines exposed to cardamonin by means of western blot analysis in the present study. Cardamonin did not inhibit P65 in these cell lines.
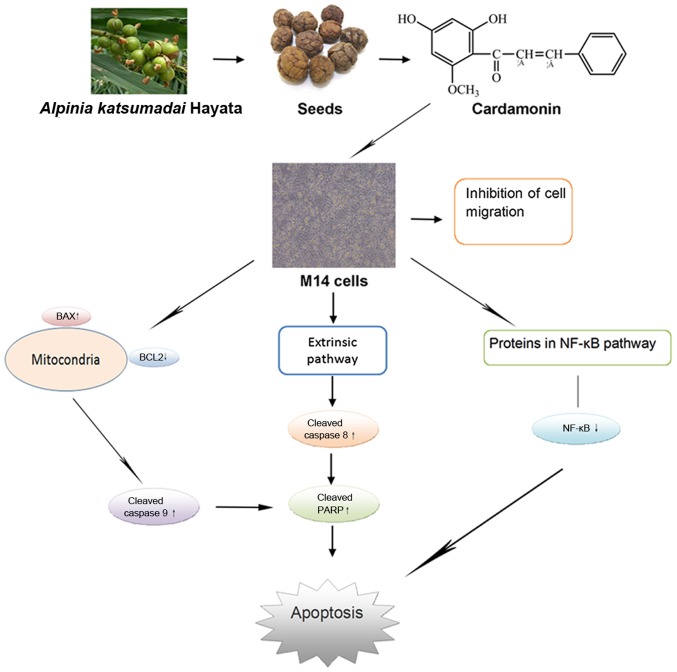
The present study demonstrated that cardamonin promotes apoptosis of melanoma cells via the regulation of proteins in the intrinsic, extrinsic and NF-κB pathways (Fig. 7). Cardamonin, as a natural compound, may be a potentially valuable factor for human melanoma treatment.
Figure 7.
Flow diagram. Cardamonin, a natural chalcone compound, can induce melanoma cell apoptosis and change the expression of apoptosis-associated proteins. PARP, poly (ADP-ribose) polymerase; BCL2, B-cell lymphoma-2.
Acknowledgements
Not applicable.
Funding
Financial assistance was provided by the Hebei Provincial Administration of Traditional Chinese Medicine (project number: 2018055).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YY, LL, YTL, PPL and YJL analyzed and collected the data regarding the MTS and the western blotting analysis. HL analyzed and collected the data regarding the flow cytometry analysis. GZ and XD helped with experimental design, interpretation of data and made major contributions to the writing of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lee C, Collichio F, Ollila D, Moschos S. Historical review of melanoma treatment and outcomes. Clin Dermatol. 2013;31:141–147. doi: 10.1016/j.clindermatol.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, Major MB, Hwang ST, Rimm DL, Moon RT. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci USA. 2009;106:1193–1198. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui C, Mao L, Chi Z, Si L, Sheng X, Kong Y, Li S, Lian B, Gu K, Tao M, et al. A phase II, randomized, double-blind, placebo-controlled multicenter trial of Endostar in patients with metastatic melanoma. Mol Ther. 2013;21:1456–1463. doi: 10.1038/mt.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan R, LoRusso P, Boerner S, Dummer R. Achievements and challenges of molecular targeted therapy in melanoma. Am Soc Clin Oncol Educ Book. 2015:177–186. doi: 10.14694/EdBook_AM.2015.35.177. [DOI] [PubMed] [Google Scholar]
- 5.Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH, et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 7.Goncalves LM, Valente IM, Rodrigues JA. An overview on cardamonin. J Med Food. 2014;17:633–640. doi: 10.1089/jmf.2013.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Qin Y, Yang C, Zhang H, Li Y, Wu B, Huang J, Zhou X, Huang B, Yang K, Wu G. Cardamonin induces ROS-mediated G2/M phase arrest and apoptosis through inhibition of NF-κB pathway in nasopharyngeal carcinoma. Cell Death Dis. 2017;8:e3024. doi: 10.1038/cddis.2017.407. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Zhang J, Sikka S, Siveen KS, Lee JH, Um JY, Kumar AP, Chinnathambi A, Alharbi SA, Basappa, Rangappa KS, et al. Cardamonin represses proliferation, invasion, and causes apoptosis through the modulation of signal transducer and activator of transcription 3 pathway in prostate cancer. Apoptosis. 2017;22:158–168. doi: 10.1007/s10495-016-1313-7. [DOI] [PubMed] [Google Scholar]
- 10.Wu N, Liu J, Zhao X, Yan Z, Jiang B, Wang L, Cao S, Shi D, Lin X. Cardamonin induces apoptosis by suppressing STAT3 signaling pathway in glioblastoma stem cells. Tumour Biol. 2015;36:9667–9676. doi: 10.1007/s13277-015-3673-y. [DOI] [PubMed] [Google Scholar]
- 11.Niu P, Shi D, Zhang S, Zhu Y, Zhou J. Cardamonin enhances the anti-proliferative effect of cisplatin on ovarian cancer. Oncol Lett. 2018;15:3991–3997. doi: 10.3892/ol.2018.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 14.Rosen LS, Lorusso P, MA WW, Goldman JW, Weise A, Colevas AD, Adjei A, Yazji S, Shen A, Johnston S, et al. A first-in-human phase I study to evaluate the MEK1/2 inhibitor, cobimetinib, administered daily in patients with advanced solid tumors. Invest New Drugs. 2016;34:604–613. doi: 10.1007/s10637-016-0374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park MK, Lee HJ, Choi JK, Kim HJ, Kang JH, Lee EJ, Kim YR, Kang JH, Yoo JK, Cho HY, et al. Novel anti-nociceptive effects of cardamonin via blocking expression of cyclooxygenase-2 and transglutaminase-2. Pharmacol Biochemistry Behav. 2014;118:10–15. doi: 10.1016/j.pbb.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caroline R, Jacob S, Georgina VL, Ana A, Jean JG, Laurent M, Adil D, Matteo SC, Catriona M, Michal L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 18.Caroline R, Georgina VL, Jacob S, Ana A, Jean JG, Laurent M, Adil D, Matteo SC, Catriona M, Michal L, et al. Long-term outcomes in patients (pts) with ipilimumab (ipi)-naïve advanced melanoma in the phase 3 KEYNOTE-006 study who completed pembrolizumab (pembro) treatment. J Clin Oncol. 2017;15(Suppl):S35–S35. [Google Scholar]
- 19.Wang S, Zhai C, Zhang Y, Yu Y, Zhang Y, Ma L, Li S, Qiao Y. Cardamonin, a novel antagonist of hTRPA1 cation channel, reveals therapeutic mechanism of pathological pain. Molecules. 2016;21:E1145. doi: 10.3390/molecules21091145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Chen X, Hu Z. Separation and determination of alpinetin and cardamonin in alpinia katsumadai hayata by flow injection-micellar electrokinetic chromatography. Talanta. 2007;71:155–159. doi: 10.1016/j.talanta.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: Cell survival and cell death. Int J Cell Biol. 2010;2010:214074. doi: 10.1155/2010/214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan SL, Yu VC. Proteins of the bcl-2 family in apoptosis signalling: From mechanistic insights to therapeutic opportunities. Clin Exp Pharmacol Physiol. 2004;31:119–128. doi: 10.1111/j.1440-1681.2004.03975.x. [DOI] [PubMed] [Google Scholar]
- 23.Hata AN, Engelman JA, Faber AC. The BCL2 family: Key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015;5:475–487. doi: 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y, Wen X, Zhao P. MicroRNA-365 inhibits cell growth and promotes apoptosis in melanoma by targeting BCL2 and cyclin D1 (CCND1) Med Sci Monit. 2018;24:3679–3692. doi: 10.12659/MSM.909633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radha G, Raghavan SC. BCL2: A promising cancer therapeutic target. Biochim Biophys Acta Rev Cancer. 2017;1868:309–314. doi: 10.1016/j.bbcan.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Monie TP, Bryant CE. Caspase-8 functions as a key mediator of inflammation and pro-IL-1β processing via both canonical and non-canonical pathways. Immunol Rev. 2015;265:181–193. doi: 10.1111/imr.12284. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 28.Break MKB, Hossan MS, Khoo Y, Qazzaz ME, Al-Hayali MZK, Chow SC, Wiart C, Bradshaw TD, Collins H, Khoo TJ. Discovery of a highly active anticancer analogue of cardamonin that acts as an inducer of caspase-dependent apoptosis and modulator of the mTOR pathway. Fitoterapia. 2018;125:161–173. doi: 10.1016/j.fitote.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Xu Y, Wei Z, Xin G, Xing Z, Niu H, Huang W. Deoxyarbutin displays antitumour activity against melanoma in vitro and in vivo through a p38-mediated mitochondria associated apoptotic pathway. Sci Rep. 2017;7:7197. doi: 10.1038/s41598-017-05416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bush JA, Cheung KJ, Jr, LI G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp Cell Res. 2001;271:305–314. doi: 10.1006/excr.2001.5381. [DOI] [PubMed] [Google Scholar]
- 31.Kaltschmidt B, Greiner JFW, Kadhim HM, Kaltschmidt C. Subunit-specific role of NF-κB in cancer. Biomedicines. 2018;6:E44. doi: 10.3390/biomedicines6020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turillazzi E, Neri M, Cerretani D, Cantatore S, Frati P, Moltoni L, Busardò FP, Pomara C, Riezzo I, Fineschi V. Lipid peroxidation and apoptotic response in rat brain areas induced by long-term administration of nandrolone: The mutual crosstalk between ROS and NF-kB. J Cell Mol Med. 2016;20:601–612. doi: 10.1111/jcmm.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D, Liu P. Ingenol-3-Angelate suppresses growth of melanoma cells and skin tumor development by downregulation of NF-κB-cox2 signaling. Med Sci Monit. 2018;24:486–502. doi: 10.12659/MSM.906049. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.