Abstract
Biological function of plasmacytoma variant translocation 1 (PVT1) in influencing the progression of non-small cell lung cancer (NSCLC) through Micro ribonucleic acid (miRNA)-526b/EZH2 regulatory loop was elucidated. Relative levels of PVT1 and miRNA-526b in NSCLC tissues were detected by quantitative real-time polymerase chain reaction (qRT-PCR). Prognostic potential of PVT1 in NSCLC was assessed by Kaplan-Meier curves. The interaction among PVT1/miRNA-526b/EZH2 regulatory loop was confirmed by dual-luciferase reporter gene assay. Regulatory effects of PVT1/miRNA-526b/EZH2 axis on viability and wound closure of A549 cells were evaluated by cell counting kit-8 (CCK-8) and wound closure assay, respectively. PVT1 was upregulated in NSCLC tissues, while miRNA-526b was downregulated. PVT1 level was negatively related to that of miR-526 in NSCLC tissues. Worse survival was seen in NSCLC patients expressing high level of PVT1 compared to those with low level. Knockdown of PVT1 attenuated viability and wound closure ability in A549 cells, which were partially reversed after miRNA-526b knockdown. miRNA-526b is the downstream target of PVT1 and its level was negatively regulated by PVT1. EZH2 is the target gene of miRNA-526b. Transfection of miRNA-526b mimic remarkably downregulated EZH2 in A549 cells. Importantly, the attenuated viability and wound closure ability in A549 cells overexpressing miRNA-526b were reversed after EZH2 overexpression. PVT1 is upregulated in NSCLC, and predicts a poor prognosis. PVT1 accelerates the progression of NSCLC via targeting miRNA-526b/EZH2 regulatory loop.
Keywords: NSCLC, PVT1, miRNA-526b, EZH2
Introduction
Lung cancer is one of the malignant tumors that seriously threaten health and lives. The morbidity and mortality rates of lung cancer rank first worldwide (1). Detection rate of early-stage lung cancer is extremely low owing to the atypical symptoms and signs (2). Non-small cell lung cancer (NSCLC), includes squamous cell carcinoma and adenocarcinoma, accounting for >80% of lung cancer cases (3). Current treatments for NSCLC, such as surgery, radiotherapy and chemotherapy have advanced greatly. The 5-year survival of NSCLC, however, is as low as 15% (4). It is urgent to uncover the pathogenesis of NSCLC, thus developing sensitive and effective approaches for clinical treatment.
Long noncoding RNAs (lncRNAs) can not encode proteins and are over 200 nucleotides in length. Functionally, lncRNAs are closely linked with gene expression, embryonic development, and systematic metabolism. Recently, vital roles of lncRNAs in human diseases have been identified. They are capable of regulating cellular behaviors, drug resistance and epithelial-mesenchymal transition (EMT) (5–7). Plasmacytoma variant translocation 1 (PVT1) is a recently discovered specific lncRNA located on the human chromosome 8q24, with a full length of 1716 bp. PVT1 is the downstream gene of proto-oncogene MYC, and its transcription products are intronic transcripts (8). PVT1 is upregulated in many types of tumors, and closely related to tumor prognosis (9,10).
MicroRNAs (miRNAs) are small noncoding RNAs 22–24 nt in length. They are of significance in maintaining metabolic functions. Through binding 3′-untranslated region (3′-UTR) of target genes, miRNAs degrade them or suppress their translation, thus downregulating target gene expression (11). Abnormally expressed miRNAs are involved in tumorigenesis (12). It is reported that miRNA-526b is downregulated in NSCLC. Overexpression of miRNA-526b remarkably inhibits lung cancer cell proliferation (13).
EZH2 locates on the human chromosome 7q35-7q36. It spans 76,939 bp, and contains 20 exons and 19 introns (14). EZH2 participates in the histone deacetylase (HDAC) process by interacting with DNA methyltransferases (DNMT), which can affect the activity of H3K27me3 (15). In breast cancer and prostate cancer, EZH2 influences tumor progression by regulating tumor growth and metastasis (16). This study investigated the function of PVT1/miRNA-526b/EZH2 regulatory loop in the progression of NSCLC.
Patients and methods
Sample collection
NSCLC tissues and adjacent normal tissues were collected from NSCLC patients undergoing radical resection in Hainan Provincial People's Hospital (Haikou, China) from May 2016 to December 2018. All tissues were pathologically confirmed. None of patients had preoperative antitumor treatment. This study was approved by the Ethics Committee of Hainan Provincial People's Hospital. Signed informed consents were obtained from all participants before the study.
Cell culture and transfection
A549 cells were cultured in Dulbeccos modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS; both from HyClone), 100 µg/ml penicillin and 0.1 mg/ml streptomycin, at 37°C, in 5% CO2 incubator. Cells pre-seeded in a 24-well plate were cultured at 70% confluence and transfected using Lipofactamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Six hours later, complete medium was replaced. Sequences of transfection vectors were as follows: Si-PVT1, forward, 5′-GCUUCUCCUGUUGCUGCATT-3′ and reverse, 5′-UAGCAGCAACAGGAGAAGCTT-3′; Si-NC, forward, 5′-GCUACGAUCUGCCCAAGAUTT-3′ and reverse, 5′-AUCUUAGGCAFGAUCGUCGCTT-3′.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Extraction of total RNA in cells was performed using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RNAs were subjected to reverse transcription. The extracted cDNA was applied for PCR using SYBR-Green method. Primer sequences were as follows: PVT1, forward, 5′-TGAGAACTGTCCTTACGTGACC-3′ and reverse, 5′-AGAGCACCAAGACTGGCTCT-3′; glyceraldheyde 3-phosphate dehydrogenase (GAPDH), forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′; miRNA-526b, forward, 5′-CTTGCTTGGAAGGGGCATGCA-3′ and reverse, 5′-GTCGTATCCAGTGCAGGGTCCGAGGTA-3′; U6, forward, 5′-AGAAAATCTGCGCTTGGTCGTCC-3′ and reverse, 5′-TAG CCGTGATATCGATGTAGCAA-3′. EZH2, forward, 5′-CGGGGTACCGAGTCATACTTGTGAAG-3′ and reverse, 5′-GCACTCGAGCCTGTTTTTGTTTGATG-3′.
Cell counting kit-8 (CCK-8)
Cells were seeded in a 96-well plate with 3×103 cells per well and cultured overnight. Absorbance (A) at 450 nm was recorded at the appointed time using the CCK-8 kit (Dojindo Laboratories) for depicting the viability curves.
Wound closure assay
Cells were inoculated in a 25 mm2 culture dish in serum-free medium. After cell adherence, a 10 µl pipette tip was utilized for scratching an artificial wound. After 24-h culture, wound closure was observed and captured (magnification, ×40).
Dual-luciferase reporter gene assay
Wild-type and mutant-type vectors were constructed based on the binding sites in the promoter regions of the genes. Cells were co-transfected with wild-type/mutant-type vectors and miRNA-526b mimic/control for 48 h. Then, cells were lysed for determining luciferase activity.
Statistical analysis
Statistical Product and Service Solutions (SPSS) 22.0 (IBM Corp.) was used for data analyses. Data were expressed as mean ± standard deviation. Intergroup differences were analyzed by the t-test. Spearman correlation test was conducted to analyze the relationship between PVT1 and miRNA-526b in NSCLC tissues. P<0.05 was considered as statistically significant.
Results
Upregulation of PVT1 in NSCLC
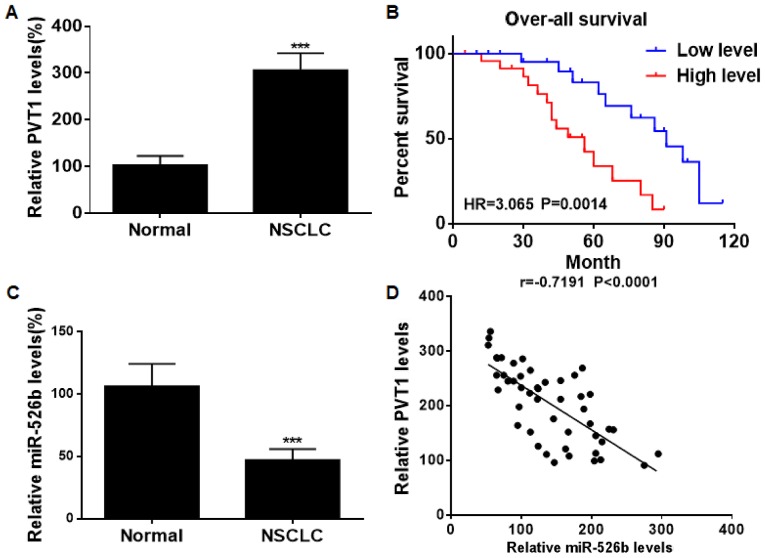
PVT1 was found to be upregulated in NSCLC tissues relative to normal ones (Fig. 1A). Kaplan-Meier analyses revealed that NSCLC patients expressing high level of PVT1 suffered worse prognosis than those with low level (Fig. 1B). On the contrary, miRNA-526b was downregulated in NSCLC tissues (Fig. 1C). Spearman correlation test showed a negative relationship between levels of PVT1 and miRNA-526b in NSCLC tissues (Fig. 1D).
Figure 1.
Upregulation of PVT1 in NSCLC. (A) PVT1 level in NSCLC and normal tissues. (B) Overall survival in NSCLC patients expressing high level and low level of PVT1. (C) miRNA-526b level in NSCLC tissues and normal tissues. (D) A negative correlation between PVT1 and miRNA-526b in NSCLC tissues. PVT1, plasmacytoma variant translocation 1; NSCLC, non-small cell lung cancer. ***P<0.001.
Silence of PVT1 attenuates viability and migration in NSCLC
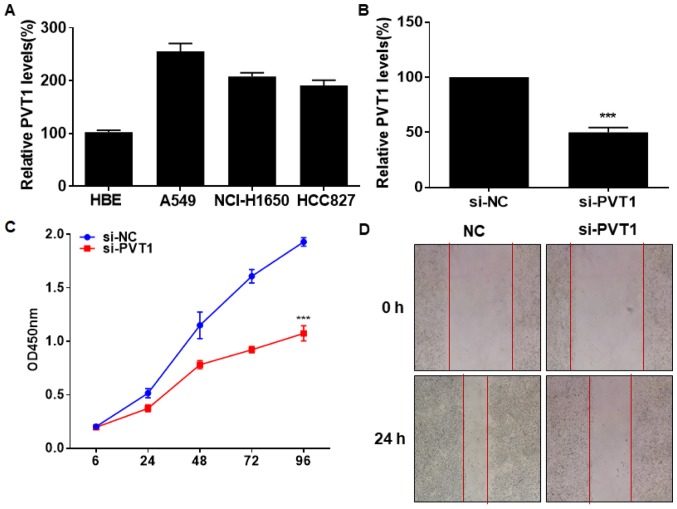
In vitro level of PVT1 was identically upregulated in lung cancer cells and normal bronchial epithelial cells (Fig. 2A). We constructed si-PVT1 and tested its transfection efficacy in A549 cells (Fig. 2B). CCK-8 assay indicated an inhibited viability in A549 cells transfected with si-PVT1 (Fig. 2C). Silence of PVT1 also reduced the wound closure percentage in A549 cells, suggesting suppressed migratory ability (Fig. 2D). It is believed that PVT1 stimulated NSCLC to proliferate and migrate.
Figure 2.
Silence of PVT1 attenuates viability and migration in NSCLC. (A) PVT1 level in normal bronchial epithelial cells HBE and lung cancer cells A549, NCI-H1650 and HCC827. (B) Transfection efficacy of si-PVT1 in A549 cells. (C) Viability in A549 cells transfected with si-NC or si-PVT1. (D) Wound closure in A549 cells transfected with si-NC or si-PVT1 at 0 and 24 h (magnification, ×40). PVT1, plasmacytoma variant translocation 1; NSCLC, non-small cell lung cancer. ***P<0.001.
PVT1 influences proliferative and migratory abilities of NSCLC by targeting miRNA-526b
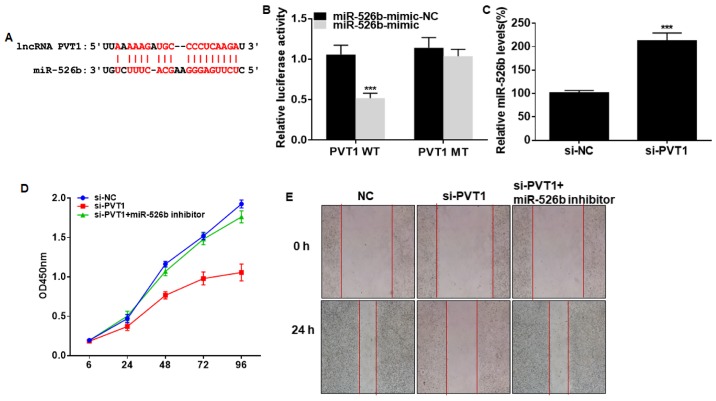
Potential binding sites were predicted in promoter regions of PVT1 and miRNA-526b (Fig. 3A). Overexpression of miRNA-526b quenched the luciferase activity in cells transfected with PVT1 WT, verifying the binding between PVT1 and miRNA-526b (Fig. 3B). By transfection of si-PVT1, miRNA-526b level in A549 cells was remarkably upregulated (Fig. 3C). Interestingly, the reduced viability and wound closure percentage in A549 cells with PVT1 knockdown were partially reversed after silencing of miRNA-526b (Fig. 3D and E). Hence, miRNA-526b was responsible for the progression of NSCLC regulated by PVT1.
Figure 3.
PVT1 influences proliferative and migratory abilities of NSCLC by targeting miRNA-526b. (A) Binding sites between PVT1 and miRNA-526b. (B) Luciferase activity in A549 cells co-transfected with miRNA-526b mimic/NC and PVT1 WT/PVT1 MT. (C) miRNA-526b level in A549 cells transfected with si-NC or si-PVT1. (D) Viability in A549 cells transfected with si-NC, si-PVT1 or si-PVT1 + miRNA-526b inhibitor. (E) Wound closure in A549 cells transfected with si-NC, si-PVT1 or si-PVT1 + miRNA-526b inhibitor at 0 and 24 h (magnification, ×40). PVT1, plasmacytoma variant translocation 1; NSCLC, non-small cell lung cancer. ***P<0.001.
miRNA-526b influences proliferative and migratory abilities of NSCLC by downregulating EZH2
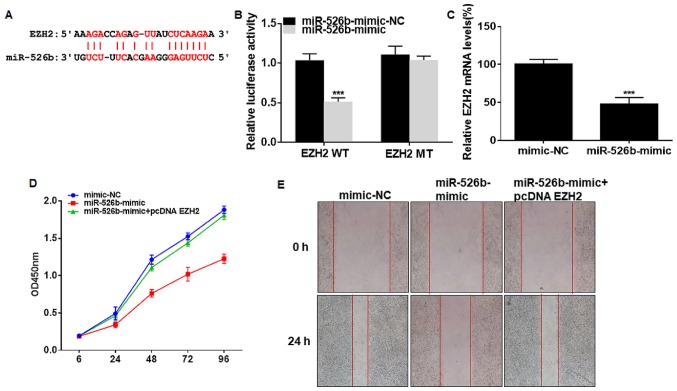
The presence of binding sites were predicted in promoter regions of EZH2 and miRNA-526b (Fig. 4A). Dual-luciferase reporter gene assay verified the online prediction that EZH2 was the downstream target of miRNA-526b (Fig. 4B). EZH2 level was downregulated in A549 cells overexpressing miRNA-526b (Fig. 4C). Transfection of miRNA-526b mimic suppressed viability and wound closure percentage in A549 cells, and were reversed by co-transfection of pcDNA EZH2 (Fig. 4D and E). As a result, EZH2 was involved in miRNA-526b-regulated progression of NSCLC.
Figure 4.
miRNA-526b influences proliferative and migratory abilities of NSCLC by downregulating EZH2. (A) Binding sites between EZH2 and miRNA-526b. (B) Luciferase activity in A549 cells co-transfected with miRNA-526b mimic/NC and EZH2 WT/EZH2 MT. (C) EZH2 level in A549 cells transfected with NC or miRNA-526b mimic. (D) Viability in A549 cells transfected with NC, miRNA-526b mimic or miRNA-526b mimic + pcDNA EZH2. (E) Wound closure in A549 cells transfected with NC, miRNA-526b mimic or miRNA-526b mimic + pcDNA EZH2 at 0 and 24 h (magnification, ×40). PVT1, plasmacytoma variant translocation 1; NSCLC, non-small cell lung cancer. ***P<0.001.
Discussion
NSCLC is a fatal malignancy with high morbidity and mortality rates. Most NSCLC patients miss the chance for surgery due to the atypical symptoms in the early stage (17). Once tumor metastasis occurs, the NSCLC patients cannot undergo radical resection. The prognosis of NSCLC is very poor, leading to extremely high mortality (2). Target inhibition of metastasis is of significance to improve the clinical outcome of advanced NSCLC patients. Accumulating evidence has proposed that dysregulated lncRNAs are closely related with the occurrence and progression of NSCLC. These lncRNAs may be utilized as diagnostic, therapeutic and prognostic markers for NSCLC (18,19). Zhu et al (20) pointed out that linc00312 is downregulated in NSCLC and exerts a tumor-suppression effect. UCA1 is identified to be highly expressed in NSCLC tissues (21). Silence of UCA1 suppresses the malignant proliferative ability of NSCLC. Our findings uncovered that PVT1 was upregulated in NSCLC tissues and cells. Knockdown of PVT1 remarkably attenuated proliferative and migratory abilities of lung cancer cells.
Regulatory mechanisms of lncRNAs in mediating cell behavior mainly include: i) lncRNAs regulate promoter transcription of target genes; ii) lncRNAs regulate activities of RNA polymerase II; iii) lncRNAs and transcripts of target genes contribute to the formation of complementary double strands; iv) lncRNAs regulate protein activities by binding to them; v) lncRNAs alter the intracellular localization of target proteins. Recently, a novel regulatory mechanism was proposed. lncRNAs can silence target miRNA-mediated gene expression through sponging these miRNAs, that is, the ceRNA hypothesis (22). Previous studies have discovered many miRNAs that are related to NSCLC metastasis (23). Hence, we speculated that PVT1 may serve as a ceRNA to target certain miRNAs, thus influencing the progression of NSCLC. Through bioinformatics prediction and dual-luciferase reporter gene assay verification, miRNA-526b was confirmed to be the downstream target of PVT1. Functional experiments confirmed that miRNA-526b was necessary for PVT1-regulated NSCLC progression.
Upregulation of EZH2 in tumor diseases markedly stimulates proliferative rate of tumor cells. During B cell differentiation in lymphoid tissues, EZH2 exerts a vital function (24). EZH2 is also a prognostic marker. High level of EZH2 predicts worse prognosis in breast cancer and bladder cancer (25). A relevant study demonstrated that overexpression of miR-138 induced apoptosis and arrested cell cycle in lung cancer cells by downregulating EZH2 (26). In this study, the presence of binding sites between EZH2 and miRNA-526b was identified. Importantly, the attenuated viability and wound closure ability in A549 cells overexpressing miRNA-526b were reversed after EZH2 overexpression.
Collectively, PVT1/miRNA-526b/EZH2 regulatory loop was discovered accelerating the malignant progression of NSCLC via influencing tumor cell proliferation and migration. Our findings provide new directions for clinical treatment of NSCLC.
In conclusion, PVT1 is upregulated in NSCLC, and predicts a poor prognosis. PVT1 accelerates the progression of NSCLC via targeting miRNA-526b/EZH2 regulatory loop.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors contributions
CQ, SL and SY designed the study and performed the experiments, CQ and DS collected the data, SL and SY analyzed the data, CQ, SL and SY prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Hainan Provincial People's Hospital (Haikou, China). Signed informed consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
References
- 1.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benderli Cihan Y. ALK inhibitors and cranial radiotherapy in brain metastasis from NSCLC. J BUON. 2018;23:1558. [PubMed] [Google Scholar]
- 3.Yang B, Zheng D, Zeng Υ, Qin A, Gao J, Yu G. Circulating tumor cells predict prognosis following secondline AZD 9291 treatment in EGFR-T790M mutant non-small cell lung cancer patients. J BUON. 2018;23:1077–1081. [PubMed] [Google Scholar]
- 4.Hu Z, Chen J, Tian T, Zhou X, Gu H, Xu L, Zeng Y, Miao R, Jin G, Ma H, et al. Genetic variants of miRNA sequences and non-small cell lung cancer survival. J Clin Invest. 2008;118:2600–2608. doi: 10.1172/JCI34934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu JY, Yao J, Li XM, Song YC, Wang XQ, Li YJ, Yan B, Jiang Q. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014;5:e1506. doi: 10.1038/cddis.2014.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng N, Li X, Zhao C, Ren S, Chen X, Cai W, Zhao M, Zhang Y, Li J, Wang Q, et al. Microarray expression profile of long non-coding RNAs in EGFR-TKIs resistance of human non-small cell lung cancer. Oncol Rep. 2015;33:833–839. doi: 10.3892/or.2014.3643. [DOI] [PubMed] [Google Scholar]
- 7.Vikram R, Ramachandran R, Abdul KS. Functional significance of long non-coding RNAs in breast cancer. Breast Cancer. 2014;21:515–521. doi: 10.1007/s12282-014-0554-y. [DOI] [PubMed] [Google Scholar]
- 8.Tseng YY, Moriarity BS, Gong W, Akiyama R, Tiwari A, Kawakami H, Ronning P, Reuland B, Guenther K, Beadnell TC, et al. PVT1 dependence in cancer with MYC copy-number increase. Nature. 2014;512:82–86. doi: 10.1038/nature13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding C, Yang Z, Lv Z, Du C, Xiao H, Peng C, Cheng S, Xie H, Zhou L, Wu J, et al. Long non-coding RNA PVT1 is associated with tumor progression and predicts recurrence in hepatocellular carcinoma patients. Oncol Lett. 2015;9:955–963. doi: 10.3892/ol.2014.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang YR, Zang SZ, Zhong CL, Li YX, Zhao SS, Feng XJ. Increased expression of the lncRNA PVT1 promotes tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol. 2014;7:6929–6935. [PMC free article] [PubMed] [Google Scholar]
- 11.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pekarsky Y, Santanam U, Cimmino A, Palamarchuk A, Efanov A, Maximov V, Volinia S, Alder H, Liu CG, Rassenti L, et al. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 2006;66:11590–11593. doi: 10.1158/0008-5472.CAN-06-3613. [DOI] [PubMed] [Google Scholar]
- 13.Zhang ZY, Fu SL, Xu SQ, Zhou X, Liu XS, Xu YJ, Zhao JP, Wei S. By downregulating Ku80, hsa-miR-526b suppresses non-small cell lung cancer. Oncotarget. 2015;6:1462–1477. doi: 10.18632/oncotarget.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsang DP, Cheng AS. Epigenetic regulation of signaling pathways in cancer: Role of the histone methyltransferase EZH2. J Gastroenterol Hepatol. 2011;26:19–27. doi: 10.1111/j.1440-1746.2010.06447.x. [DOI] [PubMed] [Google Scholar]
- 15.Tonini T, DAndrilli G, Fucito A, Gaspa L, Bagella L. Importance of Ezh2 polycomb protein in tumorigenesis process interfering with the pathway of growth suppressive key elements. J Cell Physiol. 2008;214:295–300. doi: 10.1002/jcp.21241. [DOI] [PubMed] [Google Scholar]
- 16.Riquelme E, Behrens C, Lin HY, Simon G, Papadimitrakopoulou V, Izzo J, Moran C, Kalhor N, Lee JJ, Minna JD, et al. Modulation of EZH2 expression by MEK-ERK or PI3K-AKT signaling in lung cancer is dictated by different KRAS oncogene mutations. Cancer Res. 2016;76:675–685. doi: 10.1158/0008-5472.CAN-15-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Li H. CircRNA circ_0067934 silencing inhibits the proliferation, migration and invasion of NSCLC cells and correlates with unfavorable prognosis in NSCLC. Eur Rev Med Pharmacol Sci. 2018;22:3053–3060. doi: 10.26355/eurrev_201805_15063. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Lin J, Liu T, Chen T, Pan S, Huang W, Li S. Analysis of lncRNA expression profiles in non-small cell lung cancers (NSCLC) and their clinical subtypes. Lung Cancer. 2014;85:110–115. doi: 10.1016/j.lungcan.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Wei MM, Zhou GB. Long non-coding RNAs and their roles in non-small-cell lung cancer. Genomics Proteomics Bioinformatics. 2016;14:280–288. doi: 10.1016/j.gpb.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Q, Lv T, Wu Y, Shi X, Liu H, Song Y. Long non-coding RNA 00312 regulated by HOXA5 inhibits tumour proliferation and promotes apoptosis in non-small cell lung cancer. J Cell Mol Med. 2017;21:2184–2198. doi: 10.1111/jcmm.13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nie W, Ge HJ, Yang XQ, Sun X, Huang H, Tao X, Chen WS, Li B. lncRNA-UCA1 exerts oncogenic functions in non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett. 2016;371:99–106. doi: 10.1016/j.canlet.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 22.Chan JJ, Tay Y. Noncoding RNA: RNA regulatory networks in cancer. Int J Mol Sci. 2018;19:19. doi: 10.3390/ijms19051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legras A, Pécuchet N, Imbeaud S, Pallier K, Didelot A, Roussel H, Gibault L, Fabre E, Le Pimpec-Barthes F, Laurent-Puig P, et al. Epithelial-to-mesenchymal transition and microRNAs in lung cancer. Cancers (Basel) 2017;9:9. doi: 10.3390/cancers9080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Garcia E, Licht JD. Deregulation of H3K27 methylation in cancer. Nat Genet. 2010;42:100–101. doi: 10.1038/ng0210-100. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Zhang H, Zhao M, Lv Z, Zhang X, Qin X, Wang H, Wang S, Su J, Lv X, et al. MiR-138 inhibits tumor growth through repression of EZH2 in non-small cell lung cancer. Cell Physiol Biochem. 2013;31:56–65. doi: 10.1159/000343349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.