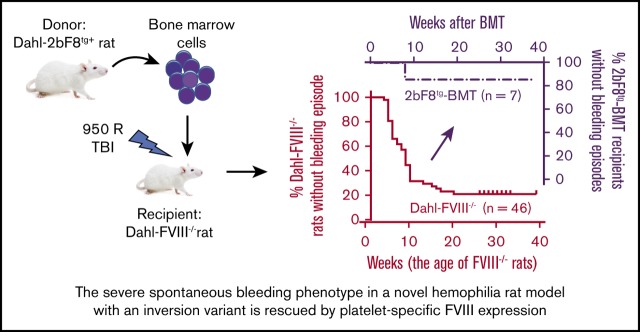
Key Points
A novel HA rat model caused by an inversion exhibits a severe spontaneous bleeding phenotype.
The severe spontaneous bleeding phenotype in HA rats is rescued by platelet-targeted FVIII expression.
Abstract
Previous studies have shown that platelet-specific factor VIII (FVIII) expression (2bF8) restores hemostasis and induces immune tolerance in hemophilia A (HA) mice even with preexisting inhibitors. Here we investigated for the first time whether platelet FVIII expression can prevent severe spontaneous bleeding in rat HA, a model mimicking the frequent spontaneous bleeding in patients with severe HA. A novel FVIII−/− rat model in a Dahl inbred background (Dahl-FVIII−/−) with nearly the entire rat FVIII gene inverted was created by using a CRISPR/Cas9 strategy. There was no detectable FVIII in plasma. Spontaneous bleeding in the soft tissue, muscles, or joints occurred in 100% of FVIII−/− rats. Sixty-one percent developed anti-FVIII inhibitors after ≥2 doses of recombinant human FVIII infusion. However, when 2bF8 transgene was crossed into the FVIII−/− background, none of the resulting 2bF8tg+FVIII−/− rats (with platelet FVIII levels of 28.26 ± 7.69 mU/108 platelets and undetectable plasma FVIII) ever had spontaneous bleeding. When 2bF8tg bone marrow (BM) was transplanted into FVIII−/− rats, only 1 of 7 recipients had a bruise at the early stage of BM reconstitution, but no other spontaneous bleeding was observed during the study period. To confirm that the bleeding diathesis in FVIII−/− rats was ameliorated after platelet FVIII expression, rotational thromboelastometry and whole-blood thrombin generation assay were performed. All parameters in 2bF8tg BM transplantation recipients were significantly improved compared with FVIII−/− control rats. Of note, neither detectable levels of plasma FVIII nor anti-FVIII inhibitors were detected in 2bF8tg BM transplantation recipients. Thus, platelet-specific FVIII expression can efficiently prevent severe spontaneous bleeding in FVIII−/− rats with no anti-FVIII antibody development.
Visual Abstract
Introduction
Hemophilia A (HA) is a genetic bleeding disorder resulting from a factor VIII (FVIII) deficiency. Protein replacement therapy is effective but requires repeatedly accessing vessels for infusion. Furthermore, up to 30% of patients develop anti-FVIII inhibitory antibodies (referred to as inhibitors) after protein replacement therapy.1-3 Gene therapy is a new approach for the treatment of HA, which may provide a cure for the disease if successful.4-6 Data from ongoing clinical trials using adeno-associated virus (AAV)–mediated liver-directed FVIII expression are very encouraging.7 However, children and adults with severe liver disease or antibodies against AAV, which are present in 40% to 50% of the population, are excluded from AAV-mediated liver-directed gene therapy.7,8
Previous studies by our group and others have shown that platelet-specific FVIII expression can rescue the bleeding phenotype in HA mice in both the inhibitor and noninhibitor models.9-17 Clinical efficacy has been further proven in a canine HA noninhibitor model.18 Although these models are informative, neither the canine nor mouse model entirely recapitulates human disease because (1) neither HA mice nor dogs exhibit the high-frequency spontaneous bleeding seen in patients with severe HA and (2) canine platelets do not contain VWF as a carrier protein for FVIII. Thus, dogs store and release FVIII differently, thereby precluding relevant examination of the efficacy of platelet gene therapy in the presence of FVIII inhibitors because the canine system is not analogous to humans, in whom platelets do contain VWF.
The efficacy of platelet FVIII gene therapy therefore needs to be examined in an animal model more representative of human disease. Recently, a HA rat model in an outbred Sprague Dawley (SD) background was developed by Nielsen et al19 using a zinc-finger nuclease strategy resulting in a 13 bp deletion in exon 16 of FVIII. The model presents with a severe spontaneous bleeding phenotype and has been used to evaluate the efficacy of novel FVIII therapeutic agents.20 Because bone marrow transplantation (BMT) is required in our platelet-specific gene therapy protocol,21 graft-versus-host immune responses could be a potential concern using an outbred colony. The current study used a CRISPR/Cas9 strategy to develop a novel HA rat model in an inbred Dahl genetic background with an inversion to simulate the pathology observed in patients with severe HA. We evaluated whether the severe spontaneous bleeding phenotype in HA could be prevented after platelet FVIII expression.
Materials and methods
Rats
All animals were kept in pathogen-free microisolator cages at the animal facilities operated by the Medical College of Wisconsin. Isoflurane or ketamine/xylazine was used for anesthesia. Buprenorphine was used for pain relief in cases of spontaneous bleeds or inflammation after bleeding. All animal studies were performed according to a protocol approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. Both male and female animals were used in our studies.
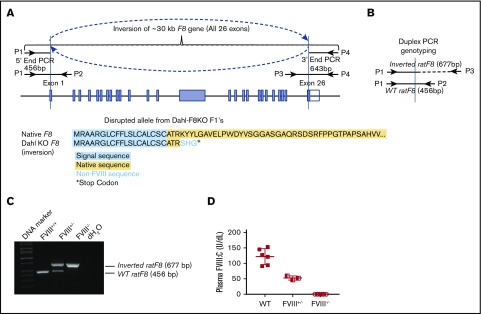
Wild-type (WT) rats were purchased from the Charles River Laboratories (Wilmington, MA). HA rats with an FVIII deficiency (FVIII−/−) in an outbred SD genetic background (SD-FVIII−/−) were a gift from Novo Nordisk (Bagsvaerd, Denmark) and were maintained in our facility. FVIII−/− rats in an inbred Dahl background (Dahl-FVIII−/−) were generated by the Medical College of Wisconsin Gene Editing Rat Resource Center using a CRISPR/Cas9 strategy as reported.22 The CRISPR guide RNA sequences used to create FVIII-deficient rats were GGCGCCAGGTAGTACTTCC (5′ end) and GAATGAGTGAATGAAGTTCA (3′ end). Disruption of the 5′- and 3′-ends of the rat F8 gene was screened by polymerase chain reaction (PCR) using primers 5′-end forward (GCGGATGTAAGGATTCGCTCA [P1]) and reverse (GAGGACCGACAGTTACCTGGA [P2]) and 3′-end forward (ATTCCACATCTGGACCCACG [P3]) and reverse (GGTCACGCAATGTCAGAAAGA [P4]). The primers used for PCR genotyping included a duplex 3-primer set of P1, P2, and P3 (Figure 1A-B). The disrupted rat FVIII variant was confirmed by sequencing. Unless otherwise specified, the animals used in this study were in a Dahl background.
Figure 1.
An inbred Dahl-FVIII−/−rat model is established by using a CRISPR/Cas9 strategy. (A) Schematic diagram of the inverted F8 gene in our Dahl-FVIII−/− rat model. All 26 exons were inverted. (B) Schematic diagram of duplex PCR genotyping. (C) PCR detection of the inverted rat F8 gene. Shown is a representative image from duplex PCR genotyping using 3 primers (P1, P2, and P3 shown in panels A and B). DNA was purified from peripheral leukocytes. A fragment of 677 bp was amplified from the inverted rat F8 gene. A fragment of 456 bp was amplified from the WT rat F8 gene. (D) Functional FVIII:C levels in plasma. Blood samples were collected from the ventral tail artery, and plasmas were isolated. FVIII:C levels were determined by using a chromogenic assay. There was no detectable FVIII:C in plasma of Dahl-FVIII−/− rats.
Platelet FVIII expressing transgenic rats (2bF8tg), in which a human B-domain–deleted FVIII expression cassette is driven by the human platelet-specific αIIb promoter (2bF8), were generated by lentivirus-mediated oocyte transduction transgenesis using a protocol similar to that described in our mouse model studies.14,23,24 Briefly, the 2bF8 lentivirus was generated as described in our previous report25 and used to transduce fertilized oocytes. The 2-cell-stage embryos were transferred into pseudo-pregnant Dahl-WT female rats. The 2bF8 transgene-positive pups (F0) were identified by using PCR as previously reported.9 2bF8 transgene-positive pups were crossed with Dahl-WT rats for 2 generations (F1 and F2) to segregate the potential multiple copies of 2bF8 transgene. The 2bF8 transgene was then subsequently crossed onto the FVIII−/− Dahl background (2bF8tg+FVIII−/−). 2bF8tg rats were used as donors for BMT.
Bone marrow transplantation
Dahl-2bF8tg rats were euthanized, and tibias and femurs were isolated. BM cells were flushed from tibias and femurs by using Dulbecco’s phosphate-buffered saline (Thermo Fisher Scientific, Waltham, MA) containing 0.5% bovine serum albumin, 2 mM EDTA (MilliporeSigma, Burlington, MA), and 1× penicillin, streptomycin, and glutamine (Thermo Fisher Scientific). A single-cell suspension was prepared by dispersing BM cell aggregates. Red blood cells were lysed by using the RBC Lysis Buffer (eBioscience, San Diego, CA). Approximately 7 to 10 × 107 nucleated cells were resuspended in 2 mL of Dulbecco’s phosphate-buffered saline; they were then transplanted via lateral tail vein injection into each Dahl-FVIII−/− recipient (aged between 8 and 16 weeks) that was preconditioned with 950 R total-body irradiation using a Gammacell 40 irradiator (Best Theratronics, Ottawa, ON, Canada). Starting at 2 weeks after BMT, blood samples were collected for assays.
Blood sample collection and assays
Animals were anesthetized by using isoflurane inhalation, and 200 to 500 μL of blood was collected from the ventral tail artery by using a 1 mL syringe attached with a 25-gauge needle. Blood samples were anticoagulated by using one-tenth volume of 4% sodium citrate (Jorgensen Laboratories, Inc., Loveland, CO). The samples were used for blood counts and plasma and platelet isolation as described in our previous reports10,26 or for whole-blood assays, including native rotational thromboelastometry (ROTEM)23 and native whole-blood thrombin generation assay (nWB-TGA).27
FVIII assays
FVIII expression levels in platelet lysates and plasmas were quantitated by a modified chromogenic assay using the Coatest VIII:C/4 Kit (DiaPharma, Franklin, OH) as previously described.9,10,15,25 Briefly, isolated platelets were lysed in 0.5% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate; MP Biomedicals, Irvine, CA) dissolved in an assay buffer with a concentration of 1 × 107 platelets per 100 μL for assay. Plasmas were diluted 1:80 with 1× Coatest buffer for assay. Recombinant human FVIII (rhFVIII; Xyntha, Wyeth Pharmaceuticals, Collegeville, PA) was used to construct a standard curve. Samples from WT rats were used as controls.
Phenotypic correction assessment and treatment
Animals were examined daily for spontaneous bleeding episodes, such as bleeding into the skin, soft tissues, muscles, and joints. All episodes observed were recorded. Animals with major bleeding episodes, such as a bruise accompanied by visible swelling or reluctance to move limbs, were treated by infusion with full-length rhFVIII (a gift from Novo Nordisk) at a dose of 50 U/kg via the lateral tail vein. Animals were euthanized if the clinical symptoms did not improve after 2 consecutive treatments. Blood samples were collected 4 weeks after rhFVIII infusion, and anti-FVIII inhibitory antibody (inhibitor) titers were determined by using a chromogenic-based Bethesda assay as described in our previous reports.9,10,25,26 Hemostatic functions in whole blood were determined according to ROTEM analysis and nWB-TGA following the procedures as described in our previous reports.23,27
Statistical analysis
Data are presented as the mean ± SD. The Gehan-Breslow-Wilcoxon test was used to compare bleeding events between groups. The Fisher's exact test was used to compare the incidence of inhibitor development in FVIII−/− rats after rhFVIII infusion or 2bF8tg-BMT. The unpaired 2-tailed Student t test was used to evaluate the statistical difference between experimental groups. The 1-way analysis of variance followed by the Tukey’s multiple comparisons test was used to determine whether there were statistically significant differences between the means of ≥3 groups. P < .05 was considered statistically significant.
Results
Establishing an inbred FVIII−/− rat model
Using a CRISPR/Cas9 strategy, we generated a novel HA rat model in a Dahl inbred congenic background (Dahl-FVIII−/−). In this model, nearly the entire rat F8 gene was inverted, causing translational stop 6 amino acids after the signal sequence (Figure 1A). It should be noted that the F8 gene in rat is located on autosomal chromosome 18.28,29 Animals with the heterozygous or homozygous inversion variant were first screened by using PCR genotyping and then confirmed according to the chromogenic FVIII activity (FVIII:C) assay. Using a duplex (3-primer) PCR reaction, a 456 bp fragment was amplified from the WT rat F8 gene by primers P1 and P2. A 677 bp fragment was amplified by primers P1 and P3 (Figure 1B) if the animal had the inverted variant. Thus, for WT (FVIII+/+) rats, only the 456 bp fragment was detected; with the homozygous FVIII inverted variant (FVIII−/−), only the 677 bp band was detected. Heterozygous animals (FVIII+/−) produce both 456 bp and 677 bp bands (Figure 1C). To determine FVIII expression levels in FVIII−/− rats, a chromogenic assay was used. As shown in Figure 1D, there was no detectable FVIII:C in plasma from Dahl-FVIII−/− rats (n = 8) (the limit of detection for the plasma FVIII:C assay is 1 U/dL). In contrast, there was 121.8 ± 25.5 U/dL (n = 6) in Dahl-WT control rats and 53.8 ± 6.7 (n = 3) in heterozygous (Dahl-FVIII+/−) animals.
Characterizing the bleeding phenotype in Dahl-FVIII−/− rats
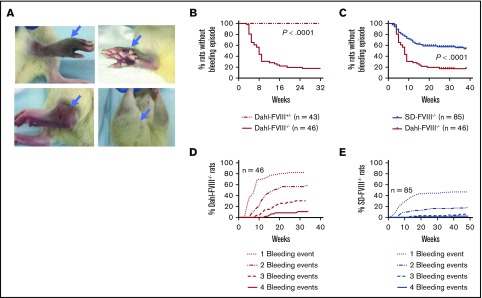
We then assessed the bleeding phenotype in our newly established Dahl-FVIII−/− rats. Spontaneous bleeding episodes, which presented as bruising and/or swelling (Figure 2A), were observed during the daily activities of the mice in a broad range of tissues, mainly in the joints but also in soft tissues, muscles, and eyes. The most frequent pathology we observed were joint injuries but only in homozygous FVIII−/− animals, which have no detectable FVIII:C in plasma. No heterozygous FVIII+/− animals ever have a spontaneous bleed. Bleeding episodes occurred in 56.5% of homozygous affected animals (n = 46) by the age of 8 weeks and 82.6% by the age of 32 weeks (Figure 2B). This finding was significantly higher (P < .0001) than the occurrence of spontaneous bleeding in the SD HA colony (SD-FVIII−/−) developed by Nielsen et al.19 In the SD-FVIII−/− rat model, spontaneous bleeds occurred in 23.3% and 42.1% of homozygous affected animals (n = 85) that were housed in our facility by the age of 8 and 32 weeks, respectively (Figure 2C). Likewise, the percentages of homozygous affected animals with 2, 3, or 4 bleeding episodes in the Dahl- FVIII−/− colony were significantly higher than those in the SD-FVIII−/− colony (Figure 2D-E). Of note, by the age of 64 weeks, all (112 of 112) homozygous affected Dahl-FVIII−/− rats experienced bleeding episodes (data not shown).
Figure 2.
Severe spontaneous bleeding occurs in Dahl-FVIII−/−rats. Affected animals were closely monitored, and bleeding episodes were recorded. (A) Representative images of spontaneous bleeding in our inbred Dahl-FVIII−/− rats. (B) Spontaneous bleeding events in homozygous (FVIII−/−) and heterozygous (FVIII+/−) Dahl rats by 32 weeks of age. (C) Comparison of the occurrence of spontaneous bleeding episodes in Dahl-FVIII−/− (inbred) to SD-FVIII−/− (outbred) rats housed in our facility. SD-FVIII−/− rats were developed by Nielsen et al19 using a zinc-finger nuclease strategy resulting in a 13 bp deletion in exon 16 of the F8 gene. (D) Percentage of Dahl-FVIII−/− rats that experienced bleeding events by 32 weeks of age. (E) Percentage of SD-FVIII−/− rats that experienced events by 32 weeks of age.
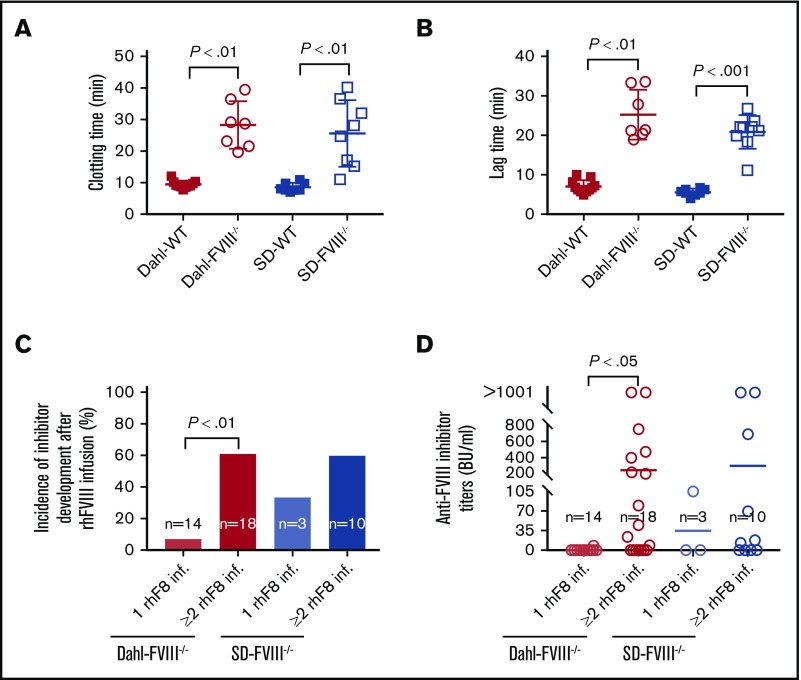
The hemostatic functions in the whole blood of FVIII−/− rats were assessed by using ROTEM and nWB-TGA. The whole-blood clotting time in Dahl-FVIII−/− rats determined by ROTEM analysis was significantly longer than that obtained in Dahl-WT rats (28.3 ± 7.5 minutes [n = 7] vs 9.5 ± 1.1 minutes [n = 11], respectively]). Likewise, the whole-blood clotting time in the SD-FVIII−/− group was significantly longer than in the SD-WT group (25.6 ± 10.5 minutes [n = 8] vs 8.6 ± 1.3 minutes [n = 8]) (Figure 3A). The lag time in FVIII−/− rats measured according to nWB-TGA was significantly longer than that obtained in WT control rats, whether in a Dahl or SD background (25.2 ± 6.3 minutes vs 7.1 ± 1.6 minutes, and 20.8 ± 4.3 minutes vs 5.6 ± 0.9 minutes) (Figure 3B). There were no statistically significant differences in the clotting time and lag time between the Dahl-FVIII−/− and SD-FVIII−/− groups as determined by using ROTEM and TGA.
Figure 3.
Dahl-FVIII−/−rats exhibit impaired blood coagulation functions and are prone to development of anti-FVIII immune responses. Pathophysiological properties in Dahl-FVIII−/− rats were characterized by native ROTEM analysis and TGA of whole blood. Blood samples were collected from the ventral tail artery by using sodium citrate as an anticoagulant. (A) Whole-blood clotting time determined by using native ROTEM analysis. Recalcified whole-blood samples were used for ROTEM analysis. (B) Lag time determined by using nWB-TGA. Recalcified whole blood samples were used for nWB-TGA. (C) Incidence of anti-FVIII inhibitor development in FVIII−/− rats that had spontaneous bleeding episodes and received human recombinant FVIII infusion. (D) Inhibitor titers in FVIII−/− rats that had spontaneous bleeding episodes and received human recombinant FVIII infusion. Blood samples were collected from animals at least 2 weeks after rhFVIII infusion, and anti-FVIII inhibitor titers were determined by using a chromogenic-based modified Bethesda assay.
To assess if FVIII−/− rats develop anti-FVIII inhibitors after rhFVIII treatment, we monitored inhibitor titers by using a chromogenic-based modified Bethesda assay. The incidence of inhibitor development significantly increased when Dahl-FVIII−/− rats were exposed to ≥2 rhFVIII infusions compared with 1 infusion. Similar to SD-FVIII−/− rats, 61% of animals developed anti-FVIII inhibitors after receiving ≥2 doses of rhFVIII treatment (Figure 3C). The inhibitor titers in animals that developed inhibitors after rhFVIII infusions varied from 8 to >1000 Bethesda units (BU)/mL, and there was no statistically significant difference between the Dahl-FVIII−/− and SD-FVIII−/− groups (Figure 3D). This finding shows that similar to SD-FVIII−/− rats, Dahl-FVIII−/− rats are likely to develop anti-FVIII inhibitory antibodies after ≥2 doses of rhFVIII treatment. Thus, our novel Dahl-FVIII−/− rat model is a unique animal model that mimics human disease with a high incidence of inhibitor development after FVIII infusion and displays the most severe spontaneous bleeding phenotype yet reported.
Targeting FVIII expression to platelets prevents spontaneous bleeding in Dahl-FVIII−/− rats
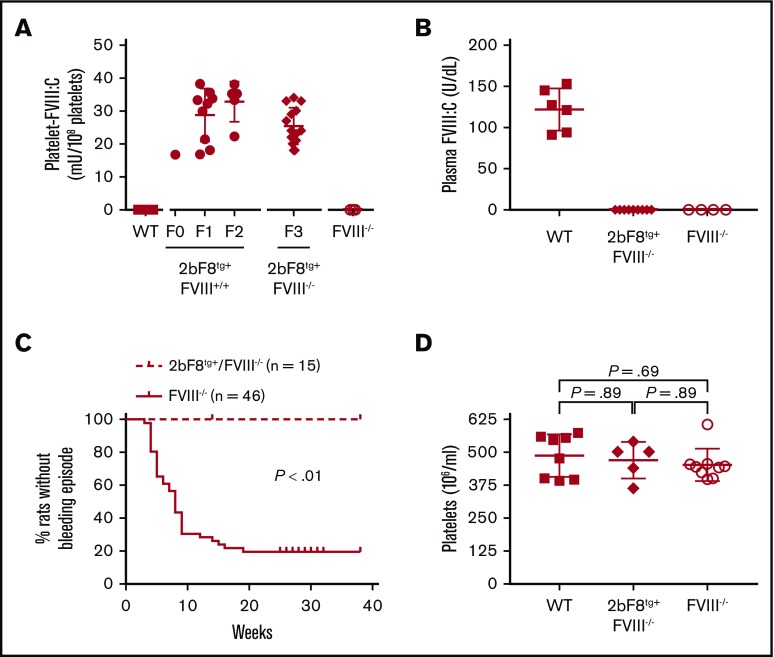
To investigate if platelet-derived FVIII can rescue the spontaneous bleeding phenotype in HA rats, we generated transgenic rats (2bF8tg) in which FVIII expression is under control of the 2bF8 in the Dahl-background using lentivirus-mediated oocyte transduction transgenesis. To achieve 2bF8tg+FVIII−/− animals, 2bF8 transgene-positive animals, which were confirmed by using PCR and platelet lysate FVIII:C assay, were crossed with Dahl-FVIII−/− rats. Platelet FVIII expression levels in 2bF8tg+FVIII−/− rats were 25.4 ± 5.6 mU/108 platelets (n = 14) (Figure 4A). There was no detectable plasma FVIII in 2bF8tg+FVIII−/− rats (Figure 4B). Importantly, none of the 2bF8tg+FVIII−/− rats had any spontaneous bleeding episodes, which was significantly different compared with the severe bleeding phenotype observed in Dahl-FVIII−/− rats (P < .01) (Figure 4C). The platelet count in the 2bF8tg+ group was similar to that in the WT or FVIII−/− groups (Figure 4D).
Figure 4.
The spontaneous bleeding phenotype in Dahl-FVIII−/−rats is rescued by 2bF8 lentivirus-mediated transgenesis. Transgenic Dahl rats were generated by 2bF8 lentivirus-mediated oocyte transduction transgenesis; 2bF8 transgene was then crossed onto the Dahl-FVIII−/− background. Animals were monitored for bleeding episodes. Platelets and plasmas were isolated from blood samples collected via ventral tail artery blood draws. FVIII expression levels in platelet lysates and plasma were determined by a modified chromogenic assay. Blood samples from WT and FVIII−/− Dahl rats were used as controls. B-domain–deleted rhFVIII (Xyntha) was used as a reference standard for FVIII:C assays. (A) FVIII expression levels in 2bF8 transgenic rat platelets. (B) FVIII expression levels in 2bF8 transgenic rat plasma. (C) Occurrence of bleeding episodes in 2bF8 transgenic rats. (D) Platelet counts in 2bF8 transgenic rats.
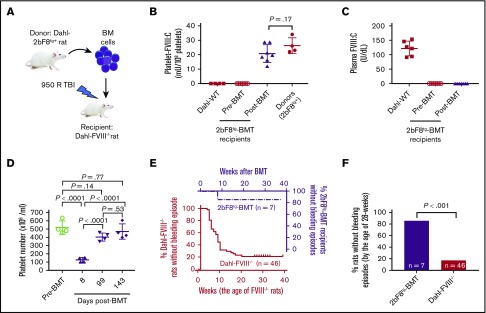
To investigate if the clinical efficacy of platelet-derived FVIII can be transferred by BMT, BM nucleated cells were isolated from femurs and tibias of 2bF8 transgenic rats and transplanted into Dahl-FVIII−/− recipients preconditioned with 950 cGy total-body irradiation as shown in a schematic diagram (Figure 5A). Animals were closely monitored after BMT, and blood samples were collected for analysis. We found that platelet FVIII was detected in recipients within 2 weeks after BMT and sustained during the study course. The average level of platelet FVIII in recipients after BMT was 20.7 ± 6.2 mU/108 platelets (n = 7), which was similar to the levels observed in their donors (Figure 5B). This amount of platelet FVIII corresponds to 14.96% of FVIII:C in whole blood in normal Dahl-WT rats, based on an average platelet number of 4.4 × 108/mL in BMT recipients (Figure 5D) and plasma FVIII:C of 121.8 U/dL in Dahl-WT (Figure 5C) [(20.7 mU/108 platelets) × (4.4 × 108 platelets/mL)]/[(1218 mU/mL)/2] × 100% = 14.96%]. There was no detectable plasma FVIII in BMT recipients. The platelet count was comparable to that obtained in the animals before BMT once BM was fully reconstituted (Figure 5D).
Figure 5.
BMT of 2bF8 genetically modified hematopoietic stem cells rescues the severe spontaneous hemorrhagic phenotype in Dahl-FVIII−/−rats. BM nucleated cells from 2bF8 transgenic rats were transplanted into 8- to 16-week-old Dahl-FVIII−/− rats preconditioned with 950 cGy total-body irradiation. Animals were closely monitored for bleeding episodes. After BM reconstitution, blood samples were collected from the ventral tail artery at various time points. Platelets and plasma were isolated for FVIII assays. Functional FVIII expression levels were determined by using a modified chromogenic assay of platelet lysates and plasmas. B-domain–deleted rhFVIII (Xyntha) was used as a reference standard for FVIII:C assays. (A) A schematic diagram for BMT. (B) Platelet FVIII expression levels in Dahl-FVIII−/− recipients after receiving BMT from 2bF8tg rats. Data shown are the average platelet FVIII:C calculated from 3 time points after BMT. Platelets isolated from Dahl-2bF8tg donors and Dahl-WT were used as controls. (C) Plasma FVIII expression levels in Dahl-FVIII−/− rats after receiving BMT from 2bF8tg animals. (D) Platelet counts in peripheral blood from recipients at various time points after BMT. Platelet counts were analyzed by using the Vet ABC Hematology Analyzer (scil animal care company, Gernee, IL). (E) Occurrence of spontaneous bleeding episodes in Dahl-FVIII−/− recipients after receiving BMT from 2bF8tg rats. The bleeding event in Dahl-FVIII−/− rats without BMT was used as a control. (F) Frequency of spontaneous bleeding episodes in 2bF8tg-BMT recipients by the age of 28 weeks compared with Dahl-FVIII−/− rats.
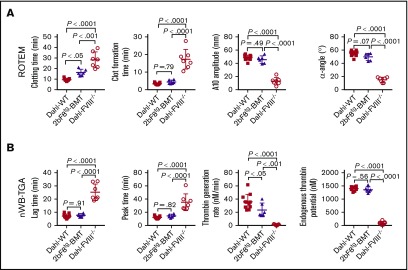
As shown in Table 1, four of seven rats before BMT experienced one or two spontaneous bleeds. However, after 2bF8 transgenic BM cells were transplanted into Dahl-FVIII−/− rats, only 1 of 7 recipients had a minor bruise (0.5 × 0.5 cm2) in the right back heel area at the early stage (8 weeks) of BM reconstitution, and the animal was treated with 1 dose of rhFVIII; no other spontaneous bleeding was observed during the study period. Thus, the frequency of spontaneous bleeds in the 2bF8tg-BMT group was significantly lower than that in the Dahl-FVIII−/− group (Figure 5E-F). To assess if the hemostatic functions in Dahl-FVIII−/− rats were ameliorated after transplantation of 2bF8 genetically modified BM cells, ROTEM was performed to analyze blood samples from 2bF8tg-BMT recipients. Whole-blood clotting time in the 2bF8tg-BMT group was significantly shorter than in the Dahl-FVIII−/− group (16.3 ± 3.1 minutes [n = 6] vs 28.3 ± 7.5 minutes [n = 7]; P < .01) but was longer than in the WT control group (9.5 ± 1.1 minutes [n = 11]) (Figure 6A). All other parameters, including clot formation time, A10 amplitude, and α-angle, determined by ROTEM analysis in the 2bF8tg-BMT recipient group were significantly different compared with the Dahl-FVIII−/− group but similar to the WT group.
Table 1.
Bleeding events and inhibitor information in Dahl-FVIII−/− rats before and after BMT
| Animal identification | No. of bleeds/treatments before BMT | No. of bleeds/treatments after BMT | Inhibitor titers (BU/mL) before BMT | Inhibitor titers (BU/mL) after BMT |
|---|---|---|---|---|
| #1 | 0 | 1 | NA | 0 |
| #2 | 1 | 0 | 0 | 0 |
| #3 | 1 | 0 | 0 | 0 |
| #4 | 0 | 0 | NA | 0 |
| #5 | 2 | 0 | 0 | 0 |
| #6 | 1 | 0 | 0 | 0 |
| #7 | 0 | 0 | NA | 0 |
NA, not available.
Figure 6.
Platelet-targeted FVIII expression restores functional hemostatic properties in Dahl-FVIII−/−rats that received 2bF8tg-BMT. Five months after receiving BMT from 2bF8tg rats, blood samples were collected from Dahl-FVIII−/− recipients via the ventral tail artery. Sodium citrate was used as an anticoagulant. Blood samples were recalcified for ROTEM analysis and TGA. Blood samples from Dahl-WT and Dahl-FVIII−/− rats were used as controls. (A) Native ROTEM analysis of the whole-blood coagulation status in Dahl-FVIII−/− recipients after receiving BMT from 2bF8tg rats. The parameters analyzed included clotting time, clot formation time, A10 amplitude, and α-angle. (B) Evaluation by nWB-TGA of the hemostatic system in Dahl-FVIII−/− recipients after receiving BMT from 2bF8tg rats. The parameters analyzed include lag time, peak time, thrombin generation rate, and endogenous thrombin potential.
To further confirm that the bleeding diathesis in FVIII−/− rats was improved after 2bF8 gene expression, nWB-TGA was performed. As shown in Figure 6B, all parameters, including lag time, peak time, thrombin generation rate, and endogenous thrombin potential, in 2bF8tg-BMT recipients were significantly different compared with Dahl-FVIII−/− control rats. However, there were no statistically significant differences between the 2bF8tg-BMT group and the WT control group in any of these parameters except thrombin generation rate, which was slightly slower than in the Dahl-WT group; this finding indicates that the overall capacity to produce thrombin in animals with platelet-targeted FVIII expression is similar to that of WT normal rats. Together, these data showed that hemostasis in Dahl-FVIII−/− rats can be restored and spontaneous bleeding can be prevented by platelet-specific FVIII expression.
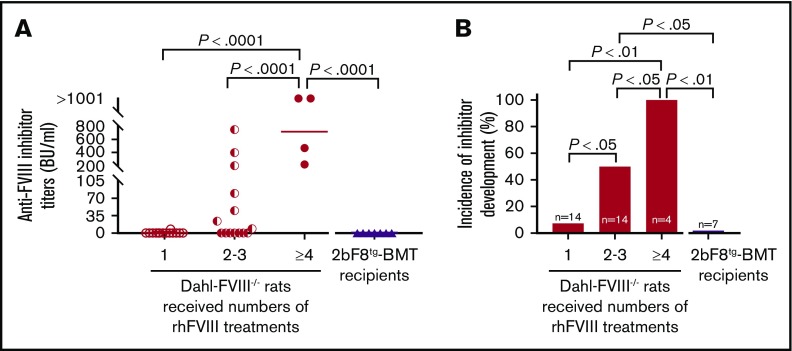
To investigate anti-FVIII immune responses in Dahl-FVIII−/− rats after receiving 2bF8 transgenic BM cells, anti-FVIII inhibitor titers were monitored by using a chromogenic-based Bethesda assay. Of note, none of the transplantation recipients (n = 7) developed anti-FVIII inhibitors during the study period despite exposure of these FVIII-naive animals to platelet-expressed human protein (Figure 7A). In contrast, both inhibitor titers and incidence significantly increased in a dose (number of treatments)-dependent manner in untransplanted FVIII−/− animals. One hundred percent of FVIII−/− animals developed anti-FVIII inhibitors when they received ≥4 rhFVIII treatments, with inhibitor titers >200 BU/mL. Fifty percent developed inhibitors when they were infused with rhFVIII 2 to 3 times, with titers ranging from 8.5 to 750 BU/mL (Figure 7B). After ≥2 rhFVIII infusions, the incidence of inhibitor development in Dahl-FVIII−/− rats was significantly higher than in BMT recipients, which, although not infused, were being continuously exposed to platelet FVIII after transplantation of 2bF8tg BM cells. Together, these data show that platelet-derived FVIII does not trigger anti-FVIII inhibitor development in Dahl-FVIII−/− rats.
Figure 7.
Dahl-2bF8tgBMT rats are tolerized to platelet-targeted FVIII expression. Blood samples were collected from Dahl-FVIII−/− recipients via ventral tail artery blood draw at various time points after BMT from 2bF8tg rats. Plasmas were isolated, and inhibitor titers were determined by using a chromogenic-based Bethesda assay. Dahl-FVIII−/− rats that did not receive BMT but received various numbers of rhFVIII infusions were used as controls for inhibitor development studies. (A) Anti-FVIII inhibitor titers. (B) Incidence of anti-FVIII inhibitor development. None of the 2bF8tg-BMT recipients developed anti-FVIII inhibitors.
Discussion
Spontaneous bleeding into the joints and muscles is one of the main problems in patients with severe HA. In the current study, we developed a novel HA rat model on an inbred Dahl background caused by a new inversion variant, with undetectable FVIII in plasma. Because the F8 gene in rat is located on autosomal chromosome 18,28,29 unlike mouse, dog, sheep, and human, in which the F8 gene is on the X chromosome, both male and female HA rats have 2 affected alleles. Homozygous animals have a severe spontaneous bleeding phenotype. Animals develop anti-FVIII inhibitors robustly when treated with rhFVIII, a model mimicking the clinical phenotype of patients with severe HA who develop inhibitors. We showed that ectopically targeting FVIII expression to platelets by oocyte transduction transgenesis can rescue the hemostatic deficiencies and prevent the spontaneous bleeding phenotype in Dahl-FVIII−/− rats without engendering anti-FVIII inhibitor development.
Inversion disruption of the F8 gene is not only the most common variant found in patients with severe HA,30-36 but it is also the most common found in canine HA models.37 Approximately one-half of cases of severe HA are caused by an inversion variant of the F8 gene, with a frequency of 40% to 50% in intron 22 and 1% to 5% in intron 1.31,32 It has been reported that an inversion variant is one of the causative genetic risk factors of inhibitor development in patients with severe HA.38 However, the incidence of inhibitor development in patients with intron 22 inversion variants seems to be much lower (∼20%)39 than that in patients with large deletion variants, which can occur in 88% to 90% of individuals.40,41 Pandey et al41 reported that patients with intron 22 inversion have endogenous FVIII muteins synthesized, which may result in tolerization to exogenous FVIII protein infusion in most patients. In contrast, patients with large deletion variants lack endogenous FVIII synthesis, and thus the immune system is less likely tolerized to FVIII and can develop inhibitors when FVIII is infused. Developing a model with an inversion variant but without truncated protein synthesized would therefore recapitulate both pathophysiological features (ie, inversion and large deletion) of the disease in patients with severe HA, which may provide new insights for disease model studies. Such a translational model could be invaluable for evaluating the efficacy and safety of novel therapeutic agents.
To avoid the potential influence of the truncated protein produced by a single-site disruption of the F8 gene on anti-FVIII immune responses or immune tolerance in our platelet-specific gene therapy, we originally intended to develop a model with the entire rat F8 gene deleted in an inbred background. In our several attempts of targeting the entire F8 gene knockout, 2 founder pups with the entire F8 gene deletion were obtained but spontaneously died of an unknown cause. However, we serendipitously obtained 1 founder pup with nearly the entire F8 gene inverted and successfully established the line. Homozygous affected animals in our Dahl-FVIII−/− model have an extremely high frequency of spontaneous bleeds, with 83% by the age of 32 weeks, and 100% of rats (112 of 112; data not shown) experienced bleeding episode(s) during their lifetime up to 64 weeks old. This is the highest frequency of spontaneous bleeds among the commonly used HA mouse, rat, and dog models reported in the literature.19,29,37,42 Importantly, our results showed that when platelet FVIII expression was introduced into these FVIII−/− rats by either lentivirus-mediated oocyte transduction transgenesis or BMT of 2bF8 genetically manipulated BM cells, the severe spontaneous bleeding phenotype was rescued. These results show that platelet FVIII is effective in preventing severe spontaneous bleeding in HA rats. Further studies to determine the threshold level of platelet FVIII required to prevent spontaneous bleeds and the clinical efficacy of 2bF8 lentiviral gene delivery to hematopoietic stem cells in such a severe HA rat model are warranted.
In addition to undetectable levels of functional FVIII:C in plasma, a severe functional hemostatic defect in Dahl-FVIII−/− rats is reflected in parameters of whole-blood clot formation and thrombin generation, as determined by native ROTEM analysis and TGA on recalcified citrated whole blood. Of note, we showed that platelet-specific FVIII expression could rescue impaired hemostatic parameters as determined by ROTEM and nWB-TGA. Regarding the coagulation state of Dahl-FVIII−/− animals with platelet FVIII expression, we found that all parameters were fully restored except the whole-blood clotting time quantified by ROTEM analysis. Although clotting time was not fully restored to WT levels, it was significantly improved compared with that of Dahl-FVIII−/− rats. We reason that this outcome could be due to delayed bioavailability of platelet-packaged FVIII. For our native ROTEM analysis, we did not add tissue factor to trigger activation of the blood coagulation cascade. For platelet-derived FVIII to participate in blood coagulation, rotation of the axis must activate platelets, which may require some time to release the sequestered FVIII from engineered platelets. In contrast, in blood samples from WT mice, soluble FVIII is readily available in plasma to participate in the blood coagulation cascade. However, once clot formation is initiated, clot formation time, A10 amplitude, and α-angle were similar to those obtained from WT control animals. Delayed bioavailability of platelet-packaged FVIII could also explain that the thrombin generation rate in the 2bF8tg-BMT group was slower than in the Dahl-WT group with nWB-TGA. Our results indicate that once FVIII is released from engineered platelets, it can fully function and effectively restore hemostatic functions.
The anti-FVIII immune response is not only a significant complication in protein replacement therapy but also a major concern in gene therapy of patients with HA. In addition to a severe spontaneous bleeding phenotype, the Dahl-FVIII−/− rats are prone to developing anti-FVIII immune responses after rhFVIII infusion. Our studies showed that 50% of animals developed anti-FVIII inhibitors after 2 doses of rhFVIII infusion and 100% developed inhibitors if they received ≥4 rhFVIII infusions. Because our protocol requires that rats be euthanized if they develop spontaneous bleeds that do not respond to a maximum of 2 treatments per episode, it is very likely that the incidence of inhibitor development reported in the current study was underestimated. This was because animals were euthanized with no opportunity to be further monitored for inhibitor development. Notably, none of the Dahl-FVIII−/− rats that received 2bF8tg BMT developed anti-FVIII inhibitors while sustained platelet FVIII expression was maintained through the study period. Our data show that FVIII is well insulated in rat platelets, avoiding triggering anti-FVIII immune responses, and Dahl-FVIII−/− rats are well tolerized to platelet-derived FVIII. Whether platelet-derived FVIII can still be tolerized and effective in preventing spontaneous bleeds in HA rats in the presence of anti-FVIII inhibitors requires further investigation.
In conclusion, we have developed a novel HA rat model with both the pathophysiology and clinical phenotype found in patients with severe HA, which could be an ideal model for evaluating the clinical efficacy of new therapeutic agents. Our studies showed that transplantation of BM cells that are genetically modified to express FVIII only in platelets can efficiently prevent severe spontaneous bleeding in HA rats with no anti-FVIII antibody development.
Acknowledgments
The authors thank Novo Nordisk for its generous gifts of SD-FVIII−/− rats and rhFVIII.
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health, grants HL-139847 (R.R.M.), HL-144457 (R.R.M.), and HL-102035 (Q.S.), and generous gifts from the Children’s Hospital of Wisconsin Foundation (Q.S.), Midwest Athletes Against Childhood Cancer Fund (Q.S.), and Versiti Blood Research Foundation (R.R.M.).
Footnotes
Data-sharing requests can be submitted to the corresponding author, Qizhen Shi (e-mail: qshi@versiti.org).
Authorship
Contribution: Q.S. designed the study, analyzed data, and wrote the manuscript; J.G.M. and S.A.F. designed the study, performed experiments, analyzed data, and made comments on the manuscript; A.M.G. and H.W. assisted in developing the FVIII−/− rat model and 2bF8 transgenic rats; and R.R.M. designed research and made critical comments on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qizhen Shi, Department of Pediatrics, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: qshi@versiti.org.
References
- 1.Schep SJ, Schutgens REG, Fischer K, Boes ML. Review of immune tolerance induction in hemophilia A. Blood Rev. 2018;32(4):326-338. [DOI] [PubMed] [Google Scholar]
- 2.Gringeri A, Mantovani LG, Scalone L, Mannucci PM; COCIS Study Group . Cost of care and quality of life for patients with hemophilia complicated by inhibitors: the COCIS Study Group. Blood. 2003;102(7):2358-2363. [DOI] [PubMed] [Google Scholar]
- 3.Walsh CE, Jiménez-Yuste V, Auerswald G, Grancha S. The burden of inhibitors in haemophilia patients. Thromb Haemost. 2016;116:S10-S17. [DOI] [PubMed] [Google Scholar]
- 4.Peyvandi F, Garagiola I. Clinical advances in gene therapy updates on clinical trials of gene therapy in haemophilia. Haemophilia. 2019;25(5):738-746. [DOI] [PubMed] [Google Scholar]
- 5.Batty P, Lillicrap D. Advances and challenges for hemophilia gene therapy. Hum Mol Genet. 2019;28:R95-R101. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguti-Hayakawa GG, Ozelo MC. Gene therapy: paving new roads in the treatment of hemophilia. Semin Thromb Hemost. 2019;45(7):743-750. [DOI] [PubMed] [Google Scholar]
- 7.Rangarajan S, Walsh L, Lester W, et al. . AAV5-factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377(26):2519-2530. [DOI] [PubMed] [Google Scholar]
- 8.VandenDriessche T, Chuah MK. Hemophilia gene therapy: ready for prime time? Hum Gene Ther. 2017;28(11):1013-1023. [DOI] [PubMed] [Google Scholar]
- 9.Shi Q, Wilcox DA, Fahs SA, et al. . Factor VIII ectopically targeted to platelets is therapeutic in hemophilia A with high-titer inhibitory antibodies. J Clin Invest. 2006;116(7):1974-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Q, Fahs SA, Wilcox DA, et al. . Syngeneic transplantation of hematopoietic stem cells that are genetically modified to express factor VIII in platelets restores hemostasis to hemophilia A mice with preexisting FVIII immunity. Blood. 2008;112(7):2713-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuether EL, Schroeder JA, Fahs SA, et al. . Lentivirus-mediated platelet gene therapy of murine hemophilia A with pre-existing anti-factor VIII immunity. J Thromb Haemost. 2012;10(8):1570-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeder JA, Chen Y, Fang J, Wilcox DA, Shi Q. In vivo enrichment of genetically manipulated platelets corrects the murine hemophilic phenotype and induces immune tolerance even using a low multiplicity of infection. J Thromb Haemost. 2014;12(8):1283-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Q, Schroeder JA, Kuether EL, Montgomery RR. The important role of von Willebrand factor in platelet-derived FVIII gene therapy for murine hemophilia A in the presence of inhibitory antibodies. J Thromb Haemost. 2015;13(7):1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumgartner CK, Mattson JG, Weiler H, Shi Q, Montgomery RR. Targeting factor VIII expression to platelets for hemophilia A gene therapy does not induce an apparent thrombotic risk in mice. J Thromb Haemost. 2017;15(1):98-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Schroeder JA, Luo X, Montgomery RR, Shi Q. The impact of GPIbα on platelet-targeted FVIII gene therapy in hemophilia A mice with pre-existing anti-FVIII immunity. J Thromb Haemost. 2019;17(3):449-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarovoi HV, Kufrin D, Eslin DE, et al. . Factor VIII ectopically expressed in platelets: efficacy in hemophilia A treatment. Blood. 2003;102(12):4006-4013. [DOI] [PubMed] [Google Scholar]
- 17.Gewirtz J, Thornton MA, Rauova L, Poncz M. Platelet-delivered factor VIII provides limited resistance to anti-factor VIII inhibitors. J Thromb Haemost. 2008;6(7):1160-1166. [DOI] [PubMed] [Google Scholar]
- 18.Du LM, Nurden P, Nurden AT, et al. . Platelet-targeted gene therapy with human factor VIII establishes haemostasis in dogs with haemophilia A. Nat Commun. 2013;4(1):2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen LN, Wiinberg B, Häger M, et al. . A novel F8 -/- rat as a translational model of human hemophilia A. J Thromb Haemost. 2014;12(8):1274-1282. [DOI] [PubMed] [Google Scholar]
- 20.Sørensen KR, Roepstorff K, Wiinberg B, et al. . The F8(-/-) rat as a model of hemophilic arthropathy. J Thromb Haemost. 2016;14(6):1216-1225. [DOI] [PubMed] [Google Scholar]
- 21.Shi Q. Platelet-targeted gene therapy for hemophilia. Mol Ther Methods Clin Dev. 2018;9:100-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao Y, Guan Y, Wang L, et al. . CRISPR/Cas-mediated genome editing in the rat via direct injection of one-cell embryos. Nat Protoc. 2014;9(10):2493-2512. [DOI] [PubMed] [Google Scholar]
- 23.Zhang G, Shi Q, Fahs SA, Kuether EL, Walsh CE, Montgomery RR. Factor IX ectopically expressed in platelets can be stored in alpha-granules and corrects the phenotype of hemophilia B mice. Blood. 2010;116(8):1235-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Schroeder JA, Chen J, et al. . The immunogenicity of platelet-derived FVIII in hemophilia A mice with or without preexisting anti-FVIII immunity. Blood. 2016;127(10):1346-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Q, Wilcox DA, Fahs SA, et al. . Lentivirus-mediated platelet-derived factor VIII gene therapy in murine haemophilia A. J Thromb Haemost. 2007;5(2):352-361. [DOI] [PubMed] [Google Scholar]
- 26.Shi Q, Fahs SA, Kuether EL, Cooley BC, Weiler H, Montgomery RR. Targeting FVIII expression to endothelial cells regenerates a releasable pool of FVIII and restores hemostasis in a mouse model of hemophilia A. Blood. 2010;116(16):3049-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgartner CK, Zhang G, Kuether EL, Weiler H, Shi Q, Montgomery RR. Comparison of platelet-derived and plasma factor VIII efficacy using a novel native whole blood thrombin generation assay. J Thromb Haemost. 2015;13(12):2210-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lozier JN, Nichols TC. Animal models of hemophilia and related bleeding disorders. Semin Hematol. 2013;50(2):175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Booth CJ, Brooks MB, Rockwell S, et al. . WAG-F8(m1Ycb) rats harboring a factor VIII gene mutation provide a new animal model for hemophilia A. J Thromb Haemost. 2010;8(11):2472-2477. [DOI] [PubMed] [Google Scholar]
- 30.Schwaab R, Brackmann HH, Meyer C, et al. . Haemophilia A: mutation type determines risk of inhibitor formation. Thromb Haemost. 1995;74(6):1402-1406. [PubMed] [Google Scholar]
- 31.Lakich D, Kazazian HH Jr, Antonarakis SE, Gitschier J. Inversions disrupting the factor VIII gene are a common cause of severe haemophilia A. Nat Genet. 1993;5(3):236-241. [DOI] [PubMed] [Google Scholar]
- 32.Bagnall RD, Waseem N, Green PM, Giannelli F. Recurrent inversion breaking intron 1 of the factor VIII gene is a frequent cause of severe hemophilia A. Blood. 2002;99(1):168-174. [DOI] [PubMed] [Google Scholar]
- 33.Zahari M, Sulaiman SA, Othman Z, Ayob Y, Karim FA, Jamal R. Mutational profiles of F8 and F9 in a cohort of haemophilia A and haemophilia B patients in the multi-ethnic Malaysian population. Mediterr J Hematol Infect Dis. 2018;10(1):e2018056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantilla-Capacho JM, Beltrán-Miranda CP, Luna-Záizar H, et al. . Frequency of intron 1 and 22 inversions of Factor VIII gene in Mexican patients with severe hemophilia A. Am J Hematol. 2007;82(4):283-287. [DOI] [PubMed] [Google Scholar]
- 35.Dutta D, Gunasekera D, Ragni MV, Pratt KP. Accurate, simple, and inexpensive assays to diagnose F8 gene inversion mutations in hemophilia A patients and carriers. Blood Adv. 2016;1(3):231-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodeve A. The incidence of inhibitor development according to specific mutations—and treatment? Blood Coagul Fibrinolysis. 2003;14:S17-S21. [DOI] [PubMed] [Google Scholar]
- 37.Nichols TC, Hough C, Agersø H, Ezban M, Lillicrap D. Canine models of inherited bleeding disorders in the development of coagulation assays, novel protein replacement and gene therapies. J Thromb Haemost. 2016;14(5):894-905. [DOI] [PubMed] [Google Scholar]
- 38.Sawecka J, Skulimowska J, Windyga J, Lopaciuk S, Kościelak J. Prevalence of the intron 22 inversion of the factor VIII gene and inhibitor development in Polish patients with severe hemophilia A. Arch Immunol Ther Exp (Warsz). 2005;53(4):352-356. [PubMed] [Google Scholar]
- 39.Luna-Záizar H, González-Alcázar JA, Evangelista-Castro N, et al. . F8 inversions of introns 22 and 1 confer a moderate risk of inhibitors in Mexican patients with severe hemophilia A. Concordance analysis and literature review. Blood Cells Mol Dis. 2018;71:45-52. [DOI] [PubMed] [Google Scholar]
- 40.Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003;9(4):418-435. [DOI] [PubMed] [Google Scholar]
- 41.Pandey GS, Yanover C, Miller-Jenkins LM, et al. ; PATH (Personalized Alternative Therapies for Hemophilia) Study Investigators . Endogenous factor VIII synthesis from the intron 22-inverted F8 locus may modulate the immunogenicity of replacement therapy for hemophilia A. Nat Med. 2013;19(10):1318-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH Jr. Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10(1):119-121. [DOI] [PubMed] [Google Scholar]