Key Points
The presence of MetS components in patients with DVT is associated with significantly higher rates of VTE recurrence.
Abstract
An improved understanding of which patients are at higher risk of recurrent venous thromboembolism (VTE) is important to designing interventions to reduce degraded quality of life after VTE. Although metabolic syndrome (MetS), the clustering of hypertension, hyperlipidemia, diabetes mellitus, and obesity has been associated with a hypofibrinolytic state, data linking VTE recurrence with MetS remain limited. The purpose of this study was to measure the prevalence of MetS in patients with deep vein thrombosis (DVT) across a large population and determine its effect on VTE recurrence. This was a retrospective analysis of a large statewide database from 2004 to 2017. We measured the frequency with which patients with DVT carried a comorbid International Coding of Diseases diagnosis of MetS components. Association of MetS with VTE recurrence was tested with a multiple logistic regression model and VTE recurrence as the dependent variable. Risk of VTE recurrence conferred by each MetS component was assessed by Kaplan-Meier curves with the log-rank statistic. A total of 151 054 patients with DVT were included in this analysis. Recurrence of VTE occurred in 17% overall and increased stepwise with each criterion for MetS. All 4 components of MetS had significant adjusted odds ratios (OR) for VTE recurrence, with hyperlipidemia having the largest (OR, 1.8), representing the 4 largest ORs of all possible explanatory variables. All 4 MetS variables were significant on Kaplan-Meier analysis for recurrence of VTE. These data imply a role for appropriate therapies to reduce the effects of MetS as a way to reduce risk of VTE recurrence.
Visual Abstract
Introduction
Venous thromboembolism (VTE), comprising both deep vein thrombosis (DVT) and pulmonary embolism (PE), poses a significant burden to public health, with an annual incidence estimated to be between 1 and 2 per 1000 of the US population.1-3 Nearly two-thirds of these events represent patients presenting with DVT.4 Recurrence of VTE remains a scourge to patients with DVT, with 1 study finding a continued risk of recurrence of 11% in the first year after cessation of anticoagulation therapy, with a 5% risk in the subsequent year.5 Further, between one-third and one-half of patients diagnosed with DVT will develop postthrombotic syndrome, which is characterized by chronic, debilitating leg pain, edema, and skin ulcerations, resulting from residual venous obstruction and valvular reflux, with the probability of postthrombotic syndrome increasing with DVT recurrence.6,7
The prevalence of obesity continues to rise, doubling worldwide over the past 3 decades.8 Increasing in parallel with obesity is the prevalence of metabolic syndrome (MetS), now estimated to afflict at least 34% of the US population.9 MetS is generally defined as a clustering of the following clinical criteria: abdominal obesity, impaired glucose metabolism, dyslipidemia, and hypertension.10 MetS is associated with a procoagulant and hypofibrinolytic state, with prior work demonstrating prolonged clot lysis times in patients with MetS compared with controls.11 Among other mechanisms, MetS is associated with a dysregulated secretion of adipokines caused by excess adipose tissue that may create a chronic low-grade inflammatory state leading to endothelial dysfunction, vascular remodeling, and thrombosis.12-14 Although prior studies have suggested the components of MetS are associated with higher risk of first VTE event, data linking MetS with VTE recurrence remain less defined and conflicted in their conclusions.15-24 An improved understanding of the causal effect of MetS on VTE and its recurrence may aid in clinical decision-making regarding appropriate treatment and prompt initiation of adjuvant therapies aimed at reducing risk factors for recurrence at the time of VTE diagnosis. The purpose of this study was to measure the prevalence of MetS in patients with DVT across a large study population and to determine its effect on VTE recurrence and mortality.
Methods
Overall study design
This was a population-based analysis using deidentified patient information from a large statewide database, the Indiana Network for Patient Care, in collaboration with the Regenstrief Institute. This database is the primary platform that enables the Indiana Health Information Exchange, 1 of the largest health information exchanges nationwide, with data collected from the combination of these institutions representing >10 billion clinical elements in total. Inclusion criteria for this retrospective study was any diagnosis of DVT based on Sequel-based query of these databases for the appropriate International Coding of Diseases (ICD) codes (ICD-9 [453.x] or ICD-10 [I82.x]) from 2004 to 2017.
The presence or absence of each of the components of MetS in patients found to have a DVT diagnosis was determined based on ICD coding for the following 4 conditions: hypertension (401-404.x, I10-I13.x), hyperlipidemia (272.x, E78.x, E88.1), diabetes mellitus (250.x0, 250.x2, E11.x), or obesity (278.x, E66.x). This ICD-based diagnosis could have been coded at any time during the study period from 2004 to 2017 and was not necessarily always at the time of index DVT. We also collected additional data including pertinent demographics (eg, sex, race, age), presence or absence of specified comorbidities (atrial fibrillation [Afib], chronic obstructive pulmonary disease [COPD], congestive heart failure [CHF], anxiety, depression, stroke, myocardial infarction [MI], chronic kidney disease [CKD], multiple cancer diagnoses [esophageal, breast, lung, liver, testicular, pancreatic, prostate, ovarian, melanoma]), medication history, smoking history, number of subsequent return visits to the emergency department (ED) within 2 years post-DVT diagnosis, history of recurrent VTE event, and current vital status. Recurrent VTE was defined as any subsequent ICD-based diagnosis of DVT or PE occurring after the index DVT.
Statistical analysis
Statistical analyses were performed using SAS software, version 9.4 (Cary, NC). We measured the prevalence of each MetS component in patients with DVT, as well as the summative frequency with which patients carried 0, 1, 2, 3, or 4 of these components. We constructed Kaplan-Meier curves to compare rates of VTE recurrence based on the presence or absence of each MetS component, as well as a composite “+MetS” group (requiring at least 3 of the 4 components) vs “−MetS.” Kaplan-Meier curves were compared using the log-rank (Mantel-Cox) test. Further, we also created a multiple logistic regression model to test the independent predictive value of age, sex, hypertension (HTN), hyperlipidemia, DM, obesity, Afib, COPD, CHF, CKD, anxiety, depression, stroke, MI, cancer, and smoking history on VTE recurrence and mortality. Both unadjusted and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for the selected variables to assess the association with both recurrence and mortality rates. In post hoc exercises, we measured the prevalence and duration of anticoagulation prescription and compared between +MetS and −MetS groups.
Results
Demographic data and descriptive statistics
This study included a total of 151 054 patients with DVT studied from 2004 to 2017, representing 0.9 million patient-years. Table 1 displays the demographic and clinical characteristics of these patients. The average age across the entire DVT cohort was 58 years. Fifty-six percent of patients were female and 69% identified as white. Of the 4 MetS components, HTN was found to be the most common comorbid condition, present in 58% of all patients. This was followed by hyperlipidemia (41%), DM (23%), and obesity (19%). Table 1 also stratifies patients according to the number of MetS criteria they were found to have (ranging from 0 to 4). Sixty-eight percent of patients with DVT had at least 1 comorbid MetS diagnosis. Specifically, 22% had 1 component, 24% had 2 components, 17% had 3 components, and 5% had all 4 components. Those with fewer MetS criteria tended to be younger, have a less frequent smoking history, and have fewer comorbidities overall.
Table 1.
Clinical characteristics and outcomes stratified by number of MetS criteria
| 0 | 1 | 2 | 3 | 4 | All | |
|---|---|---|---|---|---|---|
| Total patients | 48 043 | 33 769 | 36 360 | 25 168 | 7714 | 151 054 |
| Average age, y | 52 | 58 | 62 | 60 | 57 | 58 |
| Female, % | 55 | 57 | 55 | 56 | 59 | 56 |
| White, % | 64 | 67 | 70 | 74 | 78 | 69 |
| African American, % | 8 | 12 | 13 | 14 | 13 | 11 |
| HTN, % | 0 | 6 | 92 | 98 | 10 | 58 |
| Hyperlipidemia, % | 0 | 16 | 72 | 92 | 100 | 41 |
| DM, % | 0 | 12 | 21 | 59 | 100 | 23 |
| Obesity, % | 0 | 7 | 15 | 51 | 100 | 19 |
| Afib, % | 4 | 16 | 26 | 30 | 32 | 18 |
| COPD, % | 8 | 24 | 35 | 43 | 48 | 26 |
| CHF, % | 5 | 21 | 33 | 43 | 46 | 24 |
| CKD, % | 3 | 17 | 29 | 38 | 38 | 20 |
| Anxiety, % | 2 | 4 | 5 | 8 | 10 | 4 |
| Depression, % | 8 | 22 | 31 | 41 | 49 | 24 |
| Stroke, % | 2 | 9 | 16 | 20 | 18 | 11 |
| MI, % | 1 | 5 | 12 | 16 | 16 | 8 |
| Cancer, % | 10 | 15 | 1 | 16 | 15 | 14 |
| Smoking history, % | 13 | 24 | 34 | 42 | 49 | 27 |
| VTE recurrence, % | 6.8 | 13.8 | 20.8 | 30.1 | 37.1 | 17.2 |
| Mortality, % | 11.6 | 20.2 | 18.1 | 16.7 | 13.0 | 16.0 |
| Average no. of return ED visits (per patient) | 1.1 | 1.7 | 2.1 | 2.7 | 3.0 | 1.9 |
Table 1 also reports outcome data for the entire DVT cohort, as well as stratified by number of comorbid MetS components, specifically displaying percent mortality, VTE recurrence rate and average number of return ED visits per patient over a 2-year span after DVT diagnosis. Across all included patients, we found a 16% mortality rate, a 17% VTE recurrence rate, and an average of 1.9 per patient return ED visits. When stratified by number of MetS components, VTE recurrence rate demonstrated a stepwise increasing association with each additional MetS criteria. Patients with 0 MetS components had a VTE recurrence rate of 7%, followed by 14% with 1 component, 21% with 2 components, 30% with 3 components, and 37% with all 4 components. A similar stepwise result was found on examination of the effect of number of MetS comorbid diagnoses on return ED visits. This stepwise association was not demonstrated in percent mortality, where no clear pattern was found when stratified by number of MetS criteria. The mortality rate for each group was as follows: 12% with 0 components, 20% with 1 component, 18% with 2 components, 17% with 3 components, and 13% with all 4 components.
Unadjusted ORs for recurrence and mortality
Table 2 demonstrates the unadjusted univariate ORs both for VTE recurrence and mortality across all MetS components and additional comorbidities of interest. Three of the 4 MetS components represented the 3 largest unadjusted ORs for VTE recurrence: hyperlipidemia 2.9 (95% CI, 2.84-3.00), HTN 2.8 (95% CI, 2.70-2.87), and obesity 2.7 (95% CI, 2.64-2.81). The 3 disease processes determined to have the largest magnitude unadjusted ORs for mortality were CHF 3.7 (95% CI, 3.54-3.75), CKD 2.8 (95% CI, 2.72-2.89), and COPD 2.6 (95% CI, 2.53-2.68). Two of the MetS criteria, hyperlipidemia (0.9; 95% CI, 0.87-0.92) and obesity (0.9; 95% CI, 0.86-0.92), had significant ORs <1, suggesting a negative relationship between these conditions and mortality.
Table 2.
ORs for VTE recurrence and mortality
| Variable | OR VTE recurrence (95% CI) | OR for mortality (95% CI) | ||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| HTN | 2.784 (2.700-2.872) | 1.470 (1.413-1.529) | 1.980 (1.922-2.041) | 1.209 (1.163-1.258) |
| Hyperlipidemia | 2.924 (2.844-3.006) | 1.779 (1.721-1.839) | 0.897 (0.872-0.922) | 0.444 (0.428-0.460) |
| DM | 2.102 (2.041-2.164) | 1.419 (1.375-1.465) | 1.082 (1.048-1.118) | 0.901 (0.869-0.934) |
| Obesity | 2.725 (2.645-2.808) | 1.535 (1.484-1.588) | 0.890 (0.858-0.922) | 0.767 (0.735-0.800) |
| Afib | 1.985 (1.924-2.048) | 1.415 (1.363-1.468) | 2.514 (2.437-2.594) | 1.205 (1.160-1.251) |
| COPD | 1.920 (1.866-1.975) | 1.064 (1.028-1.101) | 2.603 (2.530-2.679) | 1.577 (1.523-1.634) |
| CHF | 1.857 (1.804-1.912) | 0.995 (0.958-1.033) | 3.647 (3.543-3.753) | 2.135 (2.056-2.218) |
| CKD | 1.879 (1.823-1.937) | 1.111 (1.072-1.152) | 2.806 (2.723-2.891) | 1.665 (1.605-1.726) |
| Anxiety | 2.238 (2.122-2.361) | 1.168 (1.102-1.239) | 0.947 (0.885-1.013) | 0.841 (0.781-0.906) |
| Depression | 2.325 (2.260-2.393) | 1.340 (1.297-1.385) | 1.504 (1.459-1.550) | 1.309 (1.262-1.357) |
| Stroke | 2.195 (2.116-2.277) | 1.408 (1.352-1.466) | 2.139 (2.060-2.221) | 1.315 (1.260-1.372) |
| MI | 1.968 (1.885-2.055) | 1.010 (0.963-1.060) | 2.479 (2.376-2.586) | 1.416 (1.348-1.486) |
| Cancer | 1.306 (1.259-1.355) | 1.255 (1.207-1.305) | 2.255 (2.179-2.333) | 1.993 (1.921-2.068) |
| Smoking history | 2.071 (2.014-2.130) | 1.266 (1.227-1.307) | 1.106 (1.073-1.140) | 0.935 (0.902-0.969) |
Multivariate analysis of recurrence data
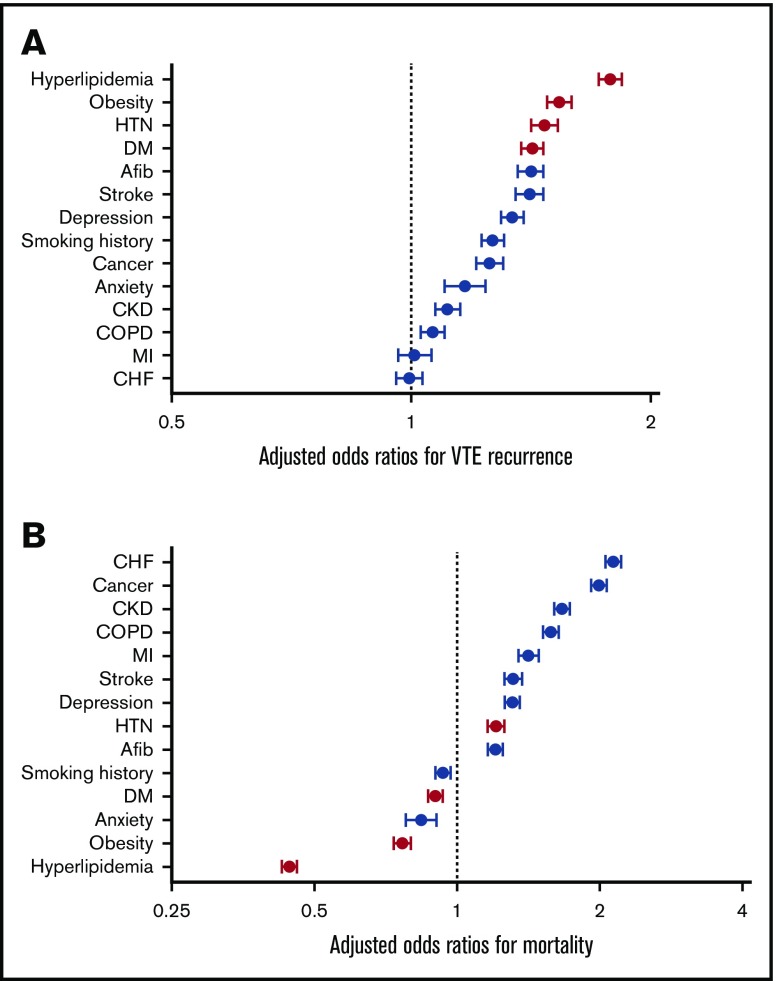
We performed multivariate logistic regression analysis with VTE recurrence as the dependent variable and several predefined predictor variables, the results of which are displayed in Tables 2 and 3. All conditions except for CHF (OR, 1.0; 95%, CI 0.96-1.03) and MI (OR, 1.0; 95% CI, 0.96-1.06) were found to be associated with significantly greater odds of VTE recurrence. The 4 MetS criteria had the 4 largest adjusted ORs for VTE recurrence, with an OR of 1.8 (95% CI, 1.72-1.84) for hyperlipidemia, 1.5 (95% CI, 1.48-1.59) for obesity, 1.5 (95% CI, 1.41-1.53) for HTN, and 1.4 (95% CI, 1.38-1.47) for DM. These adjusted ORs for recurrence are displayed in Figure 1.
Table 3.
Multivariate logistic regression analysis for VTE recurrence and mortality
| Variable | VTE recurrence | Mortality | ||
|---|---|---|---|---|
| Parameter estimate | P | Parameter estimate | P | |
| Age | −0.0128 | <.0001 | 0.0195 | <.0001 |
| Sex | 0.0144 | .3296 | −0.00664 | .6688 |
| HTN | 0.3853 | <.0001 | 0.1900 | <.0001 |
| Hyperlipidemia | 0.5760 | <.0001 | −0.8118 | <.0001 |
| DM | 0.3501 | <.0001 | −0.1045 | <.0001 |
| Obesity | 0.4287 | <.0001 | −0.2650 | <.0001 |
| Afib | 0.3468 | <.0001 | 0.1862 | <.0001 |
| COPD | 0.0619 | .0004 | 0.4558 | <.0001 |
| CHF | −0.00542 | .7793 | 0.7586 | <.0001 |
| CKD | 0.1051 | <.0001 | 0.5095 | <.0001 |
| Anxiety | 0.1557 | <.0001 | −0.1730 | <.0001 |
| Depression | 0.2930 | <.0001 | 0.2690 | <.0001 |
| Stroke | 0.3421 | <.0001 | 0.2737 | <.0001 |
| MI | 0.0101 | .6804 | 0.3476 | <.0001 |
| Cancer | 0.2271 | <.0001 | 0.6897 | <.0001 |
| Smoking history | 0.2360 | <.0001 | −0.0674 | .0003 |
Figure 1.
Results from multivariate logistic regression analyses. Adjusted odds ratios for VTE recurrence (A) and mortality (B).
Survival analysis of recurrence data
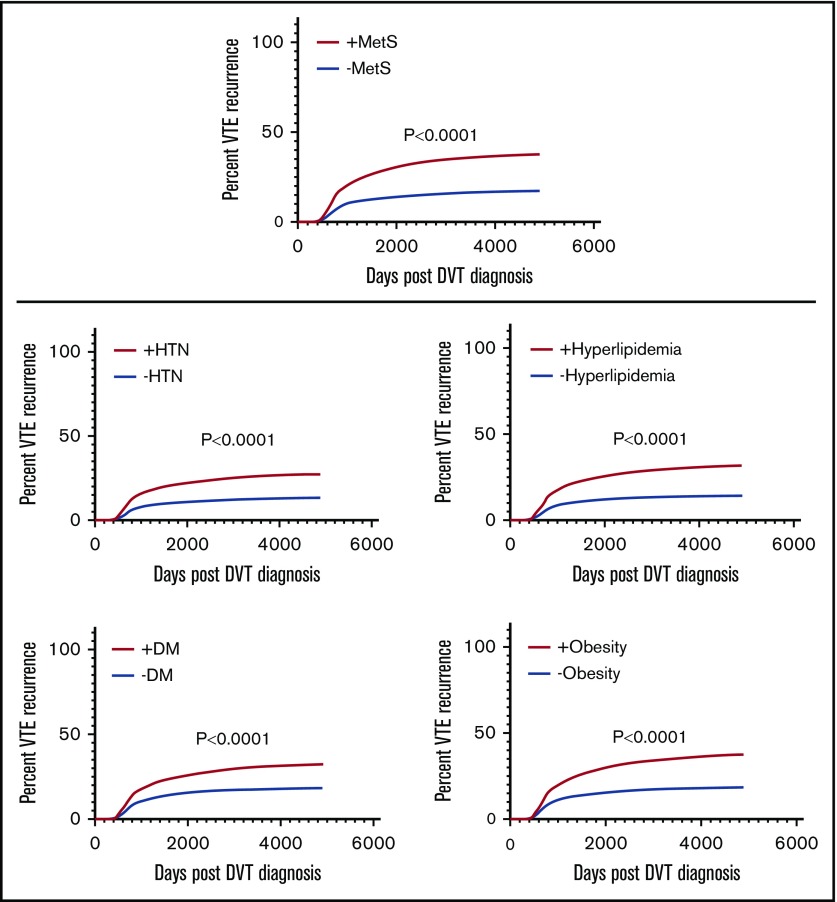
Figure 2 displays the individual Kaplan-Meier curves for VTE recurrence over time based on the presence or absence of each MetS component. These were all significant (P < .0001), with survival analysis yielding the following hazard ratios (HR): HR, 2.2 (95% CI, 2.12-2.23) for HTN; HR, 2.3 (95% CI, 2.24-2.36) for hyperlipidemia; HR, 1.8 (95% CI, 1.76-1.86) for DM; and HR 2.2 (95% CI, 2.11-2.24) for obesity. Kaplan-Meier analysis was also performed to compare rates of VTE in patients classified as having MetS (composite +MetS group, requiring at least 3 of the 4 MetS components) vs those without MetS (−MetS group). The curves were significantly different (P < .0001) between these 2 groups, with an HR of 2.4 (95% CI, 2.32-2.46) for VTE recurrence in +MetS (Figure 2).
Figure 2.
Kaplan-Meier curves for VTE recurrence based on ± composite MetS and individual components.
Multivariate analysis of mortality data
Similar logistic regression analysis was performed with mortality as the dependent variable, as demonstrated in Tables 2 and 3. The variables associated with the greatest odds of mortality were CHF (OR, 2.1; 95% CI, 2.06-2.22), cancer (OR, 2.0; 95% CI, 1.92-2.07), and CKD (OR, 1.7; 95% CI, 1.61-1.73). The ORs for the following variables exhibited a significant negative association with mortality: hyperlipidemia (OR, 0.4; 95% CI, 0.43-0.46), obesity (OR, 0.8; 95% CI, 0.74-0.80), anxiety (OR, 0.8; 95% CI, 0.78-0.91), DM (OR, 0.9; 95% CI, 0.87-0.93), and smoking history (OR, 0.9; 95% CI, 0.90-0.97). These adjusted ORs for mortality are displayed in Figure 1.
Analysis of anticoagulation data
In post hoc exercise, we measured the prevalence and duration of anticoagulation prescription and compared between +MetS and −MetS groups. Patients with the composite +MetS profile had a documented prescription for an anticoagulation medication in 23% of cases, compared with 18% of patients without MetS (P < .0001), with the initial anticoagulation prescription occurring 5 days after index DVT in those with MetS compared with 1 day in those without MetS (P < .0001). We also measured days from index DVT to the most recent documented prescription for anticoagulation as a surrogate measure of duration of therapy, finding a longer duration in those with MetS (92 days vs 50 days, P < .0001).
Discussion
To our knowledge, this is the largest cohort of DVT patients that has found that MetS plays an important role in VTE recurrence. The descriptive data show a clear positive concordance between the number of MetS criteria and recurrence rate. Percent recurrence increased in a stepwise fashion with each additional MetS component, ranging from 7% recurrence in patients without any MetS comorbidities to 37% recurrence in those with all 4 components. In multivariate analyses, the MetS components represented the 4 most important risk factors for VTE recurrence across all diseases, generating the 4 largest adjusted ORs. This was highest for hyperlipidemia, followed by obesity, HTN, and DM. Results of the Kaplan-Meier curve analysis suggest that both the composite +MetS diagnosis as well as the presence of each individual MetS component are important contributors to this recurrence risk. Further, in our analysis of anticoagulation data, this association of MetS with higher risk of VTE recurrence persisted despite those with MetS having higher documented rates and duration of anticoagulation therapy. Thus, as anticoagulation therapy is known to be very effective at reducing the risk of recurrent VTE, it is possible that the effect of MetS on recurrence is actually underestimated in our dataset. Taken together, these data strongly support the hypothesis that MetS increases risk of VTE recurrence after DVT. Prior studies have suggested an increased risk of VTE in patients with MetS, although results have varied and remain inconclusive.15-24 The underlying pathophysiology explaining the role of MetS in VTE is unclear. One proposed mechanism involves obesity-induced adipocyte dysregulation and oxidative stress, resulting in increased circulating proinflammatory adipocytokines including interleukin-6, tumor necrosis factor-α, and PAI-1. This creates a low-grade proinflammatory and prothrombotic state, which may contribute to a higher risk of recurrent VTE.11-14
Somewhat contrary to the observation that MetS increased VTE recurrence, patients with 3 of the 4 MetS components (hyperlipidemia, DM, and obesity) survived longer than patients without these conditions. The potential explanation of longer survival allowing for a longer period of surveillance and therefore greater chance of recurrent VTE seems unlikely. The hazard curves in Figure 2 show a clear separation in VTE recurrence based upon MetS status starting at about 800 days, before the time of separation of survival curves (data not shown). Further, these curves correct for loss of patients because they censor patients after death. Our data do not allow a clear explanation for the lower mortality rate for DVT patients with hyperlipidemia, DM, and obesity, but we are not the first to observe a survival benefit of obesity in cardiovascular disease. Indeed, this phenomenon has been described frequently enough in other cardiovascular diseases to receive the descriptive label of the “obesity paradox.”25-28 Additionally, similar to this obesity paradox, results from multiple studies have shown high cholesterol levels to be associated with improved outcomes and long-term survival after acute ischemic stroke, independent of statin therapy.29-31 As with obesity, the protective mechanism of elevated cholesterol remains speculative.32,33
We believe this study to be novel in its size and scope because it includes >150 000 patients with DVT, allowing for small CIs. We found the components of MetS to be prevalent in patients diagnosed with DVT, with 68% of patients having at least 1 of these comorbid conditions. Because these diagnoses were reliant on ICD coding, it is very possible that they make up even larger proportions of the population than reported here. Further research is needed to better elucidate the role of MetS in VTE, particularly in its effect on morbidity-related outcomes such as recurrence and health care utilization because this could aid in decision-making regarding implementation of the most appropriate adjuvant therapies in those diagnosed with DVT. We believe this to be an area of significant importance in the treatment of DVT because the components of MetS represent common, often undertreated, and potentially modifiable risk factors in these patients. Potential adjuvant interventions in patients with the components of MetS diagnosed with DVT might include systems-based efforts aimed toward the implementation of exercise programs, dietary education, or targeted nonfibrinolytic pharmacologic agents. Exercise has been previously shown to carry with it an antithrombotic effect through the reduction of plasma fibrinogen and increased endogenous tPA activity, weight loss, and improved exercise capacity in patients with VTE.34-37 Several nonfibrinolytic agents, including statins and nonsteroidal anti-inflammatory medications, have been proposed as a method of attenuating the acute inflammatory response after PE.38-40
The additional non-MetS comorbidities chosen for inclusion in this analysis represented common disease processes that may be frequently encountered in patients with a new diagnosis of DVT. Included in these selected comorbidities were several accepted risk factors of VTE including cancer, CKD, and age. Because of the design of this study and limitations based on reliance on ICD coding, we were unable to include all traditional major risk factors for VTE such as immobility, trauma, or recent surgery. We also selected comorbidities without a previously established association with VTE, including atrial fibrillation, COPD, CHF, anxiety, depression, stroke, and MI. Multivariate analysis yielded several associations between these predictor variables and VTE, which we found to be somewhat surprising and of unclear significance. Specifically, we found Afib, stroke, depression, and anxiety to have significant adjusted ORs for recurrence, even higher than that of CKD and cancer, for all but anxiety. Although this work provides no biological mechanism to understand these observed associations, prior studies have demonstrated anxiety and depression to be associated with a pro-inflammatory phenotype.41,42 A large, prospective population-based study has previously reported an apparent association between both Afib and ischemic stroke with VTE risk.43-45 However, the potential link between these risk factors and VTE requires further investigation.
There are several limitations of this study. The overall size and reach of this study resulted in challenges with data collection. First, the identification of the presence or absence of the MetS components was dependent on ICD coding. It is likely that these diagnoses are undercoded and actually represent a larger proportion of patients included in the dataset. Specifically, we noted discrepancies between patient weight data and the diagnosis of obesity, suggesting that this may be a diagnosis that is not consistently formally coded for across health care providers. A 19% overall prevalence of obesity is lower than what we would expect based on the study population. Because of these limitations, we defined MetS based on a surrogate collection of ICD codes for hypertension, hyperlipidemia, diabetes, and obesity. This differs from many of the more specific guidelines traditionally used to define MetS, including specific cutoffs for triglycerides, high-density lipoprotein, systolic and diastolic blood pressure, fasting glucose, and waist circumference.10 It is possible that the prevalence of patients diagnosed with MetS may have differed if we had been able to follow these more stringent guidelines. Given this limitation, it may be more precise to consider this a study aimed at the evaluation of the association of VTE with traditional cardiovascular risk factors, taken both individually and as a cluster. The lack of discrete data points, including specific blood pressure readings, fasting lipid panels for those with hyperlipidemia and hemoglobin A1c for those with DM, limits interpretation of results. Additionally, diagnosis of index DVT was also based on ICD coding. We did not have access to specific ultrasound results to verify these diagnoses. Therefore, diagnoses of DVT likely differ based on location and overall size of thrombus burden and may include more superficial thromboses if coded as such by individual providers. Prior database studies have reported only a modest predictive value of ICD codes in the identification of VTE, citing a positive predictive value of 49%, with this being even more challenging when attempting to identify recurrent VTE.46 It is possible that a portion of the coded “recurrent” events in our dataset actually represents the previously diagnosed DVT instead of a true recurrence.
Another limitation involves our ability to determine the particular treatment (or lack thereof) that patients received after DVT diagnosis and how this affected outcomes. Although oral anticoagulation therapy is very effective at reducing the risk of recurrent VTE while on therapy, this protection is not maintained after treatment cessation.5 We attempted to account for the role of anticoagulation therapy by comparing prevalence and duration of anticoagulation prescription in patients with and without MetS. That our database was able to identify only 18% and 23% of patients without and with MetS, respectively, who received outpatient prescriptions for anticoagulation suggests inadequate access of the Indiana Health Information Exchange database to prescription data. However, we have no reason to believe that the scope of reach for the data repository differed for patients based upon MetS status. Additional adjuvant pharmacologic therapies such as statins and aspirin may also reduce one’s risk of recurrent VTE.40,47-50 Finally, it should be noted that, given the extended duration of this study, our results may have been influenced by changes in clinical practice over time. For instance, the American College of Chest Physicians started to recommend the use of indefinite anticoagulation in patients with unprovoked VTE in 2008.51 In this same year, multiple studies reported obesity to be a risk factor for VTE recurrence and recommended appropriate risk stratification in this group.52,53 In a subgroup comparison of patients with DVT diagnosed before vs after 2008, we found those with obesity to have a higher likelihood of anticoagulation prescription if diagnosed after 2008. However, these patients remained at higher risk of recurrence despite more frequent anticoagulation therapy.
In conclusion, in a large cohort with long-term follow-up, we found the presence of any 1 component of MetS in patients with DVT to independently increase the risk of VTE recurrence; this risk of VTE recurrence increased successively with each additional component of MetS. These findings support the importance of recognizing MetS components in patients diagnosed with acute DVT and initiating appropriate therapies to reduce their effect on VTE recurrence risk.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgment
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute K12 Program in Emergency Care Research (5K12HL133310-03) (L.K.S.).
Footnotes
The complete data dictionary illustrating the specific ICD codes used to define each variable may be found in the supplemental Data. For original deidentified data, please contact jefkline@iu.edu.
Authorship
Contribution: L.K.S. and J.A.K. participated in the study concept, design, analysis, writing, and revision of the manuscript.
Conflict-of-interest disclosure: Indiana University has received funding from Janssen and Pfizer pharmaceuticals for investigator-initiated research by J.A.K. L.K.S. declares no competing financial interests.
Correspondence: Jeffrey A. Kline, Department of Emergency Medicine, Indiana University School of Medicine, 720 Eskenazi Ave, Indianapolis, IN 46202; e-mail: jefkline@iu.edu.
References
- 1.Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25(3):235-242. [DOI] [PubMed] [Google Scholar]
- 2.Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117(1):19-25. [DOI] [PubMed] [Google Scholar]
- 3.Park B, Messina L, Dargon P, Huang W, Ciocca R, Anderson FA. Recent trends in clinical outcomes and resource utilization for pulmonary embolism in the United States: findings from the nationwide inpatient sample. Chest. 2009;136(4):983-990. [DOI] [PubMed] [Google Scholar]
- 4.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-I8. [DOI] [PubMed] [Google Scholar]
- 5.Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152(9):578-589. [DOI] [PubMed] [Google Scholar]
- 6.Jaff MR, McMurtry MS, Archer SL, et al. ; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology . Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788-1830. [DOI] [PubMed] [Google Scholar]
- 7.Kahn SR. The post-thrombotic syndrome. Hematology Am Soc Hematol Educ Program. 2010;2010:216-220. [DOI] [PubMed] [Google Scholar]
- 8.WHO obesity and overweight fact sheet no. 311. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed May 2019.
- 9.Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. J Am Coll Cardiol. 2013;62(8):697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43(1):1-23. [DOI] [PubMed] [Google Scholar]
- 11.Stubblefield WB, Alves NJ, Rondina MT, Kline JA. Variable resistance to plasminogen activator initiated fibrinolysis for intermediate-risk pulmonary embolism. PLoS One. 2016;11(2):e0148747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molica F, Morel S, Kwak BR, Rohner-Jeanrenaud F, Steffens S. Adipokines at the crossroad between obesity and cardiovascular disease. Thromb Haemost. 2015;113(3):553-566. [DOI] [PubMed] [Google Scholar]
- 13.Summer R, Walsh K, Medoff BD. Obesity and pulmonary arterial hypertension: Is adiponectin the molecular link between these conditions? Pulm Circ. 2011;1(4):440-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Husseny MW, Mamdouh M, Shaban S, et al. Adipokines: potential therapeutic targets for vascular dysfunction in type II diabetes mellitus and obesity. J Diabetes Res. 2017;2017:8095926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ageno W, Prandoni P, Romualdi E, et al. The metabolic syndrome and the risk of venous thrombosis: a case-control study. J Thromb Haemost. 2006;4(9):1914-1918. [DOI] [PubMed] [Google Scholar]
- 16.Jang MJ, Choi WI, Bang SM, et al. Metabolic syndrome is associated with venous thromboembolism in the Korean population. Arterioscler Thromb Vasc Biol. 2009;29(3):311-315. [DOI] [PubMed] [Google Scholar]
- 17.Vayá A, Martínez-Triguero ML, España F, Todolí JA, Bonet E, Corella D. The metabolic syndrome and its individual components: its association with venous thromboembolism in a Mediterranean population. Metab Syndr Relat Disord. 2011;9(3):197-201. [DOI] [PubMed] [Google Scholar]
- 18.Ay C, Tengler T, Vormittag R, et al. Venous thromboembolism--a manifestation of the metabolic syndrome. Haematologica. 2007;92(3):374-380. [DOI] [PubMed] [Google Scholar]
- 19.Ageno W, Dentali F, Grandi AM. New evidence on the potential role of the metabolic syndrome as a risk factor for venous thromboembolism. J Thromb Haemost. 2009;7(5):736-738. [DOI] [PubMed] [Google Scholar]
- 20.Borch KH, Braekkan SK, Mathiesen EB, et al. Abdominal obesity is essential for the risk of venous thromboembolism in the metabolic syndrome: the Tromsø study. J Thromb Haemost. 2009;7(5):739-745. [DOI] [PubMed] [Google Scholar]
- 21.Steffen LM, Cushman M, Peacock JM, et al. Metabolic syndrome and risk of venous thromboembolism: Longitudinal Investigation of Thromboembolism Etiology. J Thromb Haemost. 2009;7(5):746-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linnemann B, Zgouras D, Schindewolf M, Schwonberg J, Jarosch-Preusche M, Lindhoff-Last E. Impact of sex and traditional cardiovascular risk factors on the risk of recurrent venous thromboembolism: results from the German MAISTHRO Registry. Blood Coagul Fibrinolysis. 2008;19(2):159-165. [DOI] [PubMed] [Google Scholar]
- 23.Eichinger S, Hron G, Bialonczyk C, et al. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch Intern Med. 2008;168(15):1678-1683. [DOI] [PubMed] [Google Scholar]
- 24.Vučković BA, Cannegieter SC, van Hylckama Vlieg A, Rosendaal FR, Lijfering WM. Recurrent venous thrombosis related to overweight and obesity: results from the MEGA follow-up study. J Thromb Haemost. 2017;15(7):1430-1435. [DOI] [PubMed] [Google Scholar]
- 25.Hainer V, Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes Care. 2013;36(2 Suppl 2):S276-S281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonopoulos AS, Tousoulis D. The molecular mechanisms of obesity paradox. Cardiovasc Res. 2017;113(9):1074-1086. [DOI] [PubMed] [Google Scholar]
- 27.Khan SS, Ning H, Wilkins JT, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3(4):280-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iliodromiti S, Celis-Morales CA, Lyall DM, et al. The impact of confounding on the associations of different adiposity measures with the incidence of cardiovascular disease: a cohort study of 296 535 adults of white European descent. Eur Heart J. 2018;39(17):1514-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen TS, Christensen RH, Kammersgaard LP, Andersen KK. Higher total serum cholesterol levels are associated with less severe strokes and 2lower all-cause mortality: ten-year follow-up of ischemic strokes in the Copenhagen Stroke Study. Stroke. 2007;38(10):2646-2651. [DOI] [PubMed] [Google Scholar]
- 30.Markaki I, Nilsson U, Kostulas K, Sjöstrand C. High cholesterol levels are associated with improved long-term survival after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23(1):e47-e53. [DOI] [PubMed] [Google Scholar]
- 31.Yeramaneni S, Kleindorfer DO, Sucharew H, et al. Hyperlipidemia is associated with lower risk of poststroke mortality independent of statin use: A population-based study. Int J Stroke. 2017;12(2):152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xi L, Ghosh S, Wang X, Das A, Anderson FP, Kukreja RC. Hypercholesterolemia enhances tolerance to lethal systemic hypoxia in middle-aged mice: possible role of VEGF downregulation in brain. Mol Cell Biochem. 2006;291(1-2):205-211. [DOI] [PubMed] [Google Scholar]
- 33.Vatassery GT, Smith WE, Quach HT, Lai JC. In vitro oxidation of vitamin E, vitamin C, thiols and cholesterol in rat brain mitochondria incubated with free radicals. Neurochem Int. 1995;26(5):527-535. [DOI] [PubMed] [Google Scholar]
- 34.Eliasson M, Asplund K, Evrin PE. Regular leisure time physical activity predicts high activity of tissue plasminogen activator: the Northern Sweden MONICA Study. Int J Epidemiol. 1996;25(6):1182-1188. [DOI] [PubMed] [Google Scholar]
- 35.el-Sayed MS. Effects of exercise on blood coagulation, fibrinolysis and platelet aggregation. Sports Med. 1996;22(5):282-298. [DOI] [PubMed] [Google Scholar]
- 36.Stratton JR, Chandler WL, Schwartz RS, et al. Effects of physical conditioning on fibrinolytic variables and fibrinogen in young and old healthy adults. Circulation. 1991;83(5):1692-1697. [DOI] [PubMed] [Google Scholar]
- 37.Lakoski SG, Savage PD, Berkman AM, et al. The safety and efficacy of early-initiation exercise training after acute venous thromboembolism: a randomized clinical trial. J Thromb Haemost. 2015;13(7):1238-1244. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez AL, Wojcik BM, Wrobleski SK, Myers DD Jr., Wakefield TW, Diaz JA. Statins, inflammation and deep vein thrombosis: a systematic review. J Thromb Thrombolysis. 2012;33(4):371-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams NB, Lutsey PL, Folsom AR, et al. Statin therapy and levels of hemostatic factors in a healthy population: the Multi-Ethnic Study of Atherosclerosis. J Thromb Haemost. 2013;11(6):1078-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt M, Cannegieter SC, Johannesdottir SA, Dekkers OM, Horváth-Puhó E, Sørensen HT. Statin use and venous thromboembolism recurrence: a combined nationwide cohort and nested case-control study. J Thromb Haemost. 2014;12(8):1207-1215. [DOI] [PubMed] [Google Scholar]
- 41.Pierce GL, Kalil GZ, Ajibewa T, et al. Anxiety independently contributes to elevated inflammation in humans with obesity. Obesity (Silver Spring). 2017;25(2):286-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salim S, Chugh G, Asghar M. Inflammation in anxiety. Adv Protein Chem Struct Biol. 2012;88:1-25. [DOI] [PubMed] [Google Scholar]
- 43.Enga KF, Rye-Holmboe I, Hald EM, et al. Atrial fibrillation and future risk of venous thromboembolism:the Tromsø study. J Thromb Haemost. 2015;13(1):10-16. [DOI] [PubMed] [Google Scholar]
- 44.Hald EM, Enga KF, Løchen ML, et al. Venous thromboembolism increases the risk of atrial fibrillation: the Tromso study. J Am Heart Assoc. 2014;3(1):e000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rinde LB, Småbrekke B, Mathiesen EB, et al. Ischemic stroke and risk of venous thromboembolism in the general population: thre Tromso study. J Am Heart Assoc. 2016;5(11):e004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Ani F, Shariff S, Siqueira L, Seyam A, Lazo-Langner A. Identifying venous thromboembolism and major bleeding in emergency room discharges using administrative data. Thromb Res. 2015;136(6):1195-1198. [DOI] [PubMed] [Google Scholar]
- 47.Smith NL, Harrington LB, Blondon M, et al. The association of statin therapy with the risk of recurrent venous thrombosis. J Thromb Haemost. 2016;14(7):1384-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunutsor SK, Seidu S, Khunti K. Statins and secondary prevention of venous thromboembolism: pooled analysis of published observational cohort studies. Eur Heart J. 2017;38(20):1608-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becattini C, Agnelli G, Schenone A, et al. ; WARFASA Investigators . Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959-1967. [DOI] [PubMed] [Google Scholar]
- 50.Simes J, Becattini C, Agnelli G, et al. ; INSPIRE Study Investigators (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism) . Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation. 2014;130(13):1062-1071. [DOI] [PubMed] [Google Scholar]
- 51.Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition) Chest. 2008454S-545S. [DOI] [PubMed] [Google Scholar]
- 52.Eichinger S, Hron G, Bialonczyk C, et al. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch Intern Med. 2008;168(15):1678-1683. [DOI] [PubMed] [Google Scholar]
- 53.Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ. 2008;179(5):417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.