Short abstract
Patients with ischemic stroke or transient ischemic attack and non-valvular atrial fibrillation have a high risk of recurrent stroke and other vascular events. The aim of this guideline is to provide recommendations on antithrombotic medication for secondary prevention of stroke and other vascular outcomes in these patients. The working group identified questions and outcomes, graded evidence, and developed recommendations according to the Grading of Recommendations Assessment, Development, and Evaluation approach and the European Stroke Organisation (ESO) standard operating procedure for guidelines. The guideline was reviewed and approved by the ESO guideline board and the ESO executive committee. In patients with atrial fibrillation and previous stroke or transient ischemic attack, oral anticoagulants reduce the risk of recurrence over antiplatelets or no antithrombotic treatment. Non-vitamin K antagonist oral anticoagulants are preferred over vitamin K antagonists because they have a lower risk of major bleeding and death. Recommendations are weak regarding timing of treatment, (re-)starting oral anticoagulants in patients with previous intracerebral haemorrhage, and treatment in specific patient subgroups of those of older age, with cognitive impairment, renal failure or small vessel disease, because of a lack of strong evidence. In conclusion, for patients with atrial fibrillation and ischemic stroke or transient ischemic attack, non-vitamin K antagonist oral anticoagulants are the preferred treatment for secondary prevention of recurrent stroke or thromboembolism. Further research is required to determine the best timing for initiating oral anticoagulants after an acute ischemic stroke, whether or not oral anticoagulants should be (re)started in patients with a history of intracerebral haemorrhage, and the best secondary preventive treatment in specific subgroups.
Keywords: Guidelines, atrial fibrillation, stroke, secondary prevention
Introduction
In Europe, 1% to 2% of the population has atrial fibrillation (AF).1,2 As a result of a steep increase in the prevalence of AF with age and the continuously ageing population,3 the projected number of people with AF in Europe is 17.9 million by the year 2060.4 The risk of stroke attributable to AF rises from 1.5% in patients aged 50 to 59 years to 23.5% for those 80 to 89 years.5 These proportions are probably underestimated as recent randomised studies have shown that the proportion of patients with stroke and AF increases with more prolonged ECG monitoring, in particular in patients with cryptogenic or embolic stroke of undetermined source.6,7 In a recent randomised study of stroke survivors without known AF, longer ECG monitoring consisting of 10-day Holter monitoring at the time of stroke, and at 3 and 6 months’ follow-up, increased the detection rate of AF to 13.5%, compared with 4.5% in the standard care group (ECG monitoring of 24 h or longer according to guidelines).8 Patients with ischemic stroke or transient ischemic attack (TIA) and AF benefit from oral anticoagulation (OAC) treatment for the prevention of stroke and other thromboembolic events, although many of the studies that demonstrated this benefit, including those investigating non-vitamin K antagonist oral anticoagulants (NOACs), were not restricted to patients with previous ischemic stroke or TIA.
The aim of this guideline is to provide recommendations to guide stroke care providers to reach clinical decisions in practice regarding antithrombotic treatment for secondary prevention of stroke and other vascular outcomes in patients with stroke or TIA and non-valvular AF.
Methods
We followed the European Stroke Organisation (ESO) guidelines standard operating procedure,9 which is based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology. In short, the ESO guideline committee invited two chairs (CJMK and MP), who established a working group consisting of five stroke specialists (EB, EK, JK, JP and DJW), two advisors (HCD and PK), and one methodologist (AL). The working group was confirmed by the guideline committee. The stroke specialists of the working group discussed and decided by consensus on the PICO (Patient, Intervention, Comparator, Outcome) questions to be addressed and on the outcomes of interest during a face-to-face meeting. Outcomes were rated for importance by all stroke specialists. PICO questions and outcomes were reviewed by the guideline committee and revised according to its recommendations.
Literature search
We performed searches of the literature in MEDLINE, EMBASE, CINAHL and the COCHRANE controlled trials register (on 15 January 2019 without any date limit) for each of the PICO questions separately (see Online Supplement). Search terms and their corresponding Medical Subject Heading (MeSH) terms used to identify the articles are described in the Online Supplement. For each PICO question, two working group members independently screened titles, abstracts and subsequently full texts for potentially relevant studies. We based our evidence synthesis on results from randomised controlled trials, systematic reviews and meta-analyses.
Data extraction and risk of bias assessment
We extracted and analysed tabular data from randomised clinical trials (RCTs) of patients with AF specifically for the population of interest, e.g. those patients who had experienced ischemic stroke or TIA. For each PICO question, data were extracted and meta-analysed by the methodologist (AL) and checked by two or three working group members. The risk of bias of RCTs was assessed using the Cochrane Collaboration’s tool. We assessed randomisation (random sequence generation), allocation concealment, blinding of participants, outcome assessment, attrition bias (incomplete outcome data), reporting bias (selective reporting) and other biases in each study and summarised findings in evidence profile tables according to the GRADE methodology.
Meta-analysis
Meta-analysis was performed using the Review Manager (RevMan, version 5.3) Cochrane Collaboration software. We calculated risk ratios (RRs) or odds ratios (ORs) and 95% confidence intervals (CIs), with a random effects model, for all outcomes. We calculated I2 statistics and p values of Q statistics to assess heterogeneity of study results. A p value of the Q statistic ≤0.05 was considered to indicate statistically significant heterogeneity. Heterogeneity was classified as moderate (I2 ≥ 30–49%), substantial (I2 ≥ 50–74%), or considerable (I2 ≥ 75%).10 Where appropriate, we performed subgroup analyses based on category of antithrombotic drug (direct thrombin or factor Xa inhibitors), dose or severity of comorbidities (e.g. renal failure). Results were summarised in GRADE evidence profiles and summary of findings tables. In addition to study design, risk of bias, directness, heterogeneity, precision and magnitude of effect, these tables include information on magnitude of effect, confounding and dose-response relationship. Directness refers to the extent by which patient populations, interventions and outcomes are similar to those of interest.
Evidence synthesis and grading, and recommendations
For each of the PICO questions, grading of the evidence and writing of the recommendations were performed by two or three working group members. All drafts were critically revised by all working group members, and discrepancies in grading and recommendations discussed during regular telephone conferences. Quality of evidence was graded as high, moderate, low, and very low as defined in Online Table 1, and strengths of recommendations were graded as strong when the desirable effect of an intervention clearly outweighed the undesirable effects or clearly did not, or weak when the trade-off was less certain, either because of low-quality evidence, or because the evidence suggested that desirable and undesirable effects were more closely balanced. Section authors generated a section of ‘additional information’ based on observational studies and ongoing RCTs if this was deemed to provide additional information beyond the results of any RCTs. Based on this additional information, we formulated Expert opinions according to the Delphi-method.9
Results
The working group identified five areas for which PICO questions were formulated: (i) medical treatment; (ii) timing of medical treatment; (iii) treatment by means of occlusion of the left atrial appendage; (iv) (re-) starting medical treatment in patients with previous intracerebral haemorrhage (ICH); and (v) medical treatment in specific patient subgroups (i.e. elderly, patients with cognitive deficits, patients with renal failure and patients with signs of small vessel disease (SVD) on MRI) for a total of 19 PICOs. Study selection for each of the PICO questions is outlined in Online Figures 1 to 5 of the Online Supplement. The risk of bias of included studies is summarised in Online Figures 6 and 7. The working group identified seven outcomes of interests, of which the composite of all stroke or thromboembolism was considered the most important (Table 1).
Table 1.
Outcomes of interest and judgement of their importance.
| Outcomes | Ranking |
|---|---|
| Stroke (all) or thromboembolism | 9 |
| Ischemic stroke | 8 |
| Intracerebral haemorrhage | 8 |
| Major bleeding complications | 8 |
| Non-fatal stroke, non-fatal myocardial infarction and vascular death | 7 |
| Death | 7 |
| Venous thromboembolism | 4 |
Medical treatment in patients with ischemic stroke
Antiplatelet agents versus control
In patients with non-valvular AF and previous ischemic stroke or TIA, does single or dual antiplatelet therapy compared to placebo lower the risk of recurrent stroke or thromboembolism and other predefined outcomes?
Aspirin versus placebo
The European Atrial Fibrillation Trial (EAFT) randomised 1007 patients with minor ischemic stroke or TIA and AF into three arms: warfarin (international normalised ratio (INR) 2.5–4.0), aspirin 300 mg, and placebo.11 The relative risk reduction for all strokes for aspirin versus placebo was 14% (relative risk 0.86, 95% CI 0.64–1.15), which was not statistically significant. In the European Stroke Prevention Study-2 (ESPS-2)12 and the United Kingdom TIA (UK-TIA) aspirin trial, a total of 260 patients with previous stroke or TIA and AF were randomised to aspirin or placebo (data on patients with previous ischemic stroke or TIA were in part obtained via personal communication with the authors).13 A pooled analysis of these three trials showed a risk reduction for stroke or thromboembolism for aspirin versus placebo of 17%, which was not statistically significant (OR 0.83, 95% CI 0.62–1.10; Online Figure 8). The risk reduction for stroke and systemic embolism was similar for any dose of aspirin in the different clinical trials. There was no significant difference in the risk of the composite of non-fatal stroke, non-fatal myocardial infarction or vascular death in patients on aspirin compared to placebo (OR 0.88, 95% CI 0.65–1.18). The risk of ICH was 0.2% in the aspirin group and 0% in the placebo group (OR 2.81, 95% CI 0.11–69.29). The risk of major bleeding was 1.5% in the aspirin group compared to 1.1% in the placebo group (OR 1.41, 95% CI 0.39–5.03; Online Table 2).
Aspirin plus dipyridamole or dipyridamole alone versus placebo
In patients with AF included in ESPS-2, patients treated with dipyridamole alone had a stroke or systemic embolism rate of 17.5% compared to 21.5% in the placebo group (OR 0.78, 95% CI 0.40–1.51).12 The stroke or thromboembolism rate of patients treated with the combination of aspirin and dipyridamole was 13.5% and 21.5% in the placebo group (OR 0.57, 95% CI 0.27–1.18; Online Figure 8). Data regarding haemorrhagic outcomes were not reported.
Any antiplatelet agents versus placebo
Pooled data from four studies including 1474 patients randomised to receive antiplatelet agents (aspirin and/or dipyridamole) in patients with AF and previous stroke or TIA did not show a significant risk reduction for stroke or thromboembolism for antiplatelets versus placebo (OR 0.79, 95% CI 0.61–1.01; Online Figure 8).
In patients with non-valvular AF and previous ischemic stroke or TIA, does dual antiplatelet therapy compared to single antiplatelet therapy lower the risk of recurrent stroke or thromboembolism, and other predefined outcomes?
Because some patients with AF cannot tolerate vitamin K antagonists (VKAs) and before NOACs were available, there has been considerable interest in the combination of different antiplatelet agents as an alternative therapy to VKAs in hope of better efficacy than single antiplatelet therapy. In the Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE A) study that compared the combination of aspirin and clopidogrel to aspirin in patients for whom VKAs therapy were deemed unsuitable, 992 patients with AF and prior ischemic stroke or TIA (secondary prevention cohort) had a stroke rate of 4.5% per year when assigned to the combination therapy, compared to 6.3% per year when assigned to aspirin.14 Data regarding haemorrhagic outcomes were not reported. Considering all the patients included in the study (primary and secondary prevention cohorts), an analysis of major vascular events combined with major haemorrhage showed no difference between the two treatments (RR 0.97, 95% CI 0.89–1.06).
In the ESPS-2, patients with AF treated with dipyridamole alone had a stroke rate of 17.5% compared to 16.3% of patients treated with the combination of aspirin and dipyridamole (OR 0.92; 95% CI 0.45–1.87).12 Data regarding haemorrhagic outcomes were not reported.
Recommendations
In patients with non-valvular AF and previous ischemic stroke or TIA, we do not recommend antiplatelet agents, either as single or dual therapy, for secondary prevention of all events.
Quality of evidence: Moderate
Strength of recommendations: Weak
Vitamin K antagonists versus control
In patients with non-valvular AF and previous ischemic stroke or TIA, do vitamin K antagonists compared to placebo lower the risk of recurrent stroke or thromboembolism and other predefined outcomes?
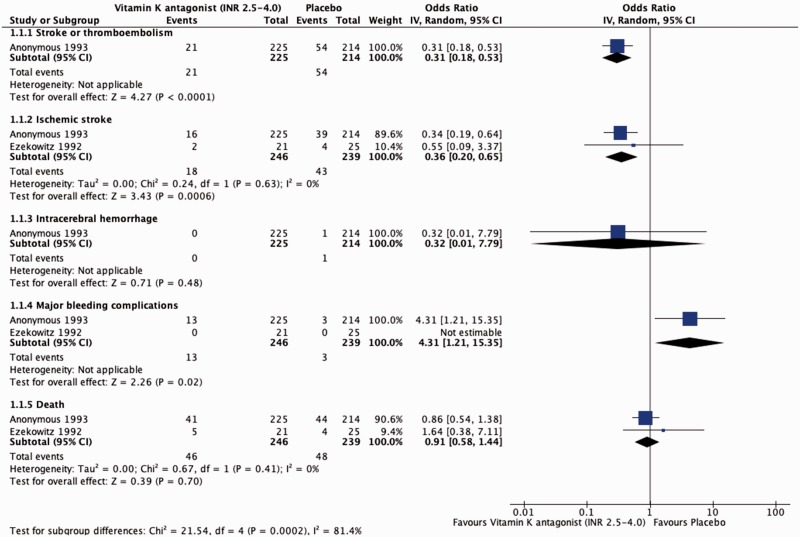
Data regarding randomised comparison of VKA with placebo in patients with prior ischemic stroke or TIA and AF come from two trials, the EAFT11 and Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation trial.15 EAFT demonstrated that adjusted-dose warfarin therapy (INR 2.5–4; target 3) reduced the risk of recurrent ischemic stroke and thromboembolism in patients with previous minor ischemic stroke or TIA or from 25.2%, compared to 9.3% in the placebo group (OR 0.31, 95% CI 0.18–0.53).11 The Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation study compared low-intensity warfarin (INR 1.2 to 1.5) to placebo. In the small secondary prevention cohort (46 patients), the risk of recurrent ischemic stroke was 9.5% in the warfarin group compared to 16% in the placebo group (OR 0.55, 95% CI 0.09–3.37).15
A pooled analysis of the results of these trials showed a risk reduction for recurrent ischemic stroke for warfarin versus placebo of 64% (OR 0.36, 95% CI 0.20–0.65). The pooled analysis showed an increase of major bleeding in patients treated with warfarin compared to placebo (OR 4.31, 95% CI 1.21–15.35) without an increase of intracranial haemorrhage (OR 0.32, 95% CI 0.01–7.79; Table 2 and Figure 1).
Table 2.
Effect of Vitamin K antagonist compared to placebo in patients with previous ischemic stroke or TIA and AF.
| Certainty assessment |
No. of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Vitamin K antagonist | Placebo | Relative (95% CI) | Absolute (95% CI) | ||
| Stroke or thromboembolism | ||||||||||||
| 1 | Randomised trials | Not serious | Not serious | Not serious | Not serious | Publication bias strongly suspecteda | 21/225 (9.3%) | 54/214 (25.2%) | OR 0.31(0.18 to 0.53) | 158 fewer per 1000 (from 101 fewer to 195 fewer) | ⨁⨁⨁MODERATE | CRITICAL |
| Ischemic stroke | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Not serious | Not serious | Publication bias strongly suspectedb | 18/246 (7.3%) | 43/239 (18.0%) | OR 0.36 (0.20 to 0.65) | 107 fewer per 1000 (from 55 fewer to 138 fewer) | ⨁⨁⨁MODERATE | CRITICAL |
| Intracerebral haemorrhage | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Not serious | Seriousc | Publication bias strongly suspectedb | 0/246 (0.0%) | 1/239 (0.4%) | OR 0.32 (0.01 to 7.79) | 3 fewer per 1000 (from 4 fewer to 28 more) | ⨁⨁LOW | CRITICAL |
| Major bleeding complications | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Not serious | Seriousc | Publication bias strongly suspectedb | 13/246 (5.3%) | 3/239 (1.3%) | OR 4.31 (1.21 to 15.35) | 39 more per 1000 (from 3 more to 151 more) | ⨁⨁LOW | CRITICAL |
| Death | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Not serious | Not serious | Publication bias strongly suspectedb | 46/246 (18.7%) | 48/239 (20.1%) | OR 0.91 (0.58 to 1.44) | 15 fewer per 1000 (from 65 more to 74 fewer) | ⨁⨁⨁MODERATE | CRITICAL |
CI: confidence interval; OR: odds ratio.
aSingle study.
bTwo studies to report this outcome.
cWide confidence intervals.
Figure 1.
Effect of vitamin K antagonists compared to placebo in patients with previous ischemic stroke or TIA and AF.
Recommendations
In patients with non-valvular AF and previous ischemic stroke or TIA, we recommend vitamin K Antagonists over no antithrombotic medication for secondary prevention of all events.
Quality of evidence: Moderate
Strength of recommendations: Strong
Vitamin K antagonists versus antiplatelet agents
In patients with non-valvular AF and previous ischemic stroke or TIA, do vitamin K antagonists lead to lower risk of recurrent stroke or thromboembolism and other predefined outcomes than antiplatelet treatment?
Adjusted-dose warfarin versus aspirin
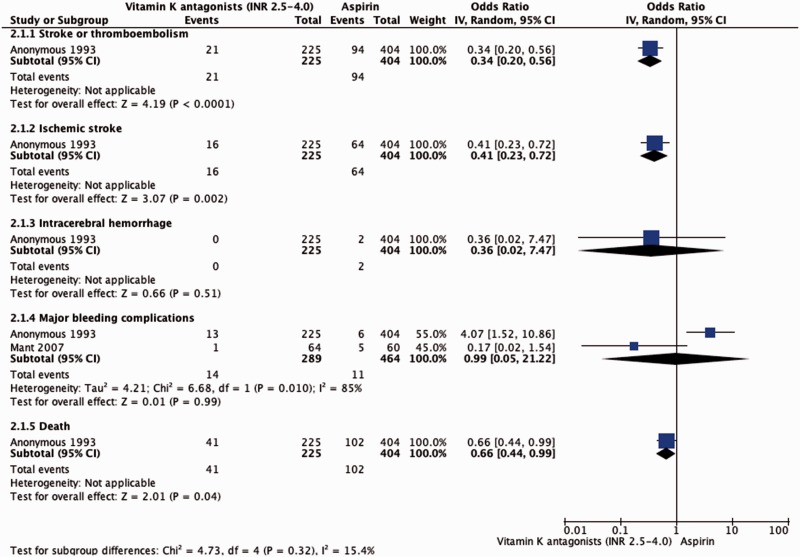
The EAFT demonstrated that OAC therapy (INR 2.5–4; target 3) reduced the risk of recurrent stroke and systemic embolism in patients with AF and TIA or minor ischemic stroke from 23.3% to 9.3% when compared to aspirin (OR 0.34, 95% CI 0.20–0.56).11 The risk of ICH was similar in the groups (OR 0.36, 95% CI 0.02–7.47). The Birmingham Atrial Fibrillation Treatment of the Aged Study (BAFTA) assessed whether warfarin reduced risk of major stroke, arterial embolism or other intracranial haemorrhage compared with aspirin in elderly patients.16 For the small secondary prevention cohort (124 patients), data were not reported regarding ischemic outcomes (ischemic stroke and systemic embolism) while the risk of major bleeding was similar in patients receiving warfarin compared to those treated with aspirin (OR 0.17, 95% CI 0.2–1.54). Pooling EAFT and BAFTA results, the risk of major bleeding was 4.8% in the VKA group compared to 2.4% in the aspirin group (OR 2.39, 95% CI 0.98–5.85; Table 3 and Figure 2).
Table 3.
Effect of Vitamin K antagonist compared to aspirin in patients with previous ischemic stroke or TIA and AF.
| Certainty assessment |
No. of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Vitamin K antagonist | Aspirin | Relative (95% CI) | Absolute (95% CI) | ||
| Stroke or thromboembolism | ||||||||||||
| 1 | Randomised trial | Seriousa | Not serious | Not serious | Not serious | Publication bias strongly suspectedStrong associationb | 21/225 (9.3%) | 94/404 (23.3%) | OR 0.34 (0.20 to 0.56) | 139 fewer per 1000 (from 88 fewer to 175 fewer) | ⨁⨁⨁MODERATE | CRITICAL |
| Ischemic stroke | ||||||||||||
| 1 | Randomised trial | Seriousa | Not serious | Not serious | Not serious | Publication bias strongly suspectedStrong associationb | 16/225 (7.1%) | 64/404 (15.8%) | OR 0.41(0.23 to 0.72) | 87 fewer per 1000(from 39 fewer to 117 fewer) | ⨁⨁⨁MODERATE | CRITICAL |
| Intracerebral haemorrhage | ||||||||||||
| 1 | Randomised trial | Seriousa | Not serious | Not Serious | Seriousc | Publication bias strongly suspectedb | 0/225 (0.0%) | 2/404 (0.5%) | OR 0.36(0.02 to 7.47) | 3 fewer per 1000(from 5 fewer to 31 more) | ⨁VERY LOW | CRITICAL |
| Major bleeding complications | ||||||||||||
| 2 | Randomised trial | Seriousa | Seriousd | Not serious | Not serious | Publication bias strongly suspectedstrong associatione | 14/289 (4.8%) | 11/464 (2.4%) | OR 2.39(0.98 to 5.85) | 31 more per 1000(from 0 fewer to 101 more) | ⨁⨁LOW | CRITICAL |
| Death | ||||||||||||
| 1 | Randomised trial | Seriousa | Not serious | Not serious | Not serious | Publication bias strongly suspectedb | 41/225 (18.2%) | 102/404 (25.2%) | OR 0.66(0.44 to 0.99) | 70 fewer per 1000(from 2 fewer to 123 fewer) | ⨁⨁LOW | CRITICAL |
CI: confidence interval; OR: odds ratio.
aData not randomised between intervention and comparator.
bSingle study.
cWide confidence intervals.
dSignificant heterogeneity.
eTwo studies to report this outcome.
Figure 2.
Effect of vitamin K antagonists compared to aspirin in patients with previous ischemic stroke or TIA and AF.
Adjusted-dose warfarin versus clopidogrel plus aspirin
The Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE W) compared warfarin with a combination of clopidogrel and aspirin in AF patients with at least one risk factor for stroke.17 This study was stopped prematurely after 3371 patients were enrolled because of clear superiority of warfarin (INR 2.0–3.0) over the antiplatelet combination (RR 1.44, 95% CI 1.18–1.76 for clopidogrel and aspirin vs. warfarin). Patients with prior stroke or TIA (510 in the warfarin group and 510 in the clopidogrel plus aspirin group) had a stroke rate of 2.99% per year when assigned to warfarin and 6.22% per year when assigned to clopidogrel plus aspirin (RR 2.13, 95% CI 1.23–3.69). In the secondary prevention cohorts, data regarding haemorrhagic outcomes were not reported.
Adjusted-dose warfarin (INR 2.0–3.0) versus fixed-dose warfarin (INR 1.2–1.5) plus aspirin
In the Stroke Prevention in Atrial Fibrillation III (SPAF III) trial, 1044 patients with AF and with at least one thromboembolic risk factor were randomly assigned to adjusted-dose warfarin (INR 2.0–3.0) or to a combination of low-intensity, fixed-dose warfarin (INR 1.2–1.5 for initial dose adjustment) and aspirin (325 mg/day).18 Of the trial arms, 36% of those receiving adjusted-dose warfarin and 40% of those receiving combination therapy had a history of prior thromboembolism. The trial was stopped after a mean follow-up of 1.1 years as an interim analysis showed that the rate of ischemic stroke and thromboembolism in patients given adjusted-dose warfarin (1.9% per year) was significantly lower than in those given combination therapy (7.9% per year), yielding an absolute reduction of 6.0% per year (95% Cl 3.4–8.6) by adjusted-dose warfarin. In patients with prior thromboembolism, the rate of ischemic stroke and systemic embolism was 3.4% per year for adjusted-dose warfarin and 11.9% per year for combination therapy. The rates of major bleeding were similar for both regimens (2.1% per year, 95% CI 1.2–3.7 with adjusted-dose warfarin vs. 2.4% per year, 95% CI 1.4–4.1 with combination therapy).
Recommendations
In patients with non-valvular AF and previous ischemic stroke or TIA we recommend vitamin K antagonists (INR 2–3) over antiplatelet therapy (single or dual) for secondary prevention of all events.
Quality of evidence: Moderate
Strength of recommendations: Strong
In patients with non-valvular AF and previous ischemic stroke or TIA, we suggest adjusted-dose vitamin K antagonists (INR 2.0–3.0) over fixed-dose vitamin K antagonists (INR 1.2–1.5) plus aspirin for secondary prevention of all events.
Quality of evidence: Moderate
Strength of recommendations: Weak
Additional information
Regarding the optimal intensity of oral anticoagulation for stroke prevention in patients with AF, an observational study of primary and secondary prevention found that the risk of ischemic stroke rose steeply at INRs below 2.0.19 In 77 patients with non-rheumatic AF, at an INR of 1.7, the adjusted OR for stroke, as compared with the risk at an INR of 2.0, was 2.0 (95% CI 1.6–2.4); at an INR of 1.5, it was 3.3 (95% CI 2.4–4.6); and at an INR of 1.3, it was 6.0 (95% CI 3.6–9.8). Another study that included 121 patients found that over the entire range of INRs, for each 0.5 increase in INR, the risk for intracranial haemorrhage doubled (OR 2.1, 95% CI 1.4–2.9) and this risk rises even more rapidly at INRs greater than 4 to 5.20
Non-vitamin K antagonist oral anticoagulants versus vitamin K antagonists
In patients with non-valvular AF and previous ischemic stroke or TIA, do non-vitamin K antagonists oral anticoagulants compared to vitamin K antagonists lead to lower risks of recurrent stroke or thromboembolism and other predefined outcomes?
We identified four randomised trials comparing NOACs to VKAs in patients with AF providing preplanned subgroup analyses for patients with prior ischemic stroke or TIA.21–24 One trial investigated the direct thrombin inhibitor dabigatran and three trials factor Xa inhibitors, rivaroxaban, apixaban and edoxaban. In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY), 18,113 patients were assigned to 110 mg or 150 mg dabigatran twice daily or warfarin dose-adjusted to INR 2.0 to 3.0.21 The regimen was open label, but patients and investigators were not aware of dabigatran dose, and events were adjudicated by investigators blinded to treatment allocation. A total of 2428 patients with previous ischemic stroke or TIA were randomised and followed for a median of 2.0 years.21 In the Rivaroxaban Once-daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation Patients (ROCKET AF), a total of 14,264 patients were randomly assigned in a double-blind manner to rivaroxaban 20 mg once daily (15 mg if creatinine clearance 30–49 mL/min) or adjusted-dose warfarin (INR 2.0–3.0).22 Of these, 7468 patients had a previous ischemic stroke or TIA and they were followed for a median of 1.85 years. In the double-blind Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE), 18,201 patients were assigned to apixaban 5 mg twice daily (2.5 mg twice daily for patients with two or more of the following: age ≥80 years, bodyweight ≤60 kg, serum creatinine ≥133 µmol/L) or warfarin (INR 2.0–3.0).23 A total of 3436 patients had a prior ischemic stroke or TIA and the median duration of follow-up was 1.8 years. In the double-blind Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) trial, 21,105 patients were randomised to once-daily edoxaban (60/30 mg in the higher dose regimen (HDER); 30/15 mg in the lower dose regimen (LDER)) or warfarin (INR 2.0–3.0). A total of 5973 patients with previous ischemic stroke or TIA were enrolled and followed for a median of 2.8 years.24 These four trials included a total of 19,305 patients with prior stroke or TIA.
Pooling the results of the four trials, NOACs were associated with a significant reduction of haemorrhagic stroke (RR 0.43, 95% CI 0.29–0.64) and death from any cause (RR 0.87, 95% CI 0.80–0.95; Online Figure 9(A) and (B)) when compared to adjusted-dose warfarin (Table 4).
Table 4.
Effect of non-vitamin K antagonist compared to vitamin K antagonist in patients with previous ischemic stroke or TIA and AF.
| Certainty assessment |
No. of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies/No. of comparisons | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | NOAC | VKA | Relative (95% CI) | Absolute (95% CI) | ||
| Stroke or thromboembolism | ||||||||||||
| 4/6 | Randomised trials | Not Serious | Not Serious | Not Serious | Not Serious | Publication bias suspecteda | 648/11858 (5.5%) | 495/8642 (5.7%) | RR 0.91(0.81 to 1.02) | 5 fewer per 1000(from 1 more to 11 fewer) | ⨁⨁⨁MODERATE | CRITICAL |
| Ischemic stroke | ||||||||||||
| 4/6 | Randomised trials | Not serious | Not serious | Not serious | Not serious | None | 557/11858 (4.7%) | 362/8642 (4.2%) | RR 1.07(0.93 to 1.22) | 3 more per 1000(from 3 fewer to 9 more) | ⨁⨁⨁⨁HIGH | CRITICAL |
| Intracerebral haemorrhage | ||||||||||||
| 1/1 | Randomised trials | Not serious | Not serious | Not serious | Not serious | Publication bias suspectedb | 26/3754 (0.7%) | 31/3714 (0.8%) | RR 0.83(0.49 to 1.39) | 1 fewer per 1000(from 3 more to 4 fewer) | ⨁⨁⨁MODERATE | CRITICAL |
| Major bleeding complications | ||||||||||||
| 4/6 | Randomised trials | Not serious | Seriousc | Not serious | Not serious | None | 649/11858 (5.5%) | 551/8642 (6.4%) | RR 0.79(0.64 to 0.96) | 13 fewer per 1000(from 3 fewer to 23 fewer) | ⨁⨁⨁MODERATE | CRITICAL |
| Death | ||||||||||||
| 4/6 | Randomised trials | Not serious | Not serious | Not serious | Not serious | None | 1048/11858 (8.8%) | 827/8642 (9.6%) | RR 0.87(0.80 to 0.95) | 12 fewer per 1000(from 5 fewer to 19 fewer) | ⨁⨁⨁⨁HIGH | CRITICAL |
CI: confidence interval; RR: risk ratio.
aFunnel plot shows slight asymmetry (Online Figure 10(B)).
bSingle study. Intracranial haemorrhage however showed large effect on pooling of four studies.
cSignificant heterogeneity I2 = 67%, p = 0.01. Subgroup analysis however did not show any publication bias for apixaban, rivaroxaban, edoxaban 60 mg and dabigatran.
There was no significant difference in the risk of stroke or thromboembolism (RR 0.91, 95% CI 0.81–1.02; Online Figure 10(A) and (B)) and ischemic stroke (RR 1.15, 95% CI 0.84–1.57; Online Figure 10(C)) in patients on NOACs compared to warfarin. There was no significant heterogeneity across the trials in the efficacy outcomes, with the exception of moderate heterogeneity for ICH (I2 34%, p = 0.18).
Analysis of efficacy outcomes using different types and doses of NOACs are summarised in Online Table 3. Of note, there was a significant reduction of stroke and systemic embolism and of stroke in favour or NOACs when higher dose regimens of dabigatran and edoxaban were included, while the reduction of haemorrhagic stroke remained similar.
Major bleeding was defined according to the International Society on Thrombosis and Hemostasis (ISTH) in all four trials.25 Pooling the results of the four trials, NOACs were associated with a significant reduction of major bleeding (RR 0.79, 95% CI 0.64–0.96; online Figure 11(A) and (B)) and, most profoundly, intracranial haemorrhage (RR 0.45, 95% CI 0.45–0.63). Specifically, ICH was only reported in patients with previous ischemic stroke or TIA allocated to rivaroxaban and there was a trend favouring NOACs over warfarin. There was substantial heterogeneity across the trials regarding major bleeding complications (I2 67%; p = 0.01).
Analysis of safety endpoints are shown in Online Tables 4 and 5. In these combinations, the key efficacy and safety results were in accordance with the main pooled analysis.
Recommendations
In patients with non-valvular AF and previous ischemic stroke or TIA, we recommend non-vitamin K antagonist oral anticoagulants over vitamin K antagonists for secondary prevention of all events.
Quality of evidence: High
Strength of recommendation: Strong
Additional information
The trials were not uniform with regard to methods of blinding, definitions of co-morbidities and endpoints (for example, definition of haemorrhagic stroke was inconsistent between the trials), baseline co-morbidities, length of follow-up and time in therapeutic INR range (TTR) in patients allocated to warfarin. Furthermore, we pooled data from trials testing NOACs with two different mechanisms of action, factor Xa inhibition and direct thrombin inhibition, and with varying dosages for some of the drugs. Nevertheless, there was no significant heterogeneity between results for the key efficacy and safety endpoints and data were fairly consistent also when mechanism of action and different dosing regimens were taken into account.
For efficacy of warfarin, it is important that TTR is high (>70%).26 In RE-LY, mean TTR was 63%, in ROCKET-AF 57%, in ARISTOTLE 65% and in ENGAGE AF-TIMI 48 68%. With respect to reduction of stroke and systemic embolism, NOACs remain superior to warfarin at TTR levels less than 70%, but this superiority no longer exists at high TTR levels.27,28 However, TTR levels do not appear to similarly modify the superiority of NOACs in safety outcomes.29
Patients with AF may also have symptomatic ischemic heart disease or intra- or extracranial atherosclerosis that can potentially increase the risk of recurrent vascular events warranting antiplatelet therapy. Currently, there are no data to inform us regarding the optimal therapeutic strategy in these patients, the combination of a NOAC and antiplatelet agents, VKAs alone, or VKAs in combination with antiplatelet therapy. Combination therapy of OACs and antiplatelets increases the risk of major bleedings but may be beneficial in some patients, e.g. those with recent ischemic heart disease or in case of recent coronary angioplasty and stenting.30 A meta-analysis of cohort studies reported that patients with ICHs related to NOACs had smaller baseline hematoma volumes and less severe acute stroke syndromes compared to patients with ICHs related to VKAs.31
Non-vitamin K antagonist oral anticoagulants vs. antiplatelet agents
In patients with non-valvular AF and previous ischemic stroke or TIA, do non-vitamin K antagonist oral anticoagulants compared to antiplatelets lower the risk of recurrent stroke or thromboembolism and other predefined outcomes?
The Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients who have Failed or are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial evaluated the efficacy and safety of factor Xa inhibitor apixaban compared with aspirin.32 A pre-specified subgroup of patients with previous stroke or TIA was randomly assigned to receive either apixaban (390 patients) 5 mg twice daily, or a reduced dose of 2.5 mg twice daily in patients aged 80 years or older, with bodyweight ≤60 kg, or with creatinine concentrations of ≥1.5 mg/dL), or to receive 81–324 mg of aspirin (374 patients). Patients and investigators were masked to treatment groups, and all outcomes were adjudicated by a masked committee. The mean duration of follow-up was 1.1 years. No other randomised controlled trials comparing NOACS to antiplatelets were identified.
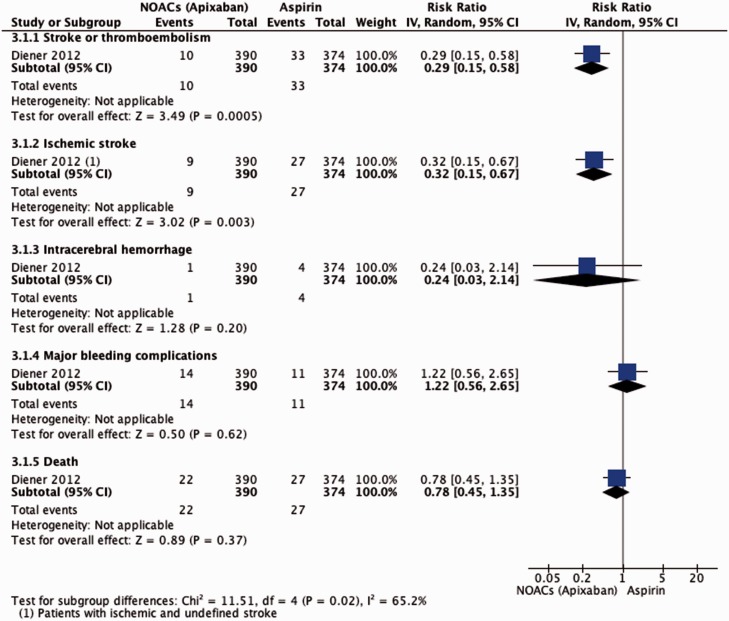
Apixaban was associated with a significant reduction of stroke and systemic embolism and a significant reduction of stroke. There was no statistically significant difference in deaths from any cause between the apixaban and aspirin groups (Figure 3).
Figure 3.
Effect of NOACs versus aspirin in patients with previous stroke or TIA and AF.
Although there were a few more ICHs in the aspirin group and major bleedings in the apixaban group during follow-up period, differences were not significant; the study was terminated prematurely because of a clear benefit in favour of apixaban.
Recommendations
In patients with non-valvular AF and previous ischemic stroke or TIA, we suggest non-vitamin K antagonist oral anticoagulants over aspirin in patients who have failed or are unsuitable for vitamin K antagonist therapy for secondary prevention of all events.
Quality of evidence: Moderate
Strength of recommendations: Weak
Additional information
Although the data favour apixaban compared to aspirin, they are based on a single RCT (AVERROES trial) with only one NOAC and a small number of patients with previous ischemic stroke or TIA. The EAFT study demonstrated that adjusted-dose anticoagulation therapy significantly reduced the risk of recurrent stroke and systemic embolism in patients with AF and TIA or minor stroke compared with aspirin (see “Vitamin K antagonists versus antiplatelet agents”).11 Moreover, the pooled analysis comparing all NOACs vs. VKAs showed that NOACs were associated with a better efficacy and safety without significant heterogeneity (see ‘Non-vitamin K antagonist oral anticoagulants versus vitamin K antagonists’ subsection).
Timing and bridging of oral anticoagulants
In patients with non-valvular AF and previous ischemic stroke or TIA, does ‘early’ compared to ‘late’ initiation of anticoagulant lower the rates of recurrent stroke or thromboembolism, without increasing the risk of intracranial bleeding?
If untreated, the risk of early recurrence of ischemic stroke in patients with AF is between 0.5% and 1.3% per day.33–36 Although warfarin has been the standard OAC therapy for decades, the optimal timing of its initiation for secondary stroke prevention in AF is based on weak evidence, mainly based on expert opinion. The RAF (Early Recurrence and Cerebral Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation: Effect of Anticoagulation and Its Timing) study was a prospective observational study that included 1029 ischemic stroke patients with AF, treated with anticoagulants (alone or in combination with antiplatelets), only antiplatelets, or no treatment.37 The main outcome was a composite of recurrent ischemic cerebrovascular events (stroke or TIA), symptomatic systemic embolisms, symptomatic intracerebral bleedings and major extracerebral bleeding at 90 days. In this non-randomised study, the optimal timing for initiating anticoagulant treatment was between 4 and 14 days. Other recent observational studies reported that, if NOACs are started early (within the first week) after an index event (ischemic stroke or TIA), the risk of intracranial bleeding appears to be low.38–42
The five large NOAC trials (RE-LY,21 ROCKET-AF,22 ARISTOTLE,23 AVERROES32 and ENGAGE AF-TIMI 48 trial24) excluded patients with stroke within the previous 7–14 days, and severe disabling stroke within 3–6 months. A recent proof-of-concept open-label trial, Acute Stroke with Xarelto to Reduce Intracranial Hemorrhage, Recurrent Embolic Stroke, and Hospital Stay (Triple AXEL) randomised South-Korean patients with AF-related mild ischemic stroke within the previous five days,43 at a median of two days from stroke onset, to rivaroxaban (10 mg/d for 5 days followed by 15 or 20 mg/d) or dose-adjusted warfarin for four weeks. The trial used the composite of new ischemic lesion or new intracranial haemorrhage on MRI at four weeks as the primary endpoint and length of hospital stay as a key secondary endpoint. Of 195 patients, 183 were included in the analysis. There was no difference in the primary endpoint between the groups (RR 0.91, 95% CI 0.69–1.12), nor any difference in the incidence of new ischemic lesions (RR 0.83, 95% CI 0.54–1.26). New intracranial haemorrhage occurred in 31.6% in the rivaroxaban group and 28.7% in the warfarin group (RR 1.10, 95% CI 0.70–1.71), but all of these were asymptomatic haemorrhagic transformations within or adjacent to the infarction. The duration of hospitalisation was significantly shorter with rivaroxaban compared with warfarin. However, this trial was not adequately powered for important clinical outcomes and there was no comparison between early initiation of anticoagulants and late initiation. Therefore, the optimal timing of treatment initiation of NOACs for secondary stroke prevention remains unknown.
Recommendations
We cannot make recommendations about the optimal time for initiating anticoagulation treatment in patients with acute ischemic stroke based on randomised trials. We encourage inclusion of patients in ongoing randomised controlled trials testing the efficacy and safety of early anticoagulation to answer this question.
Quality of evidence: Low
Strength of recommendation: Weak
Additional information
Although we found only a single inadequately powered RCT relevant to this PICO question (Triple AXEL trial), some observational evidence suggests an optimal 4- to 14-day window for anticoagulation post-acute ischemic stroke.37 However, the largest study on this question had limitations, including mixed treatment protocols with low molecular-weight heparin (LMWH) and warfarin as well as NOACs, and insufficient statistical power to determine benefit of earlier anticoagulation with NOACs.37 Moreover, index infarct size and severity of stroke may need to be taken into account before making any decision to minimise the risk of haemorrhagic transformation of the infarct or other intracranial bleeding. Although haemorrhagic transformation and infarct size have been associated with poorer outcome 44,45 there are no randomised data to confirm that early anticoagulation is more hazardous with potential for net harm in those patients with larger infarcts. Nevertheless, in the absence of definitive data, many expert clinicians currently suggest that stroke severity and infarct size should be considered when deciding on optimal timing for anticoagulation: for example, in patients with mild stroke and small infarcts (<1.5 cm) anticoagulation treatment has been suggested to be appropriate at day three or four from the index stroke; for moderate infarcts, it is suggested that anticoagulation treatment may be started at day 7 from index stroke; for large infarcts, anticoagulation treatment might be best delayed for 14 days after the index stroke.46 The European Heart Rhythm Association guidelines suggest that for patients with TIA and AF, VKAs or NOACs can be initiated on day one, and for those already receiving VKAs or NOACs, treatment can be continued because of a low risk of ICH. For patients with mild stroke (National Institutes of Health Stroke Scale (NIHSS) <8), NOACs can be initiated at three days, or after ICH has been excluded by imaging (computed tomography or magnetic resonance imaging).47 For patients with moderate stroke (NIHSS 8–16), anticoagulation can be initiated at 5–7 days, and in severe stroke (NIHSS > 16) at 12–14 days.47
The results of two large randomised, non-blinded intervention studies (IST and CAST) indicate that aspirin given within 48 h of stroke occurrence reduces case fatality and rate of recurrent stroke only minimally.35,48 A meta-analysis showed a reduction in the combined outcome of death or non-fatal recurrent stroke of nine per 1000 patients treated. This benefit was present also in patients with AF. Aspirin may therefore reasonably be given within 48 h (100–300 mg/day) after acute ischemic stroke or TIA for short-term treatment, pending the introduction of anticoagulation.
RCTs failed to produce any evidence supporting the administration of anticoagulants in patients with acute ischemic stroke within 48 h from stroke onset.36 For this reason, in patients already on VKAs one may consider to stop anticoagulant therapy, repeat a second brain CT scan after 24–72 h and decide the time to re-initiate treatment based on the size of the lesion. Thus, pending further evidence, aspirin should be administered in this acute time frame to all patients.49
There are several ongoing randomised controlled studies on the best timing of initiation of medical treatment after ischemic stroke: The Timing of oral anticoagulant therapy in acute ischemic stroke with AF (TIMING study; NCT02961348), the Early Versus Late Initiation of Direct Oral Anticoagulants in Post-ischemic Stroke Patients With Atrial fibrillatioN (ELAN trial; NCT03148457), the Optimal Delay Time to Initiate Anticoagulation After Ischemic Stroke in Atrial Fibrillation Trial (START; NCT03021928) and the OPtimal TIMing of Anticoagulation after AF-associated acute ischemic Stroke (OPTIMAS, Werring; EudraCT, 2018 003859–38).
Expert opinion (Delphi vote: 6/7 agree, 1/7 disagree)
We suggest antiplatelet therapy in the first 48 h after ischemic stroke associated with AF.
We consider it reasonable to start anticoagulant therapy at day 3 or 4 from the index stroke in patients with mild stroke and small infarcts (<1.5 cm) and at day 7 for moderate infarcts.
For large infarcts, anticoagulation treatment might be best delayed for 14 days after the index stroke.
In patients with non-valvular AF and previous ischemic stroke or TIA, does bridging with heparin or heparinoids lead to better outcomes than avoiding bridging in the time-window between symptom onset and start of oral antiocoagulation?
The risk of early recurrent ischemic stroke occurring within the first 2 weeks, is higher in patients with AF than in patients with stroke resulting from other causes.33–36 In patients with AF and acute ischemic stroke, unfractionated heparin (UFH), LMWH, or heparinoids are commonly used in routine clinical practice outside clinical trials while awaiting the commencement or effect of OAC. However, RCTs indicate that in patients with acute cardioembolic stroke, early anticoagulation with UFH or LMWH is associated with increased intracranial bleeding, a non-significant reduction in recurrence of ischemic stroke, and no substantial reduction in death and disability.36 Furthermore, observational studies reported that patients who had received VKA alone had a significantly lower risk of bleeding events, compared with patients treated with LMWH followed by OAC.50–53
Recommendations
In patients with non-valvular AF and previous ischemic stroke or TIA, we suggest avoiding routine bridging therapy prior to anticoagulation with vitamin K antagonists or non-vitamin K antagonist oral anticoagulants for secondary prevention of all events.
Quality of evidence: Low
Strength of recommendation: Weak
Additional information
Although we did not find any RCTs relevant to this PICO question, results of some observational studies indicate that bridging therapy should be avoided. Several studies found that patients who had received OACs alone had a significantly lower risk of bleeding events than patients treated with full-dose LMWH followed by OAC.50–53 The results of these studies should not be applied in a generalised manner because patients who received LMWH were more likely to have dysphagia and perhaps be at inherently greater risk of adverse outcomes.
Left atrial appendage occlusion versus oral anticoagulants
In patients with non-valvular AF and previous ischemic stroke or TIA, does left atrial appendage closure reduce risk of recurrent stroke or thromboembolism and other predefined outcomes compared to oral anticoagulant treatment?
Because in patients with non-valvular AF the majority of thrombi leading to ischemic stroke originate from blood stasis in the left atrial appendage (LAA), endovascular LAA occlusion (LAAO) provides a potential treatment to reduce the risk of ischemic stroke.54 Two RCTs have tested LAAO in comparison to dose-adjusted warfarin but the majority of patients did not have a prior ischemic stroke or TIA (PROTECT-AF and PREVAIL).55,56 For the primary outcome of stroke (ischemic or haemorrhagic) and thromboembolism, both these trials showed non-inferiority to dose-adjusted warfarin. PROTECT AF randomised 707 patients with non-valvular AF and an additional stroke risk factor (19% with previous ischemic stroke or TIA) to dose-adjusted warfarin or LAA closure in a 2:1 ratio.56 The primary outcome of stroke, thromboembolism or cardiovascular/unexplained death occurred in 3% in the WATCHMAN group versus 4.9% in the warfarin group (RR 0.62, 95% CI 0.35–1.25). In PREVAIL (Evaluation of the WATCHMAN LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy), 407 patients (with a mean CHADS2 score of 2.6; 28% with previous ischemic stroke or TIA) were randomised 2:1 to LAAO or warfarin. LAAO was non-inferior to warfarin for the primary outcome of stroke or thromboembolism > 7 days post-randomisation.55 Observational data from 1047 patients using another device, the AMPLATZER cardiac plug, showed an annual stroke risk of 2.3%.57
However, the procedure of LAAO device implantation carries a procedural risk of complications including device embolisation, arteriovenous fistula, cardiac perforation and pericardial effusion with cardiac tamponade. The rate of procedural complications was 8.7% in PROTECT-AF and 4.2% in PREVAIL.
Recommendations
For patients with non-valvular AF and previous ischemic stroke or TIA, we cannot make any recommendation on whether left atrial appendage occlusion should be preferred over long-term vitamin K antagonists for secondary prevention of all events.
Quality of evidence: Low
Strength of recommendation: Weak
Additional information
For clinicians and patients, a major attraction of LAAO is that patients do not need to take long-term oral anticoagulants. This makes LAAO potentially beneficial for patients deemed to be at high long-term risk of bleeding complications.58,59 A meta-analysis of five-year outcome data from PREVAIL and PROTECT-AF (comparing LAAO vs. Warfarin) showed similar rates of the composite coprimary endpoint of stroke, systemic embolism or cardiovascular/unexplained death (hazard ratio (HR) 0.82, 95% CI 0.58–1.17) and for all-stroke/systemic embolism (HR 0.96, 95% CI 0.60–1.54). The rate of haemorrhagic stroke was lower in LAAO compared to Warfarin (HR 0.20, 95% CI 0.07–0.56).60 No data from randomised studies exist on the optimal duration of antiplatelet therapy after LAAO. The benefit of LAAO in comparison to OACs or no medical treatment in patients with previous ICH is now being tested in RCTs (A3ICH, NCT03243175; and STROKECLOSE, NCT 02830152; see also ‘Oral anticoagulants versus no oral anticoagulants in patients with ICH’).
Expert opinion (Delphi vote: 7/7 agree)
LAAO can be considered in individual patients as an alternative to life-long oral anticoagulation after careful weighing of risks and benefits.
Oral anticoagulants versus no oral anticoagulants in patients with ICH
In patients with non-valvular AF who have experienced ICH, does starting or restarting oral anticoagulants reduce the risk of recurrent stroke or systemic embolism and other predefined outcomes in comparison to not (re-)starting oral anticoagulants?
Around 15–25% of patients presenting with ICH use OAC,61–63 in the majority because of AF.61 This proportion rises to >30% in patients 65 years and older61 and has increased over time.63 So far, most patients in studies on OAC associated ICH were treated with VKA.61–63 Adoption of NOACs for secondary prevention in AF might result in a decreasing incidence of OAC associated ICH in the coming years, at least in high-income countries, but increased uptake of OAC in older people might counteract this trend. In a recent Cochrane review, no randomised controlled trials could be identified that analysed efficacy and safety of administration of OAC or antiplatelet drugs after ICH.64 As a result, there is large variation in clinical practice, as illustrated in a cohort study providing observational data from four different countries, in which the proportion of patients who restarted antithrombotic drugs varied between 11% and 45%.65 In a recent systematic review and meta-analysis specifically addressing patients with AF (7 studies; 2452 patients), survivors of intracranial haemorrhage who restarted OAC had a lower risk of ischemic stroke in comparison to those in whom anticoagulants were not recommenced (pooled RR 0.46, 95% CI 0.29–0.72), whereas the risk of recurrent ICH was comparable (pooled RR 1.23, 95% CI 0.80–1.87).66 A recent study of 1012 patients with ICH and AF, that was not included in the previous meta-analysis, found that both in patients with lobar ICH (379 patients; OAC restarted in 23%) and in patients with non-lobar ICH (633 patients; OAC restarted in 28%), OAC resumption was associated with a decreased risk of death (adjusted (a) HR 0.29, 95% CI 0.17–0.45 for lobar and aHR 0.25, 95% CI 0.14–0.44 for non-lobar ICH), and with a decreased risk of ischemic stroke (aHR 0.48, 95% CI 0.25–0.75 for lobar and aHR 0.39, 95% CI 0.21–0.74 for non-lobar ICH).67 Restarting OAC was not associated with an increase in the risk of ICH recurrence for both lobar (aHR, 1.26, 95% CI, 0.88–1.71) and non-lobar (aHR, 1.17, 95% CI, 0.89–1.54) ICH patients.67 In 190 patients who fulfilled the modified Boston criteria for cerebral amyloid angiopathy (CAA),68 OAC resumption was associated with decreased risk of death both in those with possible CAA (aHR, 0.27, 95% CI 0.08–0.86; 136 patients) and in those with probable CAA (aHR 0.30, 95% CI 0.10–0.92; 54 patients); moreover, the presence of ≥2 cerebral microbleeds or cortical superficial siderosis did not modify these associations.67 In these previous studies, OAC consisted predominantly or exclusively of VKA and no information is available yet on the safety and efficacy of the use of NOACs in patients who have experienced ICH.
The results of these observational studies should be interpreted with caution as they are prone to selection biases and confounding by indication. Several randomised controlled trials are now addressing the clinical dilemma of whether or not to restart OACs in patients with AF who have had an ICH, APACHE-AF (NCT02565693),69 NASPAF-ICH (NCT02998905), SoSTART (NCT03153150), A3ICH (NCT03243175), STATICH (NCT03186729), STROKECLOSE (NCT 02830152), PRESTIGE-AF and ASPIRE, with most of them testing the use of NOACs against other strategies, including no OAC.70
Recommendations
In patients with AF who have experienced an ICH, we cannot make recommendations regarding whether or not oral anticoagulation should be (re-)started or not.
Quality of evidence: Low
Strength of recommendation: Weak
Expert opinion (Delphi vote: 7/7 agree)
In patients with AF who have experienced an ICH, restarting oral anticoagulation can be considered after careful weighing of risks and benefits.
Medical treatment in subgroups of patients with ischemic stroke
Elderly patients
In elderly patients with non-valvular AF and previous ischemic stroke or TIA, does oral anticoagulant treatment reduce the risk of recurrent stroke or systemic embolism and other predefined outcomes compared to antiplatelet treatment or no anticoagulant treatment?
We found no data to suggest that the benefit-RR is different in elderly patients with ischemic stroke or TIA receiving OAC treatment for AF. The absolute risk of intracranial haemorrhage is increased, as indicated by the HAS-BLED score, but so is also the absolute risk of ischemic events, as indicated by the CHA2DS2Vasc score. In the trials of anticoagulant treatment vs. no OAC treatment (EAFT11 and Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation trial15) and in the trials of OAC vs. antiplatelet treatment (EAFT,11 BAFTA,16 ACTIVE W17 and AVERROES32), there was no evidence of effect modification by age. Since there are no direct comparisons in elderly patients with previous ischemic stroke or TIA, the quality of evidence is low and the strength of the recommendation is weak.
Recommendations
In elderly patients with non-valvular AF and a history of ischemic stroke or TIA, we suggest oral anticoagulant treatment over antiplatelet treatment or no oral anticoagulant treatment for secondary prevention of all events.
Quality of evidence: Low
Strength of recommendation: Weak
In elderly patients with non-valvular AF and previous ischemic stroke or TIA, does non-vitamin K antagonist oral anticoagulants reduce the risk of recurrent stroke or systemic embolism and other predefined outcomes compared to vitamin K antagonists?
In the trials of NOACs vs. VKAs (RE-LY,21 ROCKET-AF,22 ARISTOTLE23 and ENGAGE AF-TIMI 48 trial24), analyses of the subgroups of elderly patients showed no evidence of any different effect in elderly patients.71 Since there are no direct comparisons in elderly patients with previous ischemic stroke or TIA, the quality of evidence is low and the strength of the recommendation is weak.
Recommendation
In elderly patients with non-valvular AF and previous ischemic stroke or TIA, we suggest non-vitamin K antagonist oral anticoagulants treatment over vitamin K antagonist treatment for secondary prevention of all events.
Quality of evidence: Low
Strength of recommendation: Weak
Patients with cognitive deficits
In patients with cognitive deficits, non-valvular AF and previous ischemic stroke, does anticoagulant treatment reduce the risk of recurrent stroke or systemic embolism and other predefined outcomes compared to antiplatelet treatment or no anticoagulant treatment?
In the trials of OAC vs. no OAC treatment (EAFT11 and Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation trial15) and in the trials of anticoagulant vs. antiplatelet treatment (EAFT,11 BAFTA16 and ACTIVE W17), there were no subgroup analyses of patients with cognitive deficits. Analyses of the subgroups of elderly patients and patients with previous ischemic stroke showed no evidence of any different effect in those patients, and this can perhaps be used as indirect evidence. In addition, many considerations must be made when offering long-term anticoagulant treatment to patients with cognitive decline, such as reduced capacity to consent, reduced life expectancy and quality of life, poorer adherence to treatment and increased risk of bleeding, so we believe that the strength of the recommendation should be ‘weak’.
Recommendation
In patients with cognitive deficits, non-valvular AF and previous ischemic stroke or TIA, we suggest oral anticoagulant treatment over antiplatelet treatment or no oral anticoagulant treatment for secondary prevention of all events.
Quality of evidence: Low
Strength of recommendation: Weak
In patients with cognitive deficits, non-valvular AF and previous ischemic stroke or TIA, does non-vitamin K antagonist oral anticoagulants reduce the risk of recurrent stroke or systemic embolism and other predefined outcomes compared to vitamin K antagonists?
In the trials of NOACs vs. VKAs (RE-LY,21 ROCKET-AF,22 ARISTOTLE23 and ENGAGE AF-TIMI 48 trial24), there was no analyses of subgroups of patients with cognitive deficits. Analyses of the subgroups of elderly patients and patients with previous stroke showed no evidence of any different effect in those patients, which can perhaps be used as indirect evidence. In addition, many factors should be taken into account when prescribing long-term OACs to patients with cognitive decline, including reduced capacity to consent, reduced life expectancy and quality of life, poor adherence to treatment and increased risk of bleeding. Therefore, the strength of the recommendation should be ‘weak’.
Recommendation
In patients with cognitive decline, non-valvular AF and previous ischemic stroke or TIA, we suggest non-vitamin K antagonist oral anticoagulants treatment over vitamin K antagonist treatment for secondary prevention of all events.
Quality of evidence: Low
Strength of recommendation: Weak
Patients with renal impairment
In patients with renal impairment, non-valvular AF, previous ischemic stroke or TIA, do non-vitamin K antagonist oral anticoagulants compared to vitamin K antagonists reduce the risk of recurrent stroke or systemic embolism and other predefined outcomes?
Impaired renal function is associated with a higher prevalence of AF and with an increased risk of ischemic stroke and systemic embolism.72–75 About 15–20% of AF patients also have chronic kidney disease (CKD).76 Renal impairment confers a substantially increased risk of major bleeding and often contributes to underuse of OAC in patients with AF.75,77 No RCT has directly investigated the efficacy and safety of NOACs compared to VKAs in AF patients with a prior stroke or TIA and impaired renal function. Available data focusing on renal function derive from pre-specified subgroup analyses of all four completed phase 3 clinical trials comparing NOACs with dose-adjusted warfarin in patients with AF.78–82 Considering that patients with prior ischemic stroke or TIA represent a substantial proportion in those studies, ranging from 20% to 63%, results may also be considered to be informative for patients with a previous stroke or TIA and renal impairment.
In all sub-group analyses, renal function was estimated using the Cockroft-Gault method.83 Renal dysfunction was defined according to the original trials based on the European Medicines Agency classification as moderate when creatinine clearance (CrCl) ranged between 30 and 50 ml/min, and as mild in the case of CrCl between 50 and 80 ml/min. Definitions of renal impairment varied across trials. In ENGAGE TIMI (HDER), mild renal impairment was defined as CrCl of 50–95 ml/min and in ENGAGE TIMI (LDER) and ROCKET AF, it was defined as CrCl > 50 ml/min.
The RELY trial tested the long-term efficacy and safety of two different doses (110 mg and 150 mg BID) of the direct thrombin inhibitor dabigatran and dose-adjusted warfarin. Renal elimination with dabigatran is approximately 80%. According to the Cockcroft-Gault equation a total of 8553 patients (47.6%) had CrCl 50 – <80 ml/min, and 3554 (19.8%) had CrCl <50 ml/min. The proportion of patients with a history of previous stroke or TIA in each group was 19.4% and 20.1%, respectively.82 ROCKET-AF compared fixed-dose rivaroxaban 20 mg daily or 15 mg daily with dose-adjusted warfarin. Of the 14,264 patients with AF, 2950 had moderate renal insufficiency defined as CrCl 30–49 ml/min at baseline. In patients with CrCl <50 ml/min, representing 20.7% of the trial cohort, the dose of rivaroxaban was reduced from 20 to 15 mg daily. Approximately, one third of rivaroxaban is excreted via the kidneys. Almost 50% of patients with CrCl < 50 ml/min and 56% of patients with preserved renal function had a history of a previous ischemic stroke/TIA.79
In the ARISTOTLE trial, apixaban was administered at 5 mg twice daily or 2.5 mg twice daily for a subgroup of patients with two or more of the following criteria: age ≥80 years, body weight ≤60 kg and serum creatinine ≥1.5 mg/dL (133 mmol/L). Renal elimination of apixaban is approximately 25%. According to the Cockcroft-Gault equation, 7587 patients had CrCl 50–80 ml/min and 3017 had severe renal impairment with CrCl ≤50 ml/min. The proportion of patients with a history of a previous stroke/TIA in each group was 21.6% and 25.1%, respectively.80
The ENGAGE AF-TIMI 48 trial evaluated the long-term efficacy and safety of two dosing regiments (60 mg and 30 mg) of edoxaban and adjusted-dose warfarin in patients with AF.78 Because of the significant renal clearance of edoxaban (50%), patients with moderate renal dysfunction (CrCl 30–50 ml/min) and low body weight (≤60 kg,) or concomitant use of a phosphorylated glycoprotein inhibitor received a dose of edoxaban reduced by 50% (30 mg and 15 mg, respectively). Of 14,071 patients in the warfarin and high-dose edoxaban arms, 2740 patients (19.5%) had a CrCl ≤50 ml/min and 8208 (58.3%) had a CrCl 50–95 ml/min at the time of randomisation. Of the 14,070 patients in the warfarin and LDER arm of the study, 2695 patients had a CrCl ≤50 ml/min and 11,375 had a CrCl > 50 ml/min. Among patients with moderate renal failure randomised to high-dose edoxaban, 30% had previous ischemic stroke or TIA.78
Patients with moderate renal failure (creatinine clearance ≤50ml/min)
We pooled a total of 13,880 patients with moderate renal impairment from five RCTs (8258 patients were assigned to NOAC and 5622 were assigned to warfarin). The reduction in risk of stroke or systemic embolism in patients with moderate renal failure did not differ significantly in patients receiving NOAC compared to those receiving warfarin (RR 0.87, 95% CI 0.74–1.04; Online Table 6 and Figure 15). Notably, there was a significant reduction of stroke or systemic embolism with the high dose of dabigatran; however, without a significant reduction in major bleeding at the same dose. There was little heterogeneity across the trials (I2 13%, p = 0.33).
Major bleeding was defined according to the ISTH criteria in all included trials.25 Pooling the results of four trials corresponding to a total population of 13,574 patients (8101 assigned to NOAC and 5473 patients to warfarin), use of NOACs was associated with a significant reduction of major bleeding by 27% (RR 0.73, 95% CI 0.54–0.99; Online Figure 16). The most significant reduction of major bleeding events was observed with apixaban and the lower dose of edoxaban, which was consistent with the main pooled analysis, whereas rivaroxaban, the higher dose of edoxaban as well as the lower and higher dose of dabigatran did not significantly reduce the rate of major bleeding compared to dose-adjusted warfarin in patients with moderate renal impairment. Heterogeneity among trials was high and statistically significant (I2 83%, p < 0.0001).
Three of the trials assessed the effect on all-cause death,78,80,82 whereas two trials assessed vascular death. 79,81 Pooling the results on all-cause death from three trials corresponding to 9299 patients (5302 assigned to NOAC group and 3997 assigned to warfarin), death was not significantly reduced with NOACs compared to warfarin (RR 0.93, 95%CI 0.80–1.07; Online Figure 17). The main pooled results were consistent across all trials with the exception of the higher dose of edoxaban, which showed a significant reduction in death (RR 0.83, 95%CI 0.71–0.96). Heterogeneity was substantial but not statistically significant across the trials (I2 50%, p = 0.11).
Recommendations
In patients with non-valvular AF and previous stroke or TIA, and moderate renal impairment, we suggest non-vitamin K antagonist oral anticoagulants over vitamin K antagonists for secondary prevention of all events.
Quality of evidence: Low
Strength of recommendation: Weak
Patients with mild renal failure (CrCl 50–80 ml/min)
Analysis of pooled data deriving from subgroup analyses of three RCTs (RE-LY,21,82 ARISTOTLE,23,80 ENGAGE AF-TIMI 48 trial low dose),24,78 corresponding to a population of 17,130 patients (9968 assigned to NOAC group and 7162 assigned to warfarin) showed that NOACs significantly reduced the risk of stroke or systemic embolism compared to warfarin in patients with mild renal failure (RR 0.73, 95% CI 0.56–0.97; Online Figure 15). Heterogeneity among studies was moderate, but not statistically significant (I2 48%, p = 0.12).
Major bleeding was defined according to the ISTH25 in all included trials. Pooling results from RELY and ARISTOTLE showed a significant reduction of major bleeding with NOAC compared to warfarin (RR 0.82, 95% CI 0.72–0.93; Online Figure 16). No heterogeneity was observed among studies (I2 0%, p = 0.51).
Pooled results from RE-LY and ARISTOTLE showed a significant reduction in death from all causes with NOAC compared to dose-adjusted warfarin (RR 0.85%, 95% CI 0.73–0.99; Online Figure 17). Heterogeneity among studies was moderate, but not statistically significant (I2 42%, p = 0.18).
Recommendations
In patients with mild renal impairment, non-valvular AF, and previous ischemic stroke or TIA, we suggest non-vitamin K antagonists oral anticoagulants over vitamin K antagonists for secondary prevention of all events.
Quality of evidence: Low
Strength of recommendation: Weak
Additional information
Extrapolation of results from predefined subgroup analyses on all AF patients with impaired renal function to AF patients with a previous ischemic stroke or TIA and renal impairment may be inadequate. Although patients with moderate renal insufficiency represent a substantial population within the RCTs, the predefined subgroup analyses on patients with renal dysfunction were not powered to demonstrate superiority or non-inferiority for the comparisons of NOAC over warfarin. Moreover, we pooled data from trials testing NOACs with different metabolism and pharmacokinetics: renal elimination is approximately 80% using dabigatran, 35% using rivaroxaban, 25% using apixaban and 50% using edoxaban. In addition, patients with impaired renal function across all trials were older and had higher event rates irrespective of study treatment. RCTs testing dabigatran, rivaroxaban and edoxaban excluded patients with CrCl < 30 ml/min, while trials of apixaban excluded those with CrCl <25 mL/min. Furthermore, patients were recruited according to the baseline GFR with no dose adjustments post-baseline for alterations in creatinine levels. The risk of drug accumulation and bleeding may have been amplified by several drug–drug interactions as well.
Anticoagulation constitutes the treatment of choice also for AF patients with moderate-to-severe renal dysfunction who have been excluded from RCTs testing NOACs over warfarin. Results from two large RCTs showed that OAC are effective and safe for AF patient with severely impaired renal function. SPAF III trial reported favourable efficacy and safety profile of adjusted-dose warfarin (target INR 2.0–3.0) compared to aspirin in high-risk AF patients with stage 3 CKD. 74 A subgroup analysis of AVERROES trial also showed that among patients with stage III CKD, apixaban significantly reduced ischemic stroke compared to aspirin without a significant increase in major bleeding.84 CKD is most prevalent in older people and OAC should be prescribed with caution. Nevertheless, even in this patient group, anticoagulation seems to be of benefit associated with lower rate of all-cause death, despite an increased rate of ischemic stroke and haemorrhage.85
Finally, there are limited data on the efficacy and safety of NOAC in patients on haemodialysis and VKAs remain the OAC of choice in those patients. A recent meta-analysis of five observational cohort studies reported outcomes on 43,850 patients with CKD stage 4–5 or end-stage renal disease on dialysis most of them (87%) using apixaban for AF.86 Apixaban was associated with reduced risk of major bleeding (pooled OR 0.42, 95% CI 0.28–0.61) compared to warfarin. In end-stage renal disease patients on dialysis, the pooled OR of major bleeding was 0.27 (95% CI 0.07–0.95). No significant difference was observed in the risk of thromboembolism in advanced CKD or end-stage renal disease patients treated with apixaban versus VKA (pooled OR 0.56, 95% CI 0.23–1.39). Multiple trials are investigating different treatment options in AF patients with end-stage renal disease and a history of stroke or at increased risk of stroke or embolism (RENAL-AF, NCT02942407, apixaban vs. warfarin AXADIA, NCT02933697, apixaban vs. phenprocoumon; STOP-HARM, NCT02885545, LAAO vs. continuation of prescribed OAC (VKA or apixaban or rivaroxaban); AVKDIAL, NCT02886962, no OAC vs. VKA).
Patients with cerebral SVD
In patients with non-valvular AF, previous ischemic stroke or TIA and SVD, does oral anticoagulant treatment reduce the risk of recurrent stroke or thromboembolism and other predefined outcomes compared to antiplatelet treatment or no anticoagulant treatment?
The term ‘cerebral small vessel disease’ describes pathological processes affecting the small arteries, arterioles, venules and capillaries of the brain.87 SVD causes about 25% of ischemic stroke (through small artery occlusion)87 and about 80% of ICH (through small artery rupture).88 The radiological lesions caused by SVD include lacunes (the commonest type of silent brain infarct), white matter hyperintensities (WMH), cerebral microbleeds (CMB), cortical superficial siderosis and perivascular spaces, as defined by the STRIVE (STandards for ReportIng Vascular changes on nEuroimaging) consensus group.89 SVD is of interest in patients with stroke or TIA and AF, because it might affect the risk of both future ischemic stroke and ICH (and different markers might differentially affect these outcomes), potentially affecting the risk-balance of antithrombotic treatment decisions. Of these imaging markers, the most widely studied in cohorts relevant for secondary stroke prevention for AF are WMH and CMBs; these are common in populations of patients with AF with previous stroke (WMH prevalence 19–23%, CMBs prevalence 7–32%), and can lead to clinical uncertainty regarding optimal antithrombotic therapy.90
In patients with non-valvular AF, previous ischemic stroke or TIA, and SVD does antiplatelet treatment compared to no antithrombotic treatment reduce the risk of recurrent stroke or thromboembolism and other predefined outcomes?
We found no randomised controlled trials investigating the efficacy and safety of antiplatelet therapy compared to no antithrombotic treatment for secondary stroke prevention in patients with non-valvular AF and SVD (WMH and CMBs).
Recommendations
In patients with non-valvular AF, previous ischemic stroke or TIA and SVD, we cannot make recommendations on whether antiplatelet therapy should be preferred over no antithrombotic treatment for secondary prevention of all events.
Quality of evidence: Low
Strength of recommendation: Weak
Additional information
In a meta-analysis including 5068 patients with ischemic stroke or TIA from 15 studies (most without AF, treated with antiplatelet agents), CMBs were associated with increased stroke risk after ischemic stroke or TIA, with a greater relative risk for ICH than ischemic stroke (for ischemic stroke, pooled RR 1.8 for CMBs vs. no CMBs, 95% CI 1.4–2.5; for ICH, pooled RR 6.3 for CMBs vs. no CMBs, 95% CI 3.5–11.4). With increasing CMB burden (compared to no CMBs), the risk of ICH increased more steeply than that of ischemic stroke. However, the ischemic stroke absolute event rate (115/1284 (9.6%)) was higher than the ICH absolute event rate (212/3781 (5.6%)) across all CMB burden categories.91
In patients with non-valvular AF, previous ischemic stroke or TIA and SVD do vitamin K antagonists reduce the risk of recurrent stroke or systemic embolism and other predefined outcomes compared to antiplatelet treatment?
We found no randomised controlled trial investigating the efficacy and safety of VKA therapy compared to antiplatelet therapy for secondary stroke prevention in patients with AF and SVD (WMH and CMBs).
Recommendations
In patients with non-valvular AF, previous ischemic stroke or TIA and SVD, we cannot make recommendations about whether vitamin K antagonists should be preferred over antiplatelet therapy for secondary prevention of all events.
Quality of evidence: Low
Strength of recommendation: Weak
Additional information
A post-hoc retrospective aggregate data meta-analysis of cohort studies including 1552 patients with prior ischemic stroke or TIA and AF treated with VKA found (CMBs prevalence 30%, 7% with ≥5 CMBs) that the pooled annual ICH incidence increased from 0.30% (95% CI 0.04–0.55) among CMB-negative patients to 0.81% (95% CI 0.17–1.45) in CMB-positive patients (p = 0.01) and 2.48% (95% CI 1.2–6.2) in patients with ≥5 CMBs (p = 0.001). There was no association between CMBs and recurrent ischemic stroke.92 This study had important methodological limitations, including being dominated by a single cohort of 550 patients from Asia (Korea), and having variable completeness and duration of follow-up.
Limited observational data suggest that SVD biomarkers detected on brain imaging, particularly if haemorrhagic (e.g. CMBs, cortical superficial siderosis) or severe, might increase the risk of ICH to a greater extent than that of recurrent ischemic stroke in patients with AF and prior ischemic stroke or TIA treated with VKA. Since conventional clinical risk scores for ICH in AF patients have limited predictive performance for ICH, the use of SVD neuroimaging biomarkers in risk scores might allow clinicians to make better informed predictions of ICH risk to personalised treatment. In the CROMIS-2 study, in patients anticoagulated with VKA (62%) or a NOAC (38%) after recent ischemic stroke or TIA associated with AF, baseline CMB presence was independently associated with an increased risk of symptomatic intracranial haemorrhage (aHR 3.67, 95% CI 1.27–10.60, p = 0.016). Another prospective observational cohort should provide further information (HERO NCT02238470). A large-scale international collaborative individual patient data meta-analysis on CMBs and future stroke risk in patients with prior ischemic stroke or TIA is also underway (the Microbleeds International Collaborative Network),93 which should help develop more accurate ICH risk prediction scores, determine patients who might be at net harm from OAC, assess the potential benefits of NOACs in populations with severe SVD and potentially inform the design of future RCTs.
In patients with non-valvular AF, previous ischemic stroke or TIA and SVD do non-vitamin K antagonist oral anticoagulants reduce the risk of recurrent stroke or systemic embolism and other predefined outcomes compared to vitamin K antagonists?
We found no randomised controlled trials or high quality observational studies investigating the efficacy and safety of NOACs compared to VKAs for secondary stroke prevention in patients with AF and SVD.
Recommendations
In patients with non-valvular AF, previous ischemic stroke or TIA and SVD, we cannot make recommendations about whether non-vitamin K antagonist oral anticoagulants should be preferred over vitamin K antagonists for reducing recurrent stroke or thromboembolism.
Quality of evidence: Low
Strength of recommendation: Weak
Additional information
In the AVERROES trial, participants with AF deemed unsuitable for long-term oral VKA were randomised to apixaban or aspirin. An MRI sub-study followed up 931 participants (of 1180 patients with MRI at baseline) reported no significant difference in mean change (baseline to follow-up) in periventricular white matter hyperintensity score in patients treated with apixaban compared to those treated with aspirin.94 The rate of new infarction detected on MRI was 2.5% in the apixaban group and 2.2% in the aspirin group (HR 1.09, 95% CI 0.47–2.52), but new infarcts were smaller in the apixaban group (p = 0.03). There was no difference in proportion with new CMBs on follow-up MRI (HR 0.92, 95% CI 0.53–1.60) between treatment groups. Only a minority of participants in this study were stroke survivors (17.8% in the apixaban group and 15.4% in the aspirin group) and the observation period was short.
Prospective cohort studies of patients with SVD have mainly included those treated with VKA. Theoretically, NOACs might be preferable to VKA in patients with severe SVD, but there are no current data to support this hypothesis. Future studies including patients treated with NOACs are awaited (HERO, NCT02238470).
Expert opinion (Delphi vote: 7/7 agree)
In patients with non-valvular AF and previous ischemic stroke or TIA, presence of absence of cerebral microbleeds cannot be used to determine if a patient should be treated with NOACs or VKAs.
All recommendations are summarised in Table 5.
Table 5.
Summary of recommendations for secondary prevention of recurrent stroke or systemic embolism and other vascular outcomes in patients with non-valvular AF and stroke or TIA.
| Quality of evidence | Strength of recommendation | |
|---|---|---|
| Medical treatment | ||
| We do not recommend antiplatelet agents (single or dual), over no antiplatelet therapy. | Moderate | Weak |
| We recommend VKA therapy over no antithrombotic medication | Moderate | Strong |
| We recommend VKAs (INR 2-3) over antiplatelet therapy. | Moderate | Strong |
| We recommend NOACs over VKAs | High | Strong |
| We suggest NOACs over aspirin in patients who have failed or are unsuitable for VKA therapy. | Moderate | Weak |
| Timing and bridging of medical treatment | ||
| We cannot make recommendations about the optimal time for initiating anticoagulation treatment in patients with acute ischemic stroke.Expert opinion: We suggest antiplatelet therapy in the first 48 h after ischemic stroke associated with AF. We consider it reasonable to start anticoagulant therapy at day 3 or 4 from the index stroke in patients with mild stroke and small infarcts (<1.5 cm) and at day 7 for moderate infarcts. For large infarcts, OACs might be best delayed for 14 days after the index stroke. | Low | Weak |
| We suggest that bridging therapy should be avoided prior to anticoagulation with VKAs or NOACs. | Low | Weak |
| Left atrial appendage occlusion | ||
| We cannot make recommendation on whether LAAO is an acceptable alternative to long-term anticoagulation with either VKAs or NOACs.Expert opinion: LAAO can be considered in individual patients as an alternative to life-long oral anticoagulation after careful weighing of risks and benefits. | Low | Weak |
| (Re-) starting treatment in patients with previous intracerebral haemorrhage | ||
| We cannot make recommendations on whether or not oral anticoagulation should be restarted in patients who have experienced intracerebral haemorrhage.Expert opinion: In patients with AF who have experienced an intracerebral haemorrhage, restarting oral anticoagulation can be considered after careful weighing of risks and benefits. | Low | Weak |
| Medical treatment in specific patient subgroups | ||
| Elderly patients | ||
| In elderly patients we suggest anticoagulant treatment over no anticoagulant treatment and over antiplatelets. | Low | Weak |
| In elderly patients with non-valvular AF and a history of ischemic stroke or TIA, we suggest NOACs over VKAs | Low | Weak |
| Patients with cognitive deficits | ||
| In patients with cognitive decline we suggest anticoagulant treatment over no anticoagulant treatment and over antiplatelets. | Low | Weak |
| In patients with cognitive decline we suggest NOACs over VKAs. | Low | Weak |
| Patients with renal impairment | ||
| In patients with mild (CrCl 50-80 mL/min) or moderate (<50 mL/min) renal impairment we suggest NOACs over VKAs. | Low | Weak |
| Patients with small vessel disease | ||
| In patients with small vessel disease, we cannot make recommendations regarding medical secondary prevention for reducing recurrent stroke or thromboembolism.Expert opinion: In patients with non-valvular AF and previous ischemic stroke or TIA, presence of absence of cerebral microbleeds cannot be used to determine if a patient should be treated with NOACs or VKAs. | Low | Weak |
Discussion
Our analyses on the currently available evidence show that in patients with non-valvular AF and previous stroke or TIA, OACs reduce the risk of recurrence over antiplatelets or no treatment. Of OACs, NOACs are the preferred treatment for secondary prevention of stroke or thromboembolism because they have a similar benefit to VKAs with regard to the prevention of stroke or thromboembolism or ischemic complications but a lower risk of major bleeding and death. Furthermore, other than for safety, NOACs offer a significant advantage over VKAs because INR measurement, dose adjustment and dietary restrictions are not required for patients who receive NOACs. The choice of a specific agent may be individualised taking into account unique individual characteristics, comorbidities (e.g. renal function, gastrointestinal bleeding risk) and concomitant medications, as well as patients expressed values and preferences. Antiplatelet agents, either as single or dual therapy, should not be used even in patients with contraindications to OAC because they have a significantly smaller effect in patients with non-valvular AF and stroke or TIA. In patients who have had ischemic stroke while treated with VKA, or are unsuitable for VKAs, we identified moderate quality of evidence to recommend NOACs over aspirin.
For the questions regarding timing of antithrombotic treatment, treatment by means of LAAO, (re-)starting OACs in patients with previous ICH and medical treatment in the specific patient subgroups of those of older age, with cognitive impairment, with renal failure, and those with SVD on brain imaging, we can only make weak recommendations.
Ongoing RCTs like ELAN, START, TIMING, and OPTIMAS will clarify the optimal timing of treatment with NOACs after ischemic stroke. In a recent prospective study, patients with acute ischemic stroke and non-valvular AF who were treated with NOACs had a 90-day rate of 2.8% for recurrent ischemic stroke or TIA and 1.6% for symptomatic ICH. The large majority of patients in this study received NOACs within 15 days.95 Another prospective cohort study reported that, despite the early start of NOACs (65% of the 155 included patients received therapy within seven days), no ICH occurred and four patients had recurrent ischemic stroke during follow-up of at least three months.40
RCTs are also ongoing that test whether or not OACs should be restarted in patients with AF who have experienced ICH. A recent meta-analysis66 of observational studies has suggested that restarting OACs (in these studies all VKAs) may in fact be safer and more effective than previously thought and might improve outcome. Even in patients with lobar ICH who have a relatively high risk of recurrent ICH, restarting OACs might be effective.67 However, as the observational studies were affected by selection by indication, RCTs are needed to provide evidence on the optimal secondary preventive treatment in patient with AF after ICH.
Available data focusing on renal function derived from pre-specified subgroup analyses of all four completed phase 3 RCTs that compared NOACs with dose-adjusted warfarin in patients with non-valvular AF. Considering that patients with previous ischemic stroke or TIA represent a substantial proportion in those studies, we have considered the pooled results from these trials to be informative for patients with a previous stroke or TIA and renal impairment. For this reason, we have suggested that in patients with non-valvular AF and previous ischemic stroke or TIA, NOACs are the preferred treatment.
Evidence is increasing that there is a modifying effect of presence of neuroimaging biomarkers of SVD on the risk of ischemic stroke and ICH, and on the risk–benefit ratio of antithrombotic treatment. However, in the absence of RCTs or large high-quality observational cohort data specifically addressing patients with SVD neuroimaging biomarkers, we cannot make firm recommendations on the optimal treatment for secondary prevention in this subgroup. The results of the AVERROES MRI study were reassuring with respect to their finding that there was no difference in proportion of patients with new CMBs on follow-up MRI after on average one year between patients treated with apixaban and those treated with aspirin.94
For all patients, the above information should result in an individualised choice for a specific (N)OAC taking into account unique individual characteristics, co-morbidities (e.g. renal function, gastrointestinal bleeding risk) and concomitant medications, as well as patients expressed values and preferences.
The strengths of this guideline include its systematic approach to searching the literature and guidance by the GRADE recommendations, including predefining outcome events and grading their importance. Furthermore, we have addressed several subgroups of patients for whom clinical practice raises additional questions regarding the secondary prevention medical therapy.
Our guideline also has limitations. First, the GRADE approach only allows for the strength of recommendations to be strong or weak. For some PICO questions, we found evidence that could have resulted in an intermediate strength of the recommendation, e.g. in case of availability of one RCT. Second, our guideline is restricted by the limited amount of evidence available for some of the PICO questions.
In conclusion, among patients with non-valvular AF and ischemic stroke or TIA, NOACs are the preferred medical treatment for secondary preventions of recurrent stroke or thromboembolism because of a lower risk of major bleeding and death compared to VKAs. Further research is required to test the best timing for initiating OACs after an acute ischemic stroke and whether or not OACs should be (re-)started in patients with who have experienced an ICH. Future RCTs should include pre-specified subgroup analyses for patients of older age, those with cognitive deficits and renal failure and should consider MRI biomarkers, which modify the effect of OACs after stroke or TIA.
Supplemental Material
Supplemental Material for Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: A European Stroke Organisation guideline by Catharina JM Klijn, Maurizio Paciaroni, Eivind Berge, Eleni Korompoki, Janika Kõrv, Avtar Lal, Jukka Putaala and David J Werring in European Stroke Journal
Acknowledgements
The Authors acknowledge the Independent Advisors Prof. Hans Christoph Diener and Prof. Paulus Kirchhof.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CJMK is supported by a clinical established investigator grant from the Dutch Heart Foundation (2012T077) and an Aspasia grant from ZonMw (015008048) and is coordinating investigator in the APACHE-AF trial. MP received honoraria as a member of the speaker bureau of Aspen, Sanofi-Aventis, Boehringer Ingelheim, Bayer, Bristol Meyer Squibb, Daiiki Sankyo and Pfizer. EB has received speaker’s honorarium from Bayer and is coordinating investigator in STATICH and national co-coordinating investigator in STROKECLOSE. EK has received speaker’s honoraria from Amgen and BMS/Pfizer, travel grants from Bayer, contributed to advisory board from BMS/Pfizer and is part of the consortium leading PRESTIGE-AF. JK has received speaker’s honorarium from Bayer, Boehringer Ingelheim and Pfizer, and served in advisory board of Boehringer-Ingelheim. JP has received research grants from St. Jude Medical (Abbott) and BMS-Pfizer, served in advisory boards of Bayer, Boehringer-Ingelheim, BMS-Pfizer and MSD, received speaker’s honorary from Abbott, Bayer, Boehringer-Ingelheim and BMS-Pfizer, and is national co-coordinating investigator in STROKECLOSE. DJW is chief investigator for CROMIS-2 and OTPIMAS studies. DJW has received personal fees from Bayer and Amgen, and grant funding from the British Heart Foundation, Stroke Association and the Rosetrees Trust.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Guarantors
Guideline Board and Executive Committee of the European Stroke Organisation.
Contributorship
All the authors made a substantial contribution to the concept or design of the work, acquisition, analysis or interpretation of data, drafted the article or revised it critically for important intellectual content, approved the version to be published. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014; 129: 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scowcroft AC, Cowie MR. Atrial fibrillation: improvement in identification and stroke preventive therapy – data from the UK Clinical Practice Research Datalink, 2000–2012. Int J Cardiol 2014; 171: 169–173. [DOI] [PubMed] [Google Scholar]
- 3.Stefansdottir H, Aspelund T, Gudnason V, et al. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace 2011; 13: 1110–1117. [DOI] [PubMed] [Google Scholar]
- 4.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013; 34: 2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22: 983–988. [DOI] [PubMed] [Google Scholar]
- 6.Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014; 370: 2478–2486. [DOI] [PubMed] [Google Scholar]
- 7.Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370: 2467–2477. [DOI] [PubMed] [Google Scholar]
- 8.Wachter R, Groschel K, Gelbrich G, et al. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AFRANDOMISED): an open-label randomised controlled trial. Lancet Neurol 2017; 16: 282–290. [DOI] [PubMed] [Google Scholar]
- 9.Ntaios G, Bornstein NM, Caso V, et al. The European Stroke Organisation Guidelines: a standard operating procedure. Int J Stroke 2015; 10: 128–135. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Green S. and Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Chichester, England: Wiley-Blackwell, 2008, p. xxi, 649 p. [Google Scholar]
- 11.Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet 1993; 342: 1255–1262. [PubMed] [Google Scholar]
- 12.Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996; 143: 1–13. [DOI] [PubMed] [Google Scholar]
- 13.Farrell B, Godwin J, Richards S, et al. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry 1991; 54: 1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Investigators A, Connolly SJ, Pogue J, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360: 2066–2078. [DOI] [PubMed] [Google Scholar]
- 15.Ezekowitz MD, Bridgers SL, James KE, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med 1992; 327: 1406–1412. [DOI] [PubMed] [Google Scholar]
- 16.Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370: 493–503. [DOI] [PubMed] [Google Scholar]
- 17.Investigators AWGotA, Connolly S, Pogue J, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006; 367: 1903–1912. [DOI] [PubMed] [Google Scholar]
- 18.Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet 1996; 348: 633–638. [PubMed]
- 19.Hylek EM, Skates SJ, Sheehan MA, et al. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996; 335: 540–546. [DOI] [PubMed] [Google Scholar]
- 20.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med 1994; 120: 897–902. [DOI] [PubMed] [Google Scholar]
- 21.Diener HC, Connolly SJ, Ezekowitz MD, et al. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol 2010; 9: 1157–1163. [DOI] [PubMed] [Google Scholar]
- 22.Hankey GJ, Patel MR, Stevens SR, et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol 2012; 11: 315–322. [DOI] [PubMed] [Google Scholar]
- 23.Easton JD, Lopes RD, Bahit MC, et al. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol 2012; 11: 503–511. [DOI] [PubMed] [Google Scholar]
- 24.Rost NS, Giugliano RP, Ruff CT, et al. Outcomes With edoxaban versus warfarin in patients with previous cerebrovascular events: findings from ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48). Stroke 2016; 47: 2075–2082. [DOI] [PubMed] [Google Scholar]
- 25.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–694. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt L, Speckman J, Ansell J. Quality assessment of anticoagulation dose management: comparative evaluation of measures of time-in-therapeutic range. J Thromb Thrombolysis 2003; 15: 213–216. [DOI] [PubMed] [Google Scholar]
- 27.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383: 955–962. [DOI] [PubMed] [Google Scholar]
- 28.Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010; 376: 975–983. [DOI] [PubMed] [Google Scholar]
- 29.Carmo J, Ferreira J, Costa F, et al. Non-vitamin K antagonist oral anticoagulants compared with warfarin at different levels of INR control in atrial fibrillation: a meta-analysis of randomized trials. Int J Cardiol 2017; 244: 196–201. 10.1016/j.ijcard.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Arnao V, Agnelli G, Paciaroni M. Direct oral anticoagulants in the secondary prevention of stroke and transient ischemic attack in patients with atrial fibrillation. Intern Emerg Med 2015; 10: 555–560. [DOI] [PubMed] [Google Scholar]
- 31.Tsivgoulis G, Wilson D, Katsanos AH, et al. Neuroimaging and clinical outcomes of oral anticoagulant-associated intracerebral hemorrhage. Ann Neurol 2018; 84: 694–704. [DOI] [PubMed] [Google Scholar]
- 32.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364: 806–817. [DOI] [PubMed] [Google Scholar]
- 33.Berge E, Abdelnoor M, Nakstad PH, et al. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial. Lancet 2000; 355: 1205–1210. [DOI] [PubMed] [Google Scholar]
- 34.Saxena R, Lewis S, Berge E, et al. Risk of early death and recurrent stroke and effect of heparin in 3169 patients with acute ischemic stroke and atrial fibrillation in the International Stroke Trial. Stroke 2001; 32: 2333–2337. [DOI] [PubMed] [Google Scholar]
- 35.CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet 1997; 349: 1641–1649. [PubMed] [Google Scholar]
- 36.Paciaroni M, Agnelli G, Micheli S, et al. Efficacy and safety of anticoagulant treatment in acute cardioembolic stroke: a meta-analysis of randomized controlled trials. Stroke 2007; 38: 423–430. [DOI] [PubMed] [Google Scholar]
- 37.Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF study. Stroke 2015; 46: 2175–2182. [DOI] [PubMed] [Google Scholar]
- 38.Arihiro S, Todo K, Koga M, et al. Three-month risk-benefit profile of anticoagulation after stroke with atrial fibrillation: the SAMURAI-Nonvalvular Atrial Fibrillation (NVAF) study. Int J Stroke 2016; 11: 565–574. [DOI] [PubMed] [Google Scholar]
- 39.Gioia LC, Kate M, Sivakumar L, et al. Early rivaroxaban use after cardioembolic stroke may not result in hemorrhagic transformation: a prospective magnetic resonance imaging study. Stroke 2016; 47: 1917–1919. [DOI] [PubMed] [Google Scholar]
- 40.Seiffge DJ, Traenka C, Polymeris A, et al. Early start of DOAC after ischemic stroke: risk of intracranial hemorrhage and recurrent events. Neurology 2016; 87: 1856–1862. [DOI] [PubMed] [Google Scholar]
- 41.Paciaroni M, Agnelli G, Caso V, et al. Prediction of early recurrent thromboembolic event and major bleeding in patients with acute stroke and atrial fibrillation by a risk stratification schema: the ALESSA score study. Stroke 2017; 48: 726–732. [DOI] [PubMed] [Google Scholar]
- 42.Wilson D, Ambler G, Banerjee G, et al. Early versus late anticoagulation for ischaemic stroke associated with atrial fibrillation: multicentre cohort study. J Neurol Neurosurg Psychiatry 2018. Epub ahead of print 21 November 2018. DOI: 10.1136/jnnp-2018-318890. [DOI] [PMC free article] [PubMed]
- 43.Hong KS, Kwon SU, Lee SH, et al. Rivaroxaban vs warfarin sodium in the ultra-early period after atrial fibrillation-related mild ischemic stroke: a randomized clinical trial. JAMA Neurol 2017; 74: 1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JH, Park KY, Shin JH, et al. Symptomatic hemorrhagic transformation and its predictors in acute ischemic stroke with atrial fibrillation. Eur Neurol 2010; 64: 193–200. [DOI] [PubMed] [Google Scholar]
- 45.Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke 2008; 39: 2249–2256. [DOI] [PubMed] [Google Scholar]
- 46.Klijn CJ, Hankey GJ, American Stroke A, et al. Management of acute ischaemic stroke: new guidelines from the American Stroke Association and European Stroke Initiative. Lancet Neurol 2003; 2: 698–701. [DOI] [PubMed] [Google Scholar]
- 47.Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2017; 38: 2137–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet 1997; 349: 1569–1581. [PubMed] [Google Scholar]
- 49.Chen ZM, Sandercock P, Pan HC, et al. Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40 000 randomized patients from the Chinese acute stroke trial and the international stroke trial. On behalf of the CAST and IST collaborative groups. Stroke 2000; 31: 1240–1249. [DOI] [PubMed] [Google Scholar]
- 50.Bouillon K, Bertrand M, Boudali L, et al. Short-term risk of bleeding during heparin bridging at initiation of vitamin K antagonist therapy in more than 90 000 patients with nonvalvular atrial fibrillation managed in outpatient care. J Am Heart Assoc 2016; 5. DOI: 10.1161/JAHA.116.004065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim TH, Kim JY, Mun HS, et al. Heparin bridging in warfarin anticoagulation therapy initiation could increase bleeding in non-valvular atrial fibrillation patients: a multicenter propensity-matched analysis. J Thromb Haemost 2015; 13: 182–190. [DOI] [PubMed] [Google Scholar]
- 52.Hallevi H, Albright KC, Martin-Schild S, et al. Anticoagulation after cardioembolic stroke: to bridge or not to bridge?. Arch Neurol 2008; 65: 1169–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feiz F, Sedghi R, Salehi A, et al. Study of the efficacy, safety and tolerability of low-molecular-weight heparin vs. unfractionated heparin as bridging therapy in patients with embolic stroke due to atrial fibrillation. J Vasc Interv Neurol 2016; 9: 35–41. [PMC free article] [PubMed] [Google Scholar]
- 54.Pison L, Potpara TS, Chen J, et al. Left atrial appendage closure-indications, techniques, and outcomes: results of the European Heart Rhythm Association Survey. Europace 2015; 17: 642–646. [DOI] [PubMed] [Google Scholar]
- 55.Holmes DR, Jr., Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014; 64: 1–12. [DOI] [PubMed] [Google Scholar]
- 56.Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 2014; 312: 1988–1998. [DOI] [PubMed] [Google Scholar]
- 57.Tzikas A, Shakir S, Gafoor S, et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention 2016; 11: 1170–1179. [DOI] [PubMed] [Google Scholar]
- 58.Boersma LV, Ince H, Kische S, et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-year follow-up outcome data of the EWOLUTION trial. Heart Rhythm 2017; 14: 1302–1308. [DOI] [PubMed] [Google Scholar]
- 59.Reddy VY, Mobius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol 2013; 61: 2551–2556. [DOI] [PubMed] [Google Scholar]
- 60.Reddy VY, Doshi SK, Kar S, et al. 5-Year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol 2017; 70: 2964–2975. [DOI] [PubMed] [Google Scholar]
- 61.Schols AM, Schreuder FH, van Raak EP, et al. Incidence of oral anticoagulant-associated intracerebral hemorrhage in the Netherlands. Stroke 2014; 45: 268–270. [DOI] [PubMed] [Google Scholar]
- 62.Samarasekera N, Fonville A, Lerpiniere C, et al. Influence of intracerebral hemorrhage location on incidence, characteristics, and outcome: population-based study. Stroke 2015; 46: 361–368. [DOI] [PubMed] [Google Scholar]
- 63.Flaherty ML, Kissela B, Woo D, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology 2007; 68: 116–121. [DOI] [PubMed] [Google Scholar]
- 64.Perry LA, Berge E, Bowditch J, et al. Antithrombotic treatment after stroke due to intracerebral haemorrhage. Cochrane Database Syst Rev 2017; 5: CD012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pasquini M, Charidimou A, van Asch CJ, et al. Variation in restarting antithrombotic drugs at hospital discharge after intracerebral hemorrhage. Stroke 2014; 45: 2643–2648. [DOI] [PubMed] [Google Scholar]
- 66.Korompoki E, Filippidis FT, Nielsen PB, et al. Long-term antithrombotic treatment in intracranial hemorrhage survivors with atrial fibrillation. Neurology 2017; 89: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biffi A, Kuramatsu JB, Leasure A, et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol 2017; 82: 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010; 74: 1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Nieuwenhuizen KM, van der Worp HB, Algra A, et al. Apixaban versus Antiplatelet drugs or no antithrombotic drugs after anticoagulation-associated intraCerebral HaEmorrhage in patients with Atrial Fibrillation (APACHE-AF): study protocol for a randomised controlled trial. Trials 2015; 16: 393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nielsen-Kudsk JE, Johnsen SP, Wester P, et al. Left atrial appendage occlusion versus standard medical care in patients with atrial fibrillation and intracerebral haemorrhage: a propensity score-matched follow-up study. EuroIntervention 2017; 13: 371–378. [DOI] [PubMed] [Google Scholar]
- 71.Sardar P, Chatterjee S, Chaudhari S, et al. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62: 857–864. [DOI] [PubMed] [Google Scholar]
- 72.Ananthapanyasut W, Napan S, Rudolph EH, et al. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clinical Journal of the American Society of Nephrology: CJASN 2010; 5: 173–181. 2009/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Go AS, Fang MC, Udaltsova N, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 2009; 119: 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hart RG, Eikelboom JW, Ingram AJ, et al. Anticoagulants in atrial fibrillation patients with chronic kidney disease. Nat Rev Nephrol 2012; 8: 569–578. [DOI] [PubMed] [Google Scholar]
- 75.Poulsen BK, Grove EL, Husted SE. New oral anticoagulants: a review of the literature with particular emphasis on patients with impaired renal function. Drugs 2012; 72: 1739–1753. [DOI] [PubMed] [Google Scholar]
- 76.Hart RG, Eikelboom JW, Brimble KS, et al. Stroke prevention in atrial fibrillation patients with chronic kidney disease. The Canadian Journal of Cardiology 2013; 29: S71–S78. [DOI] [PubMed] [Google Scholar]
- 77.Marinigh R, Lane DA, Lip GY. Severe renal impairment and stroke prevention in atrial fibrillation: implications for thromboprophylaxis and bleeding risk. J Am Coll Cardiol 2011; 57: 1339–1348. [DOI] [PubMed] [Google Scholar]
- 78.Bohula EA, Giugliano RP, Ruff CT, et al. Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 Trial. Circulation 2016; 134: 24–36. [DOI] [PubMed] [Google Scholar]
- 79.Fox KA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. European Heart Journal 2011; 32: 2387–2394. [DOI] [PubMed] [Google Scholar]
- 80.Hohnloser SH, Hijazi Z, Thomas L, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. European Heart Journal 2012; 33: 2821–2830. [DOI] [PubMed] [Google Scholar]
- 81.Hori M, Matsumoto M, Tanahashi N, et al. Safety and efficacy of adjusted dose of rivaroxaban in Japanese patients with non-valvular atrial fibrillation: subanalysis of J-ROCKET AF for patients with moderate renal impairment. Circ J. 2013; 77: 632–638. [DOI] [PubMed] [Google Scholar]
- 82.Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation 2014; 129: 961–970. [DOI] [PubMed] [Google Scholar]
- 83.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 84.Eikelboom JW, Connolly SJ, Gao P, et al. Stroke risk and efficacy of apixaban in atrial fibrillation patients with moderate chronic kidney disease. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association. 2012; 21: 429–435. [DOI] [PubMed] [Google Scholar]
- 85.Kumar S, de Lusignan S, McGovern A, et al. Ischaemic stroke, haemorrhage, and mortality in older patients with chronic kidney disease newly started on anticoagulation for atrial fibrillation: a population based study from UK primary care. BMJ 2018; 360. [DOI] [PubMed] [Google Scholar]
- 86.Chokesuwattanaskul R, Thongprayoon C, Tanawuttiwat T, et al. Safety and efficacy of apixaban versus warfarin in patients with end-stage renal disease: meta-analysis. Pacing Clin Electrophysiol 2018; 41: 627–634. [DOI] [PubMed] [Google Scholar]
- 87.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701. [DOI] [PubMed] [Google Scholar]
- 88.Wilson D, Adams ME, Robertson F, et al. Investigating intracerebral haemorrhage. BMJ 2015; 350: h2484. [DOI] [PubMed] [Google Scholar]
- 89.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haeusler KG, Wilson D, Fiebach JB, et al. Brain MRI to personalise atrial fibrillation therapy: current evidence and perspectives. Heart 2014; 100: 1408–1413. [DOI] [PubMed] [Google Scholar]
- 91.Wilson D, Charidimou A, Ambler G, et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: a meta-analysis. Neurology 2016; 87: 1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Charidimou A, Karayiannis C, Song TJ, et al. Brain microbleeds, anticoagulation, and hemorrhage risk: meta-analysis in stroke patients with AF. Neurology 2017; 89: 2317–2326. [DOI] [PubMed] [Google Scholar]
- 93.Microbleeds International Collaborative N. Worldwide collaboration in the Microbleeds International Collaborative Network. Lancet Neurol 2016; 15: 1113–1114. [DOI] [PubMed] [Google Scholar]
- 94.O'Donnell MJ, Eikelboom JW, Yusuf S, et al. Effect of apixaban on brain infarction and microbleeds: AVERROES-MRI assessment study. Am Heart J 2016; 178: 145–150. [DOI] [PubMed] [Google Scholar]
- 95.Paciaroni M, Agnelli G, Falocci N, et al. Early Recurrence and Major Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation Treated With Non-Vitamin-K Oral Anticoagulants (RAF-NOACs) study. J Am Heart Assoc 2017; 6: DOI: 10.1161/JAHA.117.007034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: A European Stroke Organisation guideline by Catharina JM Klijn, Maurizio Paciaroni, Eivind Berge, Eleni Korompoki, Janika Kõrv, Avtar Lal, Jukka Putaala and David J Werring in European Stroke Journal