Abstract
The adaptive immune response has recently emerged as an important factor in a wide variety of cardiovascular disorders including atherosclerosis, hypertension, cardiac remodeling, and heart failure; however, its role is not fully understood. Since an assortment of innate responsive cells, e.g., neutrophils and monocytes/macrophages, coordinate with adaptive immunity, e.g., T cells, dendritic cells, and B cells, the temporal response and descriptions pertinent to the cellular phenotype and inflammation processes, in general, need additional investigation, clarification, and consensus particularly in cardiovascular disease. This Perspectives article reviews the contributions of 15 articles (including 7 reviews) published in the American Journal of Physiology-Heart and Circulatory Physiology in response to the Call for Papers: Adaptive Immunity in Cardiovascular Disease. Here, we summarize the crucial reported findings at the cardiac, vascular, immune, and molecular levels and discuss the translational feasibility and benefits of future prospective research into the adaptive immune response. Readers are encouraged to evaluate the data and learn from this collection of novel studies.
Keywords: adaptive immunity, atherosclerosis, eosinphilia, hypertension, myocardial infarction
Activation of both the innate and adaptive immune cells and their infiltration into affected tissues has been shown for various cardiovascular diseases: they are found in atherosclerotic plaques, hearts after ischemic and nonischemic cardiomyopathy, and kidneys in hypertension (2–5, 11, 18, 19, 22, 28). The infiltrated immune cells play an important role in altering the mechanical, structural, and functional attributes of the murine/human heart and kidney, often facilitating the progression of these diseases. Cross talk between leukocyte-directed innate response and the adaptive immune system composed of lymphocytes including B and T cells has only recently become a focus in research on cardiovascular disease (2, 20, 21).
One of the latest collections of papers in this journal (https://bit.ly/32Ol5Tb) helps to shed some light on the role the adaptive immune system plays in cardiovascular disease development and prevention. The 15 papers in this collection address the mechanisms by which immune-cell populations infiltrate, activate, and regulate inflammation and fibrosis in the atherosclerotic plaque formation, cardiovascular remodeling, and initiation and progression of hypertension and preeclampsia. Below we summarize the major findings of interest to the readership and provide a frame of reference for future studies.
Monocytes and macrophages are known regulators of atherosclerotic plaque formation and rupture (4, 8, 18, 23, 25, 28). Xu et al. (27) identified a novel mechanism for recruitment and retention of CD8+ T cells to atherosclerotic lesions. The authors identified that costimulation of CD137 on CD8+ T cells is an impetus for infiltration and, interestingly, happens independently of atherosclerotic-antigen recognition. The key findings support a model where generation of effector CD8+ T cells in circulation, potentially induced by systemic inflammation, drives their infiltration into atherogenic intima where they persist, secrete proinflammatory cytokines, and mediate recruitment of other immune cells. Together, these infiltrated cells create a vulnerable plaque and potentially facilitate the process of development of atherosclerotic plaque and subsequent thrombovascular events.
In another study evaluating CD8+ T cells, Ilatovskaya et al. (12) implicated CD8+ T cells in the cardiac healing process of injured myocardium post-myocardial infarction (MI). At post-MI day 7, mice lacking functional CD8+ T cells exhibit improved cardiac physiology and less mortality compared with wild-type animals. Despite better overall survival, animals lacking CD8+ T cells had delayed removal of necrotic tissue leading to impaired collagen scar formation and increased cardiac rupture. These data suggested that CD8+ T cells have a dual role in the post-MI wound healing process. Similar to what was observed in Xu et al. (27), this study demonstrates a strong role of CD8+ T cell-mediated action on monocyte/macrophage recruitment and activation.
Some individuals are at higher risk for cardiovascular disease; for instance, HIV-positive cohorts show an increased cardiovascular disease occurrence, in part due to their dysfunctional T-cell populations (26). Teer et al. (9, 24) reported that HIV-positive patients exhibit coexpression of coagulation marker CD142 and activation marker CD38 on both CD4+ and CD8+ T cells. During chronic HIV infection, the dysregulation of T-cell activation was specifically due to upregulation of glycoprotein A repetitions predominant (GARP) and special AT-rich sequence-binding protein-1 (SATB-1). Furthermore, a subset of proatherogenic monocytes was shown to correlate with T-cell activation. This study identified a unique link between immune activation and lipid subclass alterations, revealing new biomarkers of HIV-induced cardiovascular disease risk that may be missed by traditional lipid marker assessments. In addition, the authors have reported that combined antiretroviral treatment (cART) may play a role in the increased cardiovascular disease risk, most likely as a result of an abnormal immune response coupled with lower diastolic blood pressure and perturbations of lipoprotein subclasses.
In addition to cross talk with monocytes, T cells are associated with increased eosinophilopoiesis and eosinophil activation (14, 15). Prows et al. (17) characterized a new mouse model for the hypereosinophilia-associated heart disease after discovering a spontaneous mutant in their colony. Similar to what is observed in patients with hypereosinophilia, these mice die prematurely, as they develop peripheral blood eosinophilia and infiltration of eosinophils into the lungs, spleen, and heart, as well as myocardial damage and remodeling. Expression analysis of heart tissue by protein multiplex assay revealed overexpression of Th1 and Th2 cytokines and chemokines, including IL-6, eotaxin, Ccl5 (RANTES), and macrophage inflammatory protein-1β. This model provides new avenues for the discovery of therapies targeting eosinophils.
The exact molecular interaction between inflammatory responses and the sympathetic drive is unclear and of much interest for the scientific community. Ahmari et al. (1) aimed to investigate the relationship between the bone marrow sympathetic drive, activation of the immune system, and increase in blood pressure. Of note, the authors found that infusion of angiotensin II (ANG II) activates microglia in the hypothalamus, and this precedes an elevation in femoral bone marrow sympathetic nerve activity. In turn, activation of this sympathetic nerve activity correlated with increased circulating Th17 cells and the onset of increased blood pressure, while infiltration of CD4+ T cells into paraventricular nucleus marked late hypertension. This study highlighted the involvement of the central nervous system in immune dysfunction and associated diseases, such as hypertension and many others.
One of the widely studied and recently acclaimed topics in the immune system physiology field is sex differences. Women are protected from cardiovascular diseases until the development of menopause, yet the mechanisms behind this observation are still unclear (7, 10). Pollow et al. (16) investigated the contribution of T cells to hypertension development in postmenopausal females. For this study, they used the novel and physiologically relevant mouse model of menopause by giving female mice the chemical 4-vinylcyclohexene diepoxide (VCD). Adoptive transfer of T cells elevated blood pressure after ANG II; however, this effect was absent in menopausal Rag-1−/− mice. The authors demonstrated that Tregs were decreased in the spleen and kidneys of Rag-1−/− menopausal mice versus premenopausal mice. It was concluded that a change in hormonal status during menopause could stimulate proinflammatory and T cell-dependent responses and eliminate protection against hypertension. This study emphasizes that the sex of the animal is a crucial parameter to consider when interpreting hypertension study results. Targeted manipulation of T-cell subsets may prove to be an effective approach to treat hypertension.
During pregnancy, women are closely monitored for development of hypertension due to the risk preeclampsia imposes on both the mother and baby. The implication of adaptive and innate immune cells in placental ischemia-induced hypertension is well known; however, the contribution of B1 versus B2 lymphocytes is unclear. Laule et al. (13) hypothesized that peritoneal B1 lymphocytes are critical for the development of placental ischemia-induced hypertension. Using the reduced uteroplacental perfusion pressure (RUPP) model of preeclampsia, they found that classic B2 lymphocytes and peritoneal and circulating B1 lymphocytes are actually not required for development of hypertension following third-trimester placental ischemia.
Taken together, all of these studies highlight the complexity of the adaptive immune system in development of cardiovascular disease. Unfortunately, there is no consensus on the optimum strategy for isolation and characterization of immune cells from the tissue, and most published protocols differ in one of the following two aspects: 1) the use of Ficoll-paque/Percoll density-based separation mechanisms to remove myocardial debris from cardiac digests and 2) the use of coronary perfusion to remove intravascular cells. The study performed by Covarrubias et al. (6) demonstrated that density-mediated mononuclear cell-isolation strategies are less precise for quantifying tissue-infiltrating immune cells, since altered cell activation status upon tissue transmigration alters their granularity, resulting in loss of mononuclear cells in the pellets. They suggested that centrifugation of finely minced tissues at a very low speed is a viable, alternate strategy to time-consuming, tissue-perfusion approaches for removing the majority of intravascular blood cells. In addition, the absolute number of cells isolated is relatively small and will require the majority of the sample to reach the gold standard of 10,000 events/sample for the accurate evaluation of flow cytometry data. Additional studies in animals and humans are needed to fully understand the role these dynamic cells have in cardiovascular disease.
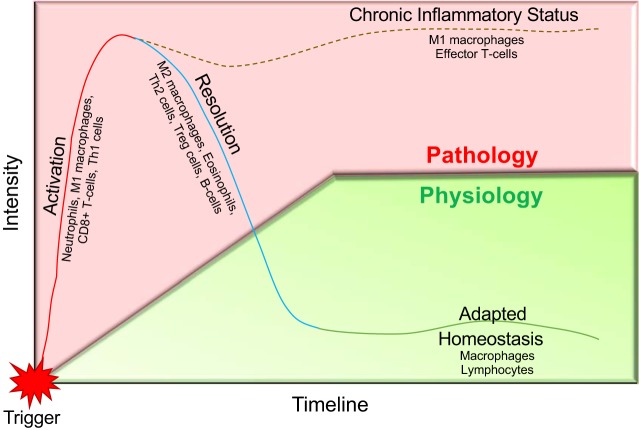
The described perspective highlights some of the adaptive immunity-driven mechanisms of cardiovascular disease. In diversified cardiovascular disease phenotype, a resolution of inflammation and restoration of homeostasis is needed for improvement in prognosis (Fig. 1). This process is dependent on the magnitude of trigger, e.g., myocardial infarction in ischemic heart failure, and complex regulation coordinated by innate and adaptive immune system that highlights the importance of cellular and molecular inflammation. Future consideration for the role that primary risk factors observed in the clinical setting like sex, aging, obesity, and drug interactions have on dysregulation of adaptive immune response is warranted. In addition, the focus of the research community should include development of a comprehensive breadth of adaptive immunity driven mechanisms in cardiovascular disease as a means to harness these cells as therapeutic targets in cardiovascular medicine.
Fig. 1.
The physiological and pathological oscillation of the adaptive immune response in cardiovascular disease is not fully understood. Response to a trigger, e.g., endothelial injury, myocyte death, or vascular injury/stress, initiates activation of the immune response, i.e., monocytes/macrophages, T cells, and B cells. Normal physiological response would be to dampen/resolve inflammation after the clearance of the trigger. A failure to initiate the adaptive immune response is what leads to the chronic inflammatory state, often resulting in cardiovascular pathology such as atherosclerosis, heart failure, and hypertension.
GRANTS
This work was supported by the National Institutes of Health (NIH) Grant R00-DK-105160 (to D. V. Ilatovskaya); NIH Grants HL-132989 and HL-144788 and University of Alabama at Birmingham Pittman Scholar Award (to G. V. Halade); and NIH Grant U54-DA-016511 and the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Award IK2BX003922 (to K. Y. DeLeon-Pennell). This work was also financially supported, in part, by the 2019 S&R Foundation Ryuji Ueno Award (to K. Y. DeLeon-Pennell) by the American Physiological Society.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Veterans Administration, or the American Physiological Society.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.V.I., G.V.H., and K.Y.D.-P. drafted manuscript; D.V.I., G.V.H., and K.Y.D.-P. edited and revised manuscript; D.V.I., G.V.H., and K.Y.D.-P. approved final version of manuscript.
REFERENCES
- 1.Ahmari N, Santisteban MM, Miller DR, Geis NM, Larkin R, Redler T, Denson H, Khoshbouei H, Baekey DM, Raizada MK, Zubcevic J. Elevated bone marrow sympathetic drive precedes systemic inflammation in angiotensin II hypertension. Am J Physiol Heart Circ Physiol 317: H279–H289, 2019. doi: 10.1152/ajpheart.00510.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansal SS, Ismahil MA, Goel M, Patel B, Hamid T, Rokosh G, Prabhu SD, Activated T. Activated T Lymphocytes are Essential Drivers of Pathological Remodeling in Ischemic Heart Failure. Circ Heart Fail 10: e003688, 2017. doi: 10.1161/CIRCHEARTFAILURE.116.003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, Prabhu SD. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation 139: 206–221, 2019. doi: 10.1161/CIRCULATIONAHA.118.036065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett B, Ludewick HP, Misra A, Lee S, Dwivedi G. Macrophages and T cells in atherosclerosis: a translational perspective. Am J Physiol Heart Circ Physiol 317: H375–H386, 2019. doi: 10.1152/ajpheart.00206.2019. [DOI] [PubMed] [Google Scholar]
- 5.Blanton RM, Carrillo-Salinas FJ, Alcaide P. T-cell recruitment to the heart: friendly guests or unwelcome visitors? Am J Physiol Heart Circ Physiol 317: H124–H140, 2019. doi: 10.1152/ajpheart.00028.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covarrubias R, Ismahil MA, Rokosh G, Hamid T, Accornero F, Singh H, Gumina RJ, Prabhu S, Bansal S. Optimized protocols for isolation, fixation and flow cytometric characterization of leukocytes in ischemic hearts. Am J Physiol Heart Circ Physiol 317: H658–H666, 2019. doi: 10.1152/ajpheart.00137.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLeon-Pennell KY, Mouton AJ, Ero OK, Ma Y, Padmanabhan Iyer R, Flynn ER, Espinoza I, Musani SK, Vasan RS, Hall ME, Fox ER, Lindsey ML. LXR/RXR signaling and neutrophil phenotype following myocardial infarction classify sex differences in remodeling. Basic Res Cardiol 113: 40, 2018. doi: 10.1007/s00395-018-0699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res 102: 240–248, 2014. doi: 10.1093/cvr/cvu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, So-Armah KA, Vasan RS, Oursler KA, Gottdiener J, Gottlieb S, Leaf D, Rodriguez-Barradas M, Tracy RP, Gibert CL, Rimland D, Bedimo RJ, Brown ST, Goetz MB, Warner A, Crothers K, Tindle HA, Alcorn C, Bachmann JM, Justice AC, Butt AA. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol 2: 536–546, 2017. doi: 10.1001/jamacardio.2017.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res 118: 1273–1293, 2016. doi: 10.1161/CIRCRESAHA.116.307547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge T, Yu Y, Cui J, Cai L. The adaptive immune role of metallothioneins in the pathogenesis of diabetic cardiomyopathy: good or bad. Am J Physiol Heart Circ Physiol 317: H264–H275, 2019. doi: 10.1152/ajpheart.00123.2019. [DOI] [PubMed] [Google Scholar]
- 12.Ilatovskaya DV, Pitts C, Clayton J, Domondon M, Troncoso M, Pippin S, DeLeon-Pennell KY. CD8+ T-cells negatively regulate inflammation post-myocardial infarction. Am J Physiol Heart Circ Physiol 317: H581–H596, 2019. doi: 10.1152/ajpheart.00112.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laule CF, Odean EJ, Wing CR, Root KM, Towner KJ, Hamm CM, Gilbert JS, Fleming SD, Regal JF. Role of B1 and B2 lymphocytes in placental ischemia-induced hypertension. Am J Physiol Heart Circ Physiol 317: H732–H742, 2019. doi: 10.1152/ajpheart.00132.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol 158: 1332–1344, 1997. [PubMed] [Google Scholar]
- 15.MacKenzie JR, Mattes J, Dent LA, Foster PS. Eosinophils promote allergic disease of the lung by regulating CD4(+) Th2 lymphocyte function. J Immunol 167: 3146–3155, 2001. doi: 10.4049/jimmunol.167.6.3146. [DOI] [PubMed] [Google Scholar]
- 16.Pollow DP Jr, Uhlorn JA, Sylvester MA, Romero-Aleshire MJ, Uhrlaub JL, Lindsey ML, Nikolich-Zugich J, Brooks HL. Menopause and FOXP3+ Treg cell depletion eliminate female protection against T cell-mediated angiotensin II hypertension. Am J Physiol Heart Circ Physiol 317: H415–H423, 2019. doi: 10.1152/ajpheart.00792.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prows DR, Klingler A, Gibbons WJ Jr, Homan SM, Zimmermann N. Characterization of a mouse model of hypereosinophilia-associated heart disease. Am J Physiol Heart Circ Physiol 317: H405–H414, 2019. doi: 10.1152/ajpheart.00133.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raddatz MA, Madhur MS, Merryman WD. Adaptive immune cells in calcific aortic valve disease. Am J Physiol Heart Circ Physiol 317: H141–H155, 2019. doi: 10.1152/ajpheart.00100.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren J, Crowley SD. Role of T-cell activation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 316: H1345–H1353, 2019. doi: 10.1152/ajpheart.00096.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sattler S, Fairchild P, Watt FM, Rosenthal N, Harding SE. The adaptive immune response to cardiac injury-the true roadblock to effective regenerative therapies? NPJ Regen Med 2: 19, 2017. doi: 10.1038/s41536-017-0022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srikakulapu P, McNamara CA. B cells and atherosclerosis. Am J Physiol Heart Circ Physiol 312: H1060–H1067, 2017. doi: 10.1152/ajpheart.00859.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suetomi T, Miyamoto S, Brown JH. Inflammation in nonischemic heart disease: initiation by cardiomyocyte CaMKII and NLRP3 inflammasome signaling. Am J Physiol Heart Circ Physiol 317: H877–H890, 2019. doi: 10.1152/ajpheart.00223.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabas I, Lichtman AH. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity 47: 621–634, 2017. doi: 10.1016/j.immuni.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teer E, Joseph DE, Driescher N, Nell TA, Dominick L, Midgley N, Deshpande G, Page MJ, Pretorius E, Woudberg NJ, Lecour S, Glashoff RH, Essop MF. HIV and cardiovascular diseases risk: exploring the interplay between T-cell activation, coagulation, monocyte subsets, and lipid subclass alterations. Am J Physiol Heart Circ Physiol 316: H1146–H1157, 2019. doi: 10.1152/ajpheart.00797.2018. [DOI] [PubMed] [Google Scholar]
- 25.van der Vorst EP, Weber C. Novel Features of Monocytes and Macrophages in Cardiovascular Biology and Disease. Arterioscler Thromb Vasc Biol 39: e30–e37, 2019. doi: 10.1161/ATVBAHA.118.312002. [DOI] [PubMed] [Google Scholar]
- 26.Wang T, Yi R, Green LA, Chelvanambi S, Seimetz M, Clauss M. Increased cardiovascular disease risk in the HIV-positive population on ART: potential role of HIV-Nef and Tat. Cardiovasc Pathol 24: 279–282, 2015. doi: 10.1016/j.carpath.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu MM, Ménoret A, Nicholas SE, Günther S, Sundberg EJ, Zhou B, Rodriguez A, Murphy PA, Vella AT. Direct CD137 costimulation of CD8 T cells promotes retention and innate-like function within nascent atherogenic foci. Am J Physiol Heart Circ Physiol 316: H1480–H1494, 2019. doi: 10.1152/ajpheart.00088.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu MM, Murphy PA, Vella AT. Activated T-effector seeds: cultivating atherosclerotic plaque through alternative activation. Am J Physiol Heart Circ Physiol 316: H1354–H1365, 2019. doi: 10.1152/ajpheart.00148.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]