Abstract
Dilated cardiomyopathy (DCM) is the most common cause of heart failure (HF) in children, resulting in high mortality and need for heart transplantation. The pathophysiology underlying pediatric DCM is largely unclear; however, there is emerging evidence that molecular adaptations and response to conventional HF medications differ between children and adults. To gain insight into alterations leading to systolic dysfunction in pediatric DCM, we measured cardiomyocyte contractile properties and sarcomeric protein phosphorylation in explanted pediatric DCM myocardium (N = 8 subjects) compared with nonfailing (NF) pediatric hearts (N = 8 subjects). Force-pCa curves were generated from skinned cardiomyocytes in the presence and absence of protein kinase A. Sarcomeric protein phosphorylation was quantified with Pro-Q Diamond staining after gel electrophoresis. Pediatric DCM cardiomyocytes demonstrate increased calcium sensitivity (pCa50 =5.70 ± 0.0291), with an associated decrease in troponin (Tn)I phosphorylation compared with NF pediatric cardiomyocytes (pCa50 =5.59 ± 0.0271, P = 0.0073). Myosin binding protein C and TnT phosphorylation are also lower in pediatric DCM, whereas desmin phosphorylation is increased. Pediatric DCM cardiomyocytes generate peak tension comparable to that of NF pediatric cardiomyocytes [DCM 29.7 mN/mm2, interquartile range (IQR) 21.5–49.2 vs. NF 32.8 mN/mm2, IQR 21.5–49.2 mN/mm2; P = 0.6125]. In addition, cooperativity is decreased in pediatric DCM compared with pediatric NF (Hill coefficient: DCM 1.56, IQR 1.31–1.94 vs. NF 1.94, IQR 1.36–2.86; P = 0.0425). Alterations in sarcomeric phosphorylation and cardiomyocyte contractile properties may represent an impaired compensatory response, contributing to the detrimental DCM phenotype in children.
NEW & NOTEWORTHY Our study is the first to demonstrate that cardiomyocytes from infants and young children with dilated cardiomyopathy (DCM) exhibit increased calcium sensitivity (likely mediated by decreased troponin I phosphorylation) compared with nonfailing pediatric cardiomyocytes. Compared with published values in adult cardiomyocytes, pediatric cardiomyocytes have notably decreased cooperativity, with a further reduction in the setting of DCM. Distinct adaptations in cardiomyocyte contractile properties may contribute to a differential response to pharmacological therapies in the pediatric DCM population.
Keywords: calcium sensitivity, cooperativity, peak tension, pediatric dilated cardiomyopathy, troponin phosphorylation
INTRODUCTION
Dilated cardiomyopathy (DCM) is the most common cause of end-stage heart failure in children, and within 5 yr of diagnosis 46% of children with DCM die or require heart transplantation (56). Notably, the incidence of pediatric DCM is highest in infants (4.4 cases per 100,000 per year in children <1 yr of age) and the median age at diagnosis is 1.5 yr [interquartile range (IQR) 0.3–11.3 yr] (56). The first hospitalization for children with DCM is often the beginning of a period of clinical decline (24). The use of proven adult heart failure pharmacotherapies (such as angiotensin-converting enzyme inhibitors and β-adrenergic receptor blockers) in pediatric DCM has resulted in only modestly improved survival outcomes (28, 50), with notably poorer outcomes compared with treated adult DCM patients (11). Therefore, the disease processes underlying DCM in children as well as the pediatric response to pharmacological interventions do not mimic what has been demonstrated in adult DCM.
At the molecular level, end-stage pediatric DCM myocardium exhibits a unique gene expression profile with a significant absence of pathological remodeling, characteristics prevalent in adult DCM myocardium (45, 54). Downregulation of both β1- and β2-adrenergic receptors also occurs in pediatric DCM myocardium (41) compared with desensitization of only the β1-adrenergic receptor subtype in adult DCM myocardium (8). In the setting of adult DCM, β-adrenergic receptor desensitization contributes to decreased levels of intracellular cyclic adenosine monophosphate (cAMP), with an associated decreased in protein kinase A (PKA)-mediated phosphorylation of sarcomeric proteins leading to increased calcium sensitivity and preserved maximal force generation (57, 64). Given the proven molecular and clinical differences between adult and pediatric DCM, the purpose of our study was to define the contractile properties of cardiomyocytes from prepubertal pediatric DCM subjects in comparison to cardiomyocytes from prepubertal nonfailing (NF) pediatric control subjects, with and without in vitro PKA treatment. Our initial hypothesis was that pediatric DCM cardiomyocyte contractile properties may differ from those demonstrated in adult DCM; establishing cardiomyocyte mechanical characteristics in infants and young children may help elucidate mechanisms contributing to systolic dysfunction specific to this patient population.
METHODS
Cardiac tissue procurement and preservation.
Explanted pediatric hearts were donated to the University of Colorado Denver Pediatric Cardiac Transplant Tissue Bank, as approved by the Institutional Review Board. Written, informed consent was obtained from parents or legal guardians of pediatric subjects. At the time of heart transplant or organ donation, the heart was rapidly dissected, flash frozen in liquid nitrogen, and stored at −80°C until future use. NF control heart tissues were obtained from pediatric organ donors with normal heart function whose hearts could not be placed for technical reasons (size or blood type mismatch). DCM heart tissues included in this study were from prepubertal pediatric subjects (<10 yr old at time of explant) who exhibited end-stage heart failure due to idiopathic DCM (echocardiographic findings of a dilated left ventricle with an ejection fraction <40%). Rapid procurement strategies employed for all cardiac specimens collected as part of the University of Colorado Tissue Bank preserved myofilament protein phosphorylation states (60).
Permeabilized myocyte preparation.
About 10 mg of frozen left ventricular tissue was used per patient sample. Samples were homogenized at 1,000 rpm in ice-cold rigor solution (Table 1) with 0.3% Triton X-100, as described previously by others and our group (4, 16, 17, 23, 26, 30, 58, 59). The resultant slurry was centrifuged at 800 g for 5 min, and the supernatant was discarded. The pellet was washed twice in rigor solution, the centrifugation was repeated, and the supernatant was discarded. The pellet was then resuspended in an ATP-containing relaxing solution and divided into two 300-μL aliquots.
Table 1.
Rigor solution composition
| Concentration, mM | |
|---|---|
| MOPS | 50 |
| KCl | 100 |
| MgCl2 | 2 |
| EGTA | 1 |
PKA treatment.
In vitro PKA treatment was performed on one aliquot of the permeabilized myocyte slurry by addition of 10 μL of a 1 U/mL solution of the PKA catalytic subunit (V5161; Promega Inc.) in 350 mM KH2PO4 buffer. Ten microliters of 350 mM KH2PO4 buffer was added to the non-PKA-treated myocyte slurry aliquot. The samples were rocked for 1 h at 20°C, centrifuged, and resuspended in 1 mL of rigor solution twice to remove ATP and PKA. The resulting myocyte suspensions were stored at 4°C and used within 3 days.
Force-pCa experiments.
Mechanical experiments were carried out at 15°C in a multiwell plate mounted on a phase-contrast inverted microscope (Nikon T2000), as previously described (59). An aliquot of the permeabilized myocyte suspension was placed on a glass slide, and myocytes that were rectangular with regular/smooth borders were selected for mounting. Representative photographs of glued myocytes are shown in Fig. 1. Briefly, each myocyte’s sarcomere length was set at 2.2 μm with a camera system (Aurora Scientific 901A HVSL). Myocyte length and width were measured with an eyepiece reticle, and a mirror was used to measure depth; cross-sectional area was calculated assuming an elliptical cross section. Myocytes were activated by moving from relaxing solution (pCa 8) to preactivating solution (pCa 8, 20 mM HDTA) for a few seconds and then to activation solution with one of several different calcium concentrations (Table 2). With each activation, the myocyte was subjected to a step shortening and restretch protocol that resulted in a transient drop followed by an exponential rise in force. The cell fragment was exposed to maximal calcium (pCa 4.3) for quantification of maximal force, and then a force-calcium dose-response curve (pCa 6.4, 6.0, 5.8, 5.7, 5.6, 5.4) was generated in random order. A second maximum test contraction (pCa 4.3) concluded the sequence. If the force generated at maximum contraction differed by >20%, the data from that myocyte were discarded. Force-pCa curves are shown as relative force generation, with each force divided by maximal force generation for an individual myocyte.
Fig. 1.
Representative photographs of glued cardiomyocytes. A: pediatric nonfailing cardiomyocyte. B: pediatric dilated cardiomyopathy cardiomyocyte.
Table 2.
Solution composition
| Concentration, mM |
|||
|---|---|---|---|
| Relaxing | Preactivating | Activating | |
| Ca-EGTA | 10−8 | 10−8 | 10−4.3 |
| HDTA | 0.0 | 20.0 | 0.0 |
| MgATP | 5.8 | 5.8 | 5.9 |
| Creatine phosphate | 10.0 | 10.0 | 10.0 |
| K-propionate | 40.1 | 40.7 | 0.5 |
| DTT | 1.0 | 1.0 | 1.0 |
HDTA, 1,5-diaminohexane-N,N,N′,N′-tetraacetic acid.
Phosphoprotein measurements.
Sarcomeric protein phosphorylation was quantified in the pediatric NF and DCM myocyte suspensions used for mechanical measurements with the phosphoprotein-specific stain Pro-Q Diamond (Invitrogen). Myocyte pellets were resuspended in 10 volumes of SDS sample buffer [1% SDS, 10% (vol/vol) glycerol, 50 mM Tris·HCl, and 30 mM dithiothreitol]. Ten-microliter aliquots were loaded onto 12% SDS-PAGE gels and separated at constant voltage (150 V) for 3 h. Gels were stained with Pro-Q Diamond and imaged with a Typhoon 9410 Gel Imager (GE Lifesciences). Gels were then stained with BioSafe Coomassie Blue (Bio-Rad) for determination of total protein. Image analysis was performed with Un-Scan-It Gel analysis software (Silk Scientific, Orem, UT). Relative phosphorylation, in arbitrary units, was calculated as the ratio of the integrated area of the Pro-Q Diamond band for each protein of interest to the integrated area of the myosin essential light chain (MLC)1 Coomassie brilliant blue band. Previous studies have shown MLC1 to be constant and suitable for the correction of Pro-Q Diamond signals for minor variations in total protein load (9, 61).
Slow skeletal troponin I protein expression.
Western blots were performed as described previously (53). Myocardial samples with various etiologies of cardiac failure (DCM, congenital heart disease) were selected from the University of Colorado Denver Pediatric Cardiac Transplant Tissue Bank on the basis of age at explant to evaluate slow skeletal (ss) troponin (Tn)I expression at early postnatal time points. Ten milligrams of frozen myocardium from each sample was homogenized at 4°C, and protein was extracted in isoelectric focusing buffer, as previously described (52). The COOH terminal of cardiac (c)TnI was detected (Abcam ab10239; epitope amino acids 190–196), with a single band at 25 kDa signifying cTnI and a second band at 20 kDa demonstrating the ssTnI isoform.
Statistical analyses.
Statistical analyses were performed with GraphPad Prism version 8.0.2. Force-pCa curves were fitted by using the Hill equation for each myocyte to get the fitted parameters of peak tension, calcium sensitivity (pCa50), and cooperativity [Hill coefficient (nH)] (59); mean or median values were then calculated for each group. Statistical significance was set a priori at P < 0.05. Nonnormally distributed data are presented as medians and IQR, and Mann–Whitney testing was used to compare two groups. Normally distributed data are presented as means ± SE, and an unpaired t test was used to compare two groups. Comparisons of samples with and without PKA treatment were performed via paired t test using the average of all the myocytes from each patient sample for each condition. Linear regression was used to determine the correlation between pCa50 and sarcomeric protein phosphorylation.
RESULTS
Subject characteristics.
Characteristics of pediatric subjects are listed in Table 3. The young median age of the pediatric DCM group is consistent with the increased incidence of pediatric DCM in infancy (56); 50% of our DCM cohort presented with heart failure at <1 yr of age. Reflecting the current era of treatment, the majority of pediatric DCM patients were on phosphodiesterase 3 (PDE3) inhibitor therapy (milrinone) while awaiting cardiac transplantation. One pediatric DCM subject was on her fifth day of extracorporeal membrane oxygenation support at the time of heart transplantation.
Table 3.
Subject characteristics
| NF | DCM | |
|---|---|---|
| N | 8 | 8 |
| Male | 4 (50%) | 5 (63%) |
| Median age (IQR), yr | 7.5 (2.8–8.6) | 2.6 (0.9–3.3) |
| Mean EF | 52% | 21% |
| PDE3i | 0 | 6 (75%) |
Values are for N subjects. DCM, dilated cardiomyopathy; EF, ejection fraction; IQR, interquartile range; NF, nonfailing; PDE3i, chronically treated with phosphodiesterase 3 inhibitor before explant.
Cardiomyocyte calcium sensitivity is increased in pediatric DCM.
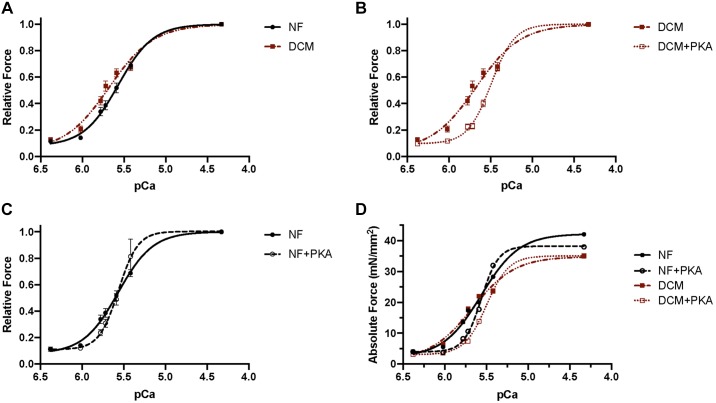
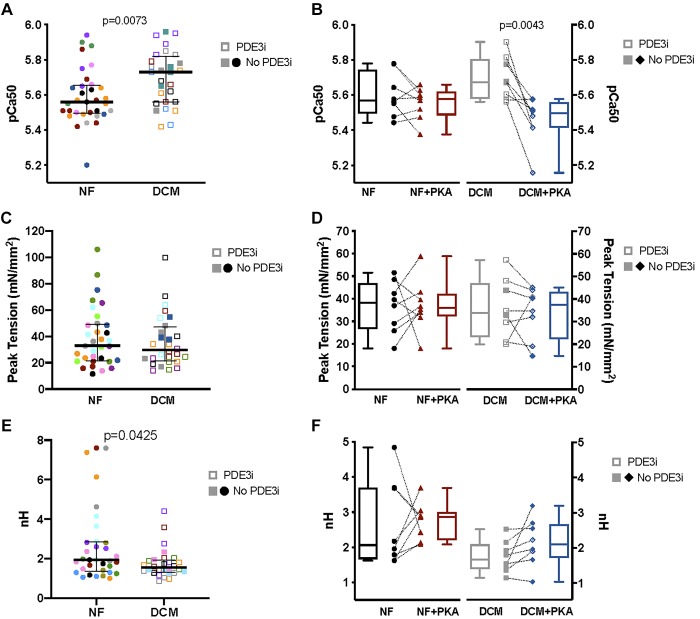
In comparison to pediatric NF myocytes, the force-pCa curve in pediatric DCM myocytes is shifted to the left, most notably at lower, physiological calcium concentrations (Fig. 2A). In vitro PKA treatment results in a rightward shift of the force-pCa curve, more prominent in DCM myocytes (Fig. 2B) compared with that in NF myocytes (Fig. 2C). Comparison between pediatric NF and DCM myocytes demonstrates decreased EC50 and increased pCa50 (Table 4; Fig. 3A; P = 0.0073) in DCM myocytes, consistent with an increase in calcium sensitivity in myocytes isolated from pediatric DCM hearts. Acute PKA treatment of myocytes decreases pCa50 in pediatric DCM (Fig. 3B; P = 0.0043); however, pCa50 is not significantly altered in NF myocytes (Fig. 3B; Table 4).
Fig. 2.
Force-pCa curves of pediatric skinned cardiomyocytes. A: relative force generation at given pCa, demonstrating a leftward shift in dilated cardiomyopathy (DCM) cardiomyocytes compared with nonfailing (NF) cardiomyocytes indicative of increased calcium sensitivity. B: there is normalization of calcium sensitivity in DCM cardiomyocytes with in vitro protein kinase A (PKA) treatment (rightward shift). C: although there is a subtle rightward shift of the relative force-pCa curve with in vitro PKA treatment of NF cardiomyocytes, there is no statistically significant difference in pCa50. D: absolute force development over a range of calcium concentrations (pCa). NF: no. of subjects (N) = 8, no. of cardiomyocytes (n) = 33; NF+PKA: N = 8, n = 29; DCM: N = 8, n = 26; DCM+PKA: N = 8, n = 26.
Table 4.
Calcium sensitivity of tension
| NF | NF+PKA | DCM | DCM+PKA | |
|---|---|---|---|---|
| N, n | 8, 33 | 8, 30 | 8, 28 | 8, 24 |
| EC50, μM | 2.75 (2.21–3.20) | 3.10 (2.15–3.50) | 1.86 (1.52–2.75)** | 3.00 (2.63–3.85)# |
| pCa50 | 5.59 ± 0.0271 | 5.53 ± 0.0281 | 5.70 ± 0.0291** | 5.46 ± 0.0430# |
| Hill coefficient nH | 1.94 (1.36–2.86) | 2.19 (1.38–3.20) | 1.56 (1.31–1.94)* | 2.14 (1.31–2.61) |
| Peak tension, mN/mm2 | 32.8 (21.5–49.2) | 31.6 (23.1–50.7) | 29.7 (21.6–47.0) | 31.6 (19.1–45.3) |
Normally distributed data are means ± SE and nonparametric data as medians (interquartile range) for N subjects and n cardiomyocytes. DCM, dilated cardiomyopathy; NF, nonfailing; PKA, protein kinase A. Different from NF:
P < 0.05,
P < 0.01. Different from DCM without PKA:
P < 0.0001.
Fig. 3.
Contractile properties of pediatric skinned cardiomyocytes. A: pCa50 of pediatric dilated cardiomyopathy (DCM) cardiomyocytes (5.70 ± 0.0291) is significantly increased (P = 0.0073) compared with that of pediatric nonfailing (NF) cardiomyocytes (5.59 ± 0.0271). B: pCa50 of NF cardiomyocytes is unchanged with acute protein kinase A (PKA) treatment (NF+PKA 5.53 ± 0.0281), whereas pCa50 of DCM cardiomyocytes decreases (P = 0.0043) with acute PKA treatment (DCM+PKA 5.46 ± 0.0430). C: median peak tension generation is similar between NF [32.8, interquartile range (IQR) 21.5–49.2] and DCM (29.7, IQR 21.6–47.0) cardiomyocytes. D: no significant changes in peak tension were present in NF or DCM samples treated with PKA. E: cooperativity, as represented by the Hill coefficient (nH), is decreased (P = 0.0425) in DCM (1.56, IQR 1.31–1.94) compared with NF (1.94, IQR 1.36–2.86). F: acute PKA treatment did not significantly alter cooperativity in either DCM (2.14, IQR 1.31–2.61) or NF (2.19, IQR 1.38–3.20). NF: no. of subjects (N) = 8, no. of cardiomyocytes (n) = 33; NF+PKA: N = 8, n = 29; DCM: N = 8, n = 26; DCM+PKA: N = 8, n = 26. PDE3i, chronically treated with a phosphodiesterase 3 inhibitor before explant.
Peak tension generation is maintained in pediatric DCM.
The median peak tension generation is similar between pediatric DCM and NF samples (Fig. 3C; Table 4); although the mean (NF 39.0 ± SE 3.36; DCM 36.0 ± SE 3.78) and median (NF 32.8, IQR 21.5–49.2; DCM 29.7, IQR 21.6–47.0) peak tensions trend lower in pediatric DCM samples, these differences are not statistically significant. Acute PKA treatment does not alter peak tension generation in NF or DCM myocytes (Fig. 3D; Table 4).
Pediatric DCM is associated with lower cooperativity.
In comparison with pediatric NF samples, cooperativity (represented by nH) in pediatric DCM myocytes is decreased (Fig. 3E, P = 0.0425; Table 4). Cooperativity was not significantly altered in NF or DCM myocytes acutely treated with PKA (Fig. 3F; Table 4).
Sarcomeric protein phosphorylation alterations in pediatric DCM.
Compared with pediatric NF samples, DCM samples demonstrate a significant decrease in phosphorylation of several sarcomeric proteins, including myosin binding protein C (MBPC; Fig. 4B; P = 0.0031), TnI (Fig. 4C; P = 0.0252), and TnT (Fig. 4F; P = 0.0254). Desmin phosphorylation is higher in pediatric DCM (Fig. 4D; P = 0.0029). In vitro PKA treatment of NF myocytes resulted in a trend toward increased MBPC (Fig. 4B; P = 0.0635) and TnI (Fig. 4C; P = 0.0571) phosphorylation, whereas PKA treatment of DCM myocytes significantly increased phosphorylation of MBPC (Fig. 4B; P = 0.0495), TnI (Fig. 4C; P = 0.0019), and tropomyosin (Tm; Fig. 4E; P = 0.039). Additionally, there is a moderate inverse correlation between MBPC (Fig. 4B), TnI (Fig. 4C), and TnT (Fig. 4F) phosphorylation and pCa50, suggesting that lower phosphorylation levels of these proteins are associated with increased calcium sensitivity.
Fig. 4.
Phosphorylation of sarcomeric proteins in pediatric cardiomyocytes. A: Pro-Q Diamond stain of nonfailing (NF) and dilated cardiomyopathy (DCM) samples. PKA, protein kinase A. B: myosin binding protein C (MBPC) phosphorylation [normalized to myosin essential light chain (MLC)1] is decreased in DCM (P = 0.0031) and increases with in vitro PKA treatment (P = 0.0495). Decreased MBPC phosphorylation correlates with increased calcium sensitivity (higher pCa50), R2 = 0.4415, P = 0.0361. C: troponin (Tn)I phosphorylation is decreased in DCM (P = 0.0252) and increases with in vitro PKA treatment (P = 0.0019). Decreased TnI phosphorylation correlates with increased calcium sensitivity, R2 = 0.5185, P = 0.0188. D: desmin phosphorylation is increased in DCM (P = 0.0029) and does not change significantly with PKA treatment. No significant correlation is demonstrated between desmin phosphorylation and calcium sensitivity. E: tropomyosin (Tm) phosphorylation is not significantly altered in DCM but is increased with PKA treatment of DCM cardiomyocytes (P = 0.039). No significant correlation is demonstrated between Tm phosphorylation and calcium sensitivity. F: TnT phosphorylation is decreased in DCM (P = 0.0254), and there is a trend toward increased phosphorylation with PKA treatment (P = 0.1060). Decreased TnT phosphorylation is correlated with increased calcium sensitivity, R2 = 0.4264, P = 0.0406. G: MLC2 phosphorylation is unchanged in DCM and does not change significantly with PKA treatment. No significant correlation is demonstrated between MLC2 phosphorylation and calcium sensitivity. NF: no. of subjects (N) = 6; NF+PKA: N = 6; DCM: N = 4; DCM+PKA: N = 4.
ssTnI is infrequently expressed, even at early postnatal ages.
As ssTnI is expressed in fetal hearts (5, 15, 25, 49), we quantified the expression of ssTnI in a survey of our pediatric heart samples across the postnatal age spectrum, which shows that ssTnI is not a prominent isoform: only 1 of 11 (9%) young myocardial samples (median age, 7.8 wk, IQR 2.86–9.88 wk) demonstrates ssTnI (Fig. 5).
Fig. 5.
Troponin (Tn)I isoforms in young pediatric cardiac explants. The slow skeletal TnI isoform (20 kDa) was present in 1 of 11 (9%) young pediatric myocardial samples (lane 3) explanted for end-stage heart failure of varying etiologies.
DISCUSSION
Our findings demonstrate that alterations in cardiomyocyte contractile properties in young children with DCM share several features with those in adult (57, 64) and older pediatric (noninfantile) (7) DCM, including increased calcium sensitivity, likely mediated by decreased TnI phosphorylation. Additionally, in comparison to pediatric NF cardiomyocytes, our cohort of pediatric DCM cardiomyocytes demonstrates preserved maximal force generation as well as a decreased cooperativity.
Functional implications of increased calcium sensitivity.
Pediatric myocardium from subjects with end-stage heart failure secondary to DCM demonstrates increased calcium sensitivity, which may favor increased inotropy at the cost of decreased lusitropy, which could result in a decreased rate in pressure relaxation (18, 35). The finding of increased calcium sensitivity in end-stage pediatric DCM is consistent with what has previously been reported in end-stage adult DCM (57, 64) as well as older pediatric DCM (7) hearts. Nevertheless, the physiological impact of increased calcium sensitivity at the level of the whole heart remains unclear. We postulate that, rather than being an etiology for DCM development in the majority of pediatric cases, increased calcium sensitivity is a compensatory mechanism in pediatric DCM cardiomyocytes to allow for increased force generation at lower calcium concentrations, prompted by alterations in calcium handling (12) and extracellular matrix proteins (45, 65) in the failing myocardium. Additionally, although specific thin filament mutations involved in DCM have been demonstrated to alter calcium sensitivity (40), all pediatric DCM samples were from cases of idiopathic DCM, specifically without a family history of DCM or known sarcomeric gene mutations. Although this adaptation in calcium sensitivity is ultimately insufficient to maintain normal cardiac output, differences in the ability to augment calcium sensitivity may play a role in the rate of disease progression.
Influence of decreased sarcomeric protein phosphorylation on calcium sensitivity.
Cardiomyocyte calcium sensitivity is affected by 1) TnC calcium binding, resulting in differences in the availability of actin for myosin binding, 2) strength of myosin-actin cross bridge formation (20), 3) modulation of TnC-TnI interactions through phosphorylation of TnI, and 4) TnT mutations. Given that cAMP levels and PKA-mediated phospholamban phosphorylation are significantly decreased in end-stage pediatric DCM myocardium (42), we evaluated calcium sensitivity of pediatric DCM cardiomyocytes with a focus on alterations of PKA-mediated phosphorylation of sarcomeric proteins. Prior studies have demonstrated that phosphorylation of TnI in cardiac muscle at the two NH2-terminal serine sites (Ser22 and Ser23) decreases calcium sensitivity two- to threefold by decreasing calcium binding to the troponin complex (48) through modulation of the TnC-TnI interaction (37) and increasing the rate of calcium dissociation from TnC (40). Consistent with this and other work (7), our results demonstrate that pediatric DCM cardiomyocytes have increased calcium sensitivity with an associated decrease in TnI phosphorylation, which is abrogated by PKA treatment. Notably, the magnitude of increase in calcium sensitivity is slightly blunted (~1.5-fold) in comparison to the change in calcium sensitivity traditionally attributed to TnI phosphorylation. This could be a result of prior pharmacological treatment [specifically PDE3 inhibition (PDE3i)] or uncoupling of calcium sensitivity and TnI phosphorylation, which has been described in in vitro models of DCM caused by sarcomeric gene mutations (40, 63). Additionally, the phosphorylation background of other myofilament proteins is known to impact the effects of TnI phosphorylation on calcium sensitivity (32). We demonstrated that MBPC phosphorylation is also decreased in pediatric DCM and is inversely correlated with calcium sensitivity, consistent with phosphorylation findings in adult end-stage DCM samples (31, 32). Phosphorylation of MBPC by PKA has been shown to accelerate cross bridge cycling kinetics (23, 51) and is thought to contribute to the inotropic response of β1-adrenergic receptor agonists (29, 55). Dissimilar from adult DCM (32), TnT phosphorylation is significantly decreased and desmin phosphorylation is increased in pediatric DCM cardiomyocytes compared with pediatric nonfailing donors. However, whether or how these differences in TnT and desmin phosphorylation contribute to changes in calcium sensitivity in pediatric DCM remains unclear.
Phosphorylation-independent factors, such as TnC calcium binding kinetics, are also known to be important contributors to calcium sensitivity of the thin filament in cardiac muscle. Prior studies have demonstrated that calcium sensitivity can be increased or decreased by mutations in TnC that modulate calcium binding kinetics (33, 44), which have also been reported in patients with hypertrophic cardiomyopathy (34, 36, 38, 43). Although TnC mutations were not the primary cause of DCM in our patient population, variants in TnC can modulate calcium-sensitive tension development through alterations in calcium binding, particularly in the presence of TnI phosphorylation following β-adrenergic stimulation (6).
Decreased cooperativity in pediatric cardiomyocytes.
The Hill coefficient (nH) denotes the steepness of the force-pCa relationship and represents the cooperativity of calcium activation. Cooperativity is an essential feature of cardiac muscle given the need for highly coordinated contraction and relaxation (39). In cardiomyocytes, possible sources of cooperativity include coupling between calcium binding sites along the thin filament and changes in calcium binding or movement of Tm induced by cross bridge formation (20). Thus, in addition to the local cooperativity within a regulatory unit (composed of Tm, a troponin complex, and 7 actin monomers) there is also longer-range cooperativity between regulatory units along a thin filament, in which Tm is thought to play a central role (39). Binding of calcium to a regulatory unit causes displacement of Tm and may induce calcium binding to the troponin complex of neighboring subunits through the end-to-end overlap that exists between Tm molecules. Functional changes in cooperative calcium-induced activation of cardiac thin filaments may arise as a consequence of Tm mutations that alter the flexural rigidity of the molecule (39). Although the underlying source of decreased cooperativity in pediatric DCM myocytes is unclear, it does appear that this finding is discordant with an increase in calcium sensitivity since factors predicted to increase cooperativity would also be expected to increase calcium sensitivity. For instance, strongly bound, cycling cross bridge attachments both facilitate more cross bridge formation at a given calcium concentration (resulting in an apparent increase in calcium sensitivity) and increase calcium binding to TnC (10, 22) (reflected as higher cooperativity). Nevertheless, despite consistently increased calcium sensitivity, nH of pediatric DCM cardiomyocytes is lower than that of pediatric nonfailing cardiomyocytes. Additionally, both mean and median nH for our pediatric DCM group appear lower than those of adult cardiomyocytes with heart failure at comparable sarcomere lengths of 2.2 μm [3.44 ± 0.28 (n = 8) (57), 3.51 IQR 2.65–6.17 (n = 8, unpublished data)], although decreased cooperativity has been reported in adult DCM at a sarcomere length of 2.1 μm (1). Interestingly, mean and median nH for our pediatric NF group are also lower than those demonstrated in adult nonfailing donors [3.65 ± 0.44 (n = 5) (57); 2.30 ± 0.81 (n = 8) (1); 2.70, IQR 2.26–3.53 (n = 8, unpublished data)]. From a technical standpoint, our pediatric samples were prepared and measured in an identical fashion to adult samples yielding results comparable to what has previously been published. Decreases in MBPC phosphorylation increase the inhibition of the thick filament, negatively impacting cooperativity of the thick filament (27). Reduced MBPC phosphorylation is a common feature between pediatric and adult DCM, however; thus it is unclear whether there are additional alterations unique to the pediatric milieu that ultimately result in decreased cooperativity.
Additional studies are required to determine the mechanism by which cardiomyocytes from children demonstrate lower cooperativity compared with those from adults, with a further decline in cooperativity in the setting of pediatric DCM. Hypothetically, both increases in calcium sensitivity and cooperativity may be compensatory mechanisms to improve contractility in the setting of end-stage heart failure. In this context, it could be that the relatively rapid progression to end-stage heart failure in infant pediatric DCM patients may be in part due to the inability to mount a compensatory cooperativity response. Furthermore, an impaired compensatory cooperativity response may also be representative of a different underlying disease process in infant and young pediatric DCM.
ssTnI expression.
Elegant studies have demonstrated the influence of the fetal TnI isoform on cardiomyocyte mechanics, specifically documenting increased calcium sensitivity in the presence of ssTnI expression (13, 62). However, the physiological relevance of this finding relies on the prevalence of ssTnI in postnatal explanted heart tissue, with variable reports in the literature (5, 15, 25, 49). In our pediatric tissue bank, ssTnI was detected in a small minority of explanted hearts, even at young postnatal ages. Additionally, we and others (49) have not seen that there is reexpression of ssTnI in the setting of heart failure. Thus, similar to reexpression of fetal cTnT (21), expression of ssTnI may be highly variable and not consistent among all heart failure etiologies. Differences in the reported prevalence of ssTnI may be secondary to 1) patchy, nonuniform distribution of ssTnI expression, 2) downregulated ssTnI expression in the setting of heart failure, or 3) detection of degraded TnI in stunned tissue (19). Furthermore, it has been demonstrated that ssTnI is not responsive to PKA treatment (18), and our DCM samples exhibited a robust normalization of calcium sensitivity with exogenous PKA treatment [similar to Bollen and colleagues (2)], suggesting that ssTnI expression is negligible in our cohort. Overall, our results indicate that the increase in calcium sensitivity demonstrated in our pediatric DCM cohort is unlikely to be related to ssTnI isoform expression.
Preserved peak tension generation.
Preserved peak tension generation in pediatric DCM is consistent with previous studies in adult DCM, which demonstrate an increase in myocyte calcium sensitivity but no difference in maximum tension between failing and normal preparations (57, 64). Although Bollen et al. describe decreased peak tension generation in pediatric DCM cardiomyocytes in comparison to adult nonfailing samples, this difference is no longer present once the peak tension is corrected for decreased myofibril density (7). Given the equivalent maximum tension generation between our pediatric NF and DCM samples, myofibril density was not assessed.
Effects of downregulation of both β1 and β2 on myocyte mechanics.
Despite the concurrent downregulation of both β1- and β2-adrenergic receptor subtypes in end-stage pediatric DCM myocardium (41), the predominant profile of cardiomyocyte mechanical properties is that of downregulation of β1-adrenergic receptors, with decreased PKA-mediated sarcomeric protein phosphorylation (specifically TnI, TnT, and MBPC). As an acute compensatory response, adrenergic stimulation results in increased cAMP- and PKA-mediated phosphorylation of TnI at Ser22 and Ser23, decreasing the affinity of TnI for TnC and the affinity of the regulatory site of TnC for calcium, resulting in a decreased pCa50 of ATPase activity and force development (14, 47). In the setting of chronic adrenergic stimulation, desensitization of β1-adrenergic receptors leads to lower cAMP levels and decreased PKA activity, resulting in decreased phosphorylation of TnI and increased calcium sensitivity (64). One contribution of β2-adrenergic receptor stimulation is to serve as a counterbalance to β1-adrenergic receptor-mediated cAMP generation through inhibition of adenylyl cyclases; downregulation of β2-adrenergic receptors in the failing pediatric heart may serve as an attempt to preserve cAMP generation. However, our results demonstrate that in the pediatric DCM heart the overall intracellular milieu is still one of cAMP depletion (42) with accompanying decreased PKA-mediated sarcomeric protein phosphorylation. Thus the downregulation of β2-adrenergic receptors in failing pediatric DCM is not sufficient to preserve PKA-mediated sarcomeric protein phosphorylation or preclude an increase in calcium sensitivity.
Limitations.
Given the robust and favorable effects of long-term PDE3 inhibition (PDE3i) in pediatric subjects with DCM (3, 46), the overwhelming majority of pediatric DCM subjects with end-stage heart failure but without ventricular assist device support are supported with PDE3i until the time of heart transplant. However, because of the scarcity of pediatric DCM tissue without PDE3i treatment, our study was not adequately powered to determine the effects of chronic PDE3i treatment on cardiomyocyte contractility and sarcomeric protein phosphorylation. Comparison of our data to those of prior human DCM studies yields similar findings, although none of the previous studies specifically comments on PDE3i use. It is notable that sarcomeric protein phosphorylation remains significantly decreased in pediatric DCM even in samples treated with PDE3i, suggesting that increased cAMP and PKA activity downstream from PDE3 is localized to the microdomain surrounding phospholamban (42) rather than that surrounding the sarcomere. Furthermore, even in myocytes exposed to chronic PDE3i, the decrease in pCa50 with acute PKA treatment is maintained. Overall, we postulate that the mechanism of action of PDE3i in increasing inotropy and lusitropy is predominantly through facilitating calcium cycling between the cytosol and sarcoplasmic reticulum rather than direct PKA-mediated phosphorylation of sarcomeric proteins, although more comprehensive investigations of the effects of PDE3i on the sarcomere are needed.
In addition to the limited conclusions regarding the effects of PDE3i treatment, several other limitations of our study merit discussion. First, although both the number of patient samples (N) and the number of cardiomyocytes (n) are included for review, assessment of mechanical differences between NF and DCM groups was performed based on each cardiomyocyte, consistent with prior literature (7, 57, 64). The variability between myocytes, even from an individual patient, suggests that each should be considered individually: the individual properties of the myocyte affected measurements to a larger degree than subject characteristics, other than the presence of DCM. To provide a paired comparison, the effects of PKA treatment were analyzed based on the average of all the cardiomyocytes from a particular subject. Second, given the differences in β-adrenergic receptor expression between pediatric and adult DCM, this study focused on the effects of PKA; however, activity of other kinases and phosphatases, such as cardiac myosin light chain kinase, protein kinase C, and protein phosphatase 1, may also influence the mechanical landscape.
Conclusions.
When compared with pediatric nonfailing cardiomyocytes, cardiomyocytes from pediatric DCM subjects demonstrate altered contractile properties, characterized by increased calcium sensitivity, preserved maximal tension generation, and decreased cooperativity. Increased calcium sensitivity mediated in part by decreased PKA-mediated TnI phosphorylation appears to be a common feature between adult and pediatric DCM, even in the setting of differential β-adrenergic receptor regulation. Nevertheless, the lower cooperativity present in NF pediatric cardiomyocytes with a further reduction in the setting of pediatric DCM is distinct from what has been demonstrated in adults and warrants further investigation as to whether this represents an impaired compensatory response contributing to poorer prognosis in infants and children with DCM.
GRANTS
This work was supported by National Institutes of Health Grants K08-HL-130592-01A1 and K12-HD-068372 (to S. J. Nakano), R21-HL-113846 and R01-HL-126928 (to S. D. Miyamoto), K08-HL-080212 and R01-HL-107715 (to B. L. Stauffer), and R21-HL-097123 (to S. D. Miyamoto, C. C. Sucharov, and B. L. Stauffer); the Addison Scott Memorial Fund; the Boedecker Foundation; the Nair Family; and the Jack Cooper Millisor Chair in Pediatric Heart Disease.
DISCLOSURES
C. C. Sucharov is scientific founder and shareholder at miRagen, Inc. C. C. Sucharov, S. D. Miyamoto, and B. L. Stauffer are scientific founders and shareholders at CoramiR, Inc. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
J.S.W., L.A.W., S.D.M., C.C.S., and B.L.S. conceived and designed research; J.S.W., L.A.W., X.L., Y.D., and M.B.M. performed experiments; S.J.N., J.S.W., L.A.W., X.L., Y.D., and A.V.A. analyzed data; S.J.N., J.S.W., L.A.W., S.D.M., C.C.S., A.M.G., A.V.A., and B.L.S. interpreted results of experiments; S.J.N., L.A.W., X.L., Y.D., A.M.G., and B.L.S. prepared figures; S.J.N., J.S.W., L.A.W., S.D.M., C.C.S., and B.L.S. drafted manuscript; S.J.N., L.A.W., X.L., Y.D., S.D.M., C.C.S., A.M.G., M.B.M., A.V.A., and B.L.S. edited and revised manuscript; S.J.N., J.S.W., L.A.W., S.D.M., C.C.S., A.M.G., M.B.M., A.V.A., and B.L.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Pediatric Heart Transplant team at the Children’s Hospital Colorado.
REFERENCES
- 1.Ambardekar AV, Walker JS, Walker LA, Cleveland JC Jr, Lowes BD, Buttrick PM. Incomplete recovery of myocyte contractile function despite improvement of myocardial architecture with left ventricular assist device support. Circ Heart Fail 4: 425–432, 2011. doi: 10.1161/CIRCHEARTFAILURE.111.961326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beqqali A, Bollen IA, Rasmussen TB, van den Hoogenhof MM, van Deutekom HW, Schafer S, Haas J, Meder B, Sørensen KE, van Oort RJ, Mogensen J, Hubner N, Creemers EE, van der Velden J, Pinto YM. A mutation in the glutamate-rich region of RNA-binding motif protein 20 causes dilated cardiomyopathy through missplicing of titin and impaired Frank-Starling mechanism. Cardiovasc Res 112: 452–463, 2016. doi: 10.1093/cvr/cvw192. [DOI] [PubMed] [Google Scholar]
- 3.Berg AM, Snell L, Mahle WT. Home inotropic therapy in children. J Heart Lung Transplant 26: 453–457, 2007. doi: 10.1016/j.healun.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Best PM, Donaldson SK, Kerrick WG. Tension in mechanically disrupted mammalian cardiac cells: effects of magnesium adenosine triphosphate. J Physiol 265: 1–17, 1977. doi: 10.1113/jphysiol.1977.sp011702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhavsar PK, Dhoot GK, Cumming DV, Butler-Browne GS, Yacoub MH, Barton PJ. Developmental expression of troponin I isoforms in fetal human heart. FEBS Lett 292: 5–8, 1991. doi: 10.1016/0014-5793(91)80820-S. [DOI] [PubMed] [Google Scholar]
- 6.Biesiadecki BJ, Kobayashi T, Walker JS, Solaro RJ, de Tombe PP. The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ Res 100: 1486–1493, 2007. doi: 10.1161/01.RES.0000267744.92677.7f. [DOI] [PubMed] [Google Scholar]
- 7.Bollen IA, van der Meulen M, de Goede K, Kuster DW, Dalinghaus M, van der Velden J. Cardiomyocyte hypocontractility and reduced myofibril density in end-stage pediatric cardiomyopathy. Front Physiol 8: 1103, 2017. doi: 10.3389/fphys.2017.01103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res 59: 297–309, 1986. doi: 10.1161/01.RES.59.3.297. [DOI] [PubMed] [Google Scholar]
- 9.Bruns DR, Buttrick PM, Walker LA. Genetic ablation of interleukin-18 does not attenuate hypobaric hypoxia-induced right ventricular hypertrophy. Am J Physiol Lung Cell Mol Physiol 310: L542–L550, 2016. doi: 10.1152/ajplung.00166.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butters CA, Tobacman JB, Tobacman LS. Cooperative effect of calcium binding to adjacent troponin molecules on the thin filament-myosin subfragment 1 MgATPase rate. J Biol Chem 272: 13196–13202, 1997. doi: 10.1074/jbc.272.20.13196. [DOI] [PubMed] [Google Scholar]
- 11.Cubbon RM, Gale CP, Kearney LC, Schechter CB, Brooksby WP, Nolan J, Fox KA, Rajwani A, Baig W, Groves D, Barlow P, Fisher AC, Batin PD, Kahn MB, Zaman AG, Shah AM, Byrne JA, Lindsay SJ, Sapsford RJ, Wheatcroft SB, Witte KK, Kearney MT. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ Heart Fail 4: 396–403, 2011. doi: 10.1161/CIRCHEARTFAILURE.110.959882. [DOI] [PubMed] [Google Scholar]
- 12.de Tombe PP. Altered contractile function in heart failure. Cardiovasc Res 37: 367–380, 1998. doi: 10.1016/S0008-6363(97)00275-7. [DOI] [PubMed] [Google Scholar]
- 13.de Tombe PP, Belus A, Piroddi N, Scellini B, Walker JS, Martin AF, Tesi C, Poggesi C. Myofilament calcium sensitivity does not affect cross-bridge activation-relaxation kinetics. Am J Physiol Regul Integr Comp Physiol 292: R1129–R1136, 2007. doi: 10.1152/ajpregu.00630.2006. [DOI] [PubMed] [Google Scholar]
- 14.Dohet C, al-Hillawi E, Trayer IP, Rüegg JC. Reconstitution of skinned cardiac fibres with human recombinant cardiac troponin-I mutants and troponin-C. FEBS Lett 377: 131–134, 1995. doi: 10.1016/0014-5793(95)01319-9. [DOI] [PubMed] [Google Scholar]
- 15.Elhamine F, Iorga B, Krüger M, Hunger M, Eckhardt J, Sreeram N, Bennink G, Brockmeier K, Pfitzer G, Stehle R. Postnatal development of right ventricular myofibrillar biomechanics in relation to the sarcomeric protein phenotype in pediatric patients with conotruncal heart defects. J Am Heart Assoc 5: e003699, 2016. doi: 10.1161/JAHA.116.003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabiato A, Fabiato F. Effects of magnesium on contractile activation of skinned cardiac cells. J Physiol 249: 497–517, 1975. doi: 10.1113/jphysiol.1975.sp011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan D, Wannenburg T, de Tombe PP. Decreased myocyte tension development and calcium responsiveness in rat right ventricular pressure overload. Circulation 95: 2312–2317, 1997. doi: 10.1161/01.CIR.95.9.2312. [DOI] [PubMed] [Google Scholar]
- 18.Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, Martin AF, Solaro RJ, Moss RL, Leiden JM. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J Physiol 517: 143–157, 1999. doi: 10.1111/j.1469-7793.1999.0143z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao WD, Atar D, Liu Y, Perez NG, Murphy AM, Marban E. Role of troponin I proteolysis in the pathogenesis of stunned myocardium. Circ Res 80: 393–399, 1997. doi: 10.1161/01.res.0000435855.49359.47. [DOI] [PubMed] [Google Scholar]
- 20.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev 80: 853–924, 2000. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 21.Hamdani N, Kooij V, van Dijk S, Merkus D, Paulus WJ, Remedios CD, Duncker DJ, Stienen GJ, van der Velden J. Sarcomeric dysfunction in heart failure. Cardiovasc Res 77: 649–658, 2008. doi: 10.1093/cvr/cvm079. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann PA, Fuchs F. Effect of length and cross-bridge attachment on Ca2+ binding to cardiac troponin C. Am J Physiol Cell Physiol 253: C90–C96, 1987. doi: 10.1152/ajpcell.1987.253.1.C90. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann PA, Hartzell HC, Moss RL. Alterations in Ca2+ sensitive tension due to partial extraction of C-protein from rat skinned cardiac myocytes and rabbit skeletal muscle fibers. J Gen Physiol 97: 1141–1163, 1991. doi: 10.1085/jgp.97.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollander SA, Bernstein D, Yeh J, Dao D, Sun HY, Rosenthal D. Outcomes of children following a first hospitalization for dilated cardiomyopathy. Circ Heart Fail 5: 437–443, 2012. doi: 10.1161/CIRCHEARTFAILURE.111.964510. [DOI] [PubMed] [Google Scholar]
- 25.Hunkeler NM, Kullman J, Murphy AM. Troponin I isoform expression in human heart. Circ Res 69: 1409–1414, 1991. doi: 10.1161/01.RES.69.5.1409. [DOI] [PubMed] [Google Scholar]
- 26.Jweied EE, McKinney RD, Walker LA, Brodsky I, Geha AS, Massad MG, Buttrick PM, de Tombe PP. Depressed cardiac myofilament function in human diabetes mellitus. Am J Physiol Heart Circ Physiol 289: H2478–H2483, 2005. doi: 10.1152/ajpheart.00638.2005. [DOI] [PubMed] [Google Scholar]
- 27.Kampourakis T, Yan Z, Gautel M, Sun YB, Irving M. Myosin binding protein-C activates thin filaments and inhibits thick filaments in heart muscle cells. Proc Natl Acad Sci USA 111: 18763–18768, 2014. doi: 10.1073/pnas.1413922112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kantor PF, Abraham JR, Dipchand AI, Benson LN, Redington AN. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol 55: 1377–1384, 2010. doi: 10.1016/j.jacc.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 29.Kensler RW, Craig R, Moss RL. Phosphorylation of cardiac myosin binding protein C releases myosin heads from the surface of cardiac thick filaments. Proc Natl Acad Sci USA 114: E1355–E1364, 2017. doi: 10.1073/pnas.1614020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, de Tombe PP. Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J Physiol 547: 951–961, 2003. doi: 10.1113/jphysiol.2002.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kooij V, Holewinski RJ, Murphy AM, Van Eyk JE. Characterization of the cardiac myosin binding protein-C phosphoproteome in healthy and failing human hearts. J Mol Cell Cardiol 60: 116–120, 2013. doi: 10.1016/j.yjmcc.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kooij V, Saes M, Jaquet K, Zaremba R, Foster DB, Murphy AM, Dos Remedios C, van der Velden J, Stienen GJ. Effect of troponin I Ser23/24 phosphorylation on Ca2+-sensitivity in human myocardium depends on the phosphorylation background. J Mol Cell Cardiol 48: 954–963, 2010. doi: 10.1016/j.yjmcc.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreutziger KL, Piroddi N, McMichael JT, Tesi C, Poggesi C, Regnier M. Calcium binding kinetics of troponin C strongly modulate cooperative activation and tension kinetics in cardiac muscle. J Mol Cell Cardiol 50: 165–174, 2011. doi: 10.1016/j.yjmcc.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landstrom AP, Parvatiyar MS, Pinto JR, Marquardt ML, Bos JM, Tester DJ, Ommen SR, Potter JD, Ackerman MJ. Molecular and functional characterization of novel hypertrophic cardiomyopathy susceptibility mutations in TNNC1-encoded troponin C. J Mol Cell Cardiol 45: 281–288, 2008. doi: 10.1016/j.yjmcc.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Love ML, Putkey JA, Cohen C. Bepridil opens the regulatory N-terminal lobe of cardiac troponin C. Proc Natl Acad Sci USA 97: 5140–5145, 2000. doi: 10.1073/pnas.090098997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang B, Chung F, Qu Y, Pavlov D, Gillis TE, Tikunova SB, Davis JP, Tibbits GF. Familial hypertrophic cardiomyopathy-related cardiac troponin C mutation L29Q affects Ca2+ binding and myofilament contractility. Physiol Genomics 33: 257–266, 2008. doi: 10.1152/physiolgenomics.00154.2007. [DOI] [PubMed] [Google Scholar]
- 37.Liao R, Wang CK, Cheung HC. Time-resolved tryptophan emission study of cardiac troponin I. Biophys J 63: 986–995, 1992. doi: 10.1016/S0006-3495(92)81685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim CC, Yang H, Yang M, Wang CK, Shi J, Berg EA, Pimentel DR, Gwathmey JK, Hajjar RJ, Helmes M, Costello CE, Huo S, Liao R. A novel mutant cardiac troponin C disrupts molecular motions critical for calcium binding affinity and cardiomyocyte contractility. Biophys J 94: 3577–3589, 2008. doi: 10.1529/biophysj.107.112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loong CK, Badr MA, Chase PB. Tropomyosin flexural rigidity and single Ca2+ regulatory unit dynamics: implications for cooperative regulation of cardiac muscle contraction and cardiomyocyte hypertrophy. Front Physiol 3: 80, 2012. doi: 10.3389/fphys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messer AE, Marston SB. Investigating the role of uncoupling of troponin I phosphorylation from changes in myofibrillar Ca2+-sensitivity in the pathogenesis of cardiomyopathy. Front Physiol 5: 315, 2014. doi: 10.3389/fphys.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyamoto SD, Stauffer BL, Nakano S, Sobus R, Nunley K, Nelson P, Sucharov CC. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J 35: 33–41, 2014. doi: 10.1093/eurheartj/ehs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakano SJ, Miyamoto SD, Movsesian M, Nelson P, Stauffer BL, Sucharov CC. Age-related differences in phosphodiesterase activity and effects of chronic phosphodiesterase inhibition in idiopathic dilated cardiomyopathy. Circ Heart Fail 8: 57–63, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neulen A, Stehle R, Pfitzer G. The cardiac troponin C mutation Leu29Gln found in a patient with hypertrophic cardiomyopathy does not alter contractile parameters in skinned murine myocardium. Basic Res Cardiol 104: 751–760, 2009. doi: 10.1007/s00395-009-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norman C, Rall JA, Tikunova SB, Davis JP. Modulation of the rate of cardiac muscle contraction by troponin C constructs with various calcium binding affinities. Am J Physiol Heart Circ Physiol 293: H2580–H2587, 2007. doi: 10.1152/ajpheart.00039.2007. [DOI] [PubMed] [Google Scholar]
- 45.Patel MD, Mohan J, Schneider C, Bajpai G, Purevjav E, Canter CE, Towbin J, Bredemeyer A, Lavine KJ. Pediatric and adult dilated cardiomyopathy represent distinct pathological entities. JCI Insight 2: e94382, 2017. doi: 10.1172/jci.insight.94382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price JF, Towbin JA, Dreyer WJ, Moffett BS, Kertesz NJ, Clunie SK, Denfield SW. Outpatient continuous parenteral inotropic therapy as bridge to transplantation in children with advanced heart failure. J Card Fail 12: 139–143, 2006. doi: 10.1016/j.cardfail.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Ray KP, England PJ. Phosphorylation of the inhibitory subunit of troponin and its effect on the calcium dependence of cardiac myofibril adenosine triphosphatase. FEBS Lett 70: 11–16, 1976. doi: 10.1016/0014-5793(76)80716-8. [DOI] [PubMed] [Google Scholar]
- 48.Robertson SP, Johnson JD, Holroyde MJ, Kranias EG, Potter JD, Solaro RJ. The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. J Biol Chem 257: 260–263, 1982. [PubMed] [Google Scholar]
- 49.Sasse S, Brand NJ, Kyprianou P, Dhoot GK, Wade R, Arai M, Periasamy M, Yacoub MH, Barton PJ. Troponin I gene expression during human cardiac development and in end-stage heart failure. Circ Res 72: 932–938, 1993. doi: 10.1161/01.RES.72.5.932. [DOI] [PubMed] [Google Scholar]
- 50.Singh RK, Canter CE, Shi L, Colan SD, Dodd DA, Everitt MD, Hsu DT, Jefferies JL, Kantor PF, Pahl E, Rossano JW, Towbin JA, Wilkinson JD, Lipshultz SE; Pediatric Cardiomyopathy Registry Investigators . Survival without cardiac transplantation among children with dilated cardiomyopathy. J Am Coll Cardiol 70: 2663–2673, 2017. doi: 10.1016/j.jacc.2017.09.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stelzer JE, Fitzsimons DP, Moss RL. Ablation of myosin-binding protein-C accelerates force development in mouse myocardium. Biophys J 90: 4119–4127, 2006. doi: 10.1529/biophysj.105.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sucharov CC, Hijmans JG, Sobus RD, Melhado WF, Miyamoto SD, Stauffer BL. β-Adrenergic receptor antagonism in mice: a model for pediatric heart disease. J Appl Physiol (1985) 115: 979–987, 2013. doi: 10.1152/japplphysiol.00627.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sucharov CC, Mariner P, Long C, Bristow M, Leinwand L. Yin Yang 1 is increased in human heart failure and represses the activity of the human alpha-myosin heavy chain promoter. J Biol Chem 278: 31233–31239, 2003. doi: 10.1074/jbc.M301917200. [DOI] [PubMed] [Google Scholar]
- 54.Tatman PD, Woulfe KC, Karimpour-Fard A, Jeffrey DA, Jaggers J, Cleveland JC, Nunley K, Taylor MR, Miyamoto SD, Stauffer BL, Sucharov CC. Pediatric dilated cardiomyopathy hearts display a unique gene expression profile. JCI Insight 2: e94249, 2017. doi: 10.1172/jci.insight.94249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tong CW, Stelzer JE, Greaser ML, Powers PA, Moss RL. Acceleration of crossbridge kinetics by protein kinase A phosphorylation of cardiac myosin binding protein C modulates cardiac function. Circ Res 103: 974–982, 2008. doi: 10.1161/CIRCRESAHA.108.177683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 296: 1867–1876, 2006. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 57.van der Velden J, de Jong JW, Owen VJ, Burton PB, Stienen GJ. Effect of protein kinase A on calcium sensitivity of force and its sarcomere length dependence in human cardiomyocytes. Cardiovasc Res 46: 487–495, 2000. doi: 10.1016/S0008-6363(00)00050-X. [DOI] [PubMed] [Google Scholar]
- 58.van der Velden J, Klein LJ, van der Bijl M, Huybregts MA, Stooker W, Witkop J, Eijsman L, Visser CA, Visser FC, Stienen GJ. Force production in mechanically isolated cardiac myocytes from human ventricular muscle tissue. Cardiovasc Res 38: 414–423, 1998. doi: 10.1016/S0008-6363(98)00019-4. [DOI] [PubMed] [Google Scholar]
- 59.Walker JS, Li X, Buttrick PM. Analysing force-pCa curves. J Muscle Res Cell Motil 31: 59–69, 2010. doi: 10.1007/s10974-010-9208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walker LA, Medway AM, Walker JS, Cleveland JC Jr, Buttrick PM. Tissue procurement strategies affect the protein biochemistry of human heart samples. J Muscle Res Cell Motil 31: 309–314, 2011. doi: 10.1007/s10974-010-9233-6. [DOI] [PubMed] [Google Scholar]
- 61.Walker LA, Walker JS, Ambler SK, Buttrick PM. Stage-specific changes in myofilament protein phosphorylation following myocardial infarction in mice. J Mol Cell Cardiol 48: 1180–1186, 2010. doi: 10.1016/j.yjmcc.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Westfall MV, Rust EM, Metzger JM. Slow skeletal troponin I gene transfer, expression, and myofilament incorporation enhances adult cardiac myocyte contractile function. Proc Natl Acad Sci USA 94: 5444–5449, 1997. doi: 10.1073/pnas.94.10.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilkinson R, Song W, Smoktunowicz N, Marston S. A dilated cardiomyopathy mutation blunts adrenergic response and induces contractile dysfunction under chronic angiotensin II stress. Am J Physiol Heart Circ Physiol 309: H1936–H1946, 2015. doi: 10.1152/ajpheart.00327.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolff MR, Buck SH, Stoker SW, Greaser ML, Mentzer RM. Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: role of altered beta-adrenergically mediated protein phosphorylation. J Clin Invest 98: 167–176, 1996. doi: 10.1172/JCI118762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woulfe KC, Siomos AK, Nguyen H, SooHoo M, Galambos C, Stauffer BL, Sucharov C, Miyamoto S. Fibrosis and fibrotic gene expression in pediatric and adult patients with idiopathic dilated cardiomyopathy. J Card Fail 23: 314–324, 2017. doi: 10.1016/j.cardfail.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]