Abstract
The severity of polycystic kidney diseases (PKD) depends on the counterbalancing of genetic predisposition and environmental factors exerting permissive or protective influence on cyst development. One poorly characterized phenomenon in the cystic epithelium is abnormal purinergic signaling. Earlier experimental studies revealed the high importance of the ionotropic P2X receptors (particularly, P2X7) in the pathophysiology of the cyst wall. To study mechanisms of P2X7 involvement in cyst growth and aspects of targeting these receptors in PKD treatment we performed a CRISPR/SpCas9-mediated global knockout of the P2rx7 gene in PCK rats, a model of autosomal recessive PKD (ARPKD). A single base insertion in exon 2 of the P2rx7 gene in the renal tissues of homozygous mutant animals leads to lack of P2X7 protein that did not affect their viability or renal excretory function. However, PCK.P2rx7 rats demonstrated slower cyst growth (but not formation of new cysts) compared with heterozygous and PCK.P2rx7+ littermates. P2X7 receptors are known to activate pannexin-1, a plasma channel capable of releasing ATP, and we found here that pannexin-1 expression in the cystic epithelium is significantly higher than in nondilated tubules. P2X7 deficiency reduces renal pannexin-1 protein expression and daily urinary ATP excretion. Patch-clamp analysis revealed that lack of P2X7 increases epithelial sodium channel activity in renal tissues and restores impaired channel activity in cysts. Interpretation of our current data in the context of earlier studies strongly suggests that P2X7 contributes to cyst growth by increasing pannexin-1-dependent pathogenic ATP release into the lumen and reduction of sodium reabsorption across the cyst walls.
Keywords: ATP, cyst, epithelial sodium channel, P2X7, pannexin
INTRODUCTION
Autosomal recessive polycystic kidney disease (ARPKD) is initiated due to mutations in the PKHD1 gene encoding fibrocystin, a protein capable of interacting with the polycystin-1/2 complex (33). This aggressive form of polycystic diseases leads to end-stage renal disease and Caroli syndrome in the first decade of life (11). Mechanisms underlying PKD development include clonal expansion of epithelial cells, abnormalities in cell polarity/matrix adhesion, extracellular matrix rearrangements, and aberrant water-electrolyte transport, which is recognized as a significant factor of tubular dilatation (1).
Abnormal purinergic signaling is recognized as a pivotal factor that contributes to the development of PKD. In normal kidneys, ATP acts by binding with P2 receptors (P2X and P2Y subfamilies) and regulates hemodynamics and epithelial ion transport (3, 34). In normal collecting ducts (CDs), P2Y receptors are responsible for ion transport regulation by limiting the activity of epithelial sodium channels (ENaC) during high salt consumption (18, 25). However, high ATP accumulation is shown in the cyst fluid collected from autosomal dominant PKD (ADPKD) patients and PCK rats, a rodent model of ARPKD (20, 30, 37). The pathogenic effect of the excessive ATP level in PKDs has been summarized in reviews by Rangan (28) and Ilatovskaya et al. (12). In particular, a remodeled P2 receptor profile (a shift from metabotropic P2Y to ionotropic P2X signaling) can cause improper intracellular calcium handling, increased chloride secretion, and impaired sodium reabsorption in the cystic epithelial cells and enhanced proliferation of cystic epithelia (9, 13, 24, 27, 30).
PCK/CrljCrl-Pkhd1pck/Crl (PCK) rats, derived from the Sprague-Dawley (SD) strain, carry a mutation in the Pck locus (orthologous gene for human PKHD1) and serve as a broadly used model of ARPKD (17, 36). Recently, we discovered the remodeling of the P2 receptor profile in cyst lining cells toward a loss of P2Y and increased expression of P2X receptors (20). In this study, confocal Ca2+ imaging in live, freshly isolated cyst monolayers and pharmacological targeting of purinergic receptors revealed a prevalent expression of P2X4 and P2X7 receptors, whereas normal, nondilated tubules express both P2X and P2Y. Western blot, RT-PCR, and immunofluorescence analyses from samples of the kidney cortex of 5-, 12-, and 40-wk-old PCK rats demonstrated an age-related increase of P2X7 and P2X4 receptors and localization of both types of receptors at the plasma membrane (20).
The importance of P2X7 receptors for ARPKD development demonstrated by Hillman et al. (8) reported high levels of this protein in cpk/cpk mouse cysts. In recent studies, we (2, 10) found that Pkd1RC/RC mice, a model of ADPKD harboring the mutation in the PKD1 p.R3277C allele seen in patients, also exhibited an increased abundance of P2X7 and pannexin-1 (PANX1) in the cystic epithelium. That is not typical for nondilated CDs, from which ~70% of cysts develop. Using patch-clamp and flow stimulation analyses, we (2) showed that P2X7 signaling activates PANX-1-mediated ATP release and reduces ENaC-dependent sodium reabsorption from the lumen. Earlier studies demonstrated that targeting P2X receptors is beneficial in PKD. Chang et al. (5) performed pharmacological and genetic targeting of P2X7 in a zebrafish model of ADPKD induced by pkd2 morpholino injection. Both genetic ablation of p2rx7 and treatment with OxATP, a P2X7 antagonist, reduced cystic phenotype of pkd2 morphants. In the current study, we investigated the effect of CRISPR/Cas9-induced P2rx7 knockout on cyst development in a mammalian model of ARPKD, the PCK rat strain.
METHODS
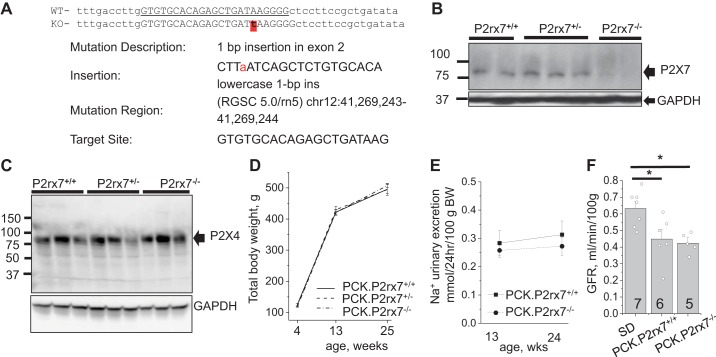
P2rx7 global knockout in the PCK strain (PCK.P2rx7em8Mcwi; RGDID: 12790679) was performed using CRISPR-SpCas9. We injected the pronucleus of PCK/CrljCrl-Pkhd1pck/Crl (Charles River Laboratories) strain embryos with the CAS9/sgRNA dual expression px330 plasmid (Addgene no. 43220) containing the gRNA sequence (5′-GTGTGCACAGAGCTGATAAG-3′) targeting exon 2, resulting in a single base insertion of a T, which will induce a frame shift (Fig. 1A). Genotyping included sequencing of PCR products produced with the following primers: forward 5′-CTAGCCAAGCATTCTACCAGTTGA-3′ and reverse 5′-AGATTGCCATCAAGCAGCGG-3′. F2 and further litters were used to establish a colony in the animal facility of Henry Ford Health System. All in vivo experiments were conducted according the ARRIVE guidelines and approved by the Institutional Animal Care and Use Committee; presented data were collected from male animals. Both parents in breeding pairs were heterozygous, and experimental animals were littermates. Control SD rats were also purchased from Charles River Laboratories. Breeding and housing of the animals was performed on standard 12:12-h light-dark cycle, with water and food (Envigo Teklad 8640) provided ad libitum.
Fig. 1.
A: schema of CRISPR-induced insertion in exon 2 of the P2X receptor (P2rx7) gene leading to a frameshift mutation. B: Western blot demonstrating P2X7 protein expression in total kidney samples of polycystic kidney (PCK) rat littermates with different genotypes. GAPDH expression was used as a loading control. C: P2X4 abundance in kidneys was not affected in heterozygous or knockout rats. D: body weight progression with aging (n = 6–14). E: 24-h urinary sodium excretion measured in young and adult rats (n = 11–21). F: glomerular filtration rate (GFR) measured in 24-wk-old Sprague-Dawley (SD), PCK.P2rx7+/+, and PCK.P2rx7−/− rats (n = 5–7). PCK.P2rx7+/+, PCK rats with intact P2rx7; PCK.P2rx7−/−, PCK rats with P2rx7−/− knockout; PCK.P2rx7+/−, PCK rats heterozygous for P2rx7−/−. Plasma FITC-inulin clearance in conscious animals was measured after a bolus intravenous administration and GFR was calculated in the 2-compartment model. *P < 0.05 vs. the SD group.
Urine analyses.
Animals were placed in metabolic cages for a 24-h period with ad libitum access to water and food. Urinary electrolytes were measured with a Nova Biomedical 1+ analyzer. A bioluminescent luciferin/luciferase assay kit (Invitrogen no. A22066) was used to measure urinary ATP level.
Chronic blood pressure measurements were performed with implantable telemetry (Data Sciences International). Similar to earlier publications (21, 23), HD-S10 transmitters were surgically installed in femoral arteries and affixed in a subcutaneous pocket in the loin area. After a recovery period, blood pressure was recorded continuously. Mean arterial blood pressure (MAP) was averaged every 30 min in each animal, and mean values in the groups were reported. For blood pressure comparison in inactive periods, the three lowest consecutive afternoon values were averaged each day.
Glomerular filtration rate in conscious rats was studied according to the methods previously described by Rieg (29) and adapted for rats (13). FITC-labeled inulin was injected in the tail vein (2 μL of 2% solution per 1 g body wt), and then a series of 10-μL blood samples were collected from conscious animals for 2 h. Plasma inulin clearance was measured using a NanoDrop 3300 Fluorospectrometer (ThermoFisher Scientific) and analyzed in the two-compartment mathematical model of Sturgeon et al. (32).
Histology and Western blot analyses.
For morphological studies, kidneys were stained with hematoxylin-eosin, and whole slice images were collected with a high-resolution Leica Aperio scanner. Cyst area was automatically calculated with the Analyze Particles module in the Fiji software package (NIH, Bethesda, MD). Objects with circularity above 0.05 were identified as cysts if their area exceeded 10,000 µm2. For morphological analysis, cysts were divided into three size groups: small early cysts (0.1–0.3 mm diameter), medium (0.3–1 mm diameter), and large mature cysts (above 1 mm diameter). Areas of cysts of each class were summarized and used as a parameter estimating their contribution to formation of total cyst area. PANX-1 distribution was characterized with anti-PANX1 (1:20, SAB1403167, Sigma-Aldrich) antibody with immunohistochemistry as reported earlier (2). In Western blot analyses, total kidney lysates were probed with anti-P2X7 or anti-P2X4 (APR-004 and APR-002, Alomone Laboratories) or anti-PANX1 and secondary antibody-horseradish peroxidase (sc-2357, Santa Cruz Biotechnology). Staining with antibody for GAPDH (G9545, Sigma) or β-actin (sc-47778, Santa Cruz Biotechnology) was used to control the protein loading. Each band represents one animal, there were two or three rats in each group. Chemiluminescence was detected with a Pierce ECL kit (ThermoFisher Scientific, no. 34094) and the Bio-Rad ChemiDoc Imaging System.
Patch-clamp experiments.
ENaC activity was assessed in freshly isolated nondilated CDs and cysts as previously described (22, 24). Briefly, kidneys from 16-wk-old rats were flushed and cut in longitudinal plane in 1- to 2-mm slices. Using fine-tipped forceps, we dissected cortical CDs or the internal epithelial layer of a cyst to obtain a monolayer area. Tissues were transferred onto poly-l-lysine-coated glass chips and analyzed under inverted Nikon Ti and Diaphot microscopes. Single-channel currents were acquired with an Axopatch 200B amplifier (Axon Instruments) and Bessel filter LPF-8 at 200 kHz (Warner Instruments), interfaced via a Digidata 1550B to a PC running the pClamp 10.6 suite of software (Axon Instruments). The bath solution contained (in mM) 150 NaCl, 1 CaCl2, 2 MgCl2 and 10 HEPES (pH 7.4). The pipette solution contained (in mM) 140 LiCl, 2 MgCl2, and 10 HEPES (pH 7.4).
Statistical analysis.
The distribution of genotypes was studied for Mendelian ratio with a χ2 test. All data are presented as means ± SE. Comparison of means was performed with one-way ANOVA. Differences among group means were considered significant when P < 0.05.
RESULTS
General characterization of the P2rx7 knockout.
Genotyping reveals Mendelian genetics as a zygosity ratio of 71 PCK.P2rx7+/+ to 121 PCK.P2rx7+/− to 60 PCK.P2rx7−/− (χ2 = 1.357; P < 0.05) with 139:113 distribution between male and female animals that indicated no prenatal mortality. Western blot analysis revealed a lack of P2X7 expression in PCK.P2rx7−/− kidneys compared with their PCK.P2rx7+/+ and heterozygous littermates (Fig. 1B). P2X7 deficiency did not result in altered abundance of P2X4 receptors (Fig. 1C). Rats of all genotypes showed similar body weight with aging (Fig. 1D). Urinary sodium excretion in knockout and P2X7-postive littermates did not differ significantly in either young or adult animals (Fig. 1E).
Because P2 signaling is a powerful regulator of renal hemodynamics (35), we studied whether P2X7 deficiency affected glomerular filtration rate (GFR) in PCK rats. PCK rats were originally derived from the SD strain (17), which can serve as a “wild-type” control line. To assess GFR in SD and PCK rats, both PCK.P2rx7+/+ and PCK.P2rx7−/− FITC-inulin clearance curves were acquired from conscious unrestrained animals. Figure 1F demonstrates that 24-wk-old polycystic rats had significantly lower GFR compared with the normal SD rats. No significant difference was found between the P2X7-positive and -deficient PCK rats.
Cardiovascular effects of P2rx7 knockout in PCK rats.
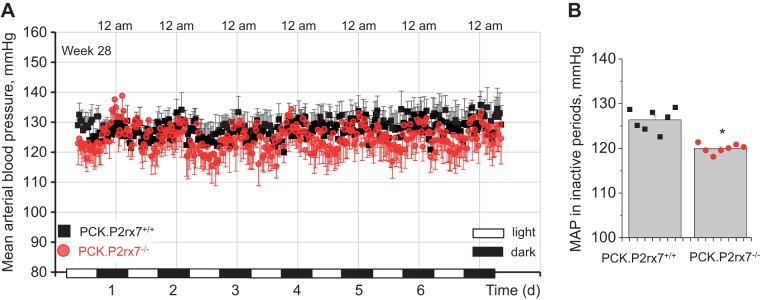
The earlier study by O’Meara and colleagues reported that male PCK rats start developing moderate hypertension after 22 wk of age (19). Within 5 wk, MAP reached 122 ± 8 mmHg as measured with the use of implantable telemetry in conscious freely moving animals. We used a similar approach to study whether P2rx7 deficiency affects blood pressure in PCK rats. Figure 2A shows continuous recording of MAP during the 28th week of life, where this parameter was averaged every 30 min. MAP in PCK.P2rx7+/+ littermates had circadian fluctuations and was stable in the ~123- to 133-mmHg range, depending on the day/night time. Values of blood pressure in animals of the PCK.P2rx7−/− group were not significantly different in the active nighttime periods, whereas they were consistently lower in the inactive periods (Fig. 2B).
Fig. 2.
A: mean arterial blood pressure (MAP) measured with implantable telemetry in conscious 28-wk-old animals (n = 5 in each group). PCK.P2rx7+/+, polycystic kidney rats with intact P2RX7 receptor gene (P2rx7); PCK.P2rx7−/−, PCK rats with P2rx7−/− knockout. Data were averaged in 30-min periods. Day- and nighttime are denoted on the x-axis. B: MAP in the inactive time averaged 7 afternoon periods (dots consecutively represent each day data). *P < 0.05.
P2rx7 knockout attenuates cyst development.
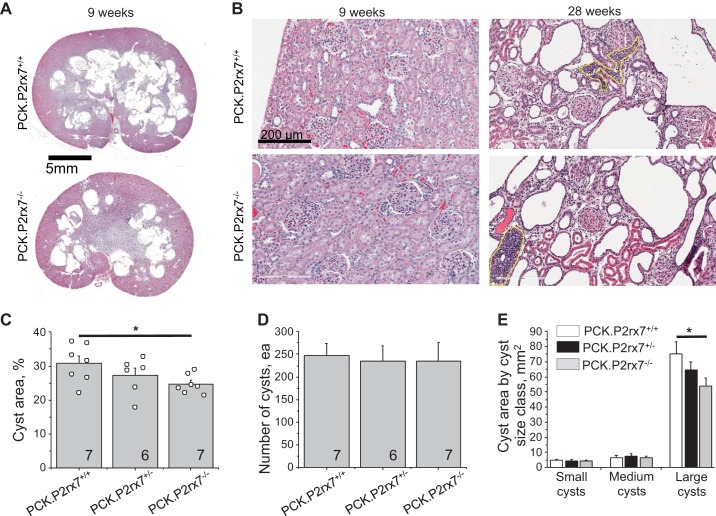
Analysis of renal morphology in 9-wk-old rats revealed that the cyst area on longitudinal cuts reached 30.8 ± 2.1% of total slice area in PCK.P2rx7+/+ male rats. In the PCK.P2rx7+/− group, the cyst area was 27.2 ± 2.2%. This parameter was statistically lower in PCK.P2rx7−/− littermates (24.7 ± 1.1%; Fig. 3, A and C). Detailed quantification of the kidney slides reveals that the number of cysts did not differ between the groups (Fig. 3D), indicating that the difference in the total cyst area did not result from an initiation of new cysts. We ranked cysts in three size categories (small, medium, and large) and found that large cysts provided the major contribution to the total cyst area and that P2X7 deficiency significantly decreased the area of large cysts (Fig. 3E). On the other hand, the cumulative areas of neither small nor medium cysts were different between genotypes. Therefore, we concluded that the protective effect of P2X7 deficiency was mediated by maturation of existing cysts, whereas the formation of new cysts was not affected. This observation is in accord with our earlier data that the expression of P2X7 is low in small cysts but increases during cyst growth (2).
Fig. 3.
A: morphology of polycystic kidneys collected from polycystic kidney (PCK) rats demonstrating reduced cyst development in P2X7 receptor (P2rx7)-deficient animals. PCK.P2rx7+/+, PCK rats with intact P2rx7; PCK.P2rx7−/−, PCK rats with P2rx7−/− knockout; PCK.P2rx7+/−, PCK rats heterozygous for P2rx7−/−. B: glomerular morphology in 9- and 28- week-old littermates showing the progressive development of glomerulopathy. Yellow line denotes massive infiltration of immune cells typical in old animals. C: summary graph of the ratio of cyst area to total slice area in littermates of all 3 genotypes. D: number of individual cysts detected in representative kidney slices. E: contribution of cysts of each of 3 sizes of total cyst area. *P < 0.05.
The development of cysts in PCK strain kidneys causes decreased GFR in adult 24-wk-old rats, as we demonstrated above (Fig. 1F). The histological analysis in Fig. 3B reveals that glomeruli were not significantly damaged in young animals but that adult rats exhibited dramatic glomerular injury that explains the low GFR compared with SD rats. No difference was found between PCK.P2rx7+/+ and PCK.P2rx7−/− groups, probably because massive cyst development by 24+ wk of age was devastating for glomeruli and mitigated the effects of P2X7 deficiency.
P2rx7 deficiency limits PANX1-dependent ATP release.
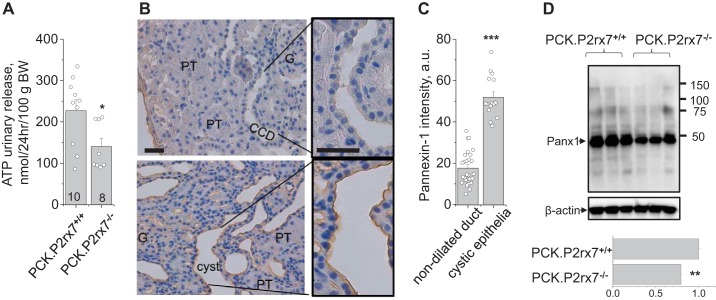
P2X7 receptors were capable of activating PANX channels, and this interaction facilitated ATP release in different tissues. Earlier, Hanner et al. (6) reported that knockout of PANX-1 resulted in reduced urinary ATP release. To test whether P2X7 deficiency alters ATP excretion, we tested urinary ATP level in 24-h urine collections. The bioluminescence assay revealed that PCK.P2rx7−/− rats excrete significantly less ATP than PCK.P2rx7+/+ rats (Fig. 4A).
Fig. 4.
A: renal excretion of ATP measured after 24-h urine collection with the bioluminescent luciferase technique. PCK.P2rx7+/+, polycystic kidney rats with intact P2rx7; PCK.P2rx7−/−, PCK rats with P2rx7−/− knockout. *P < 0.05 vs. PCK.P2rx7+/+ littermates. B: pannexin-1 (PANX-1) distribution in the renal cortex of a PCK rat. The nondilated cortical collecting duct (CCD; top) had no immunohistochemical signal, whereas developing cysts expressed a sufficient level of PANX-1 (brown). G, glomerulus; PT, proximal tubules. Scale bar, 50 µm. C: summary graph of PANX-1 abundance in nondilated duct and cystic epithelia (n = 3 rats). D: Western blot of total kidney lysate demonstrating that P2rx7 knockout decreases PANX-1 expression. Bottom: summary densitometry. **P < 0.01, ***P < 0.001.
Our recent study indicated that ADPKD cysts exhibited an abnormally high expression of PANX-1 and P2X7 that contributed to ATP release and reduced ENaC activity and therefore promoted cyst development. Here, we assume that the lack of P2X7 downregulates PANX-1 channel function in the cyst wall and ATP accumulation in the PCK rat cyst space. To study involvement of PANX-1 in the development of cysts, its distribution was characterized with immunohistochemical analysis in PCK rat renal tissues. Figure 4B shows cortical tissue of a PCK rat stained against PANX-1. As seen in the top panel, nondilated cortical CDs did not exhibit significant PANX-1 signal. However, during cyst progression, PANX-1 abundance dramatically increases in dilating cortical CDs, especially in the luminal membrane of the cysts. Importantly, the Western blot in Fig. 4D revealed a significant reduction of PANX-1 level in total kidney lysates of PCK.P2rx7−/− compared with PCK.P2rx7+/+ rats. We suggest that the growing P2X7 level during progressive tubule dilation (20) is capable of maintaining high PANX-1 abundance in the cyst wall.
Lack of P2X7 improves impaired sodium reabsorption in cystic epithelium.
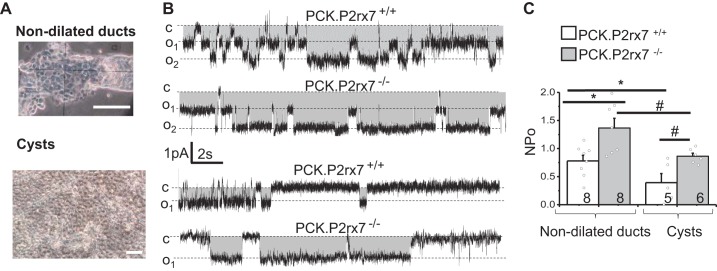
High luminal concentration of ATP reduces ENaC activity in normal CDs (2). Earlier, we showed the prevalence of P2X7 and P2X4 over P2Y receptors in PCK rat cysts. Relying on this, we hypothesized that P2X7 deficiency might restore impaired ENaC activity in cysts. Figure 5A illustrates preparations of nondilated CDs and cystic monolayers that are suitable for patch-clamp experiments. ENaC activity recorded in these tissues confirmed the earlier findings (24) that the cystic epithelium of PCK.P2rx7+/+ rats exhibited lower channel activity compared with the CDs. Importantly, a lack of P2X7 receptors significantly increased ENaC activity in both dilated and nondilated ducts, indicating a negative effect of P2X7 signaling on ENaC-dependent reabsorption.
Fig. 5.
A: freshly isolated tissues used in patch-clamp experiments: nondilated cortical collecting ducts and cyst wall (cellular monolayers). Patch pipettes were attached to the apical membrane surface of split-open ducts and cyst sheets. Single-channel activity was monitored at a holding potential of –40 mV. B: representative current traces of epithelial sodium channel (ENaC) activity. Openings are downward; gray area represents the current through ENaC; c and on levels denote closed and open states of channel under the pipette. Scale bars show time and current amplitude. C: summary graph demonstrating that ENaC activity (NPo) in cysts is lower than in nondilated collecting ducts and that P2X receptor 7 (P2X7) deficiency significantly increases channel activity (n = 3–4 rats in a group). PCK.P2rx7+/+, polycystic kidney rats with intact P2rx7; PCK.P2rx7−/− PCK rats with P2rx7−/− knockout. *P < 0.05 vs. nondilated ducts of PCK.P2rx7+/+ rats; #P < 0.05 vs. cysts of PCK.P2rx7−/− rats.
DISCUSSION
Published data have indicated that the expression of P2X7 receptors and PANX-1 in healthy kidney tubular tissues is low, whereas in pathological conditions their levels increase dramatically (7, 26). Purinergic signaling is currently being intensively explored in relation to PKD development (14, 28). The role of P2X7 receptors in PKD is also highlighted in the review by Hillman et al. (7), which features an increased renal abundance of this protein in human and rodent ARPKD and ADPKD, as well as “trapping” of ATP and other nucleotides to induce a solute and water influx. Our previous studies (20, 24) revealed a high content of ATP in the PCK cysts and a dramatic shift toward P2X (and loss of P2Y) signaling in the cystic epithelium, which could lead to impaired ENaC activity and cyst progression. Here, we obtained important evidence indicating that P2X7 receptors are involved in this pathway and may serve as a therapeutic target to control PKD progression.
We found that the tubule-to-cyst transition was accompanied with the robust expression of these proteins (Fig. 4B and Refs. 2 and 20). Knockout of the P2rx7 gene did not produce any developmental abnormalities in PCK rats, which may indicate a noncritical role, or compensatory effects of other mechanisms. For instance, sodium handing remains unchanged, probably due to a stabilization with the differential regulation by various transporters along the nephron. However, we have not tested functions of the nervous and immune systems that were shown to be affected in P2rx7 knockout mice. Implantable telemetry allowed detailed blood pressure monitoring and confirmed the earlier data that the PCK rats developed moderate hypertension. Interestingly, P2rx7 knockout animals exhibited lower blood pressure in sleeping periods and more prominent circadian dipping. A mechanism of this difference remains unclear and may include altered sympathetic/parasympathetic cardiac regulation or vascular effects, as P2X7 is involved in vascular tone maintenance (4, 16).
In the kidney, ARPKD impairs GFR to the same extent in both the P2rx7-postitve and knockout animals. Morphological analysis revealed the progressive development of massive glomerular damage in both genotypes. We hypothesize that glomeruli in young animals were exposed to the barotrauma due to the cyst development that led to histopathological abnormalities and a reduction of GFR with aging. Besides, GFR depends on other factors: the tone of afferent and efferent arterioles and tubular-glomerular feedback. Although P2X7-deficient rats had the lower cyst index, the GFR fell to the same extent by the 24th week. Interestingly, morphological analysis revealed that the contribution of P2X7 receptors in ARPKD progression occurred as a maturation of large cysts but did not affect the initiation of new cysts.
In the abnormal cystic epithelium, the role of P2X7 receptors seems to be important due to its high prevalence compared with normal tissues (20). We observed a similar process earlier in the Pkd1RC/RC mouse strain (2) that may be an indicative model of a common PANX-1/P2X-dependent mechanism of cyst progression in both autosomal dominant and recessive PKD. The high significance of P2X7 receptors in the cyst development is closely associated with their role in the regulation of PANX-1. Involvement of PANX-1 in PKD was suggested in a recent study revealing its high abundance on the luminal side of cultured Madin-Darby canine kidney cell cysts (31). The ability of PANX-1 to release ATP was recently implicated in the control of acute kidney injury, when the knockout of this protein resulted in retaining ATP in the cells and protected renal tissues against ischemia-reperfusion injury (15, 26). Similarly to our observations, reduction of PANX-1 expression was found to decrease urinary ATP excretion (6).
The present study indicates that massive ATP release and its accumulation reported previously in PCK rats (20) can be partially explained by the upregulation of P2X7 in the cysts in combination with the high PANX-1 expression. We hypothesize that the mechanism of the protective effect of P2rX7 knockout on the cyst formation may include limiting of PANX-1 permeability and, subsequently, ATP accumulation in the luminal space. In addition, P2X7 regulates electrolyte transport (as shown in Fig. 5), and potentially stabilizes and/or increases the expression of PANX-1. Restoration of ENaC activity due to P2X7 ablation in the cystic epithelium can normalize sodium reabsorption in cysts and prevent their growth. Determination of a specific mechanism of interaction between P2X7 and PANX-1 in the cystic epithelium, as well as a role of a high expression of P2X4 receptors, requires future studies.
GRANTS
Funding support came from American Society of Nephrology Carl W. Gottschalk Award, PKD Foundation (221G18a), and NIH Grants R00-HL-116603, R00-DK-105160, P30-DK-090868 via a Pilot and Feasibility Grant, and R24-HL-114474 via the Medical College of Wisconsin Gene Editing Rat Resource Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.N.A., A.M.G., and T.S.P. conceived and designed research; S.N.A., D.L.P., A.M.G., and T.S.P. performed experiments; S.N.A., D.L.P., and T.S.P. analyzed data; S.N.A. and T.S.P. interpreted results of experiments; S.N.A., A.M.G., and T.S.P. prepared figures; S.N.A. and T.S.P. drafted manuscript; S.N.A., D.L.P., A.M.G., and T.S.P. edited and revised manuscript; S.N.A., D.L.P., A.M.G., and T.S.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. D. V. Ilatovskaya for helpful discussion of the project and manuscript preparation. We appreciate technical assistance from the HFHS Histology Core.
REFERENCES
- 1.Antignac C, Calvet JP, Germino GG, Grantham JJ, Guay-Woodford LM, Harris PC, Hildebrandt F, Peters DJM, Somlo S, Torres VE, Walz G, Zhou J, Yu ASL. The future of polycystic kidney disease research–as seen by the 12 Kaplan awardees. J Am Soc Nephrol 26: 2081–2095, 2015. doi: 10.1681/ASN.2014121192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arkhipov SN, Pavlov TS. ATP release into ADPKD cysts via pannexin-1/P2X7 channels decreases ENaC activity. Biochem Biophys Res Commun 513: 166–171, 2019. doi: 10.1016/j.bbrc.2019.03.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birch RE, Schwiebert EM, Peppiatt-Wildman CM, Wildman SS. Emerging key roles for P2X receptors in the kidney. Front Physiol 4: 262, 2013. doi: 10.3389/fphys.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnstock G. Purinergic signaling in the cardiovascular system. Circ Res 120: 207–228, 2017. doi: 10.1161/CIRCRESAHA.116.309726. [DOI] [PubMed] [Google Scholar]
- 5.Chang M-Y, Lu J-K, Tian Y-C, Chen Y-C, Hung C-C, Huang Y-H, Chen Y-H, Wu M-S, Yang C-W, Cheng Y-C. Inhibition of the P2X7 receptor reduces cystogenesis in PKD. J Am Soc Nephrol 22: 1696–1706, 2011. doi: 10.1681/ASN.2010070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanner F, Lam L, Nguyen MTX, Yu A, Peti-Peterdi J. Intrarenal localization of the plasma membrane ATP channel pannexin1. Am J Physiol Renal Physiol 303: F1454–F1459, 2012. doi: 10.1152/ajprenal.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillman KA, Burnstock G, Unwin RJ. The P2X7 ATP receptor in the kidney: a matter of life or death? Nephron Exp Nephrol 101: e24–e30, 2005. doi: 10.1159/000086036. [DOI] [PubMed] [Google Scholar]
- 8.Hillman KA, Johnson TM, Winyard PJ, Burnstock G, Unwin RJ, Woolf AS. P2X(7) receptors are expressed during mouse nephrogenesis and in collecting duct cysts of the cpk/cpk mouse. Exp Nephrol 10: 34–42, 2002. doi: 10.1159/000049896. [DOI] [PubMed] [Google Scholar]
- 9.Hooper KM, Unwin RJ, Sutters M. The isolated C-terminus of polycystin-1 promotes increased ATP-stimulated chloride secretion in a collecting duct cell line. Clin Sci (Lond) 104: 217–221, 2003. doi: 10.1042/cs1040217. [DOI] [PubMed] [Google Scholar]
- 10.Hopp K, Hommerding CJ, Wang X, Ye H, Harris PC, Torres VE. Tolvaptan plus pasireotide shows enhanced efficacy in a PKD1 model. J Am Soc Nephrol 26: 39–47, 2015. doi: 10.1681/ASN.2013121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoyer PF. Clinical manifestations of autosomal recessive polycystic kidney disease. Curr Opin Pediatr 27: 186–192, 2015. doi: 10.1097/MOP.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 12.Ilatovskaya D, Palygin O, Levchenko V, Pavlov T, Staruschenko A. Remodeling of the purinergic receptors profile in the ARPKD cystic epithelia (Abstract). FASEB J 30: 968.929, 2016. [Google Scholar]
- 13.Ilatovskaya DV, Levchenko V, Pavlov TS, Isaeva E, Klemens CA, Johnson J, Liu P, Kriegel AJ, Staruschenko A. Salt-deficient diet exacerbates cystogenesis in ARPKD via epithelial sodium channel (ENaC). EBioMedicine 40: 663–674, 2019. doi: 10.1016/j.ebiom.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilatovskaya DV, Palygin O, Staruschenko A. Functional and therapeutic importance of purinergic signaling in polycystic kidney disease. Am J Physiol Renal Physiol 311: F1135–F1139, 2016. doi: 10.1152/ajprenal.00406.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jankowski J, Perry HM, Medina CB, Huang L, Yao J, Bajwa A, Lorenz UM, Rosin DL, Ravichandran KS, Isakson BE, Okusa MD. Epithelial and endothelial pannexin1 channels mediate AKI. J Am Soc Nephrol 29: 1887–1899, 2018. doi: 10.1681/ASN.2017121306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji X, Naito Y, Weng H, Endo K, Ma X, Iwai N. P2X7 deficiency attenuates hypertension and renal injury in deoxycorticosterone acetate-salt hypertension. Am J Physiol Renal Physiol 303: F1207–F1215, 2012. doi: 10.1152/ajprenal.00051.2012. [DOI] [PubMed] [Google Scholar]
- 17.Katsuyama M, Masuyama T, Komura I, Hibino T, Takahashi H. Characterization of a novel polycystic kidney rat model with accompanying polycystic liver. Exp Anim 49: 51–55, 2000. doi: 10.1538/expanim.49.51. [DOI] [PubMed] [Google Scholar]
- 18.Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE. P2Y(2) receptors and water transport in the kidney. Purinergic Signal 5: 491–499, 2009. doi: 10.1007/s11302-009-9151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Meara CC, Hoffman M, Sweeney WE Jr, Tsaih S-W, Xiao B, Jacob HJ, Avner ED, Moreno C. Role of genetic modifiers in an orthologous rat model of ARPKD. Physiol Genomics 44: 741–753, 2012. doi: 10.1152/physiolgenomics.00187.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palygin O, Ilatovskaya DV, Levchenko V, Klemens CA, Dissanayake L, Williams AM, Pavlov TS, Staruschenko A. Characterization of purinergic receptor expression in ARPKD cystic epithelia. Purinergic Signal 14: 485–497, 2018. doi: 10.1007/s11302-018-9632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Pochynyuk OM, Jacob HJ, Geurts AM, Hodges MR, Staruschenko A. Essential role of Kir5.1 channels in renal salt handling and blood pressure control. JCI Insight 2: e92331, 2017. doi: 10.1172/jci.insight.92331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavlov TS, Ilatovskaya DV, Palygin O, Levchenko V, Pochynyuk O, Staruschenko A. Implementing patch clamp and live fluorescence microscopy to monitor functional properties of freshly isolated PKD epithelium. J Vis Exp 103: e53035, 2015. doi: 10.3791/53035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavlov TS, Levchenko V, Ilatovskaya DV, Li H, Palygin O, Pastor-Soler NM, Hallows KR, Staruschenko A. Lack of effects of metformin and AICAR chronic infusion on the development of hypertension in Dahl salt-sensitive rats. Front Physiol 8: 227, 2017. doi: 10.3389/fphys.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavlov TS, Levchenko V, Ilatovskaya DV, Palygin O, Staruschenko A. Impaired epithelial Na+ channel activity contributes to cystogenesis and development of autosomal recessive polycystic kidney disease in PCK rats. Pediatr Res 77: 64–69, 2015. doi: 10.1038/pr.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pochynyuk O, Rieg T, Bugaj V, Schroth J, Fridman A, Boss GR, Insel PA, Stockand JD, Vallon V. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone. FASEB J 24: 2056–2065, 2010. doi: 10.1096/fj.09-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poudel N, Okusa MD. Pannexins in acute kidney injury. Nephron 143: 158–161, 2019. doi: 10.1159/000501278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajagopal M, Wallace DP. Chloride secretion by renal collecting ducts. Curr Opin Nephrol Hypertens 24: 444–449, 2015. doi: 10.1097/MNH.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rangan G. Role of extracellular ATP and P2 receptor signaling in regulating renal cyst growth and interstitial inflammation in polycystic kidney disease. Front Physiol 4: 218, 2013. doi: 10.3389/fphys.2013.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieg T. A High-throughput method for measurement of glomerular filtration rate in conscious mice. J Vis Exp 75: e50330, 2013. doi: 10.3791/50330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwiebert EM, Wallace DP, Braunstein GM, King SR, Peti-Peterdi J, Hanaoka K, Guggino WB, Guay-Woodford LM, Bell PD, Sullivan LP, Grantham JJ, Taylor AL. Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol Renal Physiol 282: F763–F775, 2002. doi: 10.1152/ajprenal.0337.2000. [DOI] [PubMed] [Google Scholar]
- 31.Shum MG, Shao Q, Lajoie P, Laird DW. Destination and consequences of Panx1 and mutant expression in polarized MDCK cells. Exp Cell Res 381: 235–247, 2019. doi: 10.1016/j.yexcr.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Sturgeon C, Sam AD II, Law WR. Rapid determination of glomerular filtration rate by single-bolus inulin: a comparison of estimation analyses. J Appl Physiol (1985) 84: 2154–2162, 1998. doi: 10.1152/jappl.1998.84.6.2154. [DOI] [PubMed] [Google Scholar]
- 33.Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2: 40–55, 2006. doi: 10.1038/ncpneph0070. [DOI] [PubMed] [Google Scholar]
- 34.Vallon V, Stockand J, Rieg T. P2Y receptors and kidney function. Wiley Interdiscip Rev Membr Transp Signal 1: 731–742, 2012. doi: 10.1002/wmts.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Beusecum J, Inscho EW. Regulation of renal function and blood pressure control by P2 purinoceptors in the kidney. Curr Opin Pharmacol 21: 82–88, 2015. doi: 10.1016/j.coph.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30: 259–269, 2002. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 37.Wilson PD, Hovater JS, Casey CC, Fortenberry JA, Schwiebert EM. ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys. J Am Soc Nephrol 10: 218–229, 1999. [DOI] [PubMed] [Google Scholar]