Abstract
Chlorinated alkyl and non-chlorinated aryl organophosphate flame retardants (OPFRs) and some brominated flame retardants (FR) were introduced as replacements for polybrominated diphenyl ethers (PBDEs) after PBDEs phase-out in 2004 and 2013. Organophosphorous (OP) insecticides are mainly used in agricultural settings since the Food Quality Protection Act of 1996 phased-out most residential uses of OP insecticides in the United States.
Urinary metabolites of FRs and OPs are known exposure biomarkers to FRs and OP insecticides, respectively. For large population-based studies, concurrent quantification of these metabolites using a small urine volume is desirable, but until now was not possible. We developed an analytical approach to quantify in 0.2 mL urine 10 FRs and six OP insecticide metabolites: diphenyl phosphate, bis(1,3-dichloro-2-propyl) phosphate, bis(1-chloro-2-propyl) phosphate, bis(2-chloroethyl) phosphate, dicresyl phosphates, dibutyl phosphate, dibenzyl phosphate, 2,3,4,5-tetrabromobenzoic acid, 2-((isopropyl)phenyl) phenyl phosphate, 4-((tert-butyl)phenyl)phenyl phosphate, dimethyl phosphate, diethyl phosphate, dimethyl thiophosphate, dimethyl dithiophosphate, diethyl thiophosphate, and diethyl dithiophosphate. The method relies on enzymatic deconjugation, automated off-line solid phase extraction, high-performance liquid chromatography, and isotope dilution tandem mass spectrometry. Detection limits ranged from 0.05 to 0.5 ng mL−1, accuracy from 89 to 118%, and imprecision was <10%. .
This method is the first to quantify simultaneously trace levels of 16 biomarkers of FRs and OP insecticides in only four drops of urine. We confirmed the method suitability for use in large epidemiological studies to assess background and occupational exposures to these classes of environmental pollutants by analyzing 303 samples collected from the general population and a group of firefighters. FR metabolite and DAPs concentrations in the general population group were lower than in the firefighters group, and within the ranges reported in the U.S. general population and other non-occupationally exposed populations.
Keywords: Flame retardants, Organophosphates, Insecticides, Metabolites, Biomarkers, Liquid chromatography mass spectrometry
1. Introduction
Flame retardants are added to consumer products such as furniture, electronics, and clothing to meet flammability standards and regulations. Persistent flame retardants such as polybrominated diphenyl ethers (PBDEs) were phased-out from the market (de Wit, 2002; Tullo, 2003), and other chemicals such as chlorinated alkyl and non-chlorinated aryl organophosphate flame retardants (OPFRs), and non-PBDE brominated flame retardants (FR) such as 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB) were introduced to maintain fire resistance requirements (Bergman et al., 2012; van der Veen and de Boer, 2012; US-EPA, 2013, 2014). EH-TBB and OPFRs are used in non-PBDE flame retardant formulations such as Firemaster 550, Firemaster BZ-54, and CN-2065.
Triphenyl phosphate (TPhP), mono-substitute isopropyl triphenyl phosphate (iPTPhP), mono-substitute tertbutyl triphenyl phosphate (tBTPhP), tris(1,3-dichloro-2-propyl) phosphate (TDCPP), tris(1-chloro-2-propyl) phosphate (TCPP), tris(2-chloroethyl) phosphate (TCEtP), tricresyl phosphates (TCP), tri-n-butyl phosphate (TBuP), and tribenzyl phosphate (TBzP) are among the most used OPFRs. Some of these compounds are also used as plasticizers and additives in lacquers, resins, lubricants, hydraulic fluids, and polyvinyl chloride (Andresen et al., 2004; Solbu et al., 2007; Wei et al., 2015). Some OPFRs and chemicals in non-PBDE brominated FRs are frequently detected in various consumer products (Stapleton et al., 2008, 2009, 2011, 2012, 2014; Dodson et al., 2012; Carignan et al., 2013a, 2013b), and can be present in higher concentrations than PBDEs (Dodson et al., 2012).
Because OPFRs are not chemically bound to the products, they can easily leach into the environment and expose humans by inhalation, ingestion, and dermal adsorption. Even though OPFRs are assumed to be safer alternatives to PBDEs, several OPFRs are carcinogenic, mutagenic, and neurotoxic (Dishaw et al., 2011; van der Veen and de Boer, 2012), with potential adverse health effects (Meeker and Stapleton, 2010; Meeker et al., 2013; Patisaul et al., 2013). OPFRs can undergo phase I and phase II bio-transformations including hydrolysis to diesters, hydroxylation, oxidative dechlorination, carboxylation, glucuronidation, sulfation and glutathione conjugation, to produce metabolites that are more hydrophilic than the parent compounds and readily excreted in urine (Van den Eede et al., 2013a; Hou et al., 2016). EH-TBB metabolizes to 2,3,4,5-tetrabromobenzoic acid (TBBA) (Roberts et al., 2012). Organophosphate diesters have been detected in people’s urine and used to assess exposure to the corresponding parent chemicals (Carignan et al., 2013a, 2013b; Butt et al., 2014; Dodson et al., 2014; Cequier et al., 2015; Hoffman et al., 2017; Jayatilaka et al., 2017; Romano et al., 2017; He et al., 2018; Ospina et al., 2018; Phillips et al., 2018).
Although organophosphorus (OP) insecticides are still used for controlling insects on many crops, most residential uses were phased-out in the United States after the Food Quality Protection Act of 1996 (US-EPA, 1996). Approximately 40 OP pesticides are still registered for use in agriculture (US-EPA, 2016), and certain OP insecticides such as malathion and naled are registered in the United States for public health applications (e.g., mosquito control).
Approximately 75% of the registered OP pesticides metabolize in the body to dialkylphosphates (DAPs) (Table S1) and yield dimethylphosphate (DMP), dimethylthiophosphate (DMTP), dimethyldithiophosphate (DMDTP), diethylphosphate (DEP), diethylthiophosphate (DETP), and diethyldithiophosphate (DEDTP), which are excreted in urine (Nutley and Cocker, 1993; Bravo et al., 2002). Quantification of theses metabolites can provide an estimate of overall exposure to OP pesticides.
Exposure to OP pesticides typically occurs by ingesting contaminated food or by hand-to-mouth contact with surfaces containing OP pesticides (Lu et al., 2008; Curl et al., 2015). Toxic effects to humans, among others, include neurological dysfunction that results from the inhibition of the enzyme acetylcholinesterase leading to excess acetylcholine in the central and peripheral nervous systems (Cocker et al., 2002; Kwong, 2002).
Several methods have been reported for the measurement of either DAPs or FR metabolites in human urine by liquid chromatography-mass spectrometry or gas chromatography-mass spectrometry after derivatization (Bardarov and Mitewa, 1989; Bravo et al., 2002; Bravo et al., 2004; De Alwis et al., 2006; Dulaurent et al., 2006; Ueyama et al., 2006; Petchuay et al., 2008; Schindler et al., 2009b; Schindler et al., 2009a; Odetokun et al., 2010; Cooper et al., 2011; Reemtsma et al., 2011; Van den Eede et al., 2013b; Jayatilaka et al., 2017; Bastiaensen et al., 2018). Even though these analytical methods are well developed, metabolites from both chemical classes are not included in one single method, and most of these methods require relatively large sample volumes. Furthermore, methods for FR metabolite analysis often require between one and 5 mL of urine. There is a need for developing multi-analyte methods which only use a minimum amount of sample, especially for studies where samples are scarce or hard to obtain such as those involving children. To fill in this gap, we developed a mass-spectrometry based method to concurrently quantify nine of the most common chlorinated and non-chlorinated OPFR metabolites, one non-PBDE brominated FR metabolite, and six DAP OP insecticide metabolites in only 200 mL human urine. We assessed the suitability of the method by analyzing samples from the general population, and from a group of firefighters after performing structural firefighting.
2. Materials and methods
2.1. Reagents and standards
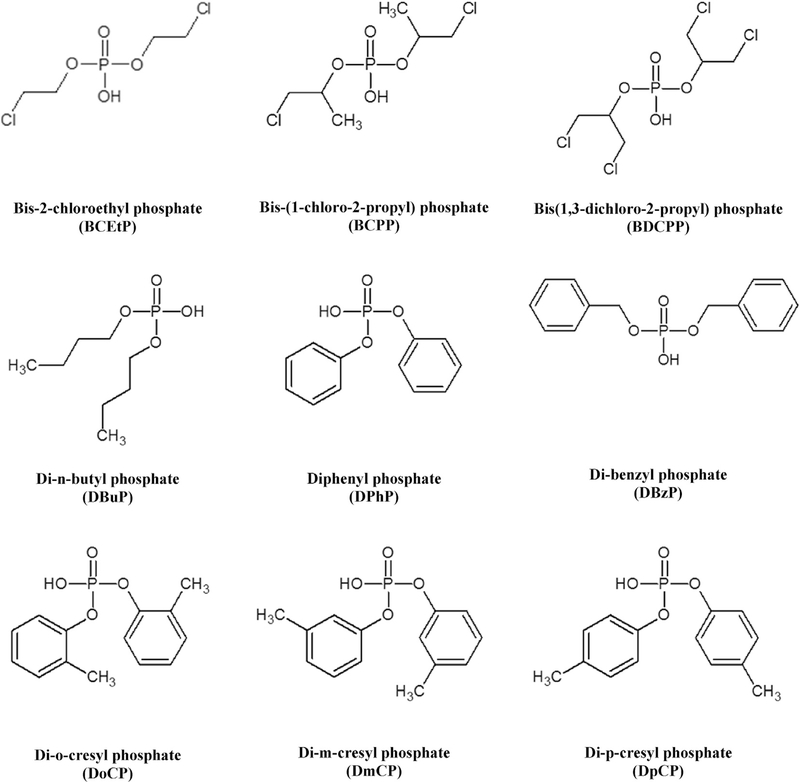
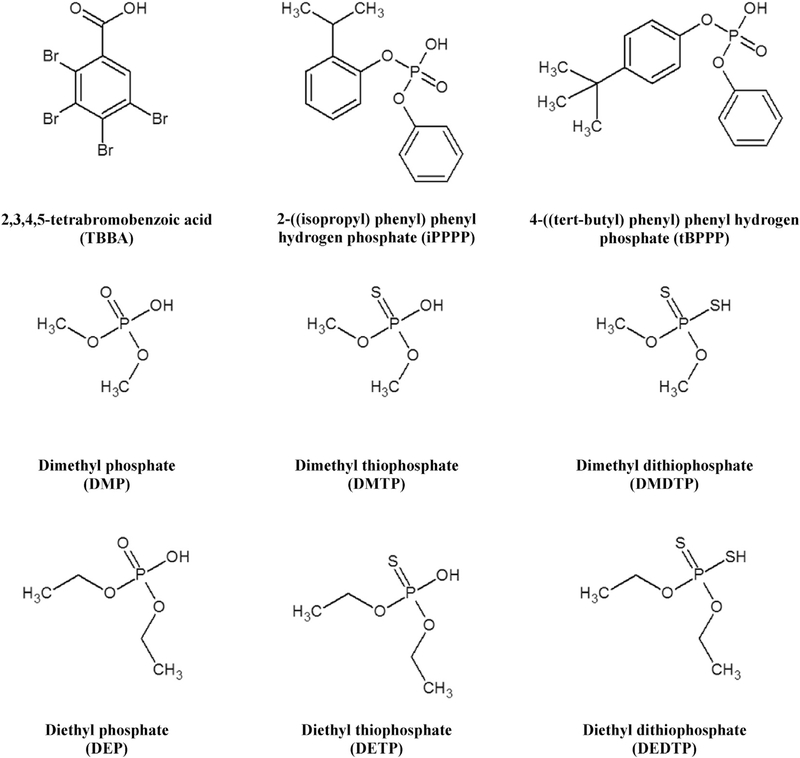
Methanol, acetonitrile, ammonium hydroxide (Fisher Scientific, Pittsburgh, PA, USA), and formic acid, acetic acid, (Sigma-Aldrich, St. Louis, MO, USA) were all HPLC-grade. Deionized water was purified using a NANOpure Infinity ultrapure water system (Barnstead/Thermolyne, IA, USA). Diphenyl phosphate (DPhP), DPhP-d10, di-m-cresyl phosphate (DmCP), di-o-cresyl phosphate (DoCP), DoCP-d14, di-p-cresyl phosphate (DpCP), DpCP-d14, bis(1-chloro-2-propyl) phosphate (BCPP), BCPP-d12, bis(2-chloroethyl) phosphate (BCEtP), and BCEtP-d8 were purchased from Toronto Research Chemicals, TRC (Toronto, Canada). Bis(1,3-dichloro-2-propyl) phosphate (BDCPP), BDCPP-d10, TBBA, and TBBA-13C6 were purchased from Wellington Laboratories (Guelph, Canada). DBuP, DBuP-d18, DBzP, DBzP-d14, β-glucuronidase Type H-1 from Helix pomatia, and 4-Methylumbelliferyl β-D-glucuronidase hydrate (UMB) were purchased from Sigma-Aldrich. DMP, DMP-d6, DMTP, DMTP-d6, DMDTP, DMDTP-d6, DEP, DEP-d10, DETP, DETP-d10, DEDTP, DEDTP-d10, UMB-13C4 were purchased from Cam-bridge Isotope Laboratories (Andover, MA, USA). 2-((isopropyl) phenyl) phenyl phosphate (iPPPP) and 4-((tert-butyl) phenyl) phenyl phosphate (tBPPP) were purchased from Duke University Small Molecule Synthesis Facility (Durham, NC, USA). All chemicals and standard materials were used without further purification. The analytes chemical structures and abbreviations are shown in Fig. 1.
Fig. 1.
Chemical structures and abbreviations for target analytes.
Individual stock solutions of standards and labeled internal standards were prepared by dissolving or diluting in appropriate solvent (according to manufacturers’ solubility recommendations). Using these individual stock solutions, three intermediate stock solutions with all target analytes were prepared in 1:1 (v/v) methanol/water giving a concentration of individual compounds of 1000 ng mL−1, 500 ng mL−1, or 50 ng mL−1. Ten calibration standard solutions containing all target analytes were prepared by diluting appropriate amounts from intermediate stock solutions in 1:4 (v/v) methanol/water. A 200 μL spike from these calibration standards onto 200 μL of urine would cover a final concentration range of 0.05 ng mL−1 to 100 ng mL−1 for DMP, DMTP, DMDTP, and DEP, and 0.05 ng mL−1 to 40 ng mL−1 for the rest of the analytes. To monitor the extent of the enzymatic reaction, 4-methylumbelliferyl β-D-glucuronide hydrate and 13C4–4-methylumbelliferone stock solutions prepared in methanol were used as deconjugation standards. By mixing appropriate amounts from isotope-labeled standards and deconjugation standards in 1:4 (v/v) methanol/water, the spiking solution of isotope-labeled standards and deconjugation standards mixture was prepared, so that a 100 μL spike onto 200 μL of urine would result in 10 ng mL−1 concentration of the individual labeled compounds, 1500 ng mL−1 of 4-methylumbelliferyl β-D-glucuronide hydrate, and 75 ng mL−1 of 13C4–4-methylumbelliferone. All stock solutions and standards were stored in amber polypropylene vials at or below −10°C.
2.2. Human urine collection for method development and validation
To prepare quality control (QC) pools and for method validation, urine samples were collected anonymously in Atlanta, GA in 2015 from a diverse group of male and female adult volunteers with no documented occupational exposure to target flame retardants or OP pesticides. CDC’s Human Subjects Institutional Review Board reviewed and approved the study protocol. A waiver of informed consent was requested under 45 CFR 46.116(d). No personal or demographic data were collected.
These individual samples were screened for endogenous amounts of target analytes. Individual samples with overall lowest concentrations of endogenous target analytes were combined to form a blank pool. The blank urine was stored at or below −20 °C in glass vials. QC materials were prepared by spiking portions of blank urine with native target compounds. The low-concentration QC (QCL) was about 4 ng mL−1; the high concentration QC (QCH) was about 15 ng mL−1. The spiked QC materials were refrigerated, mixed for over 24 h, aliquoted into polypropylene vials (1 mL portions), and stored at or below −20 °C until use.
We also used 145 urine samples collected in 2010–2011 from firefighters after performing structural firefighting while wearing full protective clothing and self-contained breathing apparatus (SCBA) (Fent et al., 2014; Pleil et al., 2014). All participants gave consent to have their residual urine stored without identifiers for future research purposes, and the study protocol was approved by the U.S. National Institute for Occupational Safety and Health’s IRB. The analysis of these de-identified specimens was determined not to constitute engagement in human subjects research.
2.3. Sample preparation and automated off-line solid phase extraction (SPE)
The extraction procedure was adapted from our previously published method (Jayatilaka et al., 2017), but adjusted for 200 μL urine. In brief, after adding 100 μL of labeled/deconjugation standard spiking mixture to each well, 200 μL of calibration standard solutions were spiked onto the wells assigned for each calibration level, 200 μL of deionized water was added to the solvent blank well, and 200 μL of QCs or study urines were added to the designated wells. Then, 400 μL of enzyme solution was dispensed to each well (a minimum of 1000 units of β-glucuronidase, 33 units of sulfatase per sample in 0.2 M sodium acetate buffer) and incubated overnight (typically 17 h) at 37 °C. Samples were cleaned up and concentrated by SPE as previously described (Jayatilaka et al., 2017), but eluted with 800 μL of freshly prepared 2% (v/v) NH4OH in methanol and reconstituted with 100 μL of 95:5 (v/v) water: acetonitrile/methanol mixture after the drying step.
2.4. Chromatographic separation and detection
High-performance liquid chromatography (HPLC) was performed on an Agilent 1290 (Agilent Technologies, Santa Clara, CA, USA) system equipped with a binary pump, an autosampler with a cooling thermostat module, and a temperature controlled column compartment. Mobile phase A was 0.1% (v/v) acetic acid in deionized water and mobile phase B was a mixture of 1:1 acetonitrile: methanol. The gradient started at 5% of mobile phase B for 0.5 min, increased to 50% B in 4.0 min, ramped to 100% in 9.5 min and held for 1.5 min, returned to 5% B in 1 min and held for 4.0 min to equilibrate the column for the next sample. All analytes eluted within 10 min. The injection volume was 10 μL and the flow rate was constant at 0.7 mL/min. Chromatographic separation was performed on a Hypersil GOLD aQ column (150 × 4.6 mm, 3 μm; Thermo scientific, San Jose, CA, USA) preceded by inline filters (2 μm and 0.5 μm, Upchurch Scientific, Oak Harbor, WA, USA). During the sample analysis, the autosampler was kept at 4 °C and the column at 45 °C.
Mass spectrometry was performed on an AB Sciex 5500 Qtrap mass spectrometer (Applied Biosystems, Foster City, CA, USA) equipped with a TurboIonSpray® source. The parameters were set equipped with a TurboIonSpray® source. The parameters were set as follows: curtain gas 20, collision gas medium option, IonSpray voltage −4500 V, temperature 450 °C, and ion source gases 45. The mass spectrometer was operated in scheduled multiple reaction monitoring (sMRM) mode using negative polarity. Table 1 shows the transitions and collision energies used for each analyte.
Table 1.
Analytes and their labeled analogues, quantitation and confirmation ions, and collision energies (CE).
| Analyte | Quantitation ion | Confirmation ion | ||||
|---|---|---|---|---|---|---|
| Precursor ion (m/z) | Product ion (m/z) | CE (eV) | Precursor ion (m/z) | Product ion (m/z) | CE (eV) | |
| BCEtP | 221 | 35 | 25 | 223 | 37 | 31 |
| BCEtP-d8 | 229 | 35 | 27 | |||
| BCPP | 249 | 35 | 33 | 251 | 37 | 27 |
| BCPP-d12 | 261 | 35 | 33 | |||
| BDCPP | 317 | 35 | 40 | 319 | 37 | 39 |
| BDCPP-d10 | 329 | 35 | 40 | |||
| DBuP | 209 | 153 | 19 | 209 | 79 | 28 |
| DBuP-d18 | 227 | 79 | 30 | |||
| DBzP | 277 | 79 | 33 | 277 | 63 | 30 |
| DBzP-d14 | 291 | 79 | 36 | |||
| DPhP | 249 | 155 | 28 | 249 | 93 | 33 |
| DPhP-d10 | 259 | 98 | 33 | |||
| DCP | 277 | 107 | 34 | 277 | 169 | 31 |
| DCP-d14 | 291 | 114 | 34 | |||
| TBBA | 436.7 | 392.7 | 14 | 434.7 | 390.7 | 13 |
| TBBA-13C6 | 442.7 | 398.7 | 14 | |||
| iPPPP | 291 | 135 | 41 | 291 | 93 | 41 |
| tBPPP | 305 | 149 | 23 | 305 | 133 | 21 |
| DMP | 125 | 63 | 24 | 125 | 110 | 22 |
| DMP-d6 | 131 | 63 | 24 | |||
| DMTP | 141 | 126 | 20 | 141 | 96 | 26 |
| DMTP-d6 | 147 | 97 | 20 | |||
| DMDTP | 157 | 112 | 28 | 157 | 142 | 22 |
| DMDTP-d6 | 163 | 145 | 22 | |||
| DEP | 153 | 125 | 14 | 153 | 79 | 26 |
| DEP-d10 | 163 | 79 | 26 | |||
| DETP | 169 | 95 | 26 | 169 | 141 | 16 |
| DETP-d10 | 179 | 95 | 26 | |||
| DEDTP | 185 | 111 | 24 | 185 | 157 | 16 |
| DEDTP-d10 | 195 | 111 | 24 | |||
The operation system and the mass spectrometry data acquisition were controlled by Analyst software version 1.6.3 (Applied Biosystems). This software controls all components of the analytical system. The data were processed and integrated with Multiquant software version 3.0.3 (Applied Biosystems).
2.5. Quality control/quality assurance
Each analytical run prepared in a 96-well plate included a solvent blank, ten calibration standards, two aliquots each from QC low-and high-concentration (QCL, QCH) samples, and the study samples. The concentrations of the QCs, averaged to obtain one measurement of high- and one of low-concentration QC for each run, were evaluated by use of standard statistical probability rules (Caudill et al., 2008). For quantification, we used 10 standard solutions to construct daily calibration curves, weighted by the reciprocal of the standard amount (1/x), of the response factor (calculated as the peak area of each analyte ion divided by the peak area of its internal standard) versus the standard amount. Calibration curves were linear up to 3e4 orders of magnitude, depending on the analyte. Samples with concentrations exceeding the highest calibration standard were diluted, re-extracted, and reanalyzed so that the measured values were within the calibration range. Appropriate dilution factors were used to get the final concentrations. Because standards and study samples went through the same extraction procedure, reagent contributions were automatically corrected by the calibration curve intercept.
3. Results and discussion
3.1. Selection of experimental conditions
The purpose of this work was to add six OP pesticide metabolites and three additional FR metabolites to an existing method which was able to quantify nine FR metabolites in urine (Jayatilaka et al., 2017). Therefore, all analytes were optimized to existing mass spectrometry parameters using negative electrospray ionization (ESI). ESI has been the method of choice for quantifying FRs and DAPs before (Moller et al., 2004; Odetokun et al., 2010; Reemtsma et al., 2011; Van den Eede et al., 2013b; Butt et al., 2014; Been et al., 2017).
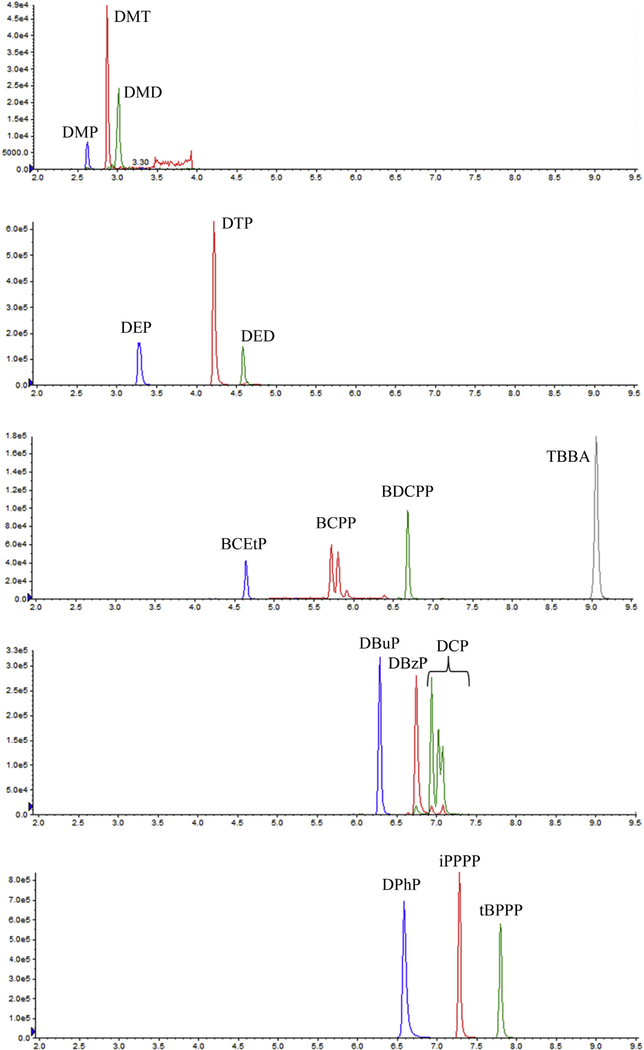
Several reversed phase HPLC columns with different dimensions and particle sizes were tested (data not shown). With most of the columns, including ZORBAX Eclipse XDB-C8 column used in our previous work (Jayatilaka et al., 2017), DMP eluted near the injection front and co-eluted with matrix interferences. Hypersil GOLD aQ (4.6 × 150 mm, 3 mm) was selected because it retained DMP a little longer than the other columns without co-eluting with matrix interferences, and had better chromatography for all the analytes considered. None of the columns evaluated separated DoCP, DmCP, and DpCP isomer peaks completely, and the isomers were quantified together (i.e., total DCP concentration). . Fig. 2 shows typical ion chromatograms for all target analytes in urine.
Fig. 2.
Extracted ion chromatograms of target analytes in urine (0.1 ng on column).
Strata-X-AW (60 mg per well, 96-well plate, Phenomenex, Torrance, CA, USA), used to quantify FR metabolites in urine before (Jayatilaka et al., 2017) was selected as the SPE column. Portions from break-through and wash steps were tested to evaluate any losses during sample loading and washing steps. Samples were eluted with 2% of NH4OH in methanol. Care was taken to control the sample pass through flow rate to as low as possible, a critical step to achieve good recoveries for DMP and DMDTP. Because Strata-X-AW extracts eluted with 2% NH4OH in methanol provided satisfying results for all new target analytes, performance for other sorbents was not evaluated.
3.2. Enzymatic treatment
Previous studies have shown that DAPs are directly excreted into urine, while hydroxylated OPFR metabolites need glucuronide conjugation before excretion (Ballesteros-Gómez al., 2015; Eede et al., 2015; Su et al., 2016). These conjugates must be hydrolyzed to measure total (conjugated plus free) concentration of the target analytes. Therefore, enzymatic treatment has been used for quantification of urinary flame retardant metabolites (Van den Eede et al., 2013a; Van den Eede et al., 2015; Su et al., 2016; He et al., 2018). Our previous work showed that the enzyme treatment only had a significant effect on DBuP and DPhP, and showed insignificant differences with the type of enzyme used (Jayatilaka et al., 2017). We tested the same enzyme, β-glucuronidase/sulfatase (Helix Pomatia, type H-1), on all new target analytes. As expected, enzyme treatment did not significantly affect the concentrations of the six DAPs. Also, no significant differences were observed for iPPPP and tBPPP with the enzyme considered. Tested samples had no detectable DCP concentrations to evaluate.
3.3. Method validation
3.3.1. Matrix effects
The composition of urine samples may vary considerably from person to person with regard to types and concentrations of solute. This complexity may cause some matrix-dependent ion enhancement or ion suppression. In most cases, matrix effects can be accounted for by utilizing stable isotope labeled internal standards or by preparing calibration standards in the same matrix as the sample. Other authors have evaluated matrix effects by comparing the spiked target analyte recoveries in urine (Odetokun et al., 2010; Cooper et al., 2011; Van den Eede et al., 2013b) and noted higher matrix effects when isotope labeled analogues were not used (Van den Eede et al., 2013b). Most of the target compounds in this method are quantified with its own deuterium or 13C labeled internal standards. Only iPPPP and tBPPP do not have their own isotope labeled internal standards, and were quantified with DPhP-d10. To evaluate matrix effects, ten sets of calibration curves spiked in ten different urines and in deionized water were analyzed in ten different days. The mean slope ± standard deviation in urine and in water for each analyte, and the percent difference between the slopes are shown in Table 2. Because no urine tested was free of all target analytes, and mean slope in urine of every analyte was not significantly different from its mean slope in water, we chose a water-based calibration curve for quantification. Percent differences between the slopes in urine and water curves for DBuP, tBPPP, DMTP, and DETP were slightly higher than those for the other analytes (>5%), but still acceptable for the intended purpose of the method (US-FDA, 2018).
Table 2.
The mean slope of calibration curve ±standard deviation in water and in urine for each analyte, and % difference between the slopes.
| Analyte | Slope ± standard deviationa | % Difference | |
|---|---|---|---|
| Water curve | Urine curve | ||
| BCEtP | 0.101 ± 0.006 | 0.103 ± 0.004 | 2.0 |
| BCPP | 0.113 ± 0.013 | 0.113 ± 0.012 | 0.0 |
| BDCPP | 0.113 ± 0.004 | 0.108 ± 0.004 | 4.4 |
| DBuP | 0.147 ± 0.003 | 0.144 ± 0.003 | 6.8 |
| DBzP | 0.111 ± 0.008 | 0.108 ± 0.010 | 2.7 |
| DPhP | 0.117 ± 0.001 | 0.116 ± 0.001 | 2.1 |
| DCP | 0.111 ± 0.001 | 0.111 ± 0.001 | 2.6 |
| TBBA | 0.092 ± 0.004 | 0.091 ± 0.003 | 1.6 |
| iPPPP | 0.030 ± 0.009 | 0.031 ±0.009 | 2.6 |
| tBPPP | 0.018 ± 0.001 | 0.020 ± 0.001 | 8.7 |
| DMP | 0.103 ± 0.010 | 0.098 ± 0.010 | 4.8 |
| DMTP | 0.715 ± 0.073 | 0.657 ± 0.063 | 8.1 |
| DMDTP | 0.071 ± 0.007 | 0.073 ± 0.004 | 1.8 |
| DEP | 0.091 ± 0.010 | 0.089 ± 0.011 | 2.3 |
| DETP | 0.103 ± 0.005 | 0.097 ± 0.006 | 5.7 |
| DEDTP | 0.176 ± 0.008 | 0.180 ± 0.011 | 2.3 |
N = 10.
3.3.2. SPE recoveries
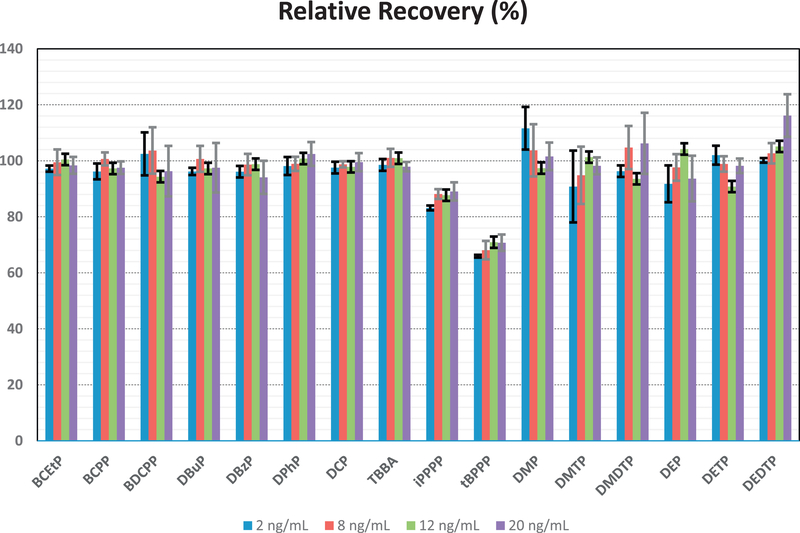
Weak anion exchange has been the optimal sorbent for SPE in previous methods to quantify separately OPFR metabolites and DAPs in urine (Odetokun et al., 2010; Cooper et al., 2011; Van den Eede et al., 2013b). After experimenting with sample loading and washing steps, Strata-X-AW cartridge afforded the best SPE recovery for most analytes. SPE recoveries were evaluated at four different concentrations (2, 8, 12, 20 ng mL−1) by using pre- and post-extraction spiked aliquots from a blank urine pool. Each concentration was analyzed in triplicate in two different days. Relative recoveries, calculated as ratio of response ratios (native/ label) for pre- and post-spiked extractions, are in Fig. 3. Recoveries of 94–110% were obtained for most analytes at all levels considered. For iPPPP and tBPPP, recoveries were 87% and 69%, respectively; these compounds do not have a corresponding labeled internal standard. Especially for tBPPP, which elutes later, this could be due to a difference in matrix effects compared to DPhP-d10. The extraction recoveries for DAPs were comparable or better than those reported before using the same SPE sorbent (Odetokun et al., 2010).
Fig. 3.
Relative recoveries, calculated as ratio of response ratios (native/label) for pre- and post-spiked extractions, at four different concentrations (2, 8, 12, 20 ng mL−1).
3.3.3. Precision and accuracy
The precision was calculated as the coefficient of variation (%CV) obtained from repeat measurements (N = 40) of quality control materials at QCL and QCH concentrations and included all sources of variability (Caudill et al., 2008). Two identical Agilent 1290 HPLC coupled to AB Sciex 5500 Qtrap mass spectrometer systems were used by two analysts over the course of a month. Inter-day CVs ranged from 2.1 to 9.8% (Table 3) and the values were within the U.S. FDA recommended limits (US-FDA, 2018). Accuracy was calculated by spike recovery at three different concentrations (0.5, 5, 20 ng mL−1) with 12 repeat measurements. Accuracy, expressed as percent error of measured value to its nominal value, ranged from 90 to 118% depending on the analyte (Table 3).
Table 3.
Method validation data for each analyte.
| Analyte | LOD (ng mL −1) | Accuracy (%)a | Precision (%)b | |||
|---|---|---|---|---|---|---|
| 0.5 (ng mL−1) |
5 (ng mL−1) |
20 (ng mL−1) |
QCL 4 (ng mL−1) |
QCH 15 (ng mL−1) |
||
| BCEtP | 0.1 | 106 | 108 | 106 | 5.4 | 5.2 |
| BCPP | 0.1 | 99 | 99 | 99 | 5.8 | 3.7 |
| BDCPP | 0.1 | 98 | 98 | 97 | 4.2 | 4.3 |
| DBuP | 0.1 | 103 | 102 | 98 | 8.5 | 5.9 |
| DBzP | 0.05 | 94 | 95 | 94 | 4.6 | 6.1 |
| DPhP | 0.1 | 100 | 102 | 102 | 3.6 | 3.9 |
| DCP | 0.5 | 101 | 102 | 102 | 3.5 | 3.5 |
| TBBA | 0.05 | 102 | 103 | 102 | 2.4 | 2.3 |
| iPPPP | 0.05 | 110 | 108 | 103 | 5.8 | 5.0 |
| tBPPP | 0.05 | 109 | 111 | 109 | 5.1 | 6.0 |
| DMP | 0.1 | 92 | 89 | 91 | 7.2 | 7.7 |
| DMTP | 0.1 | 99 | 100 | 96 | 10.1 | 10.8 |
| DMDTP | 0.1 | 111 | 116 | 114 | 7.5 | 8.5 |
| DEP | 0.1 | 103 | 102 | 99 | 7.1 | 6.9 |
| DETP | 0.1 | 112 | 112 | 107 | 6.2 | 5.6 |
| DEDTP | 0.1 | 116 | 118 | 118 | 8.4 | 5.2 |
N = 12.
N = 40, two identical instrument set-ups used by two analysts over one month.
The blank urine pool contained endogenous amounts of some of the target analytes. These background levels were subtracted for the calculation of accuracy. All values, except the values for DEDTP, were within the U.S. FDA recommendations, 85–115% (US-FDA, 2018). For all three concentrations considered, DEDTP accuracies were 116–118%, just above the recommended values. Our accuracies (except for DEDTP) and precision values were comparable with those reported before (Odetokun et al., 2010; Cooper et al., 2011; Van den Eede et al., 2013b; Jayatilaka et al., 2017).
3.3.4. Analytical sensitivity and stability
The limits of detection (LODs) were estimated by 10 repeated measurements of low concentration standards and by plotting the standard deviation of the measured concentration versus the standard concentration. The expected standard deviation at the zero concentration, S0 was determined by the y-intercept of a linear regression analysis of the above plot. The LODs, calculated as 3 times S0 (Taylor, 1987), ranged from 0.05 to 0.1 ng mL−1 for most of the analytes; LOD for DCP was 0.5 ng mL−1 (Table 3).
Our method LODs for BCEtP, BCPP, DBuP, and DPhP were comparable or lower than values reported earlier (Cooper et al., 2011; Van den Eede et al., 2013b; Butt et al., 2014; Kosarac et al., 2016; Jayatilaka et al., 2017). The LOD for BDCPP improved slightly in this method compared to that of our previous method (0.11 ng mL−1) and was lower than the values reported by Van den Eede et al., 2013b and Kosarac et al., (2016) (0.52 and 0.25 ng mL−1 respectively), but still higher than the values reported by Butt et al., (2014) (0.02 ng mL−1). LOD of TBBA in the current method is comparable to that of our previous method (0.05 ng mL−1), but about an order of magnitude higher than that was reported by Butt et al., (2014) ng mL−1). The LODs for two of the newly added flame retardant analytes, iPPPP and tBPPP, were lower than the values reported by Butt et al., (2014) (0.09 ng mL−1). Our method’s detection limit of DCP three isomers (DoCP, DmCP, and DpCP) as a sum was 0.5 ng mL−1. However, our previous method had better LODs (0.05 ng mL−1) for the individual DoCP and DpCP isomers. A UPLC-MS/MS method by Kosarac et al., (2016) in positive ESI mode has an LOD of 0.13 ng mL−1 for the sum of two DCP isomers, DoCP and DpCP, and a GC-MS/MS method by Schindler et al., (2009a) has an LOD of 1 ng mL−1 for the sum of two DCP isomers, DmCP and DpCP (Schindler et al., 2009a). Our LODs for the six DAPs (0.1 ng mL−1) were comparable or better than the previously published values (Bravo et al., 2004; Odetokun et al., 2010; Reemtsma et al., 2011).
Most existing methods for quantification of urinary FR metabolites use 1e5 mL of sample volume (Cooper et al., 2011; Van den Eede et al., 2013b; Kosarac et al., 2016) and our method yields comparable, and in some cases better, sensitivity using a fraction of their volume. A small sample volume may also improve SPE recoveries and reduce matrix interferences. Although urine is considered to be readily available sample matrix, it is not easy to obtain for studies involving infants or small children. Sample volume can also be a critical factor for studies involving the assessment of multiple chemical classes as well as clinical and/or nutritional biomarkers (e.g., hormones, vitamins).
A considerable degradation of the target analytes in urine was not observe during freeze-thaw cycles, on the bench-top, or after extraction. Details of this short-term stability assay are provided in Table S2 of the Supplementary data.
3.4. Method application
We assessed the suitability of the method by analyzing 158 urine samples collected anonymously from a convenience group of adults without known occupational exposure to these chemicals. We also tested the method by analyzing 145 urine samples collected from firefighters after performing structural firefighting while wearing full protective clothing and SCBA respirators. All of these samples were tested once with our previous method (Jayatilaka et al., 2017). With the current method, we were able to quantify concentrations of additional compounds, including DAPs. None of the samples tested had detectable DCP or DBzP (Table 4).
Table 4.
Method application data for the analytes considered in urine from anonymous adult volunteers (general population), and from occupational firefighters (exposed population)a.
| Analyte | General population (N = 158) | Firefighters (N = 145) | ||||
|---|---|---|---|---|---|---|
| Detection Frequency (%) | Median (ng mL −1) | Range (ng mL −1) | Detection Frequency (%) | Median (ng mL −1) | Range (ng mL −1) | |
| BCEtP | 49 | < LOD | < LOD − 41 | 87 | 0.84 | < LOD − 9.8 |
| BCPP | 51 | 0.11 | < LOD − 3.0 | 63 | 0.24 | < LOD − 3.0 |
| BDCPP | 78 | 0.30 | < LOD − 64 | 97 | 3.3 | < LOD − 42 |
| DBuP | 9 | < LOD | < LOD − 4.3 | 53 | 0.12 | < LOD − 2.9 |
| DPhP | 70 | 0.26 | < LOD − 12 | 100 | 4.0 | 0.14–32 |
| TBBA | 2 | < LOD | < LOD − 0.38 | 4 | 0.10 | < LOD − 0.13 |
| iPPPP | 16 | < LOD | < LOD − 0.45 | 35 | 0.11 | < LOD − 0.49 |
| tBPPP | 9 | < LOD | < LOD − 0.19 | 83 | 0.17 | < LOD − 1.1 |
| DMP | 80 | 0.68 | < LOD − 130 | 88 | 9.9 | < LOD − 190 |
| DMTP | 73 | 0.39 | < LOD − 190 | 90 | 15 | < LOD − 300 |
| DMDTP | 35 | < LOD | < LOD − 14 | 57 | 1.2 | < LOD − 11 |
| DEP | 92 | 0.73 | < LOD − 52 | 88 | 4.2 | < LOD − 60 |
| DETP | 56 | 0.12 | < LOD − 11 | 68 | 0.74 | < LOD − 5.0 |
| DEDTP | 4 | < LOD | < LOD − 0.74 | 4 | 0.22 | < LOD − 0.35 |
DBzP and DCP were not detected in any of the samples tested.
FR metabolite and DAPs concentrations in the general population group are within the ranges reported in the U.S. general population from NHANES (US-CDC, 2009; Ospina et al., 2018), and with those reported from previous research involving non- occupationally exposed populations (Cooper et al., 2011; McKelvey et al., 2013; Meeker et al., 2013; Butt et al., 2014; Dodson et al., 2014; Jayatilaka et al., 2017). For example, BDCPP and DPhP were also the FR biomarkers most frequently detected and at the highest concentrations in studies of 33 men from couples seeking fertility treatment in Massachusetts, USA (Meeker et al., 2013), 21 North Carolinian mothers and their toddlers (Butt et al., 2014), and nine other North Carolinian adults (Cooper et al., 2011), and 16 Californian adults (Dodson et al., 2014).
Compared to the general population samples, both DAPs and FR metabolite concentrations were higher in the firefighters group, and varied between two times (BCPP: 0.11 vs 0.24 ng mL−1) and 37 times (DMTP: 0.39 vs 15 ng mL−1) the concentrations of the non-occupationally exposed group. A recent study reported that U.S. fire stations were contaminated with higher levels of flame retardants than residences and other occupational settings (Shen et al., 2018), which can result in higher concentrations of flame retardant metabolites in firefighters. Polyurethane spray foam workers have also higher levels of urinary flame retardant metabolites than general population groups (Estill et al., 2019). Although dietary exposure to organophosphate insecticides or their degradation products (e.g., DAPs) influences the measured concentrations of DAPs (Lu et al., 2008; Curl et al., 2015), the reason(s) for the differences between the two populations examined are unknown. We did not have access to diet or other lifestyle related information for these groups of samples. It is possible that DEP and DMP also resulted from metabolism of trimethylphosphate (TMP) and triethylphosphate (TEP). TMP has been used as a gasoline additive for controlling surface ignition and spark-plug fouling; it is also used as a methylating agent, as a catalyst in the preparation of resins and polymers and as a flame retardant in paints and polymers (Connor, 1979). TEP is mostly used as flame retardant and plasticizer. TMP and TEP are metabolized in rats and mice to DMP and DEP, respectively and excreted in urine (Jones, 1970).
4. Conclusions
We developed a sensitive HPLC-isotope dilution tandem mass spectrometry method for the concurrent measurement of ten flame retardants metabolites and six DAPs in urine. The method uses a semi-automated SPE procedure for sample cleanup and a relatively small sample volume (200 μL), and has relatively high sample throughput (72 samples/day). To our knowledge, this is the first method that can quantify metabolites from commonly used FRs and all six DAP metabolites from OP pesticides in one single analysis. Preliminary data suggest that this multianalyte method is sensitive, precise, and accurate enough to assess exposures using urinary biomarkers in large-scale studies such as the National Health and Nutrition Examination Survey.
Supplementary Material
HIGH LIGHTS.
First assay to quantify 10 flame retardants & 6 organophosphate urinary metabolites.
Method uses 0.2 mL urine and is sensitive, reproducible, accurate, high throughput.
Suitable to assess background exposures in large-scale population studies.
Acknowledgments
We gratefully acknowledge Dr. Kenneth Fent (NIOSH, CDC) for providing firefighters’ de-identified urine specimens for method validation. This work was supported in part by an appointment (ZD) to the CDC Foundation.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chemosphere.2019.06.181.
Conflicts of interests
The authors declare they have no competing financial or other conflicts of interests.
References
- Andresen JA, Grundmann A, Bester K, 2004. Organophosphorus flame retardants and plasticisers in surface waters. Sci. Total Environ. 332, 155–166. [DOI] [PubMed] [Google Scholar]
- Ballesteros-Gómez A, Erratico CA, Eede NV, Ionas AC, Leonards PE, Covaci A, 2015. In vitro metabolism of 2-ethylhexyldiphenyl phosphate (EHDPHP) by human liver microsomes. Toxicol. Lett. 232, 203–212. [DOI] [PubMed] [Google Scholar]
- Bardarov V, Mitewa M, 1989. High-performance liquid and gas chromatography of dialkylphosphates, dialkylthiophosphates and dialkyldithiophosphates as their pentafluorobenzyl derivatives. J. Chromatogr. B 462, 233–241. [DOI] [PubMed] [Google Scholar]
- Bastiaensen M, Xu F, Been F, Eede N.V.d., Covaci A, 2018. Simultaneous determination of 14 urinary biomarkers of exposure to organophosphate flame retardants and plasticizers by LC-MS/MS. Anal. Bioanal. Chem. 410, 7871–7880. [DOI] [PubMed] [Google Scholar]
- Been F, Bastiaensen M, Lai FY, van Nuijs ALN, Covaci A, 2017. Liquid chromatography–tandem mass spectrometry analysis of biomarkers of exposure to phosphorus flame retardants in wastewater to monitor community- wide exposure. Anal. Chem. 89, 10045–10053. [DOI] [PubMed] [Google Scholar]
- Bergman A, Ryden A, Law RJ, de BJ, Covaci A, Alaee M, Birnbaum L, Petreas M, Rose M, Sakai S, Van den Eede N, Van der Veen I, 2012. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environ. Int. 49, 57–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Driskell WJ Jr, R DW, Needham LL.Barr DB.2002. Quantitation of dialkyl phosphate metabolites of organophosphate pesticides in human urine using GC-MS-MS with isotopic internal standards. J. Anal. Toxicol. 26, 245–252. [DOI] [PubMed] [Google Scholar]
- Bravo R, Caltabiano LM, Weerasekera G, Whitehead RD, Fernandez C, Needham LL, Bradman A, Barr DB, 2004. Measurement of dialkyl phosphate metabolites of organophosphorus pesticides in human urine using lyophilization with gas chromatography-tandem mass spectrometry and isotope dilution quantification. J. Expo. Anal. Environ. Epidemiol. 14, 249–259. [DOI] [PubMed] [Google Scholar]
- Butt CM, Congleton J, Hoffman K, Fang ML, Stapleton HM, 2014. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ. Sci. Technol. 48, 10432–10438. [DOI] [PubMed] [Google Scholar]
- Carignan C, Heiger-Bernays W, McClean MD, Roberts SC, Stapleton HM, Sjodin A, Webster TF, 2013a. Flame retardant exposure among collegiate United States gymnasts. Environ. Sci. Technol. 47, 13848–13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan CC, McClean MD, Cooper EM, Watkins DJ, Fraser AJ, Heiger-Bernays W, Stapleton HM, Webster TF, 2013b. Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environ. Int. 55, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudill SP, Schleicher RL, Pirkle JL, 2008. Multi-rule quality control for the age- related eye disease study. Stat. Med. 27, 4094–4106. [DOI] [PubMed] [Google Scholar]
- Cequier E, Sakhi AK, Marce RM, Becher G, Thomsen C, 2015. Human exposure pathways to organophosphate triesters - a biomonitoring study of mother-child pairs. Environ. Int. 75, 159–165. [DOI] [PubMed] [Google Scholar]
- Cocker J, Mason HJ, Garfitt SJ, Jones K, 2002. Biological monitoring of exposure to organophosphate pesticides. Toxicol. Lett. 134, 97–103. [DOI] [PubMed] [Google Scholar]
- Connor TH, 1979. The mutagenicity of trimethylphosphate. Mutat. Res. 65, 121–131. [DOI] [PubMed] [Google Scholar]
- Cooper EM, Covaci A, van Nuijs ALN, Webster TF, Stapleton HM, 2011. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 401, 2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curl C, Beresford S, Fenske R, Fitzpatrick A, Lu C, Nettleton J, Kaufman J, 2015. Estimating pesticide exposure from dietary intake and organic food choices: the Multi-Ethnic Study of Atherosclerosis (MESA). Environ. Health Perspect. 123, 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Alwis GK, Needham LL, Barr DB, 2006. Measurement of human urinary organophosphate pesticide metabolites by automated solid-phase extraction derivation and gas chromatography-tandem mass spectromy. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 843, 34–41. [DOI] [PubMed] [Google Scholar]
- de Wit CA, 2002. An overview of brominated flame retardants in the environment. Chemosphere 46, 583–624. [DOI] [PubMed] [Google Scholar]
- Dishaw LV, Powers CM, Ryde IT, Roberts SC, Seidler FJ, Slotkin TA, Stapleton HM, 2011. Is the PentaBDE replacement, tris (1,3-dichloropropyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol. Appl. Pharmacol. 256, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA, 2012. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol. 46, 13056–13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Van den Eede N, Covaci A, Perovich LJ, Brody JG, Rudel RA, 2014. Urinary biomonitoring of phosphate flame retardants: levels in California adults and recommendations for future studies. Environ. Sci. Technol. 48, 13625–13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulaurent S, Saint-Marcoux F, Marquet P, Lachâtre G, 2006. Simultaneous determination of six dialkylphosphates in urine by liquid chromatography tandem mass spectrometry. J. Chromatogr. B 831, 223–229. [DOI] [PubMed] [Google Scholar]
- Eede N.V.d., Heffernan AL, Aylward LL, Hobson P, Neels H, Mueller JF, Covaci A, 2015. Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ. Int. 74, 1–8. [DOI] [PubMed] [Google Scholar]
- Estill CF, Slone J, Mayer AC, Phillips Kaitlyn, Lu J, Chen I-C, Christianson A, Streicher R, Guardia MJL, Jayatilaka NK, Ospina M, Calafat AM, 2019. Assessment of spray polyurethane foam worker exposure to organophosphate flame retardants through measures in air, hand wipes, and urine. J. Occup. Environ. Hyg. 16, 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Examination Survey. Environ. Int. 110, 32 – 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent KW, Eisenberg J, Snawder J, Sammons D, Pleil JD, Stiegel MA, Mueller C, Horn GP, Dalton J, 2014. Systemic exposure to PAHs and benzene in firefighters suppressing controlled structure fires. Ann. Occup. Hyg. 58, 830–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, English K, Baduel C, Thai P, Jagals P, Ware RS, Li Y, Wang X, Sly PD, Mueller JF, 2018. Concentrations of organophosphate flame retardants and plasticizers in urine from young children in Queensland, Australia and associations with environmental and behavioural factors. Environ. Res. 164, 262–270. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Lorenzo A, Butt CM, Adair L, Herring AH, Stapleton HM, Daniels JL, 2017. Predictors of urinary flame retardant concentration among pregnant women. Environ. Int. 98, 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou R, Xu Y, Wang Z, 2016. Review of OPFRs in animals and humans: absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere 153, 78–90. [DOI] [PubMed] [Google Scholar]
- Jayatilaka NK, Restrepo P, Williams L, Ospina M, Valentin-Blasini L, Calafat AM, 2017. Quantification of three chlorinated dialkyl phosphates, diphenyl phosphate, 2,3,4,5-tetrabromobenzoic acid, and four other organophosphates in human urine by solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 409, 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AR, 1970. The metabolism of tri-alkyl phosphates. Experientia 26, 492–493. [DOI] [PubMed] [Google Scholar]
- Kosarac I, Kubwaboa C, Foster WG, 2016. Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction andultra performance liquid chromatography coupled to tandem mass-spectrometry (UPLC-MS/MS). J. Chromatogr. B 1014, 24–30. [DOI] [PubMed] [Google Scholar]
- Kwong, 2002. Organophosphate pesticides: biochemistry and clinical toxicology. Ther. Drug Monit. 24, 144–149. [DOI] [PubMed] [Google Scholar]
- Lu C, Barr D, Pearson M, Waller L, 2008. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ. Health Perspect. 116, 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey W, Jacobson J, Kass D, Barr D, Davis M, Calafat A, Aldous K, 2013. Population-based biomonitoring of exposure to organophosphate and pyrethroid pesticides in New York City. Environ. Health Perspect. 121, 1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Stapleton HM, 2010. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ. Health Perspect. 118, 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Cooper EM, Stapleton HM, Hauser R, 2013. Exploratory analysis of urinary metabolites of phosphorus-containing flame retardants in relation to markers of male reproductive health. Endocr. Disruptors 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller K, Crescenzi C, Nilsson U, 2004. Determination of a flame retardant hydrolysis product in human urine by SPE and LC-MS. Comparison of molecularly imprinted solid-phase extraction with a mixed-mode anion exchanger. Anal. Bioanal. Chem. 378, 197–204. [DOI] [PubMed] [Google Scholar]
- Nutley B, Cocker J, 1993. Biological monitoring of workers occupationally exposed to organophosphorus pesticides. Pestic. Sci. 38, 315–322. [Google Scholar]
- Odetokun MS, Montesano MA, Weerasekera G Jr, R DW, Needham LL, Barr DB, 2010. Quantification of dialkylphosphate metabolites of organophosphorus insecticides in human urine using 96-well plate sample preparation and high-performance liquid chromatography–electrospray ionization-tandem mass spectrometry. J. Chromatogr. B 878, 2567–2574. [DOI] [PubMed] [Google Scholar]
- Ospina M, Jayatilaka NK, Wong L-Y, Restrepo P, Calafat AM, 2018. Exposure to Organophosphate Flame Retardant Chemicals in the U.S. General Population: Data from the 2013–2014 National Health and Nutrition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM, 2013. Accumulation and endocrine disrupting effects of the flame retardant mixture firemaster (R) 550 in rats: an exploratory assessment. J. Biochem. Mol. Toxicol. 27, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petchuay C, Thoumsang S, Visuthismajarn P, Vitayavirasak B, Buckley B, Hore P, Borjan M, Robson M, 2008. Analytical method developed for measurement of dialkylphosphate metabolites in urine collected from children non- occupationally exposed to organophosphate pesticides in an agricultural community in Thailand. Bull. Environ. Contam. Toxicol. 81, 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Hammel SC, Hoffman K, Lorenzo AM, Chen A, Webster TF, Stapleton HM, 2018. Children’s residential exposure to organophosphate ester flame retardants and plasticizers: investigating exposure pathways in the TESIE study. Environ. Int. 116, 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil JD, Stiegel MA, Fent KW, 2014. Exploratory breath analyses for assessing toxic dermal exposures of firefighters during suppression of structural burns. J. Breath Res. 8, 037107. [DOI] [PubMed] [Google Scholar]
- Reemtsma T, Lingott J, Roegler S, 2011. Determination of 14 monoalkyl phosphates, dialkyl phosphates and dialkyl thiophosphates by LC-MS/MS in human urinary samples. Sci. Total Environ. 409, 1990–1993. [DOI] [PubMed] [Google Scholar]
- Roberts SC, Macaulay LJ, Stapleton HM, 2012. In vitro metabolism of the brominated flame retardants 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB) and bis(2-ethylhexyl) 2,3,4,5-tetrabromophthalate (TBPH) in human and rat tissues. Chem. Res. Toxicol. 25, 1435–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, Hawley NL, Eliot M, Calafat AM, Jayatilaka NK, Kelsey K, McGarvey S, Phipps MG, Savitz DA, Werner EF, Braun JM, 2017. Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode Island . Environ. Heal. 16, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler BK, Foerster K, Angerer J, 2009a. Determination of human urinary organophosphate flame retardant metabolites by solid-phase extraction and gas chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 877, 375–381. [DOI] [PubMed] [Google Scholar]
- Schindler BK, Foerster K, Angerer J, 2009b. Quantification of two urinary metabolites of organophosphorus flame retardants by solid-phase extraction and gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 395, 1167–1171. [DOI] [PubMed] [Google Scholar]
- Shen B, Whitehead TP, Gill R, Dhaliwal J, Brown FR, Petreas M, Patton S, Hammond SK, 2018. Organophosphate flame retardants in dust collected from United States fire stations. Environ. Int. 112, 41–48. [DOI] [PubMed] [Google Scholar]
- Solbu K, Thorud S, Hersson M, Ovrebo S, Ellingsen D, Lundanes E, Molander P, 2007. Determination of airborne trialkyl and triaryl organophosphates originating from hydraulic fluids by gas chromatography-mass spectrometry-development of methodology for combined aerosol and vapor sampling. J. Chromatogr. A 1161, 275–283. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF, 2008. Alternate and new brominated flame retardants detected in US house dust. Environ. Sci. Technol. 42, 6910–6916. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF, 2009. Detection of organophosphate flame retardants in furniture foam and US house dust. Environ. Sci. Technol. 43, 7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A, 2011. Identification of flame retardants in polyurethane foam collected from baby products. Environ. Sci. Technol. 45, 5323–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A, 2012. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ. Sci. Technol. 46, 13432–13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Misenheimer J, Hoffman K, Webster TF, 2014. Flame retardant associations between children’s handwipes and house dust. Chemosphere 116, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G, Letcher RJ, Yu H, Gooden DM, Stapleton HM, 2016. Determination of glucuronide conjugates of hydroxyl triphenylphosphate (OH-TPHP) metabolites in human urine and its use as abiomarker of TPHP exposure. Chemosphere 149, 314–319. [DOI] [PubMed] [Google Scholar]
- Taylor JK, 1987. Quality Assurance of Chemical Measurements. Lewis Publishers, Chelsea, MI. [Google Scholar]
- Tullo A, 2003. Great Lakes to phase out flame retardants. Chem. Eng. News 81, 13–13. [Google Scholar]
- Ueyama J, Saito I, Kamijima M, Nakajima T, Gotoh M, Suzuki T, Shibata E, Kondo T, Takagi K, Miyamoto K, Takamatsu J, Hasegawa T, Takagi K, 2006. Simultaneous determination of urinary dialkylphosphate metabolites of organophosphorus pesticides using gas chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 832, 58–66. [DOI] [PubMed] [Google Scholar]
- US-CDC, 2009. The Fourth National Report on Human Exposure to Environmental Chemicals. [PubMed] [Google Scholar]
- US-EPA, 1996. Summary of the Food Quality Protection Act. https://www.epa.gov/laws-regulations/summary-food-quality-protection-act.
- US-EPA, 2013. Polybrominated Diphenyl Ethers (PBDEs) Significant New Use Rules (SNUR). http://www.epa.gov/oppt/existingchemicals/pubs/qanda.html.
- US-EPA, 2014. Flame Retardants Used in Flexible Polyurethane Foam: an Alternatives Assessment Update. http://www.epa.gov/dfe/pubs/projects/flameret/ffr-update-complete.pdf.
- US-EPA, 2016. Pesticides Industry Sales and Usage. https://www.epa.gov/sites/production/files/2017-01/documents/pesticides-industry-sales-usage-2016_0.pdf.
- US-FDA, 2018. Bioanalytical Method Validation Guidance for Industry. https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf.
- Van den Eede N, Maho W, Erratico C, Neels H, Covaci A, 2013a. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol. Lett. 223, 9–15. [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Neels H, Jorens PG, Covaci A, 2013b. Analysis of organophosphate flame retardant diester metabolites in human urine by liquid chromatography electrospray ionisation tandem mass spectrometry. J. Chromatogr. A 1303, 48–53. [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Cuykx M, Rodrigues RM, Laukens K, Neels H, Covaci A, Vanhaecke T, 2015. Metabolomics analysis of the toxicity pathways of triphenyl phosphate in HepaRG cells and comparison to oxidative stress mechanisms caused by acetaminophen. Toxicol. Vitro 29, 2045–2054. [DOI] [PubMed] [Google Scholar]
- van der Veen I, de Boer J, 2012. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88, 1119–1153. [DOI] [PubMed] [Google Scholar]
- Wei G-L, Li D-Q, Zhuo M-N, Liao Y-S, Xie Z-Y, Guo T-L, Li J-J, Zhang S-Y, Liang Z-Q, 2015. Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environ. Pollut. 196, 29–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.