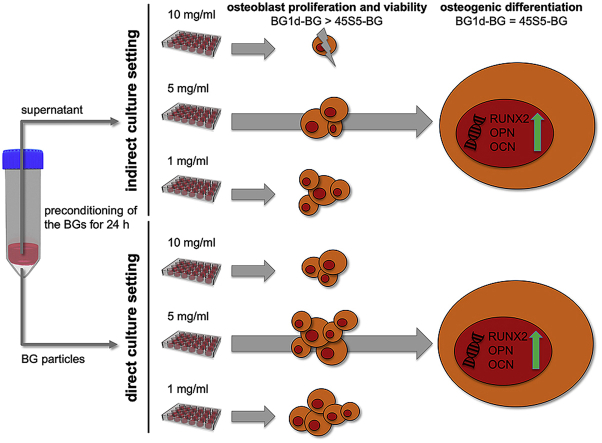
Abstract
Bioactive glasses (BGs) are promising bone substitute materials. However, under certain circumstances BGs such as the well-known 45S5 Bioglass® (composition in wt%: 45.0 SiO2, 24.5 Na2O, 24.5 CaO, 6.0 P2O5) act cytotoxic due to a strong increase in pH caused by a burst release of sodium ions. A potential alternative is a sodium-reduced fluoride-containing BG belonging to the CaO–MgO–SiO2 system, namely BG1d-BG (composition in wt%: 46.1 SiO2, 28.7 CaO, 8.8 MgO, 6.2 P2O5, 5.7 CaF2, 4.5 Na2O), that has already been evaluated in-vitro, in-vivo and in preliminary clinical trials. Before further application, however, BG1d-BG should be compared to the benchmark amongst BGs, the 45S5 Bioglass® composition, to classify its effect on cell viability, proliferation and osteogenic differentiation of human mesenchymal stem cells (MSCs). Therefore, in this study, the biocompatibility and osteogenic potential of both BGs were investigated in an indirect and direct culture setting to assess the effect of the ionic dissolution products and the BGs’ physical presence on the cells. The results indicated an advantage of BG1d-BG over 45S5 Bioglass® regarding cell viability and proliferation. Both BGs induced an earlier onset of osteogenic differentiation and accelerated the expression of late osteoblast marker genes compared to the control group. In conclusion, BG1d-BG is an attractive candidate for further experimental investigation. The basic mechanisms behind the different impact on cell behavior should be assessed in further detail, e.g. by further alteration of the BG compositions.
Keywords: 45S5 Bioglass®, Sodium-reduced bioactive glass, Fluoride-containing bioactive glass, Bone tissue engineering, Human mesenchymal stromal cells
Graphical abstract
Highlights
-
•
45S5 Bioglass® is considered to be the benchmark amongst bioactive glasses (BGs).
-
•
Sodium-reduced fluoride-containing BG1d BG was compared to 45S5-Bioglass®.
-
•
Both BGs induced osteogenic differentiation of human MSCs.
-
•
BG1d had an advantageous impact on cell viability and proliferation.
-
•
BG1d-BG is an attractive candidate for further experimental investigation.
1. Introduction
Bioactive glasses (BGs) show attractive properties making them suitable bone substitute materials [1]. The era of BGs began with the development of 45S5 Bioglass® (composition in wt%: 45.0 SiO2, 24.5 Na2O, 24.5 CaO, 6.0 P2O5; in the following referred to as 45S5-BG) in the late 1960s by the group of Hench [2,3]. In contact to body fluids, 45S5-BG induces the formation of hydroxyapatite or hydroxyl carbonated apatite (similar to the mineral phase of bone) on the glass surface, thus providing strong bonding of the material towards the surrounding (bone) tissue. Furthermore, it has been demonstrated that the ions liberated from the 45S5-BG composition support precursor cells such as mesenchymal stromal cells (MSCs) towards osteogenic differentiation [[4], [5], [6]].
Despite numerous favorable properties, 45S5-BG suffers from some shortcomings including: (i) initial burst liberation of sodium (Na) ions causing a dramatic increase in local pH that can result in cell death at least under in-vitro conditions [[7], [8], [9]] and (ii) high tendency to crystallize leading to difficulties in processing it into three-dimensional scaffolds, inhibiting protein adsorption and enforcing inappropriate protein conformation for cell adhesion [[10], [11], [12]].
In order to overcome the limitations of the 45S5-BG composition, efforts have been undertaken to develop new BG compositions with varying properties [13]. Ferreira et al. achieved significant improvements with low alkali contents in the SiO2–B2O3–MgO–CaO–Na2O–P2O5–CaF2 system [[13], [14], [15], [16], [17], [18], [19], [20], [21]]. Among them, a sodium-reduced fluoride-containing BG belonging to the CaO–MgO–SiO2 system, namely BG1d-BG (composition in wt%: 46.1 SiO2, 28.7 CaO, 8.8 MgO, 6.2 P2O5, 5.7 CaF2, 4.5 Na2O), stands out for its excellent performance in-vitro and in-vivo [16,18,19]. Interestingly, BG1d-BG's composition not only differs from 45S5-BG in the alkali content, but also in the content of MgO and CaF2. The presence of MgO promotes the formation and evolution of the newly formed apatite layers. Furthermore, MgO-containing BGs were also reported to favor cell adhesion, proliferation and the differentiation of osteoblast cells in several studies carried out in-vitro [[22], [23], [24]]. The addition of fluoride to the BG systems reduced melting and glass transition temperatures and modified the bioactivity of the glass [25,26]. The incorporation of CaF2 was shown to reduce the increase in pH, induced by BGs when immersed in simulated body fluids, leading to a more suitable environment for bone cells [27]. Moreover, biological studies revealed that the presence of fluoride enhances osteoblast proliferation [28] and increases bone density [29,30].
For newly developed BG compositions, it is of certain interest to be compared and referenced to 45S5-BG as the well-known standard amongst BGs in order to predict and understand their properties [31]. BG1d-BG already demonstrated similar rates of crystalline apatite formation during incubation in simulated body fluid in direct comparison to 45S5-BG [18]. However, neither data describing the influence of the ions liberated from BG1d-BG nor data on the effect of the physical presence of BG1d-BG on viability, proliferation and osteogenic differentiation of osteoblast precursor cells in direct comparison to 45S5-BG are available.
This study aimed to classify the properties of BG1d-BG in direct comparison to 45S5-BG regarding their influence on the viability, proliferation and osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. For that, the BGs have been produced exhibiting similar structural properties and were introduced in two different settings to the MSCs [8,32]: in physical (direct) contact to the cells and in an indirect culture setting with the MSCs only being subjected to the ionic dissolution products of the BGs. For that, the ion release from the BG particles was measured via inductively coupled plasma optical emission spectroscopy (ICP-OES). Cell viability and proliferation were quantitatively assessed by the resazurin-based PrestoBlue assay and by quantification of dsDNA amount. Furthermore, cell growth patterns and viability were visualized by a fluorescence microscopy-based live/dead assay. Osteogenic differentiation was evaluated by quantification of gene expression using quantitative polymerase chain reaction (qPCR).
2. Materials and methods
2.1. BG synthesis
BG1d-BG [[17], [18], [19]] was produced from powders of technical grade of silicon oxide (purity 99.5%) and calcium carbonate (99.5%) and of reagent grade 4MgCO3·Mg(OH)2·5H2O, Na2CO3, CaF2 and NH4H2PO4. Homogeneous mixtures of batches were preheated at 1000 °C for 1 h for decarbonization and then thoroughly melted in Pt-crucibles at 1400 °C for 1 h in air. Glass-frits were obtained by quenching of the melts into cold deionized water. The frits were dried and then milled in a high-speed planetary mill (Pulverisette 6; Fritsch, Idar-Oberstein, Germany) and passed through a 32 μm sieve to obtain a final particle size below 32 μm.
45S5-BG was produced from powders of technical grade of silicon oxide (purity 99.5%) and calcium carbonate (99.5%) and of reagent grade Na2CO3. Glass batches were preheated at 1000 °C for 1 h for decarbonization and then thoroughly melted in Pt-crucibles at 1400 °C for 1 h in air. Solid glass blocks were produced by casting glass melt into the copper molds and cooling down the samples to room temperature. The glass blocks were then crushed in a mortar followed by milling and passing through a 56 μm sieve to obtain powders with a particle size below 56 μm.
2.2. Study ethics and cell origin
MSCs were harvested of n = 10 patients that underwent surgery at the proximal femur for medical reasons at the Heidelberg Orthopedic University Hospital. Written consent was obtained from all patients prior to collection of the material. The responsible ethic committee of the Medical Faculty of the University of Heidelberg approved the study (S-443/2015)
2.3. MSC isolation, cultivation and characterization
Isolation of MSCs from bone marrow was conducted as published previously [[33], [34], [35]]. In short, directly after harvesting and washing with 1x phosphate-buffered saline (PBS; Life Technolgies, Darmstadt, Germany), bone marrow was transferred to 0.1% gelatin-coated (Sigma-Aldrich, Steinheim, Germany) culture flasks (Life Technolgies, Darmstadt, Germany) with expansion medium containing 83% Dulbecco's modified Eagle's medium (DMEM) high glucose, 12.5% fetal calf serum (FCS), 2 mM l-glutamine, 1% non-essential amino acids (NEAA), 50 μM β-mercaptoethanol (all Life Technologies, Darmstadt, Germany), 100 μg/ml penicillin/streptomycin, 2.5 mg/ml amphotericin B (both Biochrom, Berlin, Germany) and 4 ng/ml fibroblast growth factor 2 (Abcam, Cambridge, U.K.). After 24 h, the medium was changed in order to remove non-adherent cells and remaining bone marrow. Cultivation was continued with medium changes twice a week until 80% confluence of the cells was reached. The MSCs were transferred to the next passage and repetitive passaging was performed each time the cells reached 80% confluence. MSCs of the donors in passage 1 were pooled in order to reduce inter-individual variances in MSC behavior [35]. The pooled cell population was cultivated and cells in passage 4 were used for the experiments.
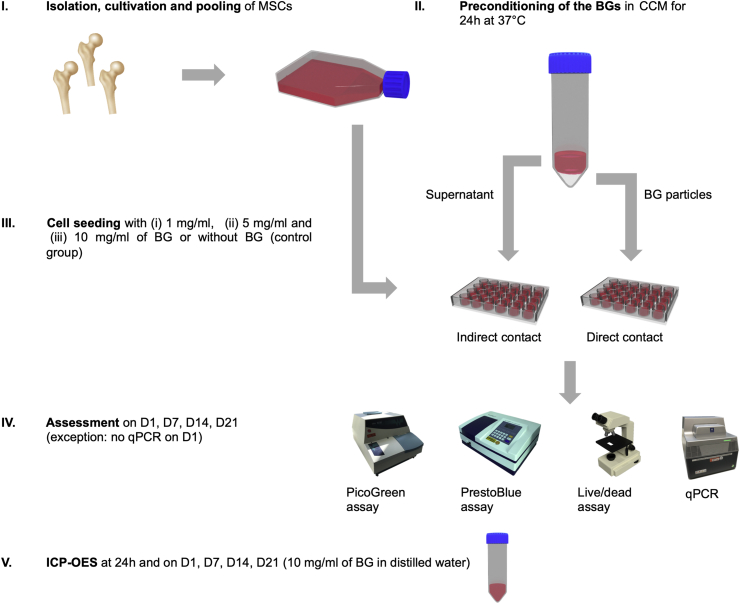
2.4. General experimental design: overview
The general experimental design is schematically represented in Fig. 1. MSCs were exposed to the BGs in two different cultivation settings in order to assess the influence of the BGs on proliferation, viability and osteogenic differentiation [8]:
-
i.
Indirect culture setting: BGs were exposed to DMEM for 24 h at 37 °C while being thoroughly mixed to allow the bioactive ions to release from the BG into the medium. After 24 h, the medium was collected, filtrated and subjected to the cell culture. The indirect approach was used to evaluate to what extent the bioactive ions released from the BGs influence the cells [36].
-
ii.
Direct culture setting: The BGs used in the indirect approach were directly applied to cell culture dishes after the 24 h incubation period in order to assess the effect of the BGs' physical presence on the MSCs [36]. The preconditioning of the BGs in DMEM before the seeding of cells on BG powders or scaffolds has recently been recommended in a study conducted by Ciraldo et al. [7] in order to reduce the local cytotoxicity in the direct environment of the BG particles.
Fig. 1.
Schematic representation of the experimental design. In order to evaluate the effect of BGs on cell viability, proliferation and osteogenic differentiation, MSCs were cultured in 24 well plates for 1 (D1), 7 (D7), 14 (D14) and 21 (D21) days. The ion release profiles were obtained via ICP-OES at 24 h, D1, D7, D14 and D21.
For both settings, cell viability and proliferation were evaluated on day 1 (D1), 7 (D7), 14 (D14) and 21 (D21) of incubation with BG concentrations of 1, 5 and 10 mg/ml. Cultivation was performed in cell culture medium (CCM; 87% DMEM, 11% FCS, 1% penicillin/streptomycin, 1% amphotericin B) at 37 °C and 5% CO2. Based on these results, the highest possible BG concentration that did not exhibit toxic effects on the cells was chosen for the evaluation of osteogenic differentiation. To cover all relevant steps of osteogenic differentiation of MSCs in the used in-vitro conditions [37], qPCR was performed on D7, D14 and D21. The ion release from BG particles was measured via ICP-OES after the 24 h preconditioning phase, as well as on D1, D7, D14 and D21. A control group of cells was cultivated without indirect nor direct presence of BG. Media changes were performed twice weekly.
2.5. Ion release from BG particles
To quantify the ion release from BG particles, the respective BGs were incubated in UltraPure DNase/RNase-Free Distilled Water (Life Technologies, Darmstadt, Germany) at a concentration of 10 mg/ml for 24 h (representing the preconditioning phase) as well as for 1, 7, 14 and 24 days. After incubation, the supernatant was collected and filtered through a 0.45 μm sterile filter (Merck, Darmstadt, Germany). The samples were acidified with 50 μl concentrated nitric acid per 10 ml sample. To avoid interference of various ions present in the CCM the measurement had to be performed in distilled water. The release of Ca, magnesium (Mg), Na, phosphate (P) and Si was measured via inductively coupled plasma optical emission spectroscopy using an Agilent 720 ICP-OES instrument (Agilent, Santa Clara, USA). All samples were measured in triplicates.
2.6. Cell viability assay
Cell viability evaluation was performed using the PrestoBlue assay (Life Technologies, Darmstadt, Germany) according to the manufacturer's instructions. In short, 50 μl PrestoBlue dye were added to 450 μl CCM and incubated for 20 min at 37 °C. Absorbance was detected at 570 nm and 600 nm using a UV-1600 PC spectrophotometer (VWR International, Darmstadt, Germany). The percentage reduction of the PrestoBlue dye positively correlates with cell viability since the blue dye resazurin is reduced to the red dye resorufin in the presence of living cells [6]. Cell viability was normalized to the viability of the control group.
2.7. Visualization of cell viability and growth patterns
In order to assess cell survival, a live/dead-staining with fluorescein diacetate (FDA) and propidium iodide (PI) was performed. FDA is cell-permeant and hydrolysed to the green fluorescent fluorescein by intracellular esterases [6]. PI is a non-cell-permeant fluorescent dye that intercalates into DNA of membrane-compromised cells [6]. After rinsing the wells with 1x PBS, staining solution containing 8 μg/ml FDA (Sigma Aldrich, Steinheim, Germany) and 20 μg/ml PI (Life Technologies, Darmstadt, Germany) was added to each well and the samples were incubated for 5 min at room temperature. The wells were rinsed with 1x PBS, then kept in PBS and evaluated immediately under an Olympus IX-81 inverted fluorescence microscope (Olympus, Hamburg, Germany).
2.8. Cell proliferation assay
The content of dsDNA directly correlates with the number of mononuclear cells such as MSCs [6]. In order to monitor MSC proliferation over time, dsDNA content was assessed using the Quant-iT PicoGreen dsDNA Assay Kit (Life Technologies, Darmstadt, Germany) according to the manufacturer's instructions. Cell proliferation was normalized to the proliferation of the control group.
2.9. qPCR
Quantitative PCR was used to monitor osteogenic differentiation of MSCs in presence of BGs on a genetic level. RNA isolation was performed using the PureLink RNA Mini Kit (Life Technologies, Darmstadt, Germany) according to the manufacturer's instructions. 100 ng RNA per sample were used for cDNA synthesis by means of High-Capacity RNA-to-cDNA Kit (Life Technologies, Darmstadt, Germany). PowerUp SYBR Green Master Mix (Life Technologies, Darmstadt, Germany) was used according to manufacturer's instructions to perform a qPCR for selected genes (Primers are shown in Table 1). Expression of the target genes was referenced to an endogenous control with stable expression and normalized to the gene expression of the control group using the ΔΔCt method.
Table 1.
Primers used for qPCR. tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ; reference gene), Runt-related transcription factor 2 (RUNX2), secreted phosphoprotein 1/osteopontin (SPP1/OPN), osteocalcin (OCN).
| Gene | Forward (5’ → 3′) | Reverse (3’ → 5′) |
|---|---|---|
| YWHAZ | TGC TTG CAT CCC ACA GAC TA | AGG CAG ACA ATG ACA GAC CA |
| RUNX2 | TGG CAG TCA CAT GGC AGA TT | CTT TTC GGG GAG GAG AGC AG |
| OPN | GCT AAA CCC TGA CCC ATC TC | ATA ACT GTC CTT CCC ACG GC |
| OCN | ACC GAG ACA CCA TGA GAG CC | GCT TGG ACA CAA AGG CTG CAC |
2.10. Statistics
Statistical analyses were conducted using SAS (Version 9.4; SAS Institute Inc., Cary, USA). Values were compared via non-parametric Brunner-Munzel rank order test and Wilcoxon test, p-values of <0.05 were regarded as significant. N = 10 single replicates were used for each quantitative method. Graphs were created using GraphPad Prism (Version 8.1.0; GraphPad Software, La Jolla, USA). Values are shown as rounded means with standard deviation where applicable.
3. Results
3.1. Ion release from BG
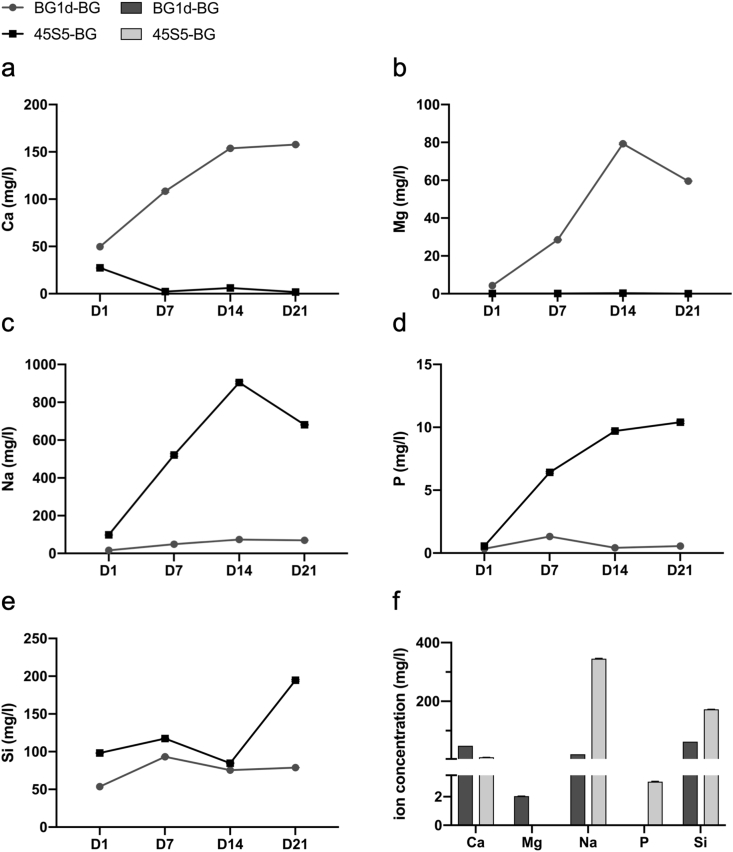
The ion release profiles of BG1d-BG and 45S5-BG are shown in Fig. 2. Comparing the two BG groups in the direct culture setting (D1-D21), the release of Ca ions appeared to be higher in the BG1d-BG group (Fig. 2a). The release of Mg ions from BG1d-BG increased from D1 to D14 and slightly decreased on D21, whereas 45S5-BG does not contain and thus did not release any Mg ions (Fig. 2b). A greater release of Na and P ions was observed in the 45S5-BG group compared to the BG1d-BG group (Fig. 2c and d). The Si release profiles were comparable in both BG groups (Fig. 2e). Ion concentrations after an incubation period of 24 h (representing the indirect cultivation setting) showed a more pronounced release of Ca and Mg ions in the BG1d-BG group, while more Na, P and Si ions were measured in the 45S5-BG group (Fig. 2f).
Fig. 2.
Ion release profiles of 10 mg/ml BG1d- and 45S5-BG. The release of Ca (a), Mg (b), Na (c), P (d) and Si (e) was measured via ICP-OES from D1 to D21 (a-e) and after 24 h (f) of incubation in distilled water.
3.2. BGs show a concentration-dependent increase of cytotoxicity
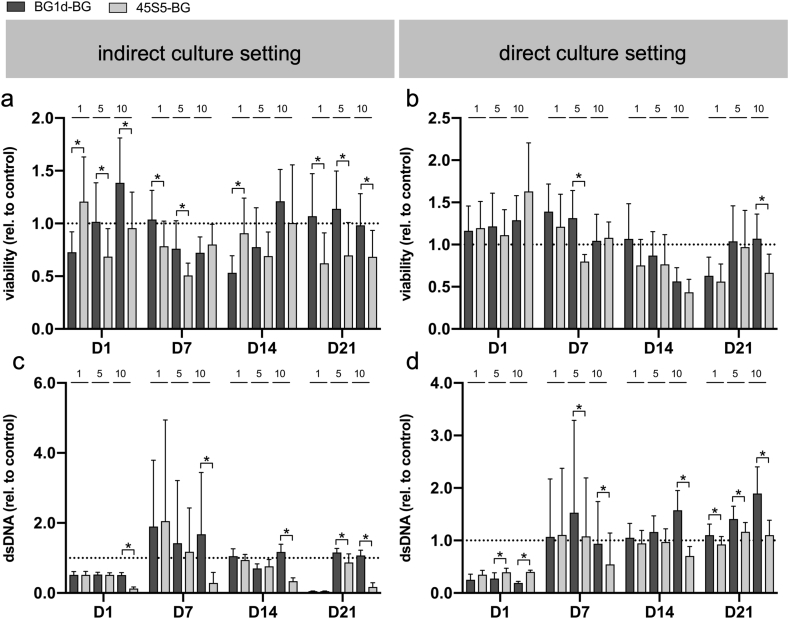
Compared to the control group, cell viability and proliferation were impaired in most of the groups in the indirect culture setting (Fig. 3a, c). In contrast, cell viability tended to be stimulated by the direct presence of BGs especially on the first days of cultivation and cell proliferation was enhanced in most direct groups from D7 onward (Fig. 3b, d). Cell viability and proliferation tended to be least impaired by 1 mg/ml of BG in both culture settings, however, 5 mg/ml of BG appeared to be tolerated by the cells (Fig. 3a–d). At certain measurement time points, however, 5 mg/ml of BG were superior to 1 mg/ml of BG, e.g. concerning proliferation at D1 and D7 in the direct culture setting (Fig. 3d). At a concentration of 10 mg/ml, the BGs seemed to affect cell viability and proliferation negatively especially in the 45S5-BG group upon indirect exposure (Fig. 3a–d). Interestingly, the difference between the two BGs appeared to be more distinct at a concentration of 5 mg/ml and 10 mg/ml of BG compared to 1 mg/ml of BG, particularly in the direct culture setting (Fig. 3a–d). The effect of the BGs on the cells thus seemed to be dependent not only on the culture setting, but also on the applied BG concentration.
Fig. 3.
Reduction of PrestoBlue reagent as a correlate of cell viability (a, b) and cell proliferation as evaluated via PicoGreen assay (c, d) in the indirect and direct cell culture setting during the incubation period (D1-D21). Values are normalized to the control group (indicated by the dotted line) and shown as means with standard deviation. (*) indicates significant difference between the BG groups.
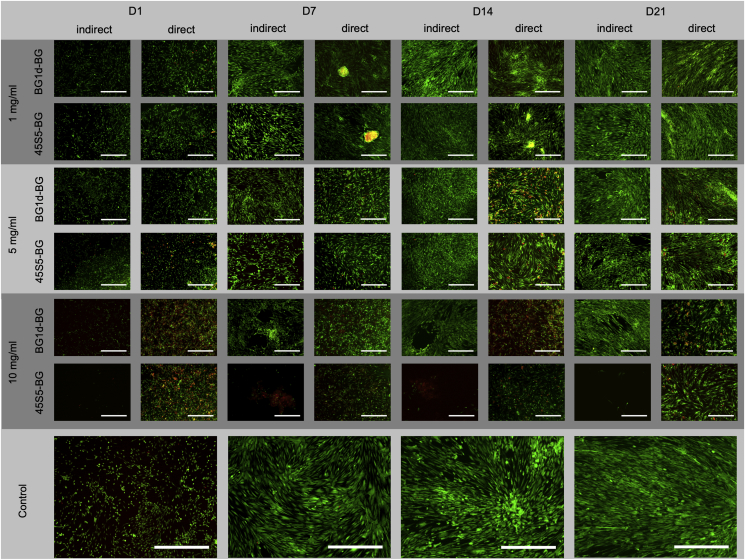
The concentration-dependent effect of the BGs on the cells could be observed in the fluorescence microscopy as well: In the indirect culture setting, cell density increased throughout the incubation period in all groups (BG groups and negative control) (Fig. 4). Comparing the BG concentrations, cell density was highest in both 1 mg/ml groups (BG1d-BG group and 45S5-BG group), however, it was comparable in the 5 mg/ml groups. In contrast to that, the MSCs did not reach confluence in any of the 10 mg/ml groups. In the direct culture setting, an increase in cell density could be observed in all groups (BG groups and negative control) during the whole incubation period (Fig. 4). Viable cells were dominating in both BG groups as well as in the control group. When comparing the BG concentrations, cell density appeared to be higher in the 1 mg/ml and 5 mg/ml groups. Red-stained DNA indicating non-viable cells was observed especially in the 10 mg/ml groups. Furthermore, MSCs did not grow to a confluent layer covering the plate in the 10 mg/ml groups as they did in the 1 mg/ml and 5 mg/ml groups as well as in the control group (Fig. 4). In both culture settings, cell density appeared to be slightly higher in the 1 mg/ml groups compared to the 5 mg/ml groups, however, no differences in shape and fluorescence signal were observed.
Fig. 4.
Representative live/dead-assay for the indirect and direct culture setting and the control group during the incubation period (D1-D21). Viable cells show green, free DNA molecules from dead cells in red fluorescence. Magnification: 40-fold. Scale bars refer to 250 μm.
Based on these results, the BG concentration of 5 mg/ml was chosen for the evaluation of osteogenic differentiation, as this concentration was not only tolerated by the MSCs but even positively influenced cell viability and proliferation at certain measurement time points (Fig. 3a–d). Compared to the 1 mg/ml groups, more significant differences were found between the 5 mg/ml groups (Fig. 3a–d). These findings suggest that the impact of the respective BGs on the cells is concentration-dependent and that their different effect on cell behavior becomes apparent especially when higher BG-concentrations are applied. The same held true for the 10 mg/ml groups, however, cell growth patterns were denser in the 5 mg/ml groups than in the 10 mg/ml groups, especially in the indirect culture setting (Fig. 4).
A closer look at the results in the 5 mg/ml groups revealed an advantage of BG1d-BG over 45S5-BG concerning cell viability and proliferation: In the indirect culture setting, cell viability was significantly higher in the BG1d-BG group compared to the 45S5-BG group on D1, D7 and D21 (Fig. 3a). On D14, higher cell viability was observed in the BG1d-BG group as well, however, the difference remained at a non-significant level (Fig. 3a). Cell proliferation was non-significantly higher in the BG1d-BG group compared to the 45S5-BG group on D1 and D7, whereas on D14 it was non-significantly higher in the 45S5-BG group (Fig. 3c). On D21, significantly higher cell viability was observed in the BG1d-BG group (Fig. 3c). When comparing the two BGs in the direct culture setting, cell viability was higher in the BG1d-BG group at all measurement time points, however the difference was not significant except for D7 (Fig. 3b). Cell proliferation was significantly higher in the 45S5-BG group compared to the BG1d-BG group on D1, while on D7 and D21 it was significantly higher in the BG1d-BG group (Fig. 3d). Non-significantly higher proliferation was observed in the BG1d-BG group on D14 (Fig. 3d).
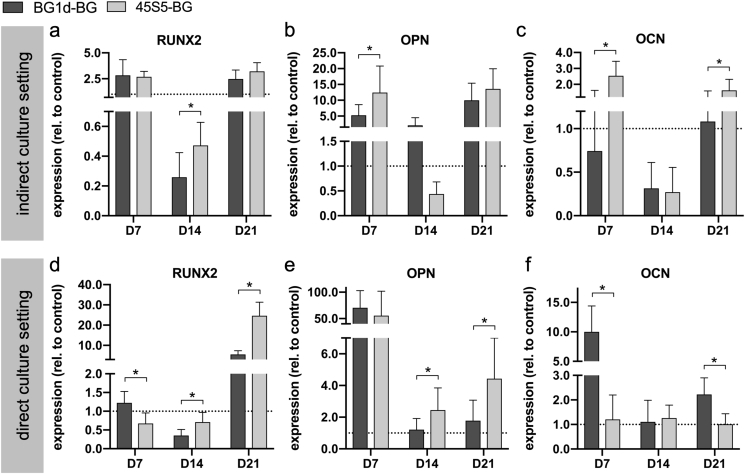
3.3. BGs induce an earlier onset of osteogenic differentiation and accelerate MSC maturation towards osteoblasts on a genetic level
Comparing the two BG groups in the indirect culture setting, significantly higher RUNX2 expression levels were observed in the 45S5-BG group on D14 (Fig. 5a). On D7, expression levels were higher in the BG1d-BG group and on D21 they were higher in the 45S5-BG group again, however the difference remained non-significant (Fig. 5a). On D7 and D21, RUNX2 expression was higher in both BG groups compared to the control group whereas on D14, it was lower than in the control group in both BG groups (Fig. 5a). OPN expression was significantly higher in the 45S5-BG group compared to the BG1d-BG group on D7 and non-significantly higher on D21 (Fig. 5b). On D14, OPN expression was non-significantly higher in the BG1d-BG group (Fig. 5b). Except for the 45S5-BG group on D14, both BG groups showed higher OPN expression than the control group at all measurement time points (Fig. 5b). OCN expression was significantly higher in the 45S5-BG group on D7 and D21 (Fig. 5c). On D14, expression values were non-significantly higher in the BG1d-BG group (Fig. 5c). OCN expression levels were higher than in the control group in the 45S5-BG group on D7 and D21 and in the BG1d-BG group on D21 (Fig. 5c). In conclusion, an advantage of 45S5-BG over BG1d-BG concerning the expression of RUNX2, OPN and OCN was observed in the indirect culture setting.
Fig. 5.
Gene expression as a correlate of osteogenic differentiation during the incubation period (D7-D21). RUNX2 (a, d) as a marker of early osteogenic differentiation. OPN (b, e) and OCN (c, f) as markers of mature osteoblasts. Values are normalized to the control group (indicated by the dotted line) and shown as means with standard deviation. (*) indicates significant difference.
Comparing the BG groups in the direct culture setting, significantly higher RUNX2 expression levels were observed in the BG1d-BG group on D7 while they were significantly higher in the 45S5-BG group on D14 and D21 (Fig. 5d). RUNX2 expression was higher in the BG1d-BG group compared to the control group on D7 and D21 and in the 45S5-BG group, RUNX2 expression was superior to the control group on D21 (Fig. 5d). OPN expression levels were significantly higher in the 45S5-BG group on D14 and D21 whereas no significant differences were detected on D7 (Fig. 5e). However, maximum expression was reached on D7 in both groups and a tendency towards higher expression levels was observed in the BG1d-BG group (Fig. 5e). Compared to the control group, OPN expression was superior in the BG groups at all measurement time points (Fig. 5e). OCN expression levels were significantly higher in the BG1d-BG group compared to the 45S5-BG group on both D7 and D21 while on D14, they were non-significantly higher in the 45S5-BG group (Fig. 5f). OCN expression levels were above the level of the control group in both BG groups at all measurement time points except for the 45S5-BG group on D21 (Fig. 5f). In summary, 45S5-BG had a stronger impact on RUNX2 and OPN expression whereas BG1d-BG was superior regarding OCN expression.
4. Discussion
The development of new BG compositions might help to overcome the limitations of the existing BGs, for instance the alkalization of the surrounding environment upon immersion of BGs in (body) fluids or their mechanical fragility [1,7]. BG1d-BG has already been evaluated in an in-vivo study and in a preliminary clinical trial conducted by Tulyaganov et al. [18]. They found BG1d-BG to be biocompatible not only upon implantation into rabbit femurs, but also when used for the treatment of jawbone defects in 45 patients. However, newly developed BG compositions ought to be compared to a benchmark or a well-known standard regarding biocompatibility on the one hand and osteogenic potential on the other hand. Consequently, this study completes the results obtained by Tulyaganov et al. by providing an in-vitro comparison of BG1d-BG to the well-known 45S5-BG [1].
In this study, MSCs were exposed to the BGs in an indirect and direct culture setting [7,8] at concentrations of 1 mg/ml, 5 mg/ml and 10 mg/ml. A concentration-dependent effect of BGs on cell proliferation and viability was reported previously [36,[38], [39], [40], [41]] and similar results were found in this study. Compared to the control group, cell proliferation was impaired when MSCs were exposed to 10 mg/ml of BG (Fig. 3c, d and Fig. 4). Cell viability decreased as well, especially after the first days of cultivation with 10 mg/ml of BG (Fig. 3a, b). Based on the evaluation of the BGs' concentration-dependent effect on cell proliferation and viability, the average BG concentration of 5 mg/ml was chosen for the evaluation of the BGs' impact on osteogenic differentiation. When comparing the two BGs at 5 mg/ml, an advantage of BG1d-BG over 45S5-BG concerning cell viability and proliferation was revealed in both culture settings (Fig. 3a–d, Fig. 4). It has been shown previously that the release of Na ions causes a cytotoxic effect and inhibits cell proliferation [42,43]. The ion release profiles obtained via ICP-OES reveal a higher release of Na ions from 45S5-BG compared to BG1d-BG (Fig. 2c) which can be attributed to the different composition of the BGs: 45S5-BG contains 24.5 wt% of Na2O while BG1d-BG contains only 4.5 wt%. Additionally, Mg ions have been shown to increase cell viability [44,45]. In contrast to 45S5-BG, BG1d-BG contains Mg, which is released into the CCM especially from D7 onward (Fig. 2b, f). Furthermore, a higher amount of Ca ions was released from BG1d-BG compared to 45S5-BG (Fig. 2a, f). Ca ions have been shown to enhance cell viability and proliferation [46] and may therefore contribute to the advantage of the BG1d-BG group. The combined effect of the lower amount of Na ions and the presence of Mg ions as well as a higher amount of Ca ions in the CCM containing BG1d-BG might thus explain the higher cell viability and stronger proliferation in the BG1d-BG group. However, based on the results found in this study, the role of the respective ions on the behavior of MSCs cannot be anticipated directly. It might thus be interesting to further investigate the respective ions’ role, e.g. by comparing BG compositions differing solely in one ion. Additionally, analyzing the ion release from BGs and its effect on cell behavior over a longer period of time might provide more information on the changes in cell behavior induced by the varying ion concentrations in the course of cultivation. The ion release assay was performed in distilled water following a recently published recommendation [36]. Ion release from BGs differs based on experimental settings (static vs. dynamic) and also depending on the media used as recently shown in a study conducted by Arango Ospina and coworkers [47]. It is likely that the ion release in DMEM is different, thus a direct comparison is not possible and the influence of the respective ions can only be anticipated indirectly.
Most studies investigating the impact of BGs on cell viability and proliferation focus on the direct contact between cells and BGs [8]. However, there are studies comparing the effect of BGs in both indirect and direct culture settings, e.g. the group of Begum [48], in order to predict the BGs' effect upon implantation in-vivo more precisely: When applied in-vivo, biomaterial will not only act on the cells present at the implantation site directly, but ions released from the BGs will also be transported to other parts of the body via body fluids and/or diffusion. The direct setting thus represents the physical contact of bone cells and BG whilst the indirect setting stands for the influence of ions released from the BGs that reach bone cells via body fluids and/or diffusion [48]. Previous studies showed varying results regarding the cytotoxicity of BG particles and their ionic dissolution products: Qazi et al. [36] compared the effect of 45S5-BG and 1393-BG on MSCs on D1, D4 and D7 in a direct culture setting and two indirect culture settings. For the indirect culture settings, transwell inserts and MSCs encapsulated in 3D alginate beads were used [36]. In their study, BG particles had a more pronounced cytotoxic effect than their ionic dissolution products [36]. In a study conducted by Bellucci et al. [49], 45S5-BG, BGCaM80 and BGCaM30 were compared in direct and indirect contact to murine fibroblasts and osteocytes. The BGs' effect on cell viability was evaluated after 24 h and 48 h [49]. In their study, Bellucci et al. [49] found that neither the direct nor the indirect contact to BGs affected cell growth and viability negatively. In contrast to the above-mentioned studies, the effect of BGs on MSCs was evaluated over a longer period of time in this study. Interestingly, a tendency towards higher cell viability and proliferation was observed in the direct culture setting compared to the indirect culture setting, particularly regarding cell viability in the BG1d-BG group (Fig. 3a–d). The advantage of the direct BG1d-BG group compared to the corresponding indirect group may partially be attributed to BG1d-BG's Mg release kinetics (Fig. 2b, f): As mentioned above, Mg ions have been reported to enhance cell viability but are released at a later stage of incubation, i.e. after more than 24 h (Fig. 2b, f) and are thus particularly present in the direct culture setting but not in the indirect setting. The effect of pure Mg and Mg alloys on cell viability and osteogenic differentiation has been investigated in several studies [50,51]: Yoshizawa et al. [50] described an increase in type X collagen and vascular endothelial growth factor gene expression upon exposure of MSCs to MgSO4. Additionally, Kim et al. [51] observed enhanced proliferation and differentiation of human fetal osteoblasts cultured in presence of Mg alloys. Furthermore, BG supplemented with Mg-doped tricalcium phosphate has been evaluated regarding its biocompatibility and osteogenic potential [52] and appeared to promote proliferation and differentiation of mouse pre-osteoblastic cells. In a study conducted by Wang et al. [53], rat bone marrow MSCs showed enhanced proliferation and upregulation of OCN, OPN and alkaline phosphatase (ALP) expression upon exposure to Mg-containing titanium surfaces. Titanium implants doped with Mg ions have also been evaluated in-vivo: Galli et al. [54] found an increase in OCN, RUNX2 and insulin-like growth factor 1 expression upon implantation of Mg-loaded titanium implants in the tibia of rabbits. Furthermore, Tao et al. [55] placed Mg-containing titanium implants in the femur of rats and observed an increase in bone formation and biomechanical strength compared to the control group. While the impact of the above-mentioned Mg-containing biomaterials has already been investigated, the effect of Mg ion incorporation into BGs has yet to be explored in further detail. In addition to that, further ions such as Ca ions have been shown to improve cell viability and proliferation [46] and could thus act synergistically to the Mg ions' positive effect on BG1d-BG's biocompatibility. Furthermore, Na ions have been reported to be cytotoxic in a cell culture setting as mentioned above [42,43] and consequently influence cell viability and proliferation as well. One may thus assume complex interactions between these ions' effect on the BGs' biocompatibility. In order to attribute the effect to the respective ions, it would be necessary to investigate the cells' behavior upon exposure to altered BG compositions.
Expression levels of RUNX2, OPN and OCN were investigated in order to evaluate the osteogenic potential of BGs. RUNX2 is a transcription factor that plays a central role in osteogenic differentiation. Its expression varies during the process of differentiation: Whilst being upregulated in the early stages of osteoblast differentiation (e.g. preosteoblasts and osteoblasts), RUNX2 expression is downregulated in mature osteoblasts and osteocytes [[56], [57], [58], [59], [60]]. However, at a later stage of osteoblast differentiation, RUNX2 stimulates growth factor expression, inducing further maturation as well as osteogenic differentiation of until then undifferentiated cells within a cell population of various stages of development [61,62]. In the indirect culture setting, RUNX2 expression levels were higher compared to the control group on D7, then declined until D14 and were elevated again on D21 (Fig. 5a). In the direct culture setting, RUNX2 expression levels were above the level of the control group in the BG1d-BG group on D7 and in both BG groups on D21, while a decline was observed on D14 (Fig. 5d). As RUNX2 is required for the transcription of further osteogenic genes, e.g. OPN and OCN [63,64], these findings indicate that osteogenic differentiation started on D7. The late increase in RUNX2 expression on D21 can be explained by an upregulation of growth factor production, leading to the differentiation of further MSCs into osteoblasts [61,62]. However, the expression of RUNX2 in the 45S5-BG group in direct contact to the BG remained below the level of the control group until D21 (Fig. 5d), suggesting that in this group, osteogenic differentiation occurred later than in the other groups (e.g. after D14). Interestingly, RUNX2 is not only involved in osteoblast maturation, but also in cell cycle regulation and thus proliferation [65,66]. According to the studies conducted by Galindo et al. [65] and Pratap et al. [66], upregulation of RUNX2 causes a delay in the G0/G1-transition in preosteoblasts and thus induces the cells’ exit from the cell cycle while activating genes that stimulate osteogenic differentiation. During the proliferative period of osteogenic differentiation, however, RUNX2 is downregulated in order to allow entry into S-phase [65]. This cell cycle-controlled downregulation of RUNX2 during the process of differentiation might be an explanation for the decrease in RUNX2 expression levels observed on D14 (Fig. 5a, d), indicating a focus on proliferation at this stage of osteoblast maturation.
Increasing OPN expression can be detected from the stage of preosteoblasts to mature osteoblasts, however, OPN expression levels are far more elevated in mature osteoblasts [67] and can thus be interpreted as an indicator of advanced osteogenic differentiation [68]. High OPN expression levels can therefore be expected between D14 and D28 in cell culture settings [37], however, OPN expression levels were already elevated above the level of the control group on D7 (Fig. 5b, e). On D14, OPN expression levels decreased before increasing again on D21 (Fig. 5b, e). These findings were observed in both BG groups and irrespective of the culture setting and suggest an earlier onset of osteogenic differentiation induced by the BGs or their ionic dissolution products. Furthermore, the re-increase of OPN expression on D21 indicates the differentiation of further MSCs into osteoblasts induced by growth factor secretion as discussed above.
OCN is a bone specific protein known to be a marker of late stage osteogenic differentiation and plays a role in extracellular matrix synthesis and mineralization [57,[68], [69], [70]]. Similar to OPN expression, OCN expression is elevated during the final stages of osteogenic differentiation, e.g. between 14 and 28 days of cultivation [37]. In the indirect culture setting, OCN expression outperformed the expression shown by the control group on D7 in the 45S5-BG group and on D21 in both BG groups (Fig. 5c). On D14, OCN expression levels were lowest in both BG groups (Fig. 5c). These observations suggest an earlier onset of osteogenic differentiation in the 45S5-BG group. In the direct culture setting, OCN expression in the BG groups was higher compared to the control group at all measurement time points (Fig. 5f). Maximum OCN expression was reached on D7 in the BG1d-BG group, whilst in the 45S5-BG group maximum expression levels were observed on D14 (Fig. 5f).
Taken together, these results indicate that both BGs do not only support osteogenic differentiation but also accelerate its onset and the maturation of the MSCs in osteoblastic lineage. The stimulation of osteogenic differentiation represented by increased expression levels of osteoblast marker genes can be attributed to the presence of BGs as no other osteogenic differentiation stimuli such as dexamethasone, ascorbic acid and/or β-glycerol phosphate [37,71] were added to the CCM. When comparing the two BGs in the indirect culture setting, 45S5-BG appeared to have a higher osteogenic potential than BG1d-BG (Fig. 5a–c). In the direct culture setting, however, this advantage of 45S5-BG over BG1d-BG was less pronounced and especially in regard to OCN expression levels, BG1d-BG outperformed 45S5-BG (Fig. 5d–f). Taking into consideration the BGs' ion release profiles, these findings may be explained by the BGs’ different composition and release kinetics: Ca, Mg, P and Si ions have been shown to promote osteogenic differentiation and bone formation in-vivo and in-vitro [72]. The data obtained via ICP-OES reveal a higher concentration of P and Si ions in the indirect medium containing 45S5-BG compared to the medium containing BG1d-BG (Fig. 2f). Several studies have shown P ions to stimulate matrix Gla protein and OPN expression via the ERK1/2 pathway making P ions relevant as a signalling molecule in osteogenic differentiation and bone mineralization [73,74]. Si ions have been shown to enhance collagen type 1 synthesis and to cause an increase in alkaline phosphatase activity as well as OCN expression, thereby stimulating osteogenic differentiation [75]. The higher amount of P and Si ions released from the 45S5-BG after 24 h of incubation in DMEM may thus be held responsible for its higher osteogenic potential in the indirect culture setting. After 24 h of incubation in DMEM, Mg ions were already detectable in the medium containing BG1d-BG, however, the Mg concentration increased more than tenfold until D7 (Fig. 2b, f). Assuming that the Mg ions released from BG1d-BG are partly responsible for its osteogenic potential [76,77], these findings might explain why BG1d-BG outperformed 45S5-BG in the direct culture setting, but not in the indirect one. The indirect tests were performed with the supernatant from DMEM that was exposed to the BGs for 24 h and the MSCs were thus exposed to smaller amounts of Mg ions than the MSCs cultured in direct contact two the BGs (Fig. 2b, f). A similar evolution can be observed regarding the Ca ion concentration: While staying at the same level from 24 h to D1, the concentration of Ca ions doubled from D1 to D7 and tripled from D1 to D14 (Fig. 2a, f). Hence the MSCs cultured in the direct culture setting were not only exposed to higher concentrations of Mg ions, but also to a higher amount of Ca ions. As Ca ions stimulate proliferation and differentiation of osteogenic progenitor cells [46], the increase in Ca ion concentration may provide an explanation for the advantage of BG1d-BG over 45S5-BG in the direct culture setting. As a consequence, a synergistic effect of Mg and Ca ions on osteogenic differentiation may lead to an advantage of BG1d-BG in the direct, but not in the indirect culture setting. The exact role of the respective ions can be evaluated by altering the BG compositions. Replacing one ion at a time and investigating the effect on cell behavior would help to understand the exact role of the single ions in a more detailed manner.
In a study conducted by Ojansivu et al. [78] it has been shown that the physical contact of MSCs to the BGs is crucial for the BGs’ osteoinductive properties. In their study, the effect of the silica-based BGs S53P4-BG and 1-06-BG on human adipose stem cells was evaluated upon direct and indirect exposure of the cells to the BGs. Whilst early osteogenic differentiation represented by an increase in ALP activity and elevated osteogenic gene expression levels was observed in the direct culture setting, these results could not be reproduced in the indirect culture setting [78]. Similar results were found in this study – gene expression levels tended to be higher in the direct culture setting compared to the indirect culture setting (e.g. OPN expression on D7, Fig. 5b, e), indicating a stronger impact of the BGs on osteogenic differentiation upon direct exposure of the cells to BG particles.
5. Conclusions
In this study, the CaO–MgO–SiO2-based BG composition BG1d-BG was compared to the established 45S5-BG regarding the BGs' effect on cell viability, proliferation and osteogenic differentiation in-vitro. Taken together, the obtained results indicate an advantage of BG1d-BG in regard to cell viability and proliferation. Osteogenic differentiation was accelerated in presence of both BGs. Compared to BG1d-BG, however, the ions released from 45S5-BG appeared to have stronger osteoinductive properties whereas no clear superiority of either of the BGs was observed upon direct cell-BG contact. Given its good osteogenic potential compared to the benchmark 45S5-BG and its higher biocompatibility, BG1d-BG seems to be an interesting alternative to 45S5-BG for bone tissue engineering applications and should also be compared to 45S5-BG using in-vivo models. In order to allow a better understanding of the mechanisms underlying the BGs’ impact on cell behavior, the effect of varying BG compositions needs to be assessed in further detail.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgements
We thank Dr. Jörg Fellenberg for technical support and inspiring scientific discussion and Sebastian Wilkesmann and Frederike Hohenbild for their support in designing the figures. This study was funded by the research fund of the Heidelberg Orthopedic University Hospital. Dr. Fabian Westhauser is supported by the “Physician Scientist Program”-scholarship introduced by the Medical Faculty of the University of Heidelberg. This study contains parts of Sarah Isabelle Schmitz's doctoral thesis.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Baino F., Hamzehlou S., Kargozar S. Bioactive glasses: where are we and where are we going? J. Funct. Biomater. 2018;9(1) doi: 10.3390/jfb9010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hench L.L., Splinter R.J., Allen W.C., Greenlee T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971;5(6):117–141. [Google Scholar]
- 3.Jones J.R. Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2013;9(1):4457–4486. doi: 10.1016/j.actbio.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Xynos I.D., Edgar A.J., Buttery L.D.K., Hench L.L., Polak J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass® 45S5 dissolution. J. Biomed. Mater. Res. 2001;55(2):151–157. doi: 10.1002/1097-4636(200105)55:2<151::aid-jbm1001>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Xynos I.D., Edgar A.J., Buttery L.D., Hench L.L., Polak J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Biophys. Res. Commun. 2000;276(2):461–465. doi: 10.1006/bbrc.2000.3503. [DOI] [PubMed] [Google Scholar]
- 6.Westhauser F., Karadjian M., Essers C., Senger A.S., Hagmann S., Schmidmaier G., Moghaddam A. Osteogenic differentiation of mesenchymal stem cells is enhanced in a 45S5-supplemented beta-TCP composite scaffold: an in-vitro comparison of Vitoss and Vitoss BA. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0212799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciraldo F.E., Boccardi E., Melli V., Westhauser F., Boccaccini A.R. Tackling bioactive glass excessive in vitro bioreactivity: preconditioning approaches for cell culture tests. Acta Biomater. 2018;75:3–10. doi: 10.1016/j.actbio.2018.05.019. ISSN 1742-7061. [DOI] [PubMed] [Google Scholar]
- 8.Karadjian M., Essers C., Tsitlakidis S., Reible B., Moghaddam A., Boccaccini A.R., Westhauser F. Biological properties of calcium phosphate bioactive glass composite bone substitutes: current experimental evidence. Int. J. Mol. Sci. 2019;20(2) doi: 10.3390/ijms20020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brito A.F., Antunes B., Dos Santos F., Fernandes H.R., Ferreira J.M.F. Osteogenic capacity of alkali-free bioactive glasses. In vitro studies. J. Biomed. Mater. Res. B Appl. Biomater. 2017;105(8):2360–2365. doi: 10.1002/jbm.b.33771. [DOI] [PubMed] [Google Scholar]
- 10.Westhauser F., Senger A.S., Obert D., Ciraldo F.E., Schuhladen K., Schmidmaier G., Moghaddam A., Boccaccini A.R. Gelatin coating increases in vivo bone formation capacity of three-dimensional 45S5 bioactive glass-based crystalline scaffolds. J. Tissue Eng. Regenerat. Med. 2019;13(2):179–190. doi: 10.1002/term.2780. [DOI] [PubMed] [Google Scholar]
- 11.Fu Q., Saiz E., Rahaman M.N., Tomsia A.P. Bioactive glass scaffolds for bone tissue engineering: state of the art and future perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2011;31(7):1245–1256. doi: 10.1016/j.msec.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchanan L.A., El-Ghannam A. Effect of bioactive glass crystallization on the conformation and bioactivity of adsorbed proteins. J. Biomed. Mater. Res. A. 2010;93(2):537–546. doi: 10.1002/jbm.a.32561. [DOI] [PubMed] [Google Scholar]
- 13.Agathopoulos S., Tulyaganov D.U., Ventura J.M., Kannan S., Karakassides M.A., Ferreira J.M. Formation of hydroxyapatite onto glasses of the CaO-MgO-SiO2 system with B2O3, Na2O, CaF2 and P2O5 additives. Biomaterials. 2006;27(9):1832–1840. doi: 10.1016/j.biomaterials.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 14.Agathopoulos S., Tulyaganov D.U., Valerio P., Ferreira J.M. A new model formulation of the SiO2-Al2O3-B2O3-MgO-CaO-Na2O-F glass-ceramics. Biomaterials. 2005;26(15):2255–2264. doi: 10.1016/j.biomaterials.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Agathopoulos S., Tulyaganov D.U., Ventura J.M.G., Kannan S., Saranti A., Karakassides M.A., Ferreira J.M.F. Structural analysis and devitrification of glasses based on the CaO–MgO–SiO2 system with B2O3, Na2O, CaF2 and P2O5 additives. J. Non-Cryst. Solids. 2006;352(4):322–328. [Google Scholar]
- 16.Tulyaganov D.U., Agathopoulos S., Valerio P., Balamurugan A., Saranti A., Karakassides M.A., Ferreira J.M. Synthesis, bioactivity and preliminary biocompatibility studies of glasses in the system CaO-MgO-SiO2-Na2O-P2O5-CaF2, Journal of materials science. Mater. Med. 2011;22(2):217–227. doi: 10.1007/s10856-010-4203-5. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalo-Juan I., Tulyaganov D.U., Balan C., Linser R., Ferreira J.M.F., Riedel R., Ionescu E. Tailoring the viscoelastic properties of injectable biocomposites: a spectroscopic assessment of the interactions between organic carriers and bioactive glass particles. Mater. Des. 2016;97:45–50. [Google Scholar]
- 18.Tulyaganov D.U., Makhkamov M.E., Urazbaev A., Goel A., Ferreira J.M.F. Synthesis, processing and characterization of a bioactive glass composition for bone regeneration. Ceram. Int. 2013;39(3):2519–2526. [Google Scholar]
- 19.Tulyaganov D.U., Reddy A.A., Siegel R., Ionescu E., Riedel R., Ferreira J.M.F. Synthesis and in vitro bioactivity assessment of injectable bioglass−organic pastes for bone tissue repair. Ceram. Int. 2015;41(8):9373–9382. [Google Scholar]
- 20.Fernandes H.R., Tulyaganov D.U., Ribeiro M.J., Ferreira J.M.F. Apatite crystallization from glasses in the Ca5(PO4)3F–CaAl2Si2O8–CaMgSi2O6–NaAlSi3O8 system. J. Non-Cryst. Solids. 2013;363:32–38. [Google Scholar]
- 21.Tulyaganov D.U., Agathopoulos S., Ventura J.M., Karakassides M.A., Fabrichnaya O., Ferreira J.M.F. Synthesis of glass–ceramics in the CaO–MgO–SiO2 system with B2O3, P2O5, Na2O and CaF2 additives. J. Eur. Ceram. Soc. 2006;26(8):1463–1471. [Google Scholar]
- 22.Saboori A., Rabiee M., Moztarzadeh F., Sheikhi M., Tahriri M., Karimi M. Synthesis, characterization and in vitro bioactivity of sol-gel-derived SiO2–CaO–P2O5–MgO bioglass. Mater. Sci. Eng. C. 2009;29(1):335–340. [Google Scholar]
- 23.Wang X., Li X., Ito A., Sogo Y. Synthesis and characterization of hierarchically macroporous and mesoporous CaO-MO-SiO(2)-P(2)O(5) (M=Mg, Zn, Sr) bioactive glass scaffolds. Acta Biomater. 2011;7(10):3638–3644. doi: 10.1016/j.actbio.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 24.Prabhu M., Kavitha K., Manivasakan P., Rajendran V., Kulandaivelu P. Synthesis, characterization and biological response of magnesium-substituted nanobioactive glass particles for biomedical applications. Ceram. Int. 2013;39(2):1683–1694. [Google Scholar]
- 25.Brauer D.S., Karpukhina N., Law R.V., Hill R.G. Structure of fluoride-containing bioactive glasses. J. Mater. Chem. 2009;19(31):5629–5636. [Google Scholar]
- 26.Fujii E., Kawabata K., Yoshimatsu H., Hayakawa S., Tsuru K., Osaka A. Structure and biomineralization of calcium silicate glasses containing fluoride ions. J. Ceram. Soc. Jpn. 2003;111(1298):762–766. [Google Scholar]
- 27.Brauer D.S., Karpukhina N., O'Donnell M.D., Law R.V., Hill R.G. Fluoride-containing bioactive glasses: effect of glass design and structure on degradation, pH and apatite formation in simulated body fluid. Acta Biomater. 2010;6(8):3275–3282. doi: 10.1016/j.actbio.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 28.Kim H.W., Kim H.E., Knowles J.C. Fluor-hydroxyapatite sol-gel coating on titanium substrate for hard tissue implants. Biomaterials. 2004;25(17):3351–3358. doi: 10.1016/j.biomaterials.2003.09.104. [DOI] [PubMed] [Google Scholar]
- 29.Aaseth J., Shimshi M., Gabrilove J.L., Birketvedt G.S. Fluoride: a toxic or therapeutic agent in the treatment of osteoporosis? J. Trace Elem. Exp. Med. 2004;17(2):83–92. [Google Scholar]
- 30.Vestergaard P., Jorgensen N.R., Schwarz P., Mosekilde L. Effects of treatment with fluoride on bone mineral density and fracture risk--a meta-analysis. Osteoporos. Int.: Journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008;19(3):257–268. doi: 10.1007/s00198-007-0437-6. [DOI] [PubMed] [Google Scholar]
- 31.Wilkesmann S., Fellenberg J., Nawaz Q., Reible B., Moghaddam A., Boccaccini A.R., Westhauser F. Primary osteoblasts, osteoblast precursor cells or osteoblast-like cell lines: which human cell types are (most) suitable for characterizing 45S5-bioactive glass? J. Biomed. Mater. Res. A. 2019:1–12. doi: 10.1002/jbm.a.36846. [DOI] [PubMed] [Google Scholar]
- 32.Hoppe A., Boccaccini A.R. Biological impact of bioactive glasses and their dissolution products. Front. Oral Biol. 2015;17:22–32. doi: 10.1159/000381690. [DOI] [PubMed] [Google Scholar]
- 33.Reible B., Schmidmaier G., Moghaddam A., Westhauser F. Insulin-like growth factor-1 as a possible alternative to bone morphogenetic protein-7 to induce osteogenic differentiation of human mesenchymal stem cells in vitro. Int. J. Mol. Sci. 2018;19(6) doi: 10.3390/ijms19061674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reible B., Schmidmaier G., Prokscha M., Moghaddam A., Westhauser F. Continuous stimulation with differentiation factors is necessary to enhance osteogenic differentiation of human mesenchymal stem cells in-vitro. Growth Factors. 2017;35(4–5):179–188. doi: 10.1080/08977194.2017.1401618. [DOI] [PubMed] [Google Scholar]
- 35.Widholz B., Tsitlakidis S., Reible B., Moghaddam A., Westhauser F. Pooling of patient-derived mesenchymal stromal cells reduces inter-individual confounder-associated variation without negative impact on cell viability, proliferation and osteogenic differentiation. Cells. 2019;8(6) doi: 10.3390/cells8060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qazi T.H., Hafeez S., Schmidt J., Duda G.N., Boccaccini A.R., Lippens E. Comparison of the effects of 45S5 and 1393 bioactive glass microparticles on hMSC behavior. J. Biomed. Mater. Res. A. 2017;105(10):2772–2782. doi: 10.1002/jbm.a.36131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birmingham E., Niebur G., McHugh P., Shaw G., Barry F., McNamara L. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone nich. Eur. Cells Mater. 2012;23:13–27. doi: 10.22203/ecm.v023a02. [DOI] [PubMed] [Google Scholar]
- 38.Labbaf S., Tsigkou O., Muller K.H., Stevens M.M., Porter A.E., Jones J.R. Spherical bioactive glass particles and their interaction with human mesenchymal stem cells in vitro. Biomaterials. 2011;32(4):1010–1018. doi: 10.1016/j.biomaterials.2010.08.082. [DOI] [PubMed] [Google Scholar]
- 39.Tsigkou O., Labbaf S., Stevens M.M., Porter A.E., Jones J.R. Monodispersed bioactive glass submicron particles and their effect on bone marrow and adipose tissue-derived stem cells. Adv. Healthc. Mater. 2014;3(1):115–125. doi: 10.1002/adhm.201300126. [DOI] [PubMed] [Google Scholar]
- 40.Rismanchian M., Khodaeian N., Bahramian L., Fathi M., Sadeghi-Aliabadi H. In-vitro comparison of cytotoxicity of two bioactive glasses in micropowder and nanopowder forms. Iran. J. Pharm. Res. (IJPR) 2013;12(3):437–443. [PMC free article] [PubMed] [Google Scholar]
- 41.Westhauser F., Widholz B., Nawaz Q., Tsitlakidis S., Hagmann S., Moghaddam A., Boccaccini A.R. Favorable angiogenic properties of the borosilicate bioactive glass 0106-B1 result in enhanced in vivo osteoid formation compared to 45S5 Bioglass. Biomater. Sci. 2019;7(12):5161–5176. doi: 10.1039/c9bm01220f. [DOI] [PubMed] [Google Scholar]
- 42.Wallace K.E., Hill R.G., Pembroke J.T., Brown C.J., Hatton P.V. Influence of sodium oxide content on bioactive glass properties. J. Mater. Sci. Mater. Med. 1999;10(12):697–701. doi: 10.1023/a:1008910718446. [DOI] [PubMed] [Google Scholar]
- 43.Kansal I., Reddy A., Munoz F., Choi S.J., Kim H.W., Tulyaganov D.U., Ferreira J.M. Structure, biodegradation behavior and cytotoxicity of alkali-containing alkaline-earth phosphosilicate glasses. Mater. Sci. Eng. C Mater. Biol. Appl. 2014;44:159–165. doi: 10.1016/j.msec.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 44.Tulyaganov D., Abdukayumov K., Ruzimuradov O., Hojamberdiev M., Ionescu E., Riedel R. Effect of alumina incorporation on the surface mineralization and degradation of a bioactive glass (CaO-MgO-SiO(2)-Na(2)O-P(2)O(5)-CaF(2))-glycerol paste. Materials. 2017;10(11):1324. doi: 10.3390/ma10111324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He L.Y., Zhang X.M., Liu B., Tian Y., Ma W.H. Effect of magnesium ion on human osteoblast activity. Braz. J. Med. Biol. Res. 2016;49(7) doi: 10.1590/1414-431X20165257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maeno S., Niki Y., Matsumoto H., Morioka H., Yatabe T., Funayama A., Toyama Y., Taguchi T., Tanaka J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials. 2005;26(23):4847–4855. doi: 10.1016/j.biomaterials.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Arango Ospina M., Hupa L., Boccaccini A. Bioactivity and dissolution behavior of boron-containing bioactive glasses under static and dynamic conditions in different media. Biomed. Glasses. 2019;5:124–139. [Google Scholar]
- 48.Begum S., Johnson W.E., Worthington T., Martin R.A. The influence of pH and fluid dynamics on the antibacterial efficacy of 45S5 Bioglass. Biomed. Mater. (Bristol, England) 2016;11(1) doi: 10.1088/1748-6041/11/1/015006. 015006. [DOI] [PubMed] [Google Scholar]
- 49.Bellucci D., Sola A., Anesi A., Salvatori R., Chiarini L., Cannillo V. Bioactive glass/hydroxyapatite composites: mechanical properties and biological evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;51:196–205. doi: 10.1016/j.msec.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 50.Yoshizawa S., Brown A., Barchowsky A., Sfeir C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014;10(6):2834–2842. doi: 10.1016/j.actbio.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Kim H.K., Han H.S., Lee K.S., Lee D.H., Lee J.W., Jeon H., Cho S.Y., Roh H.J., Kim Y.C., Seok H.K. Comprehensive study on the roles of released ions from biodegradable Mg-5 wt% Ca-1 wt% Zn alloy in bone regeneration. J. Tissue Eng. Regenerat. Med. 2017;11(10):2710–2724. doi: 10.1002/term.2166. [DOI] [PubMed] [Google Scholar]
- 52.Bellucci D., Sola A., Cacciotti I., Bartoli C., Gazzarri M., Bianco A., Chiellini F., Cannillo V. Mg- and/or Sr-doped tricalcium phosphate/bioactive glass composites: synthesis, microstructure and biological responsiveness. Mater. Sci. Eng. C Mater. Biol. Appl. 2014;42:312–324. doi: 10.1016/j.msec.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 53.Wang G., Li J., Zhang W., Xu L., Pan H., Wen J., Wu Q., She W., Jiao T., Liu X., Jiang X. Magnesium ion implantation on a micro/nanostructured titanium surface promotes its bioactivity and osteogenic differentiation function. Int. J. Nanomed. 2014;9:2387–2398. doi: 10.2147/IJN.S58357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galli S., Naito Y., Karlsson J., He W., Miyamoto I., Xue Y., Andersson M., Mustafa K., Wennerberg A., Jimbo R. Local release of magnesium from mesoporous TiO2 coatings stimulates the peri-implant expression of osteogenic markers and improves osteoconductivity in vivo. Acta Biomater. 2014;10(12):5193–5201. doi: 10.1016/j.actbio.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 55.Tao Z.-S., Zhou W.-S., He X.-W., Liu W., Bai B.-L., Zhou Q., Huang Z.-L., Tu K.-k., Li H., Sun T., Lv Y.-X., Cui W., Yang L. A comparative study of zinc, magnesium, strontium-incorporated hydroxyapatite-coated titanium implants for osseointegration of osteopenic rats. Mater. Sci. Eng. C. 2016;62:226–232. doi: 10.1016/j.msec.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 56.Bruderer M., Richards R.G., Alini M., Stoddart M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cells Mater. 2014;28:269–286. doi: 10.22203/ecm.v028a19. [DOI] [PubMed] [Google Scholar]
- 57.Miron R.J., Zhang Y.F. Osteoinduction: a review of old concepts with new standards. J. Dent. Res. 2012;91(8):736–744. doi: 10.1177/0022034511435260. [DOI] [PubMed] [Google Scholar]
- 58.Komori T. Regulation of osteoblast differentiation by Runx2. Adv. Exp. Med. Biol. 2010;658:43–49. doi: 10.1007/978-1-4419-1050-9_5. [DOI] [PubMed] [Google Scholar]
- 59.Xu J., Li Z., Hou Y., Fang W. Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am. J. Transl. Res. 2015;7(12):2527–2535. [PMC free article] [PubMed] [Google Scholar]
- 60.Vimalraj S., Arumugam B., Miranda P.J., Selvamurugan N. Runx2: structure, function, and phosphorylation in osteoblast differentiation. Int. J. Biol. Macromol. 2015;78:202–208. doi: 10.1016/j.ijbiomac.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 61.Deschaseaux F., Sensebe L., Heymann D. Mechanisms of bone repair and regeneration. Trends Mol. Med. 2009;15(9):417–429. doi: 10.1016/j.molmed.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Helvering L.M., Sharp R.L., Ou X., Geiser A.G. Regulation of the promoters for the human bone morphogenetic protein 2 and 4 genes. Gene. 2000;256(1–2):123–138. doi: 10.1016/s0378-1119(00)00364-4. [DOI] [PubMed] [Google Scholar]
- 63.Ling M., Huang P., Islam S., Heruth D.P., Li X., Zhang L.Q., Li D.-Y., Hu Z., Ye S.Q. Epigenetic regulation of Runx2 transcription and osteoblast differentiation by nicotinamide phosphoribosyltransferase. Cell Biosci. 2017;7 doi: 10.1186/s13578-017-0154-6. 27-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ducy P. Cbfa1: a molecular switch in osteoblast biology. Dev. Dynam. Off. Publ. Am. Assoc. Anatomists. 2000;219(4):461–471. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1074>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 65.Galindo M., Pratap J., Young D.W., Hovhannisyan H., Im H.J., Choi J.Y., Lian J.B., Stein J.L., Stein G.S., van Wijnen A.J. The bone-specific expression of Runx2 oscillates during the cell cycle to support a G1-related antiproliferative function in osteoblasts. J. Biol. Chem. 2005;280(21):20274–20285. doi: 10.1074/jbc.M413665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pratap J., Galindo M., Zaidi S.K., Vradii D., Bhat B.M., Robinson J.A., Choi J.Y., Komori T., Stein J.L., Lian J.B., Stein G.S., van Wijnen A.J. Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res. 2003;63(17):5357–5362. [PubMed] [Google Scholar]
- 67.Sodek J., Chen J., Nagata T., Kasugai S., Todescan R., Jr., Li I.W., Kim R.H. Regulation of osteopontin expression in osteoblasts. Ann. N. Y. Acad. Sci. 1995;760:223–241. doi: 10.1111/j.1749-6632.1995.tb44633.x. [DOI] [PubMed] [Google Scholar]
- 68.Wang L., Li Z.Y., Wang Y.P., Wu Z.H., Yu B. Dynamic expression profiles of marker genes in osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Chin. Med. Sci. J. 2015;30(2):108–113. doi: 10.1016/s1001-9294(15)30021-3. [DOI] [PubMed] [Google Scholar]
- 69.Lian J.B., Stein G.S., Stewart C., Puchacz E., Mackowiak S., Aronow M., Von Deck M., Shalhoub V. Osteocalcin: characterization and regulated expression of the rat gene. Connect. Tissue Res. 1989;21(1–4):61–68. doi: 10.3109/03008208909049996. discussion 69. [DOI] [PubMed] [Google Scholar]
- 70.Ryoo H.M., Hoffmann H.M., Beumer T., Frenkel B., Towler D.A., Stein G.S., Stein J.L., van Wijnen A.J., Lian J.B. Stage-specific expression of Dlx-5 during osteoblast differentiation: involvement in regulation of osteocalcin gene expression. Mol. Endocrinol. 1997;11(11):1681–1694. doi: 10.1210/mend.11.11.0011. [DOI] [PubMed] [Google Scholar]
- 71.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 72.Hoppe A., Guldal N.S., Boccaccini A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 73.Khoshniat S., Bourgine A., Julien M., Petit M., Pilet P., Rouillon T., Masson M., Gatius M., Weiss P., Guicheux J., Beck L. Phosphate-dependent stimulation of MGP and OPN expression in osteoblasts via the ERK1/2 pathway is modulated by calcium. Bone. 2011;48(4):894–902. doi: 10.1016/j.bone.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 74.Beck G.R., Jr., Zerler B., Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc. Natl. Acad. Sci. U.S.A. 2000;97(15):8352–8357. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reffitt D.M., Ogston N., Jugdaohsingh R., Cheung H.F., Evans B.A., Thompson R.P., Powell J.J., Hampson G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003;32(2):127–135. doi: 10.1016/s8756-3282(02)00950-x. [DOI] [PubMed] [Google Scholar]
- 76.Zheng J., Mao X., Ling J., Chen C., Zhang W. Role of magnesium transporter subtype 1 (MagT1) in the osteogenic differentiation of rat bone marrow stem cells. Biol. Trace Elem. Res. 2016;171(1):131–137. doi: 10.1007/s12011-015-0459-4. [DOI] [PubMed] [Google Scholar]
- 77.Li R.W., Kirkland N.T., Truong J., Wang J., Smith P.N., Birbilis N., Nisbet D.R. The influence of biodegradable magnesium alloys on the osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. A. 2014;102(12):4346–4357. doi: 10.1002/jbm.a.35111. [DOI] [PubMed] [Google Scholar]
- 78.Ojansivu M., Hyvari L., Kellomaki M., Hupa L., Vanhatupa S., Miettinen S. Bioactive glass induced osteogenic differentiation of human adipose stem cells is dependent on cell attachment mechanism and mitogen-activated protein kinases. Eur. Cells Mater. 2018;35:54–72. doi: 10.22203/eCM.v035a05. [DOI] [PubMed] [Google Scholar]