Abstract
This work contains original data supporting our research paper “Advances in cartilage repair: the influence of inorganic clays to improve mechanical and healing properties of antibacterial Gellan gum-Manuka honey hydrogels”, by Maria A. Bonifacio, Andrea Cochis, Stefania Cometa, Annachiara Scalzone, Piergiorgio Gentile, Giuseppe Procino, Serena Milano, Alessandro C. Scalia, Lia Rimondini, Elvira De Giglio [1]. The main paper describes how four different clays (i.e., mesoporous silica, bentonite and halloysite nanotubes, coded as MS, BE and HNT) as cheap, abundant and versatile feed materials can be used for the preparation of highly performant hydrogels as cartilage substitutes, based on Gellan Gum (GG) and Manuka Honey (MH).
Here the composites were further examined by means of Thermogravimetric Analysis (TGA), histological analysis (Alcian blue and Safranin-O) and static compression tests.
This set of data strengthens the evidence that these hydrogels possess biological and physicochemical characteristics suitable for their application as reinforcing inorganic fillers in composite materials designed for cartilage regeneration.
Keywords: Composite hydrogels, Gellan gum, Inorganic clays, Thermal characterization, Swelling, Mechanical behaviour, Cytocompatibility
Specifications Table
| Subject area | Material science. Chemistry. |
| More specific subject area | Biomaterials for cartilage regeneration |
| Type of data | Raw data, table and figures |
| How data was acquired | TGA was performed using Perkin Elmer TGA-400 instrument (PerkinElmer Inc., Waltham, MA) Histological analysis were performed by cryo-sectioning using a cryostat Leica CM3050 S. Images were acquired by a Leica LMD7000 microscope. The stress-strain curves were obtained compressing the samples by using EZ-SX mechanical testing machine (Shimadzu, Japan) equipped with a 20 N load. |
| Data format | Raw |
| Experimental factors | TGA and water uptake measurements were carried out on freeze-dried hydrogels. The freeze-drying procedure is the following: as prepared hydrogels were frozen for 24h at −20 °C, then freeze-dried at −55 °C for 48h with an ALPHA1-2/LDPlus (Martin-Christ, Germany). Glycosaminoglycans staining with Alcian Blue and Safranin O were performed on hydrogels cryosections. Samples were fixed with formaldehyde (4%, 15 minutes at room temperature) and stored at −80 °C. Cryo-sections of 8 μm were obtained by cutting at −20 °C. Staining was carried out at room temperature. For static compression tests, the 20 N load was applied while the sample was compressed until break (∼35–40% of its original height), with a crosshead speed of 1 mm∙min−1. |
| Experimental features | For TGA analyses, no further specific sample preparation was required. Thermograms and derivative thermograms have been presented. For water uptake evaluations, at predetermined time intervals, hydrogels immersed in PBS were removed and weighted to determine the water uptake percentage. Safranin-O and Alcian blue do not require further counterstaining; data have been presented as representative images of the cells seeded as spheroids into the scaffold pores. Static compression tests were performed on samples rehydrated in PBS for 1h. |
| Data source location | Bari (Italy), Novara (Italy), Newcastle upon Tyne (UK) |
| Data accessibility | Raw data are available in this article |
| Related research article | “Advances in cartilage repair: the influence of inorganic clays to improve mechanical and healing properties of antibacterial Gellan-Manuka honey hydrogels”, by Maria A. Bonifacio, Andrea Cochis, Stefania Cometa, Annachiara Scalzone, Piergiorgio Gentile, Giuseppe Procino, Serena Milano, Alessandro C. Scalia, Lia Rimondini, Elvira De Giglio |
Value of the Data
|
1. Data
1.1. TGA analysis
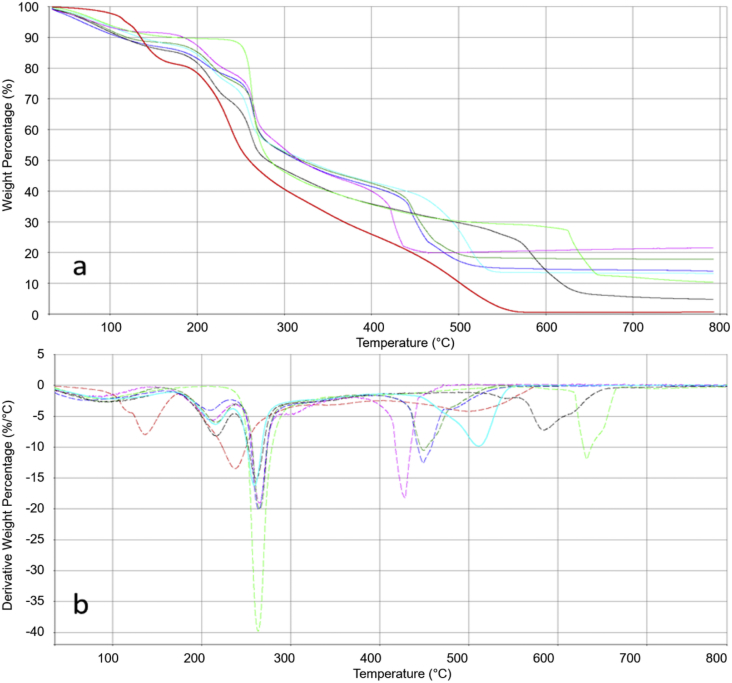
In Fig. 1, the raw thermograms and the derivative thermograms in oxidizing atmosphere of GG, MH, unreinforced and reinforced GG MH with BE, MS and HNT clays are reported.
Fig. 1.
TGA (a) and DTGA (b) of GG (light green line), MH (red line), GG MH Mg (black line), GG MH BE Mg (blue line), GG MH MS Mg (fuchsia line) and GG MH HNT Mg (turquoise line).
This analysis was carried out on freeze-dried hydrogels and revealed that the reinforced hydrogels mass percentage remaining at 800 °C resulted superior to unreinforced GG MH sample (see Table 1): this higher residue was attributable to the presence of the inorganic fillers. The amount of filler in the composites, obtainable by the residue at 800 °C, resulted MS > BENT > HNT.
Table 1.
Summary of TG data of all the investigated samples and their organic raw materials, GG and MH. The analyses have been performed on three replicates.
| Sample | Residue at 800 °C (%) | Water/volatiles content (%) | Main MH degradation (T°C/%) | Main GG degradation (T°C/%) |
|---|---|---|---|---|
| GG | 10.5 | 10.3 | – | 263.58/59.27 |
| 9.8 | 8.0 | – | 264.80/60.1 | |
| 10.7 | 12.4 | – | 260.80/59.2 | |
| MH | 0.69 | 12.8 | 237.3/42.5 | – |
| 0.91 | 16.0 | 242.6/47.9 | – | |
| 0.52 | 20.6 | 240.0/38.7 | – | |
| GG-MH | 11.1 | 9.6 | 214.1/15.5 | 259.7/34.5 |
| 5.12 | 13.0 | 218.3/15.5 | 260.1/34.9 | |
| 5.29 | 14.6 | 216.9/16.0 | 262.0/33.6 | |
| GG-MH-BE | 16.8 | 9.1 | 203.7/8.8 | 259.5/37.9 |
| 15.2 | 14.3 | 206.5/9.2 | 264.0/34.8 | |
| 14.1 | 10.9 | 208.6/9.0 | 263.8/34.0 | |
| GG-MH-MS | 11.5 | 9.8 | 209.2/13.2 | 267.4/35.7 |
| 19.1 | 8.6 | 212.9/12.9 | 266.7/35.8 | |
| 22.8 | 7.7 | 213.0/12.7 | 268.8/35.4 | |
| GG MH HNT | 13.3 | 9.2 | 216.3/13.1 | 260.6/32.8 |
| 13.7 | 13.1 | 215.7/12.4 | 260.1/32.4 | |
| 13.6 | 12.0 | 215.2/12.2 | 259.4/32.6 | |
| BE | 88.7 | 8.5 | – | – |
| 90.1 | 10.2 | – | – | |
| 87.7 | 7.2 | – | – | |
| MS | 90.7 | – | – | – |
| 87.1 | – | – | – | |
| 91.2 | – | – | – | |
| HNT | 83.2 | 2.9 | – | – |
| 82.0 | 2.2 | – | – | |
| 84.5 | 3.3 | – | – |
TGA evidenced also that the freeze-drying did not lead to a complete removal of water, both for GG MH sample and for samples reinforced with all the inorganic fillers (water or volatiles content between 9 and 12%), as also observed in GG-glycerol scaffolds already reported [2].
The initial mass loss, ascribable to the organic moieties, occurring at temperatures higher than 170 °C, indicated a high thermal stability of all the samples, which makes them suitable for long-term storage or possible heat involving processing technologies.
Even if both GG and MH degradations did not occur in a unique degradation event, two distinct main degradation steps occurred for these two organic materials at sufficiently different temperatures, i.e., 240 and 263 °C for MH and GG, respectively. In this respect, a distinction of these two contributions could be performed also in the composite hydrogels. As far as the MH content in the freeze-dried samples is concerned, an amount equal to 30% in MS- and HNT-reinforced hydrogels was estimated. On the other hand, for GG MH BE Mg samples, a lower MH percentage was detected (i.e., 21%).
MH showed a complex thermal decomposition process involving several steps that begins at room temperature and finishes near 600 °C. The TGA/DTGA traces show two main regions. The first region, from 50 °C to 170 °C, was mainly related both to the evaporation of water and to the melting of mono- and disaccharides contained in honey. The second region, from 170 °C to 300 °C, was due to decomposition of the aromatic bonds of honey [3].
When MH was entrapped into the GG-based hydrogels, the first region seemed to disappear, while a shift of the second region at lower temperatures occurred. The addition of the inorganic fillers seemed to further anticipate the MH thermal decomposition only when BE was used (206 °C). On the other hand, GG moieties in the composite hydrogels degraded at temperatures similar to those of GG alone.
1.2. Water uptake evaluation
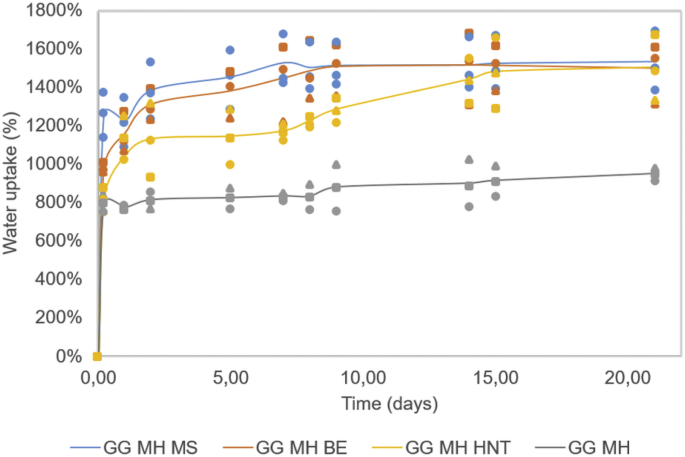
The experiments reported in this section were performed in order to shed light on the impact of the three different clays on the water uptake properties of the GG-MH-based hydrogels (See Fig. 2).
Fig. 2.
Water uptake kinetics up to 21 days in PBS of GG-MH hydrogels, with or without clays (i.e., MS, BE and HNT). The measurements were performed on three sample replicates.
The obtained swelling data evidenced that, even if with different kinetics over time, the water uptake at t = 21 days was similar for both MS, BE and HNT clays whereas, in the case of the unreinforced system, a markedly lower water uptake was reached.
1.3. GAGs staining through Alcian Blue and Safranin O
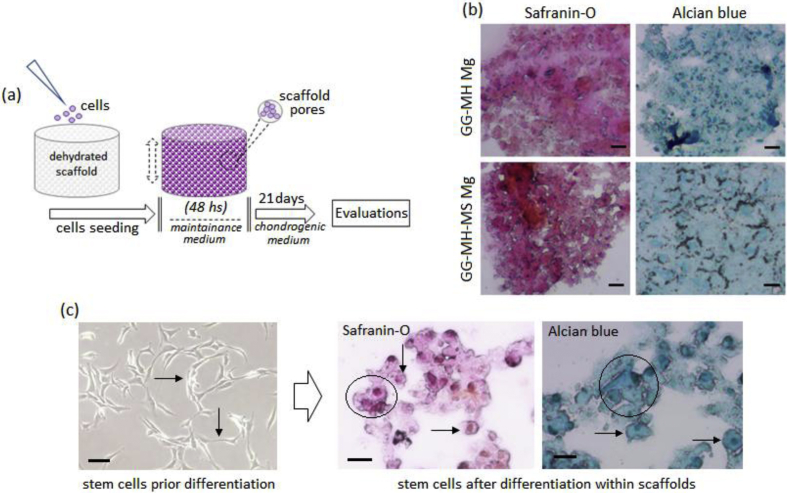
In order to investigate GG-MH and GG-MH-MS devices [1] ability to support stem cells chondrogenesis, cells were 3D seeded into scaffold's pores and cultivated used chondrogenic medium for 21 days (as schematized in Fig. 3a). Afterwards, the cartilage-like matrix staining Alcian blue and Safranin-O were applied to verify cells differentiation. In fact, Alcian blue holds specificity to sulphated proteoglycans while Safranin-O is directly proportional to the proteoglycan content, thus making them very specific marker for cartilage.
Fig. 3.
a) 3D composites protocol schematization; b) GAGs stainings (Alcian Blue and Safranin O) performed on GG-MH and GG-MH-MS scaffolds; c) cells morphology comparison prior and after differentiation onto GG-MH-MS scaffolds. Bar scale = 50 μm.
The stainings showed that cells produced a cartilage-like matrix rich in GAGs within the scaffold's pores (Fig. 3b) due to their changing in morphology from an undifferentiated fibroblasts-like shape to a round chondrocytes-like one as showed in Fig. 3c (spheroids aggregates are highlighted by circles while cells shape is indicated by the arrows). This is a fundamental step to undergo chondrogenesis confirming that devices' porosity was correctly designed to fit with the target tissue, which represents a crucial step for device design [4]. Moreover, both applied markers revealed an intense staining thus confirming that chondrogenesis correctly occurred and that the newly formed cartilage-like tissue was successfully hosted into the gellan scaffold.
1.4. Mechanical compression test
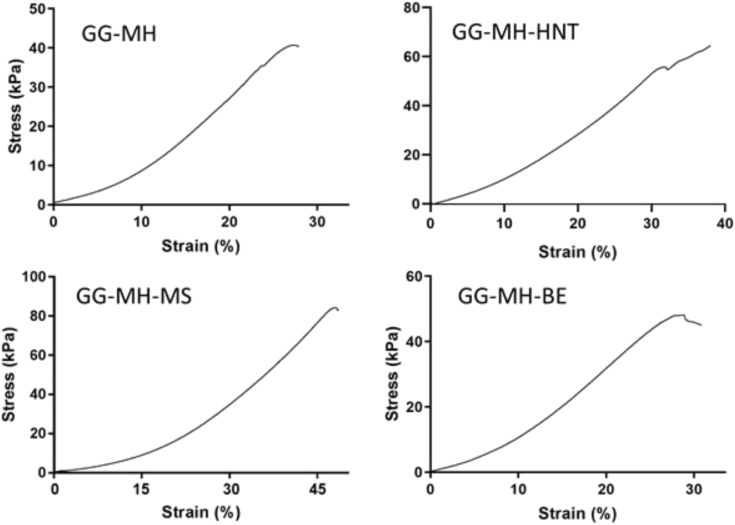
Examples of stress-strain curves recorded during static compression test are shown in Fig. 4, where the compressive Young's moduli (E) were measured as the slope of the linear region of the stress–strain curve ranging from 0 to 10% of the strain. The samples were compressed until the break (around 40% of the strain).
Fig. 4.
Typical stress-strain curves obtained by compression test for the bare GG-MH hydrogel and the reinforced GG MH with HNT, MS and BE.
2. Experimental design, materials, and methods
2.1. Thermogravimetric analysis (TGA)
Determination of thermal behaviour of freeze-dried unreinforced and clay-reinforced GG-MH hydrogels, as well as their feed materials, was obtained heating 5–10 mg of samples in air-saturated atmosphere using PerkinElmer TGA-400 instrument (PerkinElmer Inc., Waltham, MA). The heat range was 30–800 °C at a flow rate of 20 °C/min. The gas flow was set at 20 ml/min. Thermograms (TG) with respective derivative (DTG) curves were recorded and data were analysed using the software TGA Pyris series.
2.2. Water uptake evaluation
Freeze-dried GG-MH composites, with or without clays, were firstly weighted (mid), successively placed in tea bags. The tea bags containing samples were sealed and incubated in PBS at 37 °C to determine the water uptake profile up to 21 days and weighted prior (mi0) and after each time point (mit) (0.21, 1, 2, 5, 7, 8, 9, 14, 15 and 21 days). Moreover, in order to guarantee that the amount of the measured water was only ascribable to the samples swelling, the weight of empty wet tea bags was also considered after each time point (mbt). Therefore, the percentage of water content (WC %) was calculated using the equation already reported [5].
2.3. GAGs staining through Alcian Blue and Safranin O
For histological analysis, cells were fixed by a sucrose-formaldehyde solution (50:50, 10 min at room temperature), embedded into Kilik medium (from BioSigma, Milan, Italy) and stored at −80 °C. Then, 8 μm cryo-slices were collected and stained with Safranin-O and Alcian blue (both from Sigma).
2.4. Mechanical characterization
Five specimens for each sample type (cylinders of 1.6 cm diameter and 2 cm height) underwent static compression tests. The load was applied during compression until sample break (∼35–40% of its original height).
Acknowledgments
This work was supported by Università degli Studi di Bari “Aldo Moro”
Contributor Information
Lia Rimondini, Email: lia.rimondini@med.uniupo.it.
Elvira De Giglio, Email: elvira.degiglio@uniba.it.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bonifacio M.A., Cochis A., Cometa S., Scalzone A., Gentile P., Procino G., Milano S., Scalia A.C., Rimondini L., De Giglio E. Advances in cartilage repair: the influence of inorganic clays to improve mechanical and healing properties of antibacterial Gellan gum-Manuka honey hydrogels. Mater. Sci. Eng. C. 2019;108(2020):110444. doi: 10.1016/j.msec.2019.110444. [DOI] [PubMed] [Google Scholar]
- 2.Bonifacio M.A., Gentile P., Ferreira A.M., Cometa S., De Giglio E. Insight into halloysite nanotubes-loaded gellan gum hydrogels for soft tissue engineering applications. Carbohydr. Polym. 2017;163:280–291. doi: 10.1016/j.carbpol.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 3.Hoseini S.J., Darroudi M., Oskuee R.K., Gholami L., Zak A.K. Honey-based synthesis of ZnO nanopowders and their cytotoxicity effects. Adv. Powder Technol. 2015;26:991–996. [Google Scholar]
- 4.Varoni E.M., Altomare L., Cochis A., Ghalayaniesfahani A., Cigada A., Rimondini L., De Nardo L. Hierarchic micro-patterned porous scaffolds via electrochemical replica-deposition enhance neo-vascularization. Biomed. Mater. 2016;11(2) doi: 10.1088/1748-6041/11/2/025018. [DOI] [PubMed] [Google Scholar]
- 5.Bonifacio M.A., Cometa S., Cochis A., Gentile P., Ferreira A.M., Azzimonti B., Procino G., Ceci E., Rimondini L., De Giglio E. Data on Manuka Honey/Gellan Gum composite hydrogels for cartilage repair. Data in Brief. 2018;20:831–839. doi: 10.1016/j.dib.2018.08.155. [DOI] [PMC free article] [PubMed] [Google Scholar]