Highlights
-
•
A reliable protocol developed for micropropagation of Morus alba L.cv. Chinese white through indirect regeneration using different explants.
-
•
Friable callus was induced in maximum amounts from leaf and nodal explants on MS media supplemented with 2,4-D and NAA respectively.
-
•
Highest frequency of regeneration was obtained from the leaf and node derived callus on MS media with BAP + TDZ and plantlets were rooted on IBA.
-
•
Genetic homogeneity of in vitro raised plants was confirmed by SCoT and ISSR primers based molecular analysis.
Keywords: Mulberry, Callus mediated regeneration, Acclimatization, Start Codon targeted and inter simple sequence repeats
Abstract
A reliable protocol was developed for in vitro micro propagation of Morus alba L.cv. Chinese white. Initially, friable callus was induced (242.8 and 128.5 mg) from in vivo leaf and nodal explants on Murashige and Skoog’s (MS) medium amended with 4.0 μM/L of 2,4-Dichlorophenoxyacetic acid (2,4-D) and 3.0 μM/L of Naphthalene acetic acid (NAA) respectively within 3 weeks. Shoot regeneration (12.2 and 8.6) was obtained from leaf and node derived callus on 6-benzylaminopurine (BAP) + Thidiazuron (TDZ) at 2.5 + 2.0 and 7.5 + 2.0 μM/L concentrations respectively, after 4 weeks of incubation. In vitro shoots were rooted (90 %) on half strength MS medium with 7.5 μM/L indole-3 butyric acid (IBA) and plantlets were hardened in plastic pots contained farmyard manure, sand and garden soil in 1:1:2 ratio. The genetic stability of plantlets were confirmed by start codon targeted (SCoT) and inter simple sequence repeats (ISSR) primers based molecular analysis.
1. Introduction
Mulberry (Morus sp.) is a fast-growing deciduous woody perennial tree in the family Moraceae with global distribution over varied agro-climatic conditions [1]. Genus Morus (with more than 68 species) is being used from ancient times for practicing the sericulture activities across the world [2,3]. Hence, this plant considered as most economical concerning its utilization in revenue generation and the empowerment of rural communities through silkworm rearing [4,5] and other activities [6].
Among the various species of Morus (M. alba, M. atropurpurea, M. australis, M. cathayana, M. japonica, M. ihoukoidz, M. indica, M. laevigata, M. mongolica, M. multicaulis, M. nigra, M. notabilis, M. serrata, M. trilobataand M. rubra); M. alba is widely used in the mori silk production in most of the silk-producing countries like China and India [7,8]. This is due to the superiority of M. alba leaves over other species in terms of yield, moisture content and biochemical contents such as protein, carbohydrate and chlorophyll contents [9].
Besides its usage as a feeding material for silkworms [10] and other animals [11] recently, this plant gained attention of environmentalists as it is good in carbon sequestering and restoration of degraded environments [12]. Mulberry is considered as a wonderful plant of the planet due to its multiple uses such as feeding material for silkworms and domesticated animals [13], edible berries [7], source of pharmaceutically important compounds [14], role in green synthesis of nanoparticles [15], environmental protection [16] and availability of this plant all over the world (Yuan and Zhao [17]).
The mulberry can be cultivated as bush, dwarf or tree types of plantations across the world based on the suitable agro-climatic and agro-ecological zones [18]. In India, sericulture is being practiced in tropical, subtropical and temperate zones [19] but the major share of the mori silk production is coming from the tropical regions, due to multiple crops practices (5–8 crops) in a year [20]. Whereas, the sericultural practices are limited in temperate regions due to predominant cold conditions [[21], [22], [23], [24]]. In general, most of the superior mulberry varieties (Goshoerami, Chinese white, Ichinose) of temperate region are good in the other parameters except poor rooting ability [[25], [26], [27]]. Therefore, the propagation of mulberry is costlier and time consuming (takes 2–3 years in raising mulberry saplings) in the temperate areas as compared to the tropical regions.
Hence, attempts have been made to raise these varieties through other conventional techniques such as root grafts, bag grafts and wedge grafts. However, the survival through these techniques ranges from 33 to 40 % only. Moreover, these techniques are time consuming, costly and require skilled man power. Therefore, in vitro propagation techniques were used in the present study to raise the saplings of this variety. One of the major advantages of this technique, particularly under temperate climatic conditions, is that a long winter dormancy period (October to March) can be effectively utilized to raise the plantlets under controlled conditions in the laboratory. As soon as the winter period is over, the saplings can be transferred to the field.
In mulberry, micro propagation can be carried out either by direct propagation through axillary bud and shoot tip explants based cultures ([[28], [29], [30]]) or through indirect regeneration methods [31]. The frequency of raising multiple shoots/number of plantlets through nodal and shoot tip explants based micro propagation was generally reported as low. On the other hand propagation through callus mediated regeneration can produce large number of plantlets [32]. Moreover, callus based micro propagation of plantlets is regarded as the most feasible alternative to other types of in vitro based cultures methods [33], as callus mediated regeneration produces higher frequency of shoots ([[34], [35], [36]]) and callus can be sub-cultured/preserved for carrying the regeneration studies at later stages based on requirement.
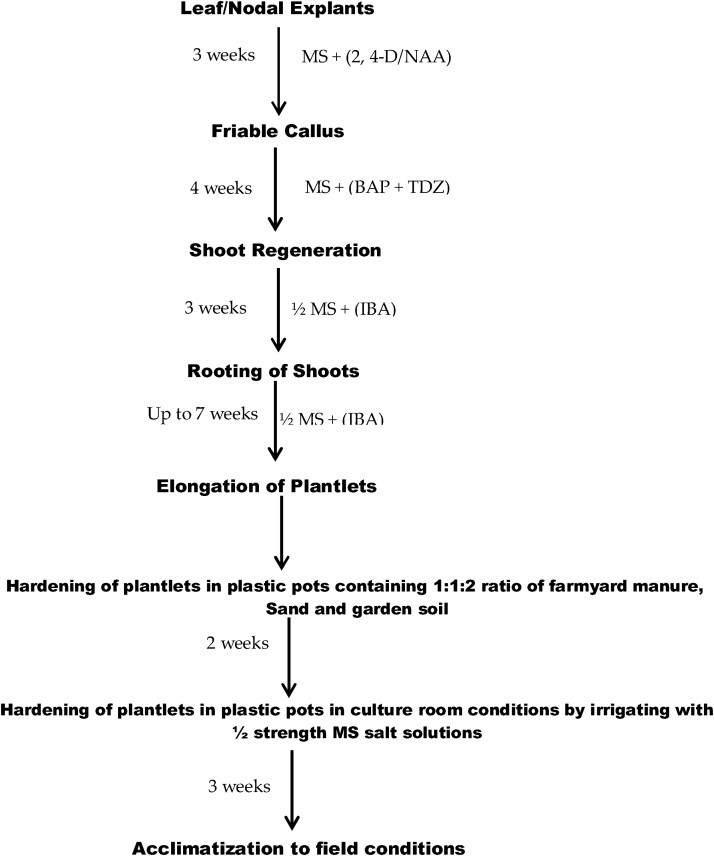
Therefore, a reliable and reproducible protocol was developed for the induction of callus and indirect regeneration in M. alba L. cv. Chinese white through this study (Fig.1). The genetic fidelity analysis of acclimated plantlets of Chinese white variety through SCoT and ISSR marker based molecular was also performed to check the homogeneity of the plantlets.
Fig. 1.
Flow chart showing stepwise protocol for in vitro clonal propagation of Morus alba L. cv. Chinese white.
2. Material and methods
2.1. Plant material and collection of explants
Morus alba L. cv. Chinese white plants (10 years old) were maintained in the mulberry field of the Central Sericultural Research & Training Institute (CSR&TI), Pampore, Jammu and Kashmir, India. Nodal segments (2−3 cm) and leaves (7−8 cm) were collected from the healthy mother plants during April month after on setting of spring season and were used as explants in the present study.
2.2. Surface sterilization of explants
The leaf and nodal explants were initially washed under running tap water and then soaked in 4 % sodium hypochlorite solution for 15−30 min (i.e.15 min for leaf and 30 min for nodal explants). Both the explants were treated with Tween-20 solution (Himedia) for 2 min followed by 45 % ethanol for 2 min and 0.1 % mercuric chloride (HgCl2) solution for 2 min. After every treatment explants were washed with sterile distilled water to remove the traces of detergents and disinfectants.After sterilization, leaf explants were cut into small segments of 0.5 × 0.5 cm in length andbreadth by using sterile scalpel blades.Additional treatment of 0.5 g/L Bavistin (BASF India Ltd.) for 20 min duration was given to nodal explants.
2.3. Culture media preparation
Murashige and Skoog’s medium [37] supplemented with 3 % sucrose was used as basal medium in this study. Full strength MS medium supplemented with different concentrations (0.10–5.0 μM/L) of auxins (2,4-D and NAA) was prepared for callus induction from leaf and nodal segments. Full strength MS medium augmented with different concentrations (BAP/Kn-2.5, 5.0, 7.5, 10.0, 12.5μM/L; TDZ-1.0, 1.5, 2.0, 2.5, 3.0, 5.0μM/L; (IAA/IBA-2.5–12.5 μM/L) of cytokinins (6-Benzyl amino purine, Kinetin and Thidiazuron) and auxins (Indole-3-Acetic acid and Indole-3-butyric acid) was used for regeneration and rooting of in vitro raised shoots. Medium was gelled with either 0.8 % agar (for callus induction, sub culturing and regeneration studies) or 0.6 % agar (rooting of in vitro raised shootlets). The pH of the medium was adjusted to 5.6 by using NaOH or HCl and sterilized in an autoclave at 121 °C at 15 lbs. pressure for 15−20 min.
2.4. Inoculation and incubation
The surface sterilized leaf and nodal explants were inoculated on MS medium (supplemented with 2, 4-D, NAA) with the help of sterile forceps. During inoculation, the abaxial side of leaf explants was inoculated in contact with the surface of media and nodal explants were inserted 0.5 cm into the media. Culture tubes with inoculated explants were maintained in a culture room at 26 ± 2 °C under 16 h photoperiod at light intensity of 3000 lx provided by cool fluorescent tubes with 60 % relative humidity. All the cultures were maintained under the controlled conditions as described above, for a period of 3 weeks duration.
2.5. Callus induction
The leaf and nodal explants were inoculated on full strength MS medium supplemented with 2, 4-D or NAA either alone or in combination (Table 1 and Table 2) for callus induction. The data with regard to percent response of explants to callusing, type of callus induced (Friable, compact, white, pale brown and bark brown) and the fresh weight of induced callus (in mg) from three randomly selected samples and ten replicates per treatment were recorded at the end of 3 weeks of incubation.
Table 1.
Effect of auxins on callus induction from leaf explants of Morus alba L. cv. Chinese white.
| Plant Growth Regulators (μM/L) |
Type of Callus induced | Percent of callusing (%) | Mean fresh weight of Callus (mg) | ||
|---|---|---|---|---|---|
| 2,4-D | NAA | 2,4-D + NAA | |||
| 0.10 | -- | 0.00 ± 0.00i | 0.00 ± 00.00l | ||
| 0.25 | -- | 0.00 ± 0.00i | 0.00 ± 00.00l | ||
| 0.50 | -- | 0.00 ± 0.00i | 0.00 ± 00.00l | ||
| 0.75 | White & Friable | 68.1 ± 5.02e | 86.1 ± 06.02h | ||
| 1.00 | White & Friable | 85.4 ± 3.22c | 100.4 ± 04.22g | ||
| 2.00 | White & Friable | 88.3 ± 4.14c | 122.3 ± 12.14f | ||
| 3.00 | White & Friable | 92.4 ± 0.28b | 182.6 ± 06.84c | ||
| 4.00 | White & Friable | 100.0 ± 0.00a | 242.8 ± 12.20a | ||
| 5.00 | White & Friable | 82.3 ± 5.12c | 232.7 ± 16.18a | ||
| 0.10 | -- | 0.00 ± 0.00i | 0.00 ± 00.00l | ||
| 0.25 | -- | 0.00 ± 0.00i | 0.00 ± 00.00l | ||
| 0.50 | White & Friable | 53.6 ± 3.22f | 62.3 ± 04.16i | ||
| 0.75 | White & Friable | 62.2 ± 4.12e | 74.5 ± 06.14h | ||
| 1.00 | White & Friable | 68.4 ± 3.56e | 88.6 ± 08.20h | ||
| 2.00 | White & Friable | 100.0 ± 0.00a | 164.6 ± 05.08d | ||
| 3.00 | White & Friable | 82.6 ± 5.45c | 138.2 ± 14.24e | ||
| 4.00 | White & Friable | 74.8 ± 3.08d | 134.8 ± 08.46e | ||
| 5.00 | White & Friable | 68.8 ± 6.25e | 128.6 ± 14.12f | ||
| 0.10 + 2.00 | White & Friable | 34.5 ± 2.28h | 48.6 ± 05.33k | ||
| 0.25 + 2.00 | White & Friable | 44.6 ± 4.60g | 52.3 ± 04.18j | ||
| 0.50 + 2.00 | White & Friable | 64.2 ± 9.80e | 65.8 ± 10.16i | ||
| 0.75 + 2.00 | White & Friable | 78.6 ± 6.36d | 104.3 ± 06.22g | ||
| 1.00 + 2.00 | White & Friable | 100.0 ± 0.00a | 162.4 ± 08.12d | ||
| 2.00 + 2.00 | Green & Friable | 88.0 ± 5.38c | 168.8 ± 07.64d | ||
| 3.00 + 2.00 | Brown & Friable | 84.9 ± 3.40c | 182.7 ± 09.04c | ||
| 4.00 + 2.00 | Brown & Compact | 68.8 ± 7.10e | 214.4 ± 10.83b | ||
| 5.00 + 2.00 | Dark Brown & Compact | 30.4 ± 4.26h | 246.2 ± 09.46a | ||
Medium: MS medium with 3 % sucrose & 0.8 % agar supplemented with different concentration of 2, 4-D and NAA.
Data represent the mean + SE of three randomly selected readings of ten replicates per treatment at the end of 3 weeks of culture. Bold characters denote significant response and values. Within columns, means followed by the same letter did not differ significantly according to Tukey’s test of SPSS Version 17 at P = 0.05.
Table 2.
Effect of auxins on callus induction from nodal explants of Morus alba L. cv. Chinese white.
| Plant Growth Regulators (μM/L) |
Type of Callus induced |
Percent of callusing (%) |
Mean fresh weight of Callus (mg) |
||
|---|---|---|---|---|---|
| 2,4-D | NAA | 2,4-D + NAA | |||
| 0.10 | -- | 0.00 ± 0.00i | 0.00 ± 0.00j | ||
| 0.25 | -- | 0.00 ± 0.00i | 0.00 ± 0.00j | ||
| 0.50 | -- | 0.00 ± 0.00i | 0.00 ± 0.00j | ||
| 0.75 | -- | 0.00 ± 0.00i | 0.00 ± 0.00j | ||
| 1.00 | White & Friable | 25.6 ± 2.48g | 38.6 ± 04.20i | ||
| 2.00 | White & Friable | 33.8 ± 5.35f | 44.2 ± 03.44h | ||
| 3.00 | White & Friable | 38.5 ± 4.64f | 45.7 ± 04.22h | ||
| 4.00 | White & Friable | 68.2 ± 6.18d | 94.8 ± 08.16f | ||
| 5.00 | Pale Brown& Friable | 44.0 ± 5.42e | 62.8 ± 05.42g | ||
| 0.10 | -- | 0.00 ± 0.00i | 0.00 ± 0.00j | ||
| 0.25 | -- | 0.00 ± 0.00i | 0.00 ± 0.00j | ||
| 0.50 | -- | 0.00 ± 0.00i | 0.00 ± 0.00j | ||
| 0.75 | -- | 0.00 ± 0.00i | 0.00 ± 0.00j | ||
| 1.00 | White & Friable | 38.6 ± 4.54f | 22.2 ± 03.55i | ||
| 2.00 | White & Friable | 48.6 ± 6.48e | 54.4 ± 04.10g | ||
| 3.00 | White & Friable | 84.4 ± 3.34b | 128.5 ± 10.32d | ||
| 4.00 | White & Friable | 62.7 ± 4.48d | 104.2 ± 04.66e | ||
| 5.00 | Brown&Compact | 46.2 ± 5.56e | 56.4 ± 05.08g | ||
| 0.10 + 2.00 | White & Friable | 12.2 ± 2.12h | 22.4 ± 02.40i | ||
| 0.25 + 2.00 | White & Friable | 18.4 ± 3.10h | 28.6 ± 02.20i | ||
| 0.50 + 2.00 | White & Friable | 45.6 ± 5.22e | 58.4 ± 05.23g | ||
| 0.75 + 2.00 | White & Friable | 64.8 ± 6.62d | 88.6 ± 04.54f | ||
| 1.00 + 2.00 | Brown & Friable | 100.0 ± 0.00a | 148.6 ± 03.34c | ||
| 2.00 + 2.00 | Pale Brown& Friable | 84.0 ± 4.46b | 158.2 ± 02.16c | ||
| 3.00 + 2.00 | Pale Brown & Friable | 70.8 ± 6.20c | 174.6 ± 05.38b | ||
| 4.00 + 2.00 | Dark Brown & Compact | 62.8 ± 4.38d | 192.6 ± 06.44a | ||
| 5.00 + 2.00 | Dark Brown & Compact | 60.8 ± 2.15d | 202.8 ± 08.12a | ||
Medium: MS medium with 3 % sucrose & 0.8 % agar supplemented with different concentration of 2, 4-D and NAA.
Data represent the mean + SE of three randomly selected readings of ten replicates per treatment at the end of 3 weeks of culture. Bold characters denote significant response and values. Within columns, means followed by the same letter did not differ significantly according to Tukey’s test of SPSS Version 17 at P = 0.05.
2.6. Multiple shoot regeneration from callus
The organogenic calli (friable type) obtained from leaf and nodal explants were separated from the explants and were made into small portions of 0.5 cm and were cultured on full strength MS medium amended with various concentrations of BAP or Kn (2.5, 5.0, 7.5, 10.0, 12.5 μM/L),TDZ (1.0, 1.5, 2.0, 2.5, 3.0, 5.0 μM/L) and combinations of cytokinins (Table 3). TDZat 2.0 μM/L was taken as constant with increasing concentrations of BAP (TDZ-2.0 μM/L + BAP-2.5, 5.0, 7.5, 10.0, 12.5 μM/L). Ten replicates were maintained for each treatment for 4 weeks. The regeneration data were collected after 4 weeks of cultures on cytokinin supplemented media from randomly selected three cultures.
Table 3.
Effect of cytokinins on shoot regeneration from leaf and node derived callus cultures in Morus alba L. cv. Chinese white.
| Plant Growth Regulators (μM/L) |
No. of shoots regenerated after 4 weeks of Culture(X*± S.E) |
||||
|---|---|---|---|---|---|
| BAP | Kn | TDZ | BAP + TDZ | From Leaf Callus | From Nodal Callus |
| 2.5 | – | – | – | 0.6 ± 0.10j | 0.00 ± 0.00f |
| 5.0 | – | – | – | 2.1 ± 0.34h | 0.00 ± 0.00f |
| 7.5 | – | – | – | 2.4 ± 0.59h | 1.8 ± 0.16e |
| 10.0 | – | – | – | 1.4 ± 0.32i | 1.6 ± 0.38e |
| 12.5 | – | – | – | 1.6 ± 0.18i | 3.2 ± 0.64d |
| 15.0 | – | – | – | 1.0 ± 0.12i | 1.0 ± 0.08e |
| – | 2.5 | – | – | 0.00 ± 0.00k | 0.00 ± 0.00f |
| – | 5.0 | – | – | 1.4 ± 0.16i | 1.2 ± 0.10e |
| – | 7.5 | – | – | 1.2 ± 0.24i | 1.6 ± 0.28e |
| – | 10.0 | – | – | 3.8 ± 0.42c | 3.0 ± 0.48d |
| – | 12.5 | – | – | 2.6 ± 0.34h | 2.4 ± 0.22e |
| – | 15.0 | – | – | 2.2 ± 0.62h | 2.1 ± 0.36e |
| – | – | 1.0 | – | 2.2 ± 0.52h | 2.0 ± 0.18e |
| – | – | 1.5 | – | 3.4 ± 0.32g | 3.2 ± 0.42d |
| – | – | 2.0 | – | 5.8 ± 0.44e | 4.6 ± 0.32c |
| – | – | 2.5 | – | 3.6 ± 0.56g | 3.2 ± 0.55d |
| – | – | 3.0 | – | 1.8 ± 0.38i | 2.4 ± 0.40e |
| – | – | 5.0 | – | 1.4 ± 0.24i | 1.2 ± 0.32f |
| – | – | – | 2.5 + 2.0 | 12.2 ± 0.68a | 3.2 ± 0.67d |
| – | – | – | 5.0 + 2.0 | 10.4 ± 0.56b | 4.2 ± 0.52c |
| – | – | – | 7.5 + 2.0 | 08.2 ± 0.46d | 8.6 ± 0.72a |
| – | – | – | 10.0 + 2.0 | 07.4 ± 0.82c | 5.8 ± 0.58b |
| – | – | – | 12.5 + 2.0 | 04.6 ± 0.67f | 3.7 ± 0.36d |
| – | – | – | 15.0 + 2.0 | 02.4 ± 0.38h | 2.8 ± 0.42e |
Medium: MS medium with 3 % sucrose & 0.8 % agar supplemented with different conc. of BAP, Kn and TDZ.
Data represent the mean + SE of three randomly selected readings of ten replicates per treatment at the end of 4 weeks of culture. Bold characters denote significant response and values. Within columns, means followed by the same letter did not differ significantly according to Tukey’s test of SPSS Version 17 at P = 0.05.
2.7. Rooting of the regenerated shoots
The healthy and elongated shoots (with minimum 1 cm) were separated from the culture vessels and transferred on half strength MS medium with different concentrations (2.5–12.5 μM/L) of IAA or IBA alone or in combination with 0.6 % agar (Table 4). Data on root induction was recorded after 3 weeks of cultures.
Table 4.
Effect of auxins on root induction from the regenerated shoots in Morus alba L. cv. Chinese white.
| Plant Growth Regulators μM/L) |
Rooting Percentage (X*± S.E) | ||
|---|---|---|---|
| IAA | IBA | IAA + IBA | After 14 days of culture |
| 2.5 | – | – | 42 ± 02.4e |
| 5.0 | – | – | 46 ± 04.3e |
| 7.5 | – | – | 62 ± 04.2d |
| 10.0 | – | – | 68 ± 03.4c |
| 12.5 | – | – | 56 ± 06.6d |
| – | 2.5 | – | 38 ± 02.6f |
| – | 5.0 | – | 48 ± 06.2e |
| – | 7.5 | – | 90 ± 05.4a |
| – | 10.0 | – | 76 ± 04.4b |
| – | 12.5 | – | 68 ± 05.8c |
| – | – | 2.5 + 7.5 | 32 ± 02.8f |
| – | – | 5.0 + 7.5 | 54 ± 02.6e |
| – | – | 7.5 + 7.5 | 69 ± 05.8c |
| – | – | 10.0 + 7.5 | 48 ± 03.2e |
| – | – | 12.5 + 7.5 | 40 ± 04.6e |
Medium: Half strength MS medium with 3 % sucrose & 0.6 % agar supplemented with different concentration of IAA and IBA.
Data represent the mean + SE of three randomly selected readings of ten replicates per treatment at the end of 3 weeks of culture. Bold characters denote significant response and values. Within columns, means followed by the same letter did not differ significantly according to Tukey’s test of SPSS Version 17 at P = 0.05.
2.8. Hardening and acclimatization of rooted plantlets
The rooting experiments were incubated for 7 weeks in rooting medium. The rooted shoots were separated carefully from the media and washed with tap water to remove the adherent traces of agar and media. The separated plantlets were kept in half strength MS salt solution for 2 h, and then transferred to the small plastic pots containing farmyard manure, sand and garden soil in the ratio of 1:1:2.
2.9. DNA isolation and assessment of genetic stability using SCoT and ISSR markers
To assess the genetic fidelity of regenerated plantlets, total genomic DNA was isolated from 500 mg of fresh leaf of mother plant and from nine randomly selected acclimated plantlets of M. alba L. cv. Chinese white (4 from each type of leaf callus and nodal callus) by following modified cetyl trimethyl ammonium bromide (CTAB) method [38]. The quantityof isolated DNA was analyzed by spectrophotometric analysisand quality of DNA by comparing the band intensities on 1 % agarose gel.
To assess the genetic stability, start codon targeted (SCoT) and inter simple sequence repeats (ISSR) primers (10 numbers from each type) were used in the present study (Table 5 and Table 6). Initially, the polymerase chain reaction experiments were standardized by using various primers, template DNA, appropriate quantity of master mix (GCC biotech), and varied temperatures of annealing and extension to determine the optimum results. The final amplification reaction mixtures with total volume of 20 μl contained 50 ng DNA, 1X PCR master mix (GCC biotech), and 10 p mole primer after standardizing the experiment. The reaction was cycled for 35 times with an initial denaturation at 94 °C for 5 min, denaturation of template DNA at 94 °C for 30 s, annealing of primer at 50 °C for 45 s, extension at 72 °C for 2 min and final extension at 72 °C for 5 min in a thermal cycler (Eppendorf, Germany). The amplified products were initially allowed to cool down with a holding temperature of 4 °C and were resolved by electrophoresis through 1 % agarose gel using TAE (Tris acetic acid EDTA) buffer (pH 8.0) with constant voltage (60 v). The gels were visualized and photographed using gel documentation system (Bio-Rad, USA) after staining with ethidium bromide. The size of amplified DNA (amplicons) was estimated by comparing with the bands on Gene Ruler of 1 kb DNA ladder (Thermo Scientific). Amplification reactions with SCoT and ISSR primers were repeated at least three times to assess the consistency of the banding profiles generated by primers.
Table 5.
List of ISSR Primers used for genetic fidelity studies.
| S. No | Primer code | Primer sequence (5′–3′) | Number of bands | Range of DNA Bands (bp) |
|---|---|---|---|---|
| 1 | R1 | AGAGAGAGAGAGAGAGG | 5 | 300-1100 |
| 2 | R2 | TCTCTCTCTCTCTCTCC | 3 | 300-900 |
| 3 | R3 | AGAGAGAGAGAGAGAGYT | 3 | 500-1500 |
| 4 | R4 | AGAGAGAGAGAGAGAGTC | 4 | 300-1800 |
| 5 | R5 | GAGAGAGAGAGAGAGAYT | 8 | 300-1500 |
| 6 | R6 | CTCTCTCTCTCTCTCTRA | 2 | 600-1200 |
| 7 | R7 | GAGAGAGAGAGAGAGAC | 4 | 300-1500 |
| 8 | R8 | ACACACACACACACACT | 4 | 600-1500 |
| 9 | R9 | CTCCTCCTCCTCCTCCTC | 2 | 1100-1400 |
| 10 | R10 | GAGAGAGAGAGAGAGAT | 5 | 250-1000 |
Table 6.
List of SCoT Primers used for genetic fidelity studies.
| S. No | Primer code | Primer sequence (5′–3′) | Number of bands | Range of DNA Bands (bp) |
|---|---|---|---|---|
| 1 | S1 | CAACAATGGCTACCACCA | 4 | 1100-2500 |
| 2 | S2 | CAACAATGGCTACCACCC | – | – |
| 3 | S3 | CAACAATGGCTACCACCG | – | – |
| 4 | S4 | CAACAATGGCTACCACCT | 1 | 2200 |
| 5 | S5 | CAACAATGGCTACCACGA | – | – |
| 6 | S6 | CAACAATGGCTACCACGC | – | – |
| 7 | S7 | CAACAATGGCTACCACGG | – | – |
| 8 | S8 | ACGACATGGCGACCAACG | 3 | 750-2000 |
| 9 | S9 | ACCATGGCTACCACCGAC | – | – |
| 10 | S10 | CCATGGCTACCACCGCAG | – | – |
2.10. Statistical analysis statistical analysis
All the experiments were repeated at least for three times with a minimum of ten replicates for experiment/treatment. The data obtained from the research study was subjected to one-way analysis of variance (ANOVA) in SPSS Version 17 (SPSS Inc. Chicago, USA) and means were compared using Tukey’s tests at the 5 % level of significance.
3. Results and discussion
3.1. Effect of auxins on callus induction
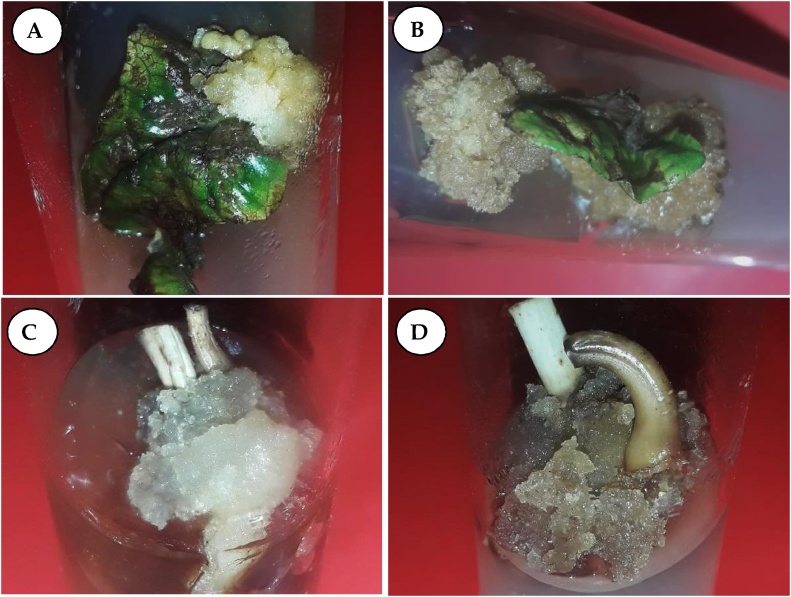
Leaf and nodal explants cultured on MS medium supplemented with auxins (2, 4-D, NAA) started callus formation from the cut ends within week at lower concentrations of auxins (0.50 μM/L). White friable organogenic callus was induced after 3 weeks of incubation with all concentrations (0.10–5.0 μM/L) of 2, 4-D and NAA alone (Fig.2A & C); whereas, same type of callus was induced at lower concentrations of combined use of auxins (< 3.0 μM/L of 2, 4-D + 2.0 μM/L of NAA) and compact type calli with dark brown color formed (Fig. 2B & D) at higher concentrations of auxins (> 3.0 μM/L of 2, 4-D + 2.0 μM/L of NAA) after 3 weeks of cultures.
Fig. 2.
Friable callus induction from various explants of Morus alba L. cv. Chinese white.
A) Friable white callus induced from leaf explants cultured on MS + 2,4-D (4μM/L).
B) Friable brown callus induced from leaf explants cultured on MS + 2,4-D (4 μM/L) + NAA (2 μM/L).Friable white callus induced from nodal explant cultured on MS + 2,4-D (4μM/L).
C) Friable brown callus induced from leaf explants cultured on MS + 2,4-D (1 μM/L) + NAA (2 μM/L).
Maximum frequency (100 %) and amounts (242.8 and 164.6 mg of fresh weight) of callus (white and friable type) induction from leaf explants was obtained at 4.0 μM/L and 2.0 μM/L concentrations of 2, 4-D and NAA respectively after 3 weeks of incubation (Table 1). Less amounts (94.8 and 128.5 mg of fresh weight) and low frequency (68.2 % and 84.4 %) of calli was obtained at 4.0 μM/L and 3.0 μM/L concentrations of 2,4-D and NAA respectively after 3 weeks of culture (Table 2). Comparatively higher amounts of callus (246.2 and 202.8 in. mg) was induced on 2,4-D (5.0 μM/L) + NAA (2.0 μM/L) containing MS medium (Table 2).
In mulberry, there is scanty information about the callus induction, as most of the previous researcher’s micro propagated other mulberry varieties through axillary buds, shoot tips and nodal explants on cytokinin amended media. Among the few reports available, the reporters used combination of media which were amended with plant growth regulators (2,4-D and BAP) supplemented with casein acid hydrolysate and coconut water [39,40]. Additionally these earlier reporters have used plant growth regulators at milligram per liter concentrations. Whereas in our study, we have induced good amount of white and friable callus by using micro molar concentrations (0.10–5.00 μM/L) of auxins only and more importantly without using any additional supplements.
Prolonged incubation of callus for more than 4 weeks turned the callus from white and friable to brown and compact type which is a common feature of tree species [41]. As brown and compact type of callus is not suitable for further in vitro studies, attempts were made to prevent turning of callus from friable to non-friable type therefore, friable and organogenic callus could be available for regeneration studies.
3.2. Effect of cytokinins on shoot regeneration
Highest number of shoots were regenerated when the callus was cultured on combination of cytokinins (BAP and TDZ) supplemented full strength MS medium after 4 weeks of culture (Figs. 3A and 4A). Among the three types of cytokinins (BAP, Kn and TDZ) used individually, good response in shoot regeneration was observed on TDZ with the maximum regeneration frequency of 5.8 and 4.6 at 2.0 μM/L concentrations from leaf and node derived calli respectively after 4 weeks of culture (Figs. 3B and 4B). Similarly, on the media amended with BAP and Kn small number of shootlets (1–3) were regenerated (Figs. 3C and 4C). Overall, the highest frequency (12.2 and 8.6) of shoot regeneration (Figs. 3D and 4D) was observed from leaf and nodal callus on combinational media of BAP + TDZ at 2.5 + 2.0 and 7.5 + 2.0 μM/L concentrations respectively after 4 weeks of culture (Table 3).
Fig. 3.
In vitro plant regeneration from leaf explant in Morus alba L. cv. Chinese white.
A) Friable white callus induction from leaf explants cultured on MS + 2,4-D (2,4-D (4 μM/L).
B) Shoots (3 no) regeneratedfrom leaf callus on MS + BAP (7.5 μM/L). C) Shoots (5 no) regenerated from leaf callus on MS + TDZ (2.0 μM/L).
D) High frequency of shoots (12 no) regenerated from leaf callus on MS + BAP (2.5 μM/L) + TDZ (2.0 μM/L).
E) Transfer of individual micro shoot on ½ MS + IBA (7.5 μM/L).
F) Root induction in single shoot cultured on MS + IBA(7.5 μM/L).
G) Complete plantlet separated for hardening.
H) Hardened complete plantlet in a plastic pot.
Fig. 4.
In vitro plant regeneration from nodal explant in Morris alba L. cv. Chinese white.
A) Friable white callus induction from nodal explants cultured on MS + 2,4-D (2,4-D (4 μM/L).
B) Shoots (5 no) regenerated from nodal callus on MS + TDZ (2.0 μM/L).
C) Shoots (3 no) regenerated from nodal callus on MS + BAP (12.5 μM/L).
D) High frequency of shoots (9 no) regenerated on MS + BAP (7.5 μM/L) + TDZ (2.0 μM/L).
E) Root induction in single shoot cultured on MS + IBA (7.5 μM/L).
F) Complete plantlet separated for hardening.
G) Hardened complete plantlet in a plastic pot.
H) One month old acclimatized plantlet in a plastic pot.
In previous study, the authors reported good number of shoots on the combined use of BAP + TDZ in mulberry variety PPR-1 [42], therefore, this study was restricted to the use of only one combination i.e. BAP and TDZ (hence, other combinations of BAP + Kn and TDZ + Kn were not considered). Thidiazuron and BAP either alone or in combination were used by several researchers as potent cytokinins in regeneration of highest number of shoots from different types of explants based calli [36,43]. It is reported that TDZ possesses more cytokinin like activity than other commonly used cytokinins [44] and gives good response of shoot regeneration in woody tree species [45].
Similarly, good number of shoots were regenerated by combined use of TDZ + BAP in Luffa cylindrica, [46]; Aloe vera [47], Rauwolfia tetraphylla [48,49]; Mirabilis jalapa [50] and Stachytarpheta jamaicensis [51] etc. There are reports on use of TDZ at higher concentrations in regeneration of shoots and roots in different species of Morus [52], but in this study, the shoots were regenerated at micro molar level concentrations of TDZ.
3.3. Rooting of in vitro regenerated shoots
The regenerated in vitro shoots were rooted when transferred (Fig. 3E) on half strength MS media supplemented with either individual or in combinations of auxins. The highest response of rooting (90 %) was observed on the media augmented individually with IBA at 7.5 μM/L concentration (Figs. 3F, 4E). On the other hand, low rooting response was observed (<70 %) on the medium containing IBA alone or with IAA (Table 4). Similar results of highest rooting (96 %) are reported in temperate mulberry variety PPR-1 at 7.5 μM/L concentration of IBA in our previous report [42].
3.4. Hardening and acclimatization of plantlets
The roots were induced from the shoots within 3 weeks of cultures on the rooting medium but the rooted shoots were allowed to grow up to 7 weeks in incubation room to maximize the formation of roots and to grow the shoots into healthy plantlets. The fully grown plantlets of M. alba L. cv. Chinese white were separated after 7 weeks of culture (Fig. 3G and 4F) and hardened in plastic pots (Figs. 3H, 4G and H) as discussed in the materials and methods section. The hardened plantlets were initially kept in culture room and irrigated with half strength MS salts for 2 weeks and then transferred to the field conditions with 84 % survivability.
3.5. Genetic fidelity analysis using ISSR markers
As the raised in vitro plantlets were passed through callus stage, always there is a chance of having soma clonal variants among the population of regenerated plantlets [53]. Hence, now-a-days it is mandatory to carry the genetic stability studies of indirectly regenerated and acclimated plantlets by primer/marker based DNA analysis to confirm their genetic fidelity with that of mother plant [54]. There are several markers or primers to confirm the genetic stability; among them the most advanced and reliable with high reproducibility rates are ISSR and SCoT. Among these SCoT markers are most advanced type which can anneal and amplify certain expressive sequences of the genome [55].
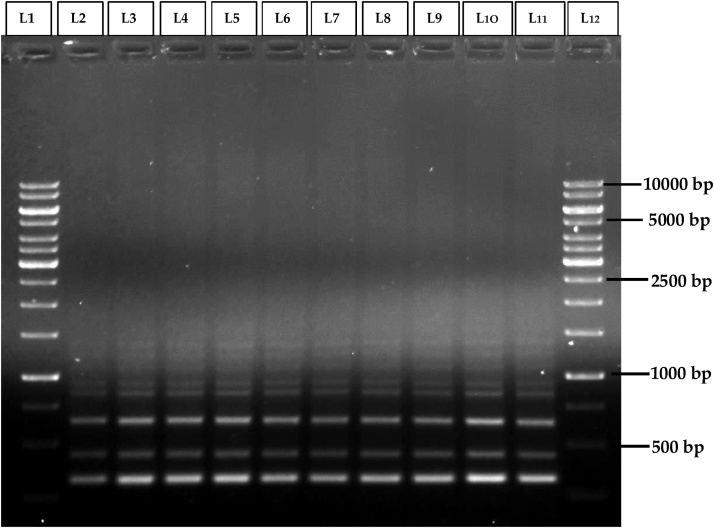
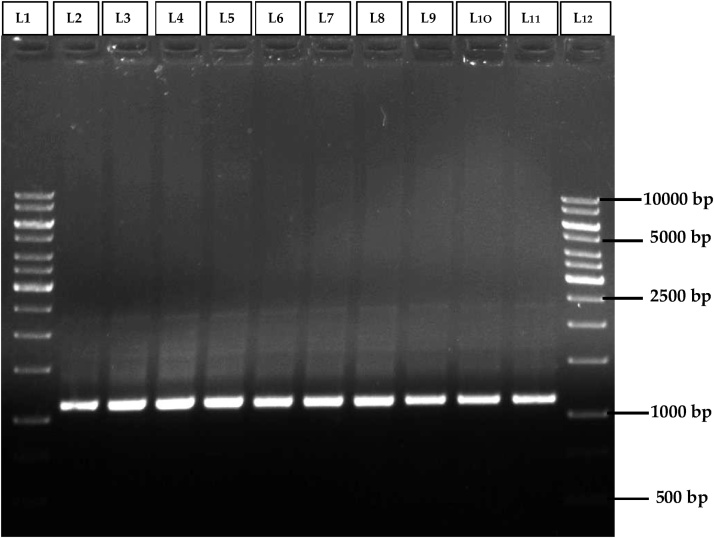
In order to assess the genetic stability of in vitro regenerated Morus alba L. cv. Chinese white plantlets, ISSR and SCoT primers based DNA fingerprinting was carried out in 9 randomly selected regenerated plantlets and their mother plant. Among the 10 ISSR primers (R1-R10) used, good response of amplification with clear, distinct and scorable DNA bands in the range of 300bp-1500bp were obtained with R5 ISSR primer (Table 5). Among 10 type of SCoT (S1-S10) primers used, only 3 primers has shown amplification with good response of amplification with clear, distinct and scorable DNA bands in the range of 1100bp-2500bp were obtained with S1 SCOT primer (Table 6). With all amplified primers of ISSR and SCoT, obtained DNA bands were found as monomorphic across the nine randomly selected in vitro raised plantlets and the mother plant (Fig. 5, Fig. 6). Hence, the in vitro regenerated Morus alba L. cv. Chinese white mulberry plantlets were confirmed as genetically stable and clonally uniform.
Fig. 5.
ISSR profiles of mother plant (Lane-2) and tissue culture raised plantlets (Lane 3–11) of Morris alba L. cv. Chinese white using R5-ISSR primer.
Lane 1 & 12: DNA ladder of 1Kbp size.
Lane 2 & 11: DNA banding pattern of mother plant.
Lane 3-6: DNA banding pattern of acclimated plants which were raised from leaf explants.
Lane 7-10: DNA banding pattern of acclimated plants which were raised from nodal explants.
Fig. 6.
SCoT profiles of mother plant (Lane-2) and tissue culture raised plantlets (Lane 3–11) of Morris alba L. cv. Chinese white using S1-SCoT primer.
Lane 1 & 12: DNA ladder of 1Kbp size.
Lane 2 & 11: DNA banding pattern of mother plant.
Lane 3-6: DNA banding pattern of acclimated plants which were raised from leaf explants.
Lane 7-10: DNA banding pattern of acclimated plants which were raised from nodal explants.
ISSR and SCoT primers were already reported for their accuracy, reliability, superiority and better resolvability over other markers [55,56]. ISSR primer based genetic stability were reported in in vitro regenerated plantlets of Musa spp. [57]; Rauwolfia tetraphylla L. [48]; Zea mays (Ramakrishnan et al., 2014); Morus alba [58], Cornus alba [59], Cucumis melo L. [60] and Solanum trilobatum [61]. Similarly, SCoT primer based genetic stability were reported in regenerated plantlets of Albizia julibrissin [62]; Pittosporum eriocarpum [63] and Rauwolfia tetraphylla [64]. This is the first report of using SCoT primers for the assessment of genetic stability of regenerated plantlets in mulberry (Morus spp.)
4. Conclusion
The developed protocol can be used effectively for the mass propagation of Chinese white mulberry variety under temperate conditions (during winter dormancy period from October to March) and raised plantlets can be transferred to the field conditions after the onset of spring season (April). This practice could reduce the time period of raising the saplings from existing 3–5 years to 1-11/2year. Further, there was no detection of any type of polymorphisms in the DNA bands across the randomly selected micro propagated and mother plants, which confirms the genetic uniformity of in vitro raised plantlets.
Author Agreement
It is to certify that all authors have seen and approved the final version of the manuscript being submitted. We confirm that this work is original and has not been published elsewhere, nor is it currently under consideration for publication elsewhere.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest in this research study.
Acknowledgement
Authors gratefully acknowledge Central Silk Board, Ministry of Textiles, Government of India for providing financial support and necessary facilities under project code: 3571 to carry out this research work. Authors also thank Dr. Mahipal Singh Shekhawat, K.M. Centre for P.G. Studies, Pondicherry, INDIA for grammatically correcting the Manuscript.
References
- 1.Yuan Q.X., Xie Y.F., Wang W., Yan Y.H., Ye H., Jabbar S., Zeng X.X. Extraction optimization, characterization and antioxidant activity in vitro of polysaccharides from mulberry (Morus alba L.) leaves. Carbohydr. Polym. 2015;128:52–62. doi: 10.1016/j.carbpol.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 2.Datta R.K. Mulberry cultivation and utilization in India. FAO Electronic Conference on Mulberry for Animal Production (Morus); Rome, Italy; 2000. pp. 45–62. www.- fao.org/DOCREP/005/x9895E/x9895e02.html.www.fao.org/livestock/agap/frg/mulberry/Papers/PDF/Datta.pdf. [Google Scholar]
- 3.Shukla P., Ramesha A.R., Kangayam M.P., Rohela G.K., Aftab A.S., Chauhan S.S., Ghosh M.K., Mishra R.K. Comparative analysis of gene expression profiles among contrasting mulberry varieties under cold stress condition. J. Exp. Biol. Agric. Sci. 2018;6(6):973–982. [Google Scholar]
- 4.Srivastava S., Kapoor R., Thathola A., Srivastava R.P. Nutritional quality of leaves of some genotypes of mulberry (Morus alba) Int. J. Food Sci. Nutri. 2006;57(5-6):305–313. doi: 10.1080/09637480600801837. [DOI] [PubMed] [Google Scholar]
- 5.Shukla P., Gulab K.R., Aftab A.S., Sharma S.P. Prospect of cold tolerant genes and its utilization in mulberry improvement. Ind. Hortic. J. 2016;6:127–129. Special. [Google Scholar]
- 6.Tewari R.K., Kumar P., Sharma P.N. Antioxidant responses to enhanced generation of superoxide anion radical and hydrogen peroxide in the copper-stressed mulberry plants. Planta. 2006;223:1145–1153. doi: 10.1007/s00425-005-0160-5. [DOI] [PubMed] [Google Scholar]
- 7.Ercisli S., Orhan E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007;103(4):1380–1384. [Google Scholar]
- 8.Butt M.S., Nazir A., Sultan M.T., Schroen K. Morus alba L. nature’s functional tonic. Trends Food Sci. Technol. 2008;19:505–512. [Google Scholar]
- 9.Ali M., Durrani Y., Ayub M. Effect of drying techniques and storage on mulberry (Morus alba) Quality. Sarhad J. Agric. 2016;32:80–88. http://10.17582/journal.sja/2016/32.2.80.88 [Google Scholar]
- 10.Rahmathulla V.K. Management of climatic factors for successful silkworm (Bombyxmori L.) crop and higher silk production: a Review. Psyche. 2012;2012 [Google Scholar]
- 11.Nguyen X.B., Vu D.G., Le D.N. Ensiling of mulberry foliage (Morus alba) and the nutritive value of mulberry foliage silage for goats in central Vietnam. Livest Res. Rural Dev. 2005;17(2) http://www.lrrd.org/lrrd17/2/ ba17015.htm. [Google Scholar]
- 12.Zhou L., Zhao Y., Wang S., Han S., Liu J. Lead in the soil–mulberry (Morus alba L.) silkworm (Bombyx mori) food chain: translocation and detoxification. Chemosphere. 2015;128:171–177. doi: 10.1016/j.chemosphere.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 13.Shukla P., Ramesha A.R., Kangayam M.P., Rohela G.K., Aftab A., Ghosh M.K., Mishra R.K. Selection of suitable reference genes for quantitative real-time PCR gene expression analysis in Mulberry (Morus alba L.) under different abiotic stresses. Mol. Biol. Rep. 2019;46(1):1–9. doi: 10.1007/s11033-019-04631-y. [DOI] [PubMed] [Google Scholar]
- 14.Raman S.T., Ganeshan A.K.P.G., Chen C., Jin C., Li S.H., Chen H.J., Gui Z.Z. In vitro and in vivo antioxidant activity of flavonoid extracted from mulberry fruit (Morus alba L.) Pharmacogn. Mag. 2016;12:128–133. doi: 10.4103/0973-1296.177910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohela G.K., Srinivasulu Y., Rathore M.S. A review paper on recent trends in bio-nanotechnology: implications and Potentials. Nanosci. Nanotech. Asia. 2019;9:12–20. [Google Scholar]
- 16.Liu Y., Willison J.M. Prospects for cultivating white mulberry (Morus alba) in the drawdown zone of the three gorges reservoir, china. Environ. Sci.Pollut. Res. 2013;20(10):7142–7151. doi: 10.1007/s11356-013-1896-2. [DOI] [PubMed] [Google Scholar]
- 17.Yuan Q., Zhao L. The Mulberry (Morus alba L.) Fruit-A review of characteristic components and health benefits. J. Agric. Food Chem. 2017;65(48):10383–10394. doi: 10.1021/acs.jafc.7b03614. [DOI] [PubMed] [Google Scholar]
- 18.Imran M., Khan H., Shah M., Khan R., Khan F. Chemical composition and antioxidant activity of certain Morus species. J. Zhejiang Univ. Sci. 2010;11(12):973–980. doi: 10.1631/jzus.B1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijayan K., Tikader A., Weiguo Z., Venugopalan N., Chirakkara E., Sezai T., Chi H. Springer-Verlag; Berlin Heidelberg: 2011. Morus. [Google Scholar]
- 20.Vijayan K., Chakraborti S.P., Roy B.N. Plant regeneration from leaf explants of mulberry: influence of sugar, genotype and 6-benzyladenine. Ind. J. Exp. Biol. 2000;38:504–508. [PubMed] [Google Scholar]
- 21.Aftab A.S., Fotadar R.K., Anil D., Bhat A.H. A comparative analysis of tropical and temperate genotypes and behaviour of five species of mulberry under temperate conditions of Kashmir. Uniswa Res. J. Agric. Sci. Technol. 2012;3(1):089–093. [Google Scholar]
- 22.Aftab A.A., Chauhan S.S., Gulab K., Pawan S., Saini P., Ghosh M.K. Mulberry breeding strategies for north and North West India. Int. J. Adv. Res. Sci. Eng. 2018;7(4):2124–2133. [Google Scholar]
- 23.Gani M., Gupta R.K., Kaul V., Zargar S.M., Bali K., Khan G. Materials Today: Proceedings. Elsevier Ltd; 2016. Management of the tent caterpillar (malacosoma indicum) using nucleopolyhedrovirus in Jammu & Kashmir, India; pp. 3914–3924. [Google Scholar]
- 24.Gani M., Chouhan S., Gupta R.K., Khan G., Kumar Bharath, N Saini, Ghosh M.K. Bombyx mori nucleopolyhedrovirus (BmBPV): its impact on silkworm rearing and management strategies. Zhongguo Mei Jie Sheng Wu Xue Ji Kong Zhi Za Zhi. 2017;31:189–193. [Google Scholar]
- 25.Anil D., Fotadar R.K., Aftab A.S., Khan M.A. Published by CSR&TI, Pampore Publications; 2011. Catalogue on Temperate Mulberry Germplasm. Book No.01, 2011. [Google Scholar]
- 26.Rohela G.K., Pawan S., Ravindra M.A., Mudasir G., Aftab A.S., Srinivasulu Y., Sharma S.P. Somatic hybridization as a potential tool for mulberry improvement: a review. Ind. Horti. J. 2016;6:46–49. Special. [Google Scholar]
- 27.Chauhan S.S., Saini P., Rohela G.K., Shukla P., Shabnam A.A., Ghosh M.K. Present postion and furture strategies of mulberry breeding for North West India: an overview. February, 2018; 2018. pp. 22–33. [Google Scholar]
- 28.Zaki M., Zahoor A.K., Mohammad S.S. Micropropagation of Morus nigra L. From nodal segments with axillary buds. World J. Agric. Sci. 2011;7(4):496–503. www.idosi.org/wjas/wjas7(4)/19.pdf [Google Scholar]
- 29.Rohela G.K., Aftab A.S., Pawan S., Ravindra A., Mudasir G., Srinivasulu Y., Sharma S.P. In vitro clonal propagation of PPR-1, a superior temperate mulberry variety. Ind. J. Biotech. 2018;17:619–625. [Google Scholar]
- 30.Rohela G.K., Aftab A.S., Pawan S., Kamili A.N., Ghosh M.K. Rapid one step protocol for the in vitro micro propagation of Morus multicaulis var. Goshoerami, an elite mulberry variety of temperate region. J. Exp. Bio. Agric. Sci. 2018;6(6):936–946. [Google Scholar]
- 31.Bhau B.S., Wakhlu A.K. Effect of genotype, explant type and growth regulators on organogenesis in Morus alba. Plant Cell Tiss. Org. Cult. 2001;66:25–29. [Google Scholar]
- 32.Magnani E., Jimenez-Gome J.M., Soubigou T.L., Lepiniec L., Fiume E. Profiling the onset of somatic embryogenesis in Arabidopsis. BMC Genomics. 2017;18:998. doi: 10.1186/s12864-017-4391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saiprasad G.V.S. Artificial seeds and their applications. Resonance. 2001;6:39–47. [Google Scholar]
- 34.Narayan P., Chakraborty S., Subba Rao S. Regeneration of plantlets from the callus of stem segments of mature plants of Morus alba L. Proc. Proc. Indian Sci. Acad. B Biol. Sci. 1989;55:469–472. [Google Scholar]
- 35.Kathiravan K., Shajahan A., Ganapathi A. Regeneration of plantlets from hypocotyl derived callus of Morus alba. Israel J. Plant Sci. 2013;43(3):259–262. [Google Scholar]
- 36.Faisal M., Anis M. Rapid in vitro propagation of Rauvolfia tetraphylla L.—an endangered medicinal plant. Physiol. Mol. Biol. Plants. 2002;8:295–299. [Google Scholar]
- 37.Murashige T., Skoog F. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- 38.Doyle J.J., Doyle J.L. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- 39.Susheelamma B.N., Raja Shekar K., Sarkar A., Rao M.R., Datta R.K. Genotype and hormonal effects on callus formation and regeneration in mulberry. Euphytica. 1996;90(1):25–29. [Google Scholar]
- 40.Ankit P., Vishwanathan A.S., Basavaraja R. Effect of nutrients on in vitro culture of Morus alba L. (White mulberry) Bioresearch Bulletin. 2010;1:008–012. http://bioresonline.org/archives/A101.pdf [Google Scholar]
- 41.Rohela G.K., Bylla P., Korra R., Reuben C. Phytochemical screening and antimicrobial activity of leaf, stem, root and their callus extracts in Rauwolfia tetraphylla. Int. J. Agric. Biol. 2016;18:21–528. [Google Scholar]
- 42.Rohela G.K., Phanikanth J., Aftab A.A., Pawan S., Sadanandam A., Mrinal K.G. In vitro regeneration and assessment of genetic fidelity of acclimated plantlets by using ISSR markers in PPR-1 (Morus sp.): an economically important plant. Sci. Horti. 2018;241:313–321. [Google Scholar]
- 43.Borchetia S., Das S.C., Handique P.J., Das S. High multiplication frequency and genetic stability for commercialization of the three varieties of micro propagated tea plants (Camellia spp.) Sci.Horti. 2009;120(4):544–550. [Google Scholar]
- 44.Mok M.C., Mok D.W.S., Armstrong D.J., Shudo K., Isogai Y., Okamoto T. Cytokinin activity of N-phenyl N′-1,2,3thiadiazol5-ylurea (thidiazuron) Phytochemistry. 1982;21(7):1509–1511. [Google Scholar]
- 45.Huetteman C.A., Preece J.E. Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tiss. Org. Cult. 1993;33(2):105–119. [Google Scholar]
- 46.Sujatha D., Ravi C.H., Raghuvardhan L., Prasad B., Gulab Khan R., Sadanandam A., Christopher R.T. In vitro plantlet regeneration and genetic transformation of sponge gourd (Luffa cylindrica L.) Afric. J. Plant. Sci. 2013;7(6):244–252. [Google Scholar]
- 47.Lavanya L., Thayamini H.S. Effect of 6-benzyl-aminopurine and thidiazuron on in vitro shoot organogenesis of Aloe vera (L.) Burm. F. Chilean J. Agric. Res. 2014;74:4. [Google Scholar]
- 48.Rohela G.K., Prasad B., Srinivas K., Sadanandam A., Ravi C.H., Christopher R.T. In vitro plantlet regeneration from leaf and stem calluses of Rauwolfia tetraphylla (R. canescens) and confirmation of genetic fidelity of plantlets using the ISSR-PCR Method. J. Her. Spi. Med. Plants. 2013;19:66–75. [Google Scholar]
- 49.Rohela G.K., Prasad B., Ravi C.H., Rajender K., Christopher R.T. In vitro clonal propagation of Rauwolfia tetraphylla, a relative of indian snake root plant. Res. J. Biotech10. 2015:23–31. [Google Scholar]
- 50.Rohela G.K., Santhosh D., Prasad B., Rajender K., Sreenu P., Christopher T.C. Somatic embryogenesis and indirect regeneration in Mirabilis jalapa Linn. Mater. Today Proc. 2016;3:3882–3891. [Google Scholar]
- 51.Korra R., Bylla P., Rohela G.K., Pendli S., Reuben T.C. In vitro micro propagation and confirmation of genetic fidelity using RAPD marker in ethno medicinal plant Stachytarpheta jamaicensis L. Vahl. Int. J. Adv. Res. (Indore) 2017;5:1494–1502. [Google Scholar]
- 52.Pavan U., Venugopal R.K., Kiranmayee K., Jaya Sree T., Sadanandam A. Plant regeneration of mulberry (Morus indica) from mesophyll-derived protoplasts. Plant Cell Tiss. Org. Cult. 2005;82:289–293. [Google Scholar]
- 53.Larkin P., Scowcroft W. Somaclonal variation-a novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 1981;60:197–214. doi: 10.1007/BF02342540. [DOI] [PubMed] [Google Scholar]
- 54.Palombi M.A., Damiano C. Comparison between RAPD and SSR molecular markers in detecting variation in kiwifruit (Actinidia deliciosa A. Chev) Plant Cell Rep. 2002;20:1061–1066. [Google Scholar]
- 55.Gorji A.M., Poczai P., Polgar Z., Taller J. Efficiency of arbitrarily amplified dominant markers (SCoT, ISSR and RAPD) for diagnostic fingerprinting in tetraploid potato. Am. J. Potato Res. 2011;88:226–237. [Google Scholar]
- 56.Rohela G.K., Bylla P., Pendli S., Rajender K., Reuben T.C. ISSR marker based DNA profiling studies in Rauwolfia species. Ann. Plant Sci. 2018;75:2289–2295. [Google Scholar]
- 57.Ray T., Dutta I., Saha P., Das S., Roy S.C. Genetic stability of three economically important micropropagated banana (Musa sp.) cultivars of lower Indo-Gangetic plains, as assessed by RAPD and ISSR markers. Plant Cell Tiss. Org. Cult. 2006;85:11–21. [Google Scholar]
- 58.Saha S., Adhikari S., Dey T., Ghosh P. RAPD and ISSR based evaluation of genetic stability of micropropagated plantlets of Morus alba L. Var. S-1. Meta Gene. 2016;7:7–15. doi: 10.1016/j.mgene.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ilczuk A., Jacygrad E. In vitro propagation and assessment of genetic stability of acclimated plantlets of Cornus alba L. Using RAPD and ISSR markers. In Vitro Cell. Dev. Biol., Plant. 2016;52:379–390. doi: 10.1007/s11627-016-9781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reza R.M., Mahmoud L., Masoud T., Bahman Z., Angela C., Loredana A. Somatic embryogenesis of muskmelon (Cucumis melo L.) and genetic stability assessment of regenerants using flow cytometry and ISSR markers. Protoplasma. 2018;255(3):873–883. doi: 10.1007/s00709-017-1194-9. [DOI] [PubMed] [Google Scholar]
- 61.Pendli S., Rohela G.K., Jogam P., Prasad B., Rajender K., Christopher T. In vitro plantlet regeneration and confirmation of genetic fidelity of R1 plantlets in Solanum trilobatum L., using ISSR-PCR Method. Vegetos. 2019;32(4):508–520. [Google Scholar]
- 62.Rahmani S.M., Paula M.P., Naghi S., Mona N. Genetic fidelity assessment of in vitro-regenerated plants of Albizia julibrissin using SCoT and IRAP fingerprinting.IN Vitro Cell. In Vitro Cell. Dev. Biol., Plant. 2015;51(4):407–419. [Google Scholar]
- 63.Thakur J., Dwivedi M.D., Sourabh P., Uniyal P.L., Pandey A.K. Genetic homogeneity revealed using SCoT, ISSR and RAPD markers in micropropagated Pittosporum eriocarpum Royle- an endemic and endangered medicinal Plant. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0159050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rohela G.K., Jogam P., Bylla P., Reuben C. Indirect regeneration and assessment of genetic fidelity of acclimated plantlets by SCoT, ISSR, and RAPD Markers in Rauwolfia tetraphylla L.: an endangered medicinal plant. Biomed Res. Int. 2019;2019:14. doi: 10.1155/2019/3698742. 3698742. [DOI] [PMC free article] [PubMed] [Google Scholar]