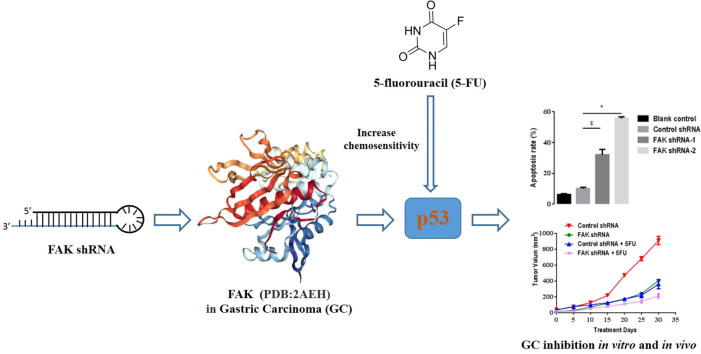
Graphical abstract
Abbreviations: GC, gastric cancer; 5-FU, 5-fluorouracil; FAK, Focal adhesion kinase; shRNA, small hairpin RNA; OS, Overall survival; FP, first progression
Keywords: Gastric carcinoma, FAK, RNA interference, 5-FU, Chemosensitivity
Abstract
The small molecule drug 5-fluorouracil (5-FU) is widely used in the treatment for gastric cancer (GC), however, it exerts poor efficacy and is associated with acquired and intrinsic resistance. Focal adhesion kinase (FAK), a non-receptor tyrosine kinase, plays a key role in adhesion, migration, and proliferation of gastric carcinoma cells, suggesting that this kinase may be a promising therapeutic target. Differentially expressed FAK in GC tissue was detected by RT-qPCR and TCGA database analysis. To investigate the biological functions of FAK, loss-of-function experiments were performed. CCK-8 assay, colony formation assay, flow cytometry, dual-luciferase reporter assays, and western blot assays were conducted to determine the underlying mechanisms of FAK in 5-FU chemosensitivity in GC. FAK is overexpressed in GC patients, and positively correlated with poor prognosis. The use of shRNA interference to target FAK decreased proliferation and increased apoptosis of GC cells in vitro. Importantly, FAK silencing enhanced the therapeutic efficacy of 5-FU, leading to reduced tumor growth in vivo. We further demonstrated that FAK silencing increased 5-FU-induced caspase-3 activity, and promoted p53 transcriptional activities. Clinical data also has shown that patients with higher levels of FAK had significantly shorter overall survival (OS) and time to first progression (FP) than those with lower levels of FAK. These findings indicate that FAK plays a critical role in 5-FU chemosensitivity in GC, and the use of FAK inhibitors as an adjunct to 5-FU might be an effective strategy for patients who undergo chemotherapy.
1. Introduction
Gastric carcinoma (GC) is the fifth most common malignancy and third leading cause of cancer-related death [1]. Surgical excision and systemic chemotherapy are the primary treatment options for metastatic gastric carcinoma [2], [3], [4]. Although the chemotherapeutant 5-fluorouracil (5-FU) is largely used for medical treatment of gastric carcinoma, it exerts chemoresistance, leading to the failure of chemotherapy [5]. Therefore, it is important to identify molecular markers and study the signal pathways that modulate gastric carcinoma chemoresistance.
Aggressive growth of tumor cells is a complex multiple-step process. Migration, invasion, and metastasis are important features of malignant tumors. Tumor cells must adhere to the extracellular matrix (ECM) and subsequently undergo migration. In recent years, investigators have investigated the modulating effects of cell-ECM interactions on the chemosensitivity of anti-cancer drugs [6], [7], [8]. Focal adhesion kinase (FAK) is a cytoplasmic non-receptor tyrosine kinase that regulates cell-cell and cell-ECM signal transduction. In response to mechanical stimulation, FAK is phosphorylated [9], [10], [11] on a catalytic loop [12], and the active form of FAK catalyzes subsequent downstream signaling [11], [13], [14], [15]. FAK is overexpressed in several cancers, including breast, oral, colon, gastric, and ovarian cancer. FAK plays an important role in tumor proliferation, migration, invasion, survival, and apoptosis [16], [17], [18], and also modulates cell and ECM adhesion, as well as cytoskeleton recombination. The ECM-integrin-cytoskeleton complex is the basic structure of integrated signal transduction [19]. FAK regulates cell adhesion and inhibits apoptosis through a variety of signaling pathways, all of which affect the sensitivity of chemotherapy. FAK might be a key factor that mediates tumor chemoresistance in gastric cancer, making it a promising therapeutic target.
In this study, we investigated the potential roles of FAK in 5-FU chemosensitivity in gastric carcinoma. We used q-PCR assay to determine that FAK was overexpressed in gastric cancer patients and the levels of overexpression correlated with poor prognosis. We found that FAK inhibition suppressed the growth of gastric cancer cells, reduced xenograft tumor growth in a nude mouse model, and enhanced the therapeutic efficacy of 5-FU in the mouse model. Mechanistically, we demonstrated that inhibition of FAK enhanced apoptosis, increased 5-FU-induced caspase-3 activity, and promoted p53 transcriptional activities. The expression levels of p53 target gene p21, Bax, and PUMA were increased. These findings showed that FAK plays a critical role in 5-FU chemosensitivity in gastric carcinoma, and suggests an effective strategy for patients who undergo chemotherapy.
2. Materials and methods
2.1. RNA interference
Recombinant vectors expressing small hairpin RNA (shRNA) against human FAK (Gene ID: 37233) were constructed by inserting chemically-synthesized double-strand DNA fragments containing FAK-targeting shRNA sequences (control 5′-shRNA GTCTCCGAACGTGTCACGT-3′); FAK shRNA-1: 5′-GAACCTCGCAGTCATTT-3′; FAK shRNA-2: 5′-GGAATGCTTCAAGTGTGCT-3′) into-plasmid pLentiLox3.7-nero at the HapI and XhoI sites, generating the following plasmids: control shRNA, FAK shRNA-1 and FAK shRNA-2. Recombinant lentiviral plasmids were cotransfected into 293 T cells with the packaging plasmids VSV-G, RSV-REV, and pMDL. After 48 h the viral supernatants were passed through 0.45-μm filters and then used to infect target cells in the presence of 6 μg/mL polybrene (Sigma-Aldrich).
2.2. Cell culture, transfection, and treatment
Human gastric cancer BGC823 and SGC7901 cells were purchased from ATCC (Manassas, VA, USA) and cultured in RPMI-1640 (Gibco, Life Technologies, NY, USA) supplemented with 10% fetal bovine serum (FBS, ExCell Bio, Shanghai, China), 100 U/ml penicillin and 100 µg/mL streptomycin (Gibco). Plasmid DNA transfection was performed with Turbofect reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. 5-FU was purchased from Sigma and was added to subconfluent cells at the indicated doses.
2.3. Real-time quantitative PCR (qPCR)
For qPCR analyses of mRNA, reverse transcription was performed with TRIzol (Invitrogen, Carlsbad, CA, USA) extracted total RNAs using a ReverTra Ace-α Kit as per the manufacturer’s instruction (Toyobo, Tokyo, Japan). qPCR was performed using the SYBR Green Real-Time PCR Master Mix (Toyobo) and the Step One Plus Real-Time PCR system (Applied Biosystems Inc., Foster City, CA, USA) according to the manufacturers’ protocols with primers as follows:
p21-forward: 5′-CTCTAAGGTTGGGCAGGGTG-3′;
p21-reverse: 5′-GAAGAAGGGTAGCTGGGGCTC-3′;
Bax-forward: 5′-GGGGACGAACTGGACAGTAAC-3′;
Bax-reverse: 5′-GGGGACGAACTGGACAGTAAC-3′;
PUMA-forward: 5′-CAGCTGCCCGCTGCCTA-3′;
PUMA-reverse: 5′- AGCGAGAGCGAGGGCTG-3′;
Actin forward: GGACTTCGAGCAAGAGATGG;
Actin reverse: AGCACTGTGTTGGCGTACAG;
FAK forward: AGCAGCCGCACCTTATAAAGA;
FAK reverse: TCTTGTGGCAGTTGCAATTA.
2.4. Cell counting kit-8 (CCK-8) assay
Relative cell viability of BGC823 cells and SGC7901 transfected with control shRNA, FAK shRNA-1 or untreated was measured with a CCK-8 kit (Dojindo, Kumamoto, Japan). Briefly, cells were plated into 96-well plates containing 100 μL of growth medium. Post-transfection for 72 h, CCK-8 reagent (10 µL/well) was added and incubated for 3 h at 37 °C in a 5% CO2 incubator. The absorbance was measured at 450 nm using a microplate reader. The cell viability was calculated as follows: relative cell viability % = [(A1 − AB)/(A0 − AB)] × 100, where A1 is the absorbance of treatment group, A0 is the absorbance of control group and AB is the absorbance of the blank group.
2.5. Colony formation assay
Cells were first infected with control shRNA, FAK shRNA-1, FAK shRNA-2, or untreated for 72 h, then 1 × 103 cells/well were seeded in 6-well plates with medium changed every two days. Cells were fixed with methanol and stained with crystal violet after 10 days. Colonies were counted and analyzed for clonogenicity.
2.6. Tumor-bearing mice model
Four- to six-week-old male nude mice were obtained from the Laboratory Animal Center of Xiamen University. The animals were maintained on standard laboratory chow under a 12 h/12 h light/dark schedule, unless otherwise indicated. All animal experiments were conducted according to protocols and guidelines approved by the Xiamen University Institutional Animal Care and Use Committee. Mice were divided into three groups, five mice each group, randomly. Mice were subcutaneously injected with 5 × 106 untransfected BGC823, FAK control shRNA BGC823 and FAK shRNA-1 stable knockdown BGC823 cells. Intratumoral administration of a dose of 10 mg/kg of 5-FU was injected every 3 days starting from the fifteenth day after inoculation of cells. The xenograft tumors of mice were monitored every 4 days with Vernier calipers. The size was calculated as follows: (V) = a × b2 × 0.5, where a is the minimum diameter, b is the maximum diameter. Mice were sacrificed 30 days later.
2.7. Western blots
Cells or tissues were lysed in a lysis buffer and protein concentrations for cells or tissues lysates were measured using the BCA protein assay kit (Thermo Fisher Scientific Inc., Waltham, MA, U.S) or G250 (Sigma-Aldrich, St. Louis, MO, USA). Thirty micrograms protein/lane whole cell lysates were electrophoresed in SDS-PAGE and transferred to a PVDF membrane (Millipore, Billerica, MA, USA). After blocking for 1 h at room temperature in TBST with 5% non-fat milk, the membranes were probed with the following primary antibodies: FAK (1:2000 CST), p-FAK (1:500 CST), Cle –PARP (1:1000 CST), actin (1:5000 Sigma). After washing three times, the membranes were incubated with HRP-conjugated goat anti-mouse or anti-rabbit secondary antibodies, 1:5000 (BD). Then, the chemiluminescence reaction was performed.
2.8. Annexin V-FITC/PI double staining assay by flow cytometry
Apoptosis was measured using Annexin V-FITC/PI (Ebioscience, San Diego, USA) dual staining by flow cytometry. Briefly, cells (1 × 105 /well) were seeded into 6-well plates and infected with lentivirus mediated FAK shRNA-1 or sh-ctrl with or without exposed to 5-FU for 24 h. Cells were harvested and washed in cold FACS buffer (PBS containing 2% FBS), and labeled with Annexin V-FITC for 30 min at 4 °C in the dark and then with PI. The stained cells were analyzed by flow cytometry (LSRFortessa, Becton Dickinson, San Jose, CA, USA). The filters used in the flow cytometry were: DAPI: 450 BP 40, FITC: 525 BP40.
2.9. Luciferase reporter assay
BGC823 cells were transfected in 6-well dishes at 80% confluence with 0.5 μg of various reporters, together with other plasmids in various combinations as indicated. Each sample was supplemented with 0.5 μg of pCMV5-LacZ expressing β-galactosidase, for monitoring transfection efficiency. The cells were collected, and luciferase activity was measured at 24 h after transfection. All transfections experiments were performed at least five times in triplicate, and the error bars represented SD of the means.
2.10. TCGA analysis
We used mRNA expression array datasets from TCGA to explore gene expression profiles in human cancer. We downloaded data from 375 tumor tissues and 32 normal tissues of mRNA expression data to determine differences in transcription levels of FAK between normal gastric tissues and GC tissues. The data regarding mRNA expression were produced on the platforms of Illumina Infinium Human Methylation450 BeadChip and IlluminaGA_RNASeqV2.1.0.0 (Illumina, Inc., San Diego, CA, USA).
2.11. Survival analysis
Overall survival (OS) and first progression (FP) curves were calculated with the Kaplan–Meier method to evaluate the prognostic value of FAK1 mRNA expression in GC (Gastric Cancer). A total of 876 GC patients were recruited from the Kaplan–Meier Plotter online database. Subjects were divided into two groups by median expression (high vs. low expression) and assessed by a Kaplan-Meier survival plots, with the hazard ratio (HR) with 95% confidence intervals (CI) and logrank P value calculated as in previous reports [20], [21].
2.12. Clinical samples
All clinical samples were collected with the informed consent of the patients and study protocols that were in accordance with the ethical guidelines of the Declaration of Helsinki (1975) and were approved by the Institutional Medical Ethics Committee of Xiamen University. GC pathological diagnosis was verified by at least two specific pathologists. 40 human GC specimens and paired adjacent epithelial tissues were obtained from the Shanghai Outdo Biotech Co., Ltd.
2.13. Statistical analysis
Values represent the mean ± standard deviation (SD) of at least three independent experiments. One-way ANOVA with Bonferroni’s post-test was used for multiple comparisons and the Student’s t test (two-tailed) was used for pair-wise comparisons. Correlation analyses were performed with Pearson’s test. p values <0.05 were considered statistically significant.
3. Results
3.1. FAK is significantly upregulated in human gastric cancer clinical samples
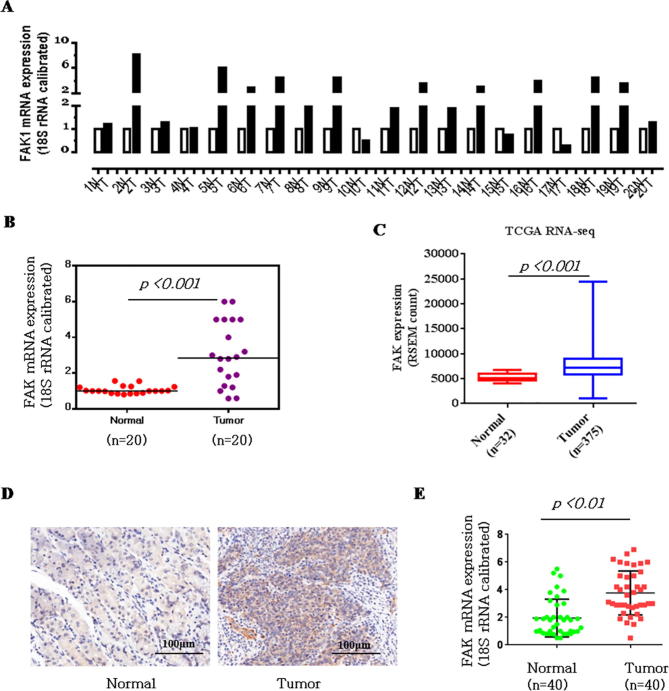
To explore the function and relationship between the expression of FAK and gastric carcinogenesis, we performed qPCR on 20 pairs of clinical samples. As shown in Fig. 1A–B, in 60%–70% of the human gastric cancer (GC) tissues, FAK was significantly upregulated compared to the levels in normal tissues (13 out of 20). We then used the TCGA cancer microarray mRNA database to identify the FAK expression levels in gastric cancer. The results showed that the FAK gene was significantly more highly expressed in gastric cancer (Fig. 1C). Immunohistochemical analysis showed that FAK was diffusely expressed in the cytoplasm. FAK was expressed in 28/40 (70%) of GC tissues, with expression levels that were higher in GC than in adjacent noncancerous gastric tissues (Fig. 1D). The relative expression levels of FAK in the GC tissue group and the corresponding pathologically noncancerous gastric tissue group (Control group) were evaluated. We observed a statistically significant increase in FAK expression in the GC group compared with the level in the control group (Fig. 1E). Further analysis in Table 1 showed that FAK level was correlated to tumor-node-metastasis TNM staging (n = 40, p < 0.05), while no apparent association was found between FAK expression with patient gender, patient age, tumor size. The detailed description of the patient information is shown in Table 2 (Age, Tumor size, Clinical staging, ect.).
Fig. 1.
FAK is significantly upregulated in GC clinical samples. (A) qPCR assay. The mRNA expression levels of FAK in 20 clinical samples were examined by qPCR assay. (B) Upregulated mRNA expression levels of FAK in the 20 gastric cancer cases shown in A. (C) Significant upregulation of FAK was observed in gastric cancer samples available in TCGA RNA-Seq dataset (p < 0. 001, Wilcoxon signed rank test). The expression of FAK was increased by 1.97-fold in gastric cancer compared with NT gastric tissue. Data are presented as RSEM (RNA-Seq expression estimation by Expectation-Maximization) normalized count. (D) Immunohistochemical analysis of FAK expression in gastric cancer tissue (Test Group) and adjacent noncancerous gastric tissue (Control Group). Typical representative immunohistochemical results are shown. Scale bar, 100 μm. (E) A bar graph representing the relative expression level of FAK in the GCT and ANLT groups, as evaluated by positive staining points (a paired-sample t-test was used to compare the data from the two groups).
Table 1.
The baseline characteristics of GC patients included in the analysis (n = 40).
| Clinicopathological parameters | FAK-expression |
p value | ||
|---|---|---|---|---|
| Case | Low | High | ||
| Age (years) | ||||
| >=50 | 22 | 8 | 14 | 0.6582 |
| <50 | 18 | 4 | 16 | |
| Gender | ||||
| Male | 22 | 7 | 16 | 0.5423 |
| Female | 18 | 5 | 13 | |
| Tumor size (diameter/cm) | ||||
| >=4 | 16 | 4 | 12 | 0.1217 |
| <4 | 24 | 8 | 16 | |
| TNM stage | ||||
| T1–T2 | 16 | 5 | 11 | 0.0356 |
| T3–T4 | 24 | 7 | 17 | |
| 12 | 28 | |||
p < 0.05 represents significant differences.
Bold type indicates statistically significant difference.
Table 2.
The detailed description of the GC patient tissues information (n = 40).
| Number | Gender | Age | Tumor size (diameter) | TNM stage | Pathological typing | Pathological typing |
|---|---|---|---|---|---|---|
| 1 | Female | 74 | ≥4 cm | III | adenocarcinoma | Tubular adenocarcinoma |
| 2 | Male | 45 | <4 cm | III | adenocarcinoma | Low adhesion adenocarcinoma |
| 3 | Female | 78 | <4 cm | Ⅱ | adenocarcinoma | Tubular adenocarcinoma |
| 4 | Male | 39 | ≥4 cm | Ⅱ | adenocarcinoma | Tubular adenocarcinoma |
| 5 | Female | 68 | <4 cm | IV | adenocarcinoma | Low adhesion adenocarcinoma |
| 6 | Male | 57 | ≥4 cm | Ⅱ | adenocarcinoma | Tubular adenocarcinoma |
| 7 | Female | 50 | <4 cm | IV | adenocarcinoma | mucosal adenocarcinoma |
| 8 | Male | 58 | <4 cm | III | adenocarcinoma | Tubular adenocarcinoma |
| 9 | Male | 80 | <4 cm | IV | adenocarcinoma | Tubular adenocarcinoma |
| 10 | Male | 57 | <4 cm | Ⅰ | adenocarcinoma | Tubular adenocarcinoma |
| 11 | Male | 43 | ≥4 cm | IV | adenocarcinoma | Low adhesion adenocarcinoma |
| 12 | Male | 76 | <4 cm | III | adenocarcinoma | Low adhesion adenocarcinoma |
| 13 | Male | 43 | <4 cm | III | adenocarcinoma | Tubular adenocarcinoma |
| 14 | Male | 66 | <4 cm | Ⅱ | adenocarcinoma | Low adhesion adenocarcinoma |
| 15 | Female | 49 | <4 cm | III | adenocarcinoma | Tubular adenocarcinoma |
| 16 | Female | 44 | ≥4 cm | Ⅱ | adenocarcinoma | Tubular adenocarcinoma |
| 17 | Male | 57 | <4 cm | IV | adenocarcinoma | Tubular adenocarcinoma |
| 18 | Female | 74 | <4 cm | Ⅱ | adenocarcinoma | Tubular adenocarcinoma |
| 19 | Male | 48 | <4 cm | IV | adenocarcinoma | Low adhesion adenocarcinoma |
| 20 | Male | 64 | ≥4 cm | Ⅱ | adenocarcinoma | Low adhesion adenocarcinoma |
| 21 | Male | 44 | <4 cm | IV | adenocarcinoma | Low adhesion adenocarcinoma |
| 22 | Male | 68 | ≥4 cm | Ⅱ | adenocarcinoma | Tubular adenocarcinoma |
| 23 | Female | 47 | ≥4 cm | IV | adenocarcinoma | Tubular adenocarcinoma |
| 24 | Female | 70 | ≥4 cm | III | adenocarcinoma | Tubular adenocarcinoma |
| 25 | Male | 46 | ≥4 cm | Ⅱ | adenocarcinoma | Tubular adenocarcinoma |
| 26 | Female | 47 | <4 cm | III | adenocarcinoma | Papillary adenocarcinoma |
| 27 | Male | 60 | <4 cm | III | adenocarcinoma | Mucosal adenocarcinoma |
| 28 | Female | 49 | <4 cm | Ⅱ | adenocarcinoma | Mucosal adenocarcinoma |
| 29 | Female | 82 | ≥4 cm | III | adenocarcinoma | Tubular adenocarcinoma |
| 30 | Male | 63 | ≥4 cm | Ⅱ | adenocarcinoma | Tubular adenocarcinoma |
| 31 | Female | 73 | ≥4 cm | III | adenocarcinoma | Papillary adenocarcinoma |
| 32 | Female | 43 | ≥4 cm | III | adenocarcinoma | Tubular adenocarcinoma |
| 33 | Male | 67 | <4 cm | Ⅱ | Adenocarcinoma | Tubular adenocarcinoma |
| 34 | Male | 59 | <4 cm | Ⅰ | adenocarcinoma | Tubular adenocarcinoma |
| 35 | Male | 49 | ≥4 cm | III | adenocarcinoma | Tubular adenocarcinoma |
| 36 | Female | 41 | <4 cm | III | adenocarcinoma | Mucosal adenocarcinoma |
| 37 | Female | 62 | ≥4 cm | IV | adenocarcinoma | Papillary adenocarcinoma |
| 38 | Female | 47 | <4 cm | Ⅱ | adenocarcinoma | Tubular adenocarcinoma |
| 39 | Female | 45 | <4 cm | Ⅱ | adenocarcinoma | Tubular adenocarcinoma |
| 40 | Male | 46 | <4 cm | III | adenocarcinoma | Mucosal adenocarcinoma |
3.2. FAK is a prognostic marker for survival of patients with gastric cancer
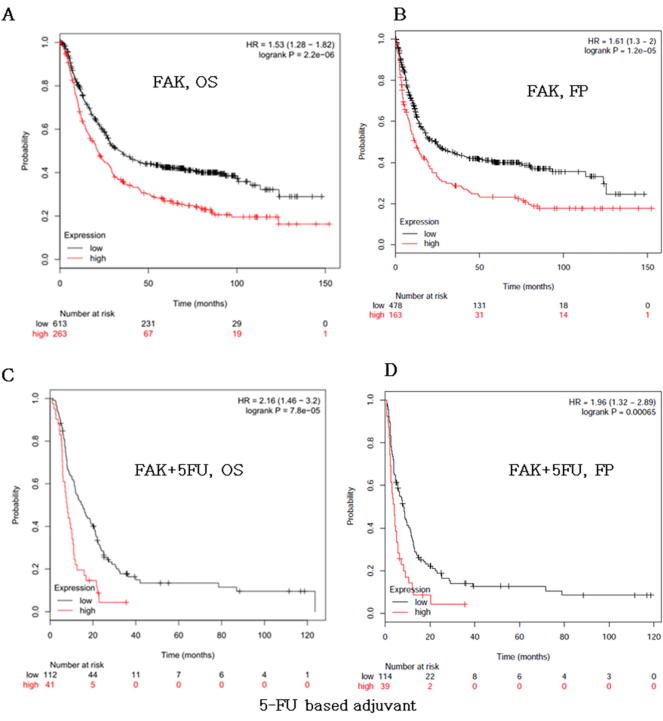
Because FAK was significantly upregulated in GC clinical samples, we asked whether the FAK gene could serve as a prognostic marker in GC patients. Therefore, overall survival (OS) and first progression (FP) curves were plotted using the Kaplan–Meier method based on the gene expression level in 882 GC samples. Patients with higher levels of FAK1 had significantly shorter OS (Fig. 2A, logrank P = 2.2 × 106) and FP (Fig. 2B, logrank P = 1.5 × 105) than those with lower levels of FAK. A similar result was also found in survival analysis according to gene expression levels in 153 GC patient samples treated with 5-FU (Fig. 2C-D). These results showed that FAK is closely related to the prognosis of the patient with gastric cancer.
Fig. 2.
FAK is a prognostic marker for survival of patients with gastric cancer. Kaplan-Meier curves stratified by the indicated mRNA levels, and tested by a log-rank test. High levels of FAK correlated with poor overall survival (OS). (A) and poor time to first progression (FP). (B) in gastric cancer. Overall survival (OS). (C) and poor time to first progression (FP). (D) in gastric cancer samples treatment with 5-FU.
3.3. Knockdown of FAK suppressed BGC823 cell proliferation
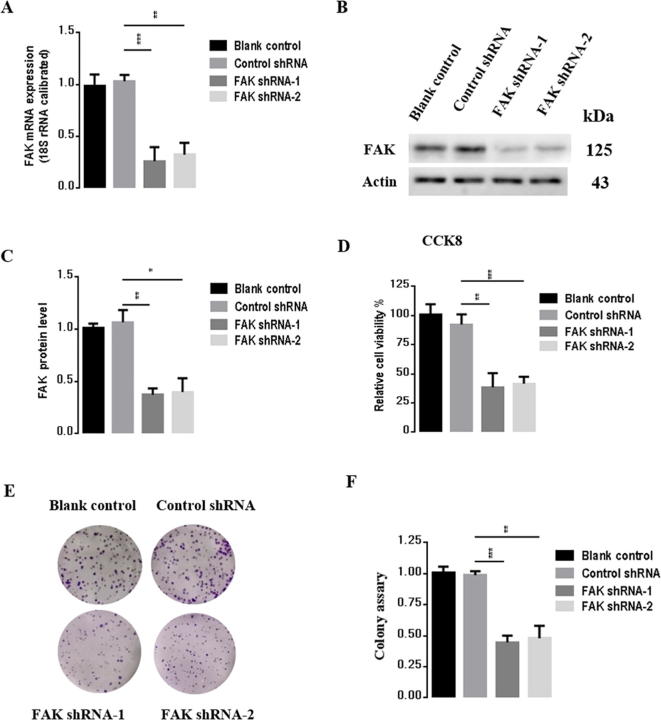
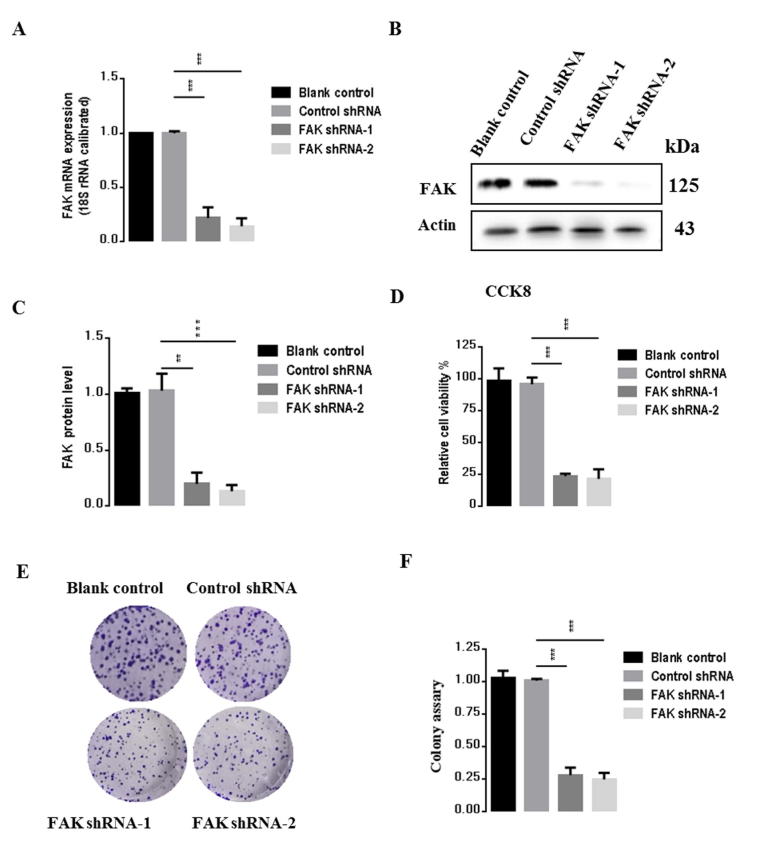
To evaluate the effects of FAK on gastric carcinogenesis, we used a lentivirus construct to knock down FAK and performed transfection studies in BGC823 gastric cancer cells with two plasmids, FAK shRNA-1 and FAK shRNA-2. After 72 h transfection, we measured mRNA and protein levels of FAK. As shown in Fig. 3A-C, compared to the control shRNA group, FAK mRNA expression was reduced by 73.4% and 62%, respectively (p < 0.001, p < 0.01), and protein level was reduced by 62.4% and 49.2%, respectively (p < 0.01, p < 0.05) in cells transfected with FAK shRNA-1 and FAK shRNA-2, respectively. To evaluate the effects of inhibition of FAK on BGC823 cells, CCK-8 assay was next used to examine the relative amount of cell proliferation after FAK knockdown. As shown in Fig. 3D, FAK knockdown significantly inhibited proliferation of BGC823 cells by 60% compared to the control group. Consistently, colony formation assay, as shown in Fig. 3E–F, indicated that the rate of proliferation of shRNA-FAK cells was reduced relative to that of shRNA-Ctrl cells (p < 0.05, vs control group). Taken together, these results demonstrated that inhibition of FAK significantly inhibited the proliferation BGC823 cells and enhanced oncogenic transformation. Because the FAK shRNA-1 has a more dramatic effect compared to shRNA-2, we selected shRNA-1 (named FAK shRNA) for subsequent experiments. Consistent results were obtained in analysis of SGC7901, another gastric cancer cell line (Supplementary Fig. 1).
Fig. 3.
FAK suppression inhibits proliferation in BGC823 cells. (A) mRNA expression levels of FAK measured by qPCR. (B) Protein expression of FAK by western blot. (C) Statistical analysis of B. (D) The viability of BGC823 cells was determined using CCK-8. (E) Colony-formation assay performed with BGC823-control and BGC823-shFAK cells. (F) Statistical analysis of E. Results are representative of three independent experiments, and the error bars represent the standard deviation (SD). *p < 0.05, ** p < 0.01, *** p < 0.001.
3.4. FAK silencing enhanced 5-FU chemosensitivity in BGC823 cells
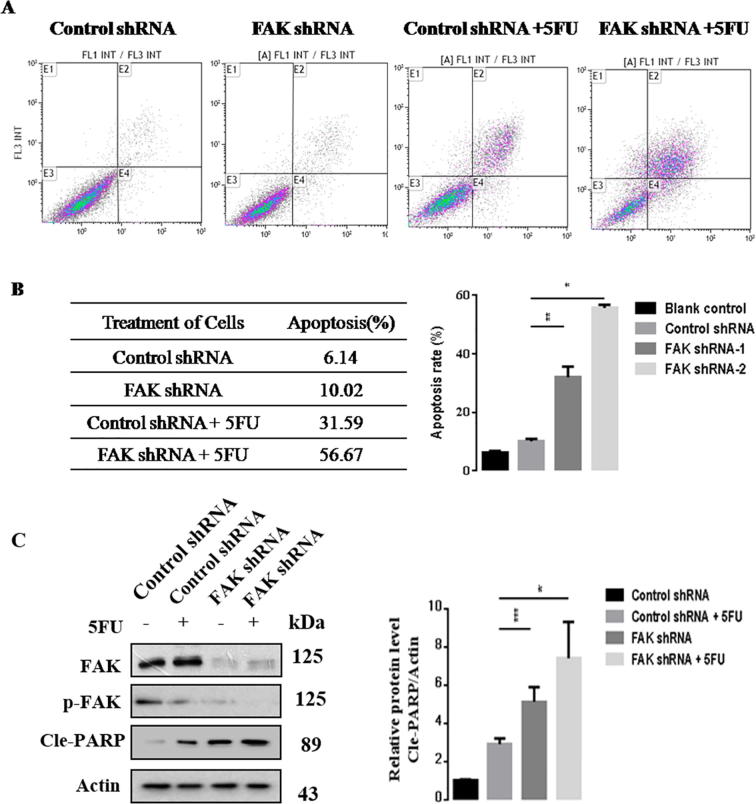
We next performed Annexin V-FITC assay to investigate the effect of FAK on 5-FU-induced BGC823 apoptosis. The flow cytometry results showed a significant increase in 5-FU-induced apoptosis compared with control cells. We found 5-FU treatment alone caused a 31.9% apoptotic rate, but the percentage of apoptotic cells induced by 5-FU increased to 56% after FAK silencing (Fig. 4A–B). The results suggest that FAK silencing enhanced 5-FU chemosensitivity in vitro. Consistently, the western blot results showed that FAK inhibition enhanced 5-FU-induced BGC823 apoptosis (Fig. 4C). In summary, these data demonstrated that FAK silencing enhanced 5-FU chemosensitivity.
Fig. 4.
FAK silencing enhances 5-FU chemosensitivity in BGC823 cells. BGC823 cells were infected with lentivirus expressing control or FAK-shRNA plasmids, and the cells were untreated or treated with 5-FU for an additional 24 h. (A) Flow cytometry to determine cell apoptosis. (B) Statistical analysis of the apoptosis ratio in the various groups as indicated. (C) Western blot analysis of the indicated proteins. The Cle-PARP expression was quantify and statistically analyzed using image analyzer. Results are representative of three independent experiments, and the error bars represent the SD. *p < 0.05, ** p < 0.01.
3.5. Downregulation of FAK enhanced p53 activation after treatment of 5-FU
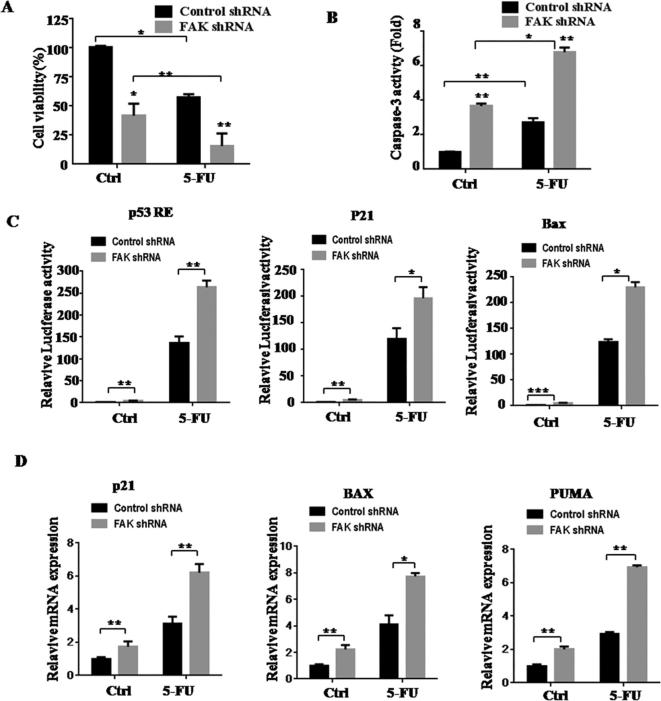
As shown in Fig. 5A, we examined the proliferation of control group cells and FAK-shRNA group cells after 5-FU treatment for 24 h. Compared with control groups, FAK-shRNA groups showed decreased proliferation and enhanced caspase-3 activity (Fig. 5A–B).
Fig. 5.
Downregulation of FAK increases 5-FU-induced apoptosis. BGC823 cells were infected with the lentivirus expressing control or FAK-shRNA plasmids, and the cells were untreated or treated with 5-FU for an additional 24 h. (A). Cell viability was determined by CCK-8. (B) Caspase-3 activation assay. (C) Luciferase reporter assays of p53RE, p21, and Bax. (D) qPCR analysis of Bax, p21, and PUMA mRNA expression. Results are representative of three independent experiments, and the error bars represent the SD. *p < 0.05, **p < 0.01.
Because knockdown of FAK increased 5-FU-induced apoptosis, we next examined the p53 signaling pathway since it is related to apoptosis. We performed luciferase reporter assays, and found that 5-FU stimulation after knockdown of FAK increased activation of several p53-target genes, including p53 RE, p21, and Bax (Fig. 5C). We also detected mRNA expression levels of p53-target genes, including Bax, p21, and PUMA. The results demonstrate that FAK-shRNA groups showed much stronger p53 transcriptional activities than control groups, both in the absence and presence of 5-FU (Fig. 5D). Overall, these results demonstrated that inhibition of FAK enhanced 5-FU-induced p53 activation and promoted the ability of 5-FU to inhibit gastric cancer proliferation.
3.6. FAK inhibition suppressed xenograft tumor growth in nude mice
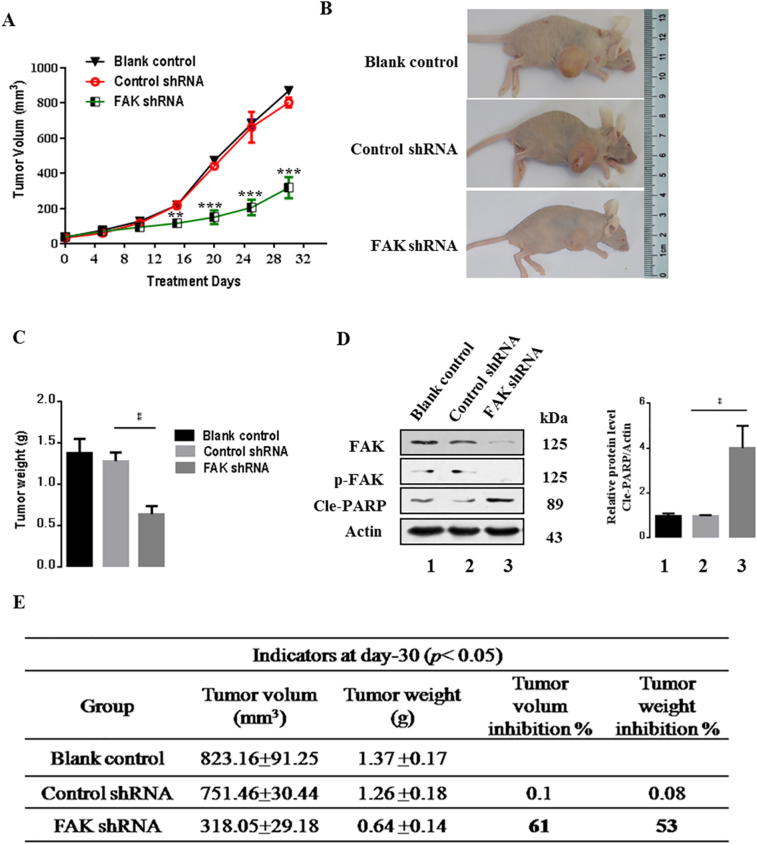
To examine the effects of FAK in gastric cancer development in vivo, xenograft tumors were induced in 6–7 weeks nude mice by a single injection of BGC823-untreated cells, BGC823-control cells, or BGC823-FAK-shRNA cells at a dosage of 5 × 106. A significant amelioration in tumor size was observed in the FAK-shRNA transfected group compared to the blank control group or the control shRNA transfected group over 16 days (p < 0.01) (Fig. 6A). At day 30, mice were sacrificed and the xenograft tumors were removed and weighed. The average tumor weight for mice injected with the cells in which FAK was inhibited was 31% lower than the tumor weight of the control group (p < 0.01). The volume inhibition rate of the FAK inhibition group was 63% and the weight inhibition was 51% compared with the control group (Fig. 6B–C, E). These results showed that inhibition of FAK decreased tumor cell growth dramatically.
Fig. 6.
FAK inhibition suppresses tumor growth in nude mice. (A) Time-dependent tumor growth alterations in nude mice. (B and C) Statistical analysis of the tumor weight of each group. (D) Expression of apoptosis-associated proteins in the tumors in each group. The Cle-PARP expression was quantify and statistically analyzed using image analyzer. (E) Measurement index of xenografts in nude mice. Results are representative of three independent experiments, and the error bars represent the SD. *p < 0.05, ** p < 0.01.
Because FAK regulated cell proliferation and tumor development, we investigated the potential signaling pathway of FAK might affect tumorigenesis. Then, we performed western blot assays to examine the apoptosis signaling pathways to further explore the underlying molecular mechanisms. We found that FAK inhibition markedly suppressed protein levels of phosphorylated FAK, but Cle-PARP was significantly upregulated (Fig. 6D). These data revealed that FAK inhibition decreased gastric cancer tumorigenesis and progression.
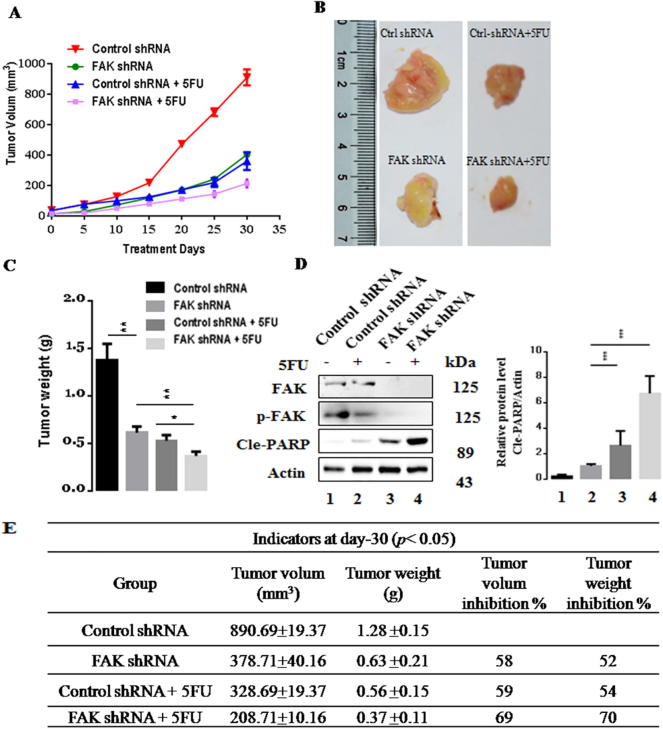
3.7. Suppression of FAK increased the 5-FU-induced inhibition of cell proliferation in vivo
To investigate whether FAK enhanced 5-FU chemosensitivity in vivo, BGC823-control cells, BGC823-FAK-shRNA cells, or the corresponding cells treated with 5-FU were subcutaneously injected into nude mice. After tumors reached approximately 100 mm3 in size, mice were randomized to receive treatment with PBS or 5-FU for 4 days of a 20-day period. The tumor size was measured every 5 days for up to 30 days (Fig. 7A). We observed that 5-FU treatment inhibited tumor development. With FAK silencing, the tumor growth inhibition was more substantial, with a significant decrease in tumor volume and weight compared with the control cells. The tumor volume inhibition was increased from 58% to 69% and the weight inhibition rate was increased from 52% to 70% (Fig. 7B–C, E). We also found that FAK inhibition markedly suppressed protein levels of phosphorylated FAK. Compared with 5-FU non-treatment group, Cle-PARP of treatment group was significantly upregulated (Fig. 6D). These results demonstrated that FAK silencing enhanced the chemosensitivity to 5-FU in vivo.
Fig. 7.
Suppressed-FAK promotes 5-FU-induced cell proliferation inhibition in vivo. (A) The tumor growth curves for various groups of nude mice treated as indicated. (B) Photographs of dissected xenograft tumors from various groups of nude mice treated as indicated. (C) Statistical analysis of the tumor weight of each group.. (D) Expression of apoptosis-associated proteins in the tumors in each group. (E) Measurement index of xenograft for various groups of nude mice. Results are representative of three independent experiments, and the error bars represent the SD. *p < 0.05, **p < 0.01, ***p < 0.001.
4. Discussion
Gastric carcinoma (GC) is one of the most common malignant tumors. The morbidity and mortality of GC have exhibited an increasing tendency in recent years. Chemotherapy is a major method used to treat GC [22], [23], [24], and 5-FU is commonly used in clinical treatment, where it causes cell death by interfering with nucleoside metabolism, DNA synthesis, and RNA dysfunction [25]. Chemoresistance is a common phenomenon and is an important factor affecting therapeutic efficacy and prognosis in cancer therapy [26]. Drug resistance of tumor cells alters the microenvironment and decreases the effects of toxic components, leading to decreased DNA repair activity and apoptosis [27], [28].
FAK is a multi-function non-receptor tyrosine kinase that plays a vital role in cell-cell and cell-ECM adhesion. FAK participates in cell cycle regulation, survival, proliferation, apoptosis, migration, invasion, metastasis, and other processes [16], [17], [18], [29], [30]. Importantly, FAK is closely associated with the development of tumors in several cancers, and recent studies reported significantly increased FAK expression levels in colon, liver, lung, gastric, breast, and ovarian cancer [19], [31], [32], [33], [34], [35]. Therefore, targeting FAK expression may be an effective therapeutic option.
In this study, we found that inhibition of FAK expression enhanced 5-FU chemosensitivity in GC. We used RNA interference to knock down FAK in gastric carcinoma BGC823 and SGC7901 cells and determined the biological changes. We found that FAK shRNA downregulated mRNA and protein expression of FAK and led to decreased cell proliferation of gastric cancer cells based on CCK-8 and colony formation assays. We also observed that FAK RNA interference reduced xenograft tumor growth in a nude mouse model. Additionally, the inhibition of FAK in BGC823 cells enhanced the therapeutic efficacy of 5-FU. Taken together, these results indicated that FAK attenuated gastric cancer cell proliferation, slowed the development of tumors, and, critically, improved the sensitivity of 5-FU treatment. FAK inhibition also increased 5-FU-induced caspase-3 activity and promoted p53 transcriptional activities. Most of our experiments were performed in BGC823 cells, and there may be some limitations of our conclusions, so these results will need to be verified in future experiments. The clinical data presented in Table 1 showed that FAK level was correlated to TNM staging lymph and node status, while no apparent association was found between FAK expression and patient gender, patient age, or tumor size. These data showed that the FAK gene may play an important role in GC development. At the same time, database analysis revealed that patients with higher levels of FAK had a significantly shorter overall survival (OS) and poor time to first progression (FP) than did those with lower levels of FAK in the absence or presence of 5-FU. High FAK expression promotes tumor recurrence and reduces patient survival, which suggested that FAK may be a prognostic marker of survival of patients with gastric cancer and used as a potential target for tumor treatment in the gastric cancers. Many early studies showed FAK contains multiple phosphorylation sites, including tyrosine, lysine, and serine residues, and together with proteins that contain SH2 and SH3 domains, FAK participates in biological processes through integrin, BRAF/MEK/ERK, Src/FAK, SFK-FAK/CSF-1R, FAK-ROCK/RhoA, FAK/PI3K/Akt, and other pathways [36], [37], [38], [39], [40]. Although our results demonstrated that p53 signaling pathways may be involved in the FAK-mediated mechanisms of gastric cancer development, whether FAK affects other signaling pathways to regulate gastric cancer progression is an important issue that requires further study.
5. Conclusion
Inhibition of FAK increased 5-FU chemoresistance and promoted apoptosis by inhibiting the p53 signaling pathway in gastric carcinoma. Our results suggest an alternative mechanism for 5-FU in gastric carcinoma and suggest that FAK silencing may serve as an effective strategy for patients undergoing chemotherapy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81902622 and 31900963), the Foundation of Fujian Provincial Department of Science and Technology (China, IDs:2019J01556, 2018-ZQN-87, and 2018-2-66), the Foundation of Xiamen Municipal Bureau of Science and Technology (China, IDs:3502Z20174080 and 3502Z20184038), the Health-Education Joint Research Project of Fujian Province (China, ID:2019-WJ-22), the Shanghai Pujiang Program (China, ID:18PJ1409400), the Scientific Research Foundation of Shanghai Municipal Commission of Health and Family Planning (China, ID:20174Y0218).
We thank Dr. Jianchun Cai and Dr. Zeyang Lin for verifying the GC pathological diagnosis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.csbj.2019.12.010.
Contributor Information
Jingjing Hou, Email: jjhou@xmu.edu.cn.
Qian Feng, Email: fengqiangrace@fjnu.edu.cn.
Zhong Wan, Email: smmuwz@126.com.
Yongsheng Yu, Email: yongshengyu@tongji.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
References
- 1.Hou X.L., Ji C.D., Tang J., Wang Y.X., Xiang D.F., Li H.Q. FPR2 promotes invasion and metastasis of gastric cancer cells and predicts the prognosis of patients. Sci Rep. 2017;7:3153. doi: 10.1038/s41598-017-03368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Jiang J., Ji J., Cai Q., Chen X., Yu Y. PKM2 promotes cell migration and inhibits autophagy by mediating PI3K/AKT activation and contributes to the malignant development of gastric cancer. Sci Rep. 2017;7:2886. doi: 10.1038/s41598-017-03031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao G.Q., Chen T., Qi X.L., Hu Y.F., Liu H., Yu J. Laparoscopic management of gastric gastrointestinal stromal tumors: a retrospective 10-year single-center experience. World J Gastroenterol. 2017;23:3522–3529. doi: 10.3748/wjg.v23.i19.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin H., Sun J., Zhu K., Liu X., Zhang Q., Shen Q. The prognostic value of neutrophil-lymphocyte ratio is superior to derived neutrophil-lymphocyte ratio in advanced gastric cancer treated with preoperative chemotherapy and sequential R0 resection: a 5-year follow-up. OncoTargets Ther. 2017;10:2655–2664. doi: 10.2147/OTT.S135641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L., Wu C., Zhao Y. miRNA-34a enhances the sensitivity of gastric cancer cells to treatment with paclitaxel by targeting E2F5. Oncol Lett. 2017;13:4837–4842. doi: 10.3892/ol.2017.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang M., Koh I., Lee S.J., Cheong J.H., Kim P. Droplet-based microtumor model to assess cell-ECM interactions and drug resistance of gastric cancer cells. Sci Rep. 2017;7:41541. doi: 10.1038/srep41541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H., Li Y., Song X., Zhao Y., Cheng L., Zhao L. CHST11/13 regulate the metastasis and chemosensitivity of human hepatocellular carcinoma cells via mitogen-activated protein kinase pathway. Dig Dis Sci. 2016;61:1972–1985. doi: 10.1007/s10620-016-4114-5. [DOI] [PubMed] [Google Scholar]
- 8.Loessner D., Stok K.S., Lutolf M.P., Hutmacher D.W., Clements J.A., Rizzi S.C. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials. 2010;31:8494–8506. doi: 10.1016/j.biomaterials.2010.07.064. [DOI] [PubMed] [Google Scholar]
- 9.Colombelli J., Besser A., Kress H., Reynaud E.G., Girard P., Caussinus E. Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J Cell Sci. 2009;122:1665–1679. doi: 10.1242/jcs.042986. [DOI] [PubMed] [Google Scholar]
- 10.Lim Y., Lim S.T., Tomar A., Gardel M., Bernard-Trifilo J.A., Chen X.L. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown M.C., Cary L.A., Jamieson J.S., Cooper J.A., Turner C.E. Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol Biol Cell. 2005;16:4316–4328. doi: 10.1091/mbc.E05-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsen S.M., Stover J.D., Nagatomi J. Examining the role of mechanosensitive ion channels in pressure mechanotransduction in rat bladder urothelial cells. Ann Biomed Eng. 2011;39:688–697. doi: 10.1007/s10439-010-0203-3. [DOI] [PubMed] [Google Scholar]
- 13.Calalb M.B., Polte T.R., Hanks S.K. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsons J.T. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 15.Zebda N., Dubrovskyi O., Birukov K.G. Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions. Microvasc Res. 2012;83:71–81. doi: 10.1016/j.mvr.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y., Huang Y., Gunst S.J. Focal adhesion kinase (FAK) and mechanical stimulation negatively regulate the transition of airway smooth muscle tissues to a synthetic phenotype. Am J Physiol Lung Cell Mol Physiol. 2016;311:L893–L902. doi: 10.1152/ajplung.00299.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessler B.E., Sharma V., Zhou Q., Jing X., Pike L.A., Kerege A.A. Not kinase activity, is a key mediator of thyroid tumorigenesis and protumorigenic processes. Mol Cancer Res. 2016;14:869–882. doi: 10.1158/1541-7786.MCR-16-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elbahesh H., Bergmann S., Russell C.J. Focal adhesion kinase (FAK) regulates polymerase activity of multiple influenza A virus subtypes. Virology. 2016;499:369–374. doi: 10.1016/j.virol.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Ding L., Wang L., Sui L., Zhao H., Xu X., Li T. Claudin-7 indirectly regulates the integrin/FAK signaling pathway in human colon cancer tissue. J Hum Genet. 2016;61:711–720. doi: 10.1038/jhg.2016.35. [DOI] [PubMed] [Google Scholar]
- 20.Cheng J., Zhuo H., Xu M., Wang L., Xu H., Peng J. Regulatory network of circRNA-miRNA-mRNA contributes to the histological classification and disease progression in gastric cancer. J Transl Med. 2018;16:216. doi: 10.1186/s12967-018-1582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szasz A.M., Lanczky A., Nagy A., Forster S., Hark K., Green J.E. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7:49322–49333. doi: 10.18632/oncotarget.10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmela C., Fonseca C., Faria R., Baptista R.B., Ribeiro S., Ferreira A.O. Increased risk for metachronous gastric adenocarcinoma following gastric MALT lymphoma-A US population-based study. United Eur Gastroenterol J. 2017;5:473–478. doi: 10.1177/2050640616671643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilson D.H. The role of radiation therapy in upper gastrointestinal cancers. Clin Adv Hematol Oncol: H&O. 2017;15:366–376. [PubMed] [Google Scholar]
- 24.Osumi H., Takahari D., Shinozaki E., Chin K., Ogura M., Wakatsuki T. Associations between early tumor shrinkage and depth of response and clinical outcomes in patients treated with 1st-line chemotherapy for advanced gastric cancer. Gastric Cancer. 2017 doi: 10.1007/s10120-017-0729-2. Official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. [DOI] [PubMed] [Google Scholar]
- 25.Ma X., Cheng Z., Jin Y., Liang X., Yang X., Dai Z. SM5-1-conjugated PLA nanoparticles loaded with 5-fluorouracil for targeted hepatocellular carcinoma imaging and therapy. Biomaterials. 2014;35:2878–2889. doi: 10.1016/j.biomaterials.2013.12.045. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J., Luo N., Tian Y., Li J., Yang X., Yin H. USP22 knockdown enhanced chemosensitivity of hepatocellular carcinoma cells to 5-Fu by up-regulation of Smad4 and suppression of Akt. Oncotarget. 2017;8:24728–24740. doi: 10.18632/oncotarget.15798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu M., Ocana A., Tannock I.F. Reversal of ATP-binding cassette drug transporter activity to modulate chemoresistance: why has it failed to provide clinical benefit? Cancer Metastasis Rev. 2013;32:211–227. doi: 10.1007/s10555-012-9402-8. [DOI] [PubMed] [Google Scholar]
- 28.Szakacs G., Paterson J.K., Ludwig J.A., Booth-Genthe C., Gottesman M.M. Targeting multidrug resistance in cancer. Nat Rev Drug Discovery. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 29.Sarode S.C., Sarode G.S., Choudhary S., Patil S. FAK is overexpressed in keratocystic odontogenic tumor: a preliminary study. J Oral Pathol Med. 2016 doi: 10.1111/jop.12532. Official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. [DOI] [PubMed] [Google Scholar]
- 30.Symeonides S.N., Anderton S.M., Serrels A. FAK-inhibition opens the door to checkpoint immunotherapy in Pancreatic Cancer. J Immunother Cancer. 2017;5:17. doi: 10.1186/s40425-017-0217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Constanzo J.D., Tang K.J., Rindhe S., Melegari M., Liu H., Tang X. PIAS1-FAK interaction promotes the survival and progression of non-small cell lung cancer. Neoplasia. 2016;18:282–293. doi: 10.1016/j.neo.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benzina S., Harquail J., Guerrette R., O'Brien P., Jean S., Crapoulet N. Breast cancer malignant processes are regulated by Pax-5 through the disruption of FAK signaling pathways. J Cancer. 2016;7:2035–2044. doi: 10.7150/jca.15200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanteti R., Batra S.K., Lennon F.E., Salgia R. FAK and paxillin, two potential targets in pancreatic cancer. Oncotarget. 2016;7:31586–31601. doi: 10.18632/oncotarget.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J.S., Li H.S., Huang J.Q., Dong S.H., Huang Z.J., Yi W. MicroRNA-379-5p inhibits tumor invasion and metastasis by targeting FAK/AKT signaling in hepatocellular carcinoma. Cancer Lett. 2016;375:73–83. doi: 10.1016/j.canlet.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 35.Zang M., Zhang Y., Zhang B., Hu L., Li J., Fan Z. CEACAM6 promotes tumor angiogenesis and vasculogenic mimicry in gastric cancer via FAK signaling. BBA. 1852;2015:1020–1028. doi: 10.1016/j.bbadis.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Baquero P., Jimenez-Mora E., Santos A., Lasa M., Chiloeches A. TGFbeta induces epithelial-mesenchymal transition of thyroid cancer cells by both the BRAF/MEK/ERK and Src/FAK pathways. Mol Carcinog. 2016;55:1639–1654. doi: 10.1002/mc.22415. [DOI] [PubMed] [Google Scholar]
- 37.Digiacomo G., Tusa I., Bacci M., Cipolleschi M.G., Dello Sbarba P., Rovida E. Fibronectin induces macrophage migration through a SFK-FAK/CSF-1R pathway. Cell Adh Migr. 2016:1–11. doi: 10.1080/19336918.2016.1221566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji Y., Wang Z., Li Z., Huang N., Chen H., Li B. Silencing IGF-II impairs C-myc and N-ras expressions of SMMC-7721 cells via suppressing FAK/PI3K/Akt signaling pathway. Cytokine. 2017;90:44–53. doi: 10.1016/j.cyto.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Pei G., Lan Y., Chen D., Ji L., Hua Z.C. FAK regulates E-cadherin expression via p-SrcY416/p-ERK1/2/p-Stat3Y705 and PPARgamma/miR-125b/Stat3 signaling pathway in B16F10 melanoma cells. Oncotarget. 2017;8:13898–13908. doi: 10.18632/oncotarget.14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song G., Xu S., Zhang H., Wang Y., Xiao C., Jiang T. TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer Res. 2016;35:148. doi: 10.1186/s13046-016-0427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.