Abstract
Background/Objectives:
Evaluation of mediastinal lymphadenopathy (MLA) is a great diagnostic challenge considering the myriad of causes. In recent years, the role of endoscopic ultrasound (EUS) has been greatly extended in evaluation of MLA due to its safety, reliability, and accuracy. The present study details the role of EUS-guided-fine-needle aspiration/fine-needle biopsy (EUS-FNA/FNB) in MLA of unknown origin.
Methods:
Seventy-two patients (34 men) with MLA of unknown etiology were studied. Mediastinum was evaluated with linear echoendoscope and FNA/FNB was performed with 22-G needle and sent for cytology, histopathological, and mycobacterial growth indicator tube/GeneXpert evaluation. EUS-FNA/FNB diagnosis was based on cytology reporting by pathologists. Patients tolerated the procedure, and insertion of needle into the lesion was always successful without any complications.
Results:
EUS-FNA/FNB established a tissue diagnosis in 66/72 patients in first sitting, while six patients underwent repeat procedure. EUS-FNA diagnoses (after second sitting) were tuberculous lymphadenitis in 45/72 (62.5%), metastatic lymph nodes 12/72 (16.7%), reactive lymphadenopathy 6/72 (8.3%), sarcoidosis 4/72 (5.6%), and lymphoma 2/72 (2.8%), while it was nondiagnostic in 3/72 (4.1%) patients. Final diagnosis was based on combined clinical presentation, EUS-FNA/FNB result and clinicoradiological response to treatment on long-term follow-up of 6 months.
Conclusion:
EUS echo features along with EUS-FNA/FNB can diagnose MLA and surgical biopsy can be avoided.
KEY WORDS: Endoscopic ultrasound-guided-fine-needle aspiration/fine needle biopsy, mediastinal lymphadenopathy, tuberculous lymphadenitis
INTRODUCTION
Evaluation of mediastinal lymphadenopathy (MLA) is a great diagnostic challenge considering the myriad of causes. Etiology of MLA ranges from benign reactive lymphadenopthy, infectious-inflammatory diseases to malignant conditions. Traditional imaging such as computed tomography (CT) scan of thorax and positron emission tomography (PET) scan are initial investigations for evaluation of MLA but do not accurately predict the nature of MLA and have a high false-positive rate (39%).[1,2,3,4,5,6,7,8] Tissue diagnosis is of utmost importance for planning treatment as well as deciding the prognosis.[9] Mediastinoscopy and/or thoracoscopy are the gold standards for sampling the mediastinal lymph nodes (LNs). Since they are invasive, costly, and require general anesthesia and hospitalization along with serious complications, their application is not feasible in all patients.[10,11,12] For the past three decades, endoscopic ultrasound-guided-fine-needle aspiration/biopsy (EUS-FNA/FNB) has been a well-established modality for diagnosis and staging of gastrointestinal and pancreaticobiliary lesions.[13,14,15,16,17] In recent years, the role of EUS has been extended in evaluation of MLA due to its safety, reliability, and accuracy which has been proved in many studies.[18] In the current era, endosonologists are frequently consulted for evaluation and tissue sampling of MLA. The aim of the present study is to detail the role and utility of EUS-FNA/FNB in patients with MLA of unknown cause.
SUBJECTS AND METHODS
This was a retrospective study, in which the EUS database was analyzed at the study site, a tertiary care center with high patients load from January 2017 to April 2018. Patients of all age groups with MLA on chest radiography or CT thorax were included in the study. In all the patients, diagnosis was not established by other means. Patients with severe coagulopathy, inaccessible LNs on EUS, and previously diagnosed patients were excluded from the present study. All EUS-FNA/FNB procedures were performed in the department of gastroenterology. Institutional review board and ethics committee permission was obtained for the study protocol. Patients data related to demographics, referring physician, procedure indication, size, location, EUS echo features, number of passes made of the LN, EUS-FNA/FNB diagnosis, and final diagnosis were entered in an Excel SpreadSheet. Diagnosis on EUS-FNA/FNB was made on cytology by the pathologists. Final diagnosis was based on combined clinical presentation, EUS-FNA/FNB result, and clinicoradiological response to treatment at follow-up of 6 months.
The diagnostic criteria for various etiologies were as per the enlisted criteria
For tuberculous lymphadenitis, the final diagnosis was based on all of the following:
Compatible clinical and radiological presentation
Necrotizing granulomatous reaction or presence of acid-fast bacilli (AFB) on smear microscopy or Mycobacterium tuberculosis (MTB) detected on cartridge-based nucleic acid amplification test, i.e., GeneXpert (GXP) or isolation of MTB complex on AFB culture
Clinical and radiological response to antituberculosis therapy (ATT)[19,20,21]
For sarcoidosis, the final diagnosis was based on all of the following:
Consistent clinical and radiological presentation
Nonnecrotizing granulomatous reaction along with negative AFB on smear microscopy, MTB not detected on GXP and no growth of MTB complex on AFB culture
Clinical and radiological response to glucocorticoid therapy.[19,20,21]
For malignancy and lymphoma: presence of typical malignant cells along with clinical and radiological response to chemotherapy or radiotherapy as appropriate.[21]
Endoscopic ultrasound-guided-fine-needle aspiration/fine-needle biopsy method
All the procedures were done at study site. Written informed consent and preprocedure preparation were done. All included patients underwent EUS-FNA/FNB by a linear Olympus GF-UCT180 echoendoscope (Olympus America Inc., Center Valley, PA, USA) using a 22-G needle. During the EUS-FNA/FNB procedure, a needle was advanced through the mucosa into the lesion under EUS guidance. The stylet was completely withdrawn, and the needle was moved to and fro in a fanning fashion inside the lesion, 10–12 times [Figure 1]. After this, the needle tube was pulled back inside the sheath. The needle was removed from the scope, and the specimen was pushed out from the needle sheath by reinserting the stylet and flushing it by air or saline. The slides were prepared after spreading the material from the needle. Slides prepared were air-dried for FNA examination, for AFB culture and GXP study material was sent in normal saline and for FNB study material was sent in formalin solution. These slides were sent to the pathology department and examined by the cytopathologist after staining. The procedure was performed on an outpatient basis. Patients were observed for immediate adverse events in the recovery room for 4 h before being discharged. Outpatients were also monitored after discharge for a minimum of 48 h for the detection of further complications. All adverse events were documented. All procedures were performed in the absence of an on-site pathologist.[22,23,24]
Figure 1.
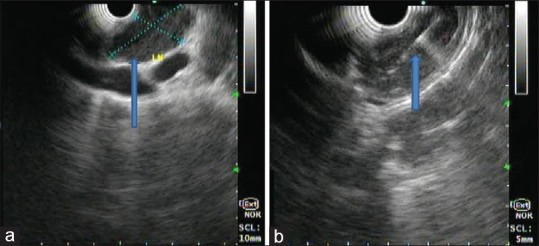
Endoscopic ultrasound examination of the mediastinum using linear echoendoscope at 7.5 mHz showing predominantly hypoechoic homogenous mass at subcarina with well-defined margin without vascularity suggestive of the lymph node (a), lesion was punctured using 22 G fine-needle aspiration needle, 2–3 passes taken (b)
Statistical analysis
SPSS Inc. Released 2009. Statistics for Windows, Version 18.0. (Chicago) SPSS Inc. and R: A Language and Environment for Statistical Computing, version 3.2.2. Released 2105, Core Team, R Foundation for Statistical Computing (Vienna, Austria) were used for the analysis of the data and Microsoft Word and Excel have been used to generate tables. Descriptive and inferential statistical analysis has been carried out in the present study. Results on continuous measurements are presented on mean ± standard deviation (minimum–maximum) and results on categorical measurements are presented in number (%). Significance is assessed at 5% level of significance. Chi-square/Fisher exact test has been used to find the significance of the study parameters on categorical scale between two or more groups.
RESULTS
Seventy-two participants were included in the study; they consisted of 38 women and 34 men with the mean age of 35.47 ± 19.93 years. Presenting complaints were dry cough in 20/72 (27.8%), fever 20/72 (27.8%), dysphagia 10/72 (13.9%), dyspnea on exertion 8/72 (11.1%), abdominal distension 4/72 (5.6%), cough with expectoration 4/72 (5.6%), weight loss 4/72 (5.6%), and chest pain in 2/72 (2.8%) patients. The predominant LN was subcarinal in 66/72 (91.7%); right paratracheal was involved in 4/72 (5.6%) and left paratracheal in 2/72 (2.8%) patients. Mean LN long-axis (LNLA) and LN short-axis (LNSA) size were 34.00 ± 8.72 mm (mm) and 17.81 ± 8.30 mm, respectively, with LNSA to LNLA ratio of 0.53 ± 0.21. Table 1 shows the EUS Echo features of the LNs studied. In 66/72 (91.66%) patients, EUS-FNA was diagnostic in the first sitting, while six subjects underwent a repeat procedure. In these cases, tissue sent for histology was reported as inadequate. However, as the final results of AFB culture take 6 weeks, so the procedure was repeated after 6 weeks as the initial EUS-FNA/FNB including GXP and AFB culture were nondiagnostic. Diagnoses on EUS FNA/FNB were tuberculous lymphadenitis [Figure 2a] in 45/72 (62.5%), metastatic lymph nodes [Figure 2b] 12/72 (16.7%), reactive lymphadenopathy [Figure 2c] 6/72 (8.3%), sarcoidosis 4/72 (5.6%), and lymphoma 2/72 (2.8%), while it was nondiagnostic in 3/72 (4.1%) patients. These three patients (after second EUS sitting) were started on empirical ATT based on their clinical presentation and additional investigations (Mantoux test positivity, normal serum calcium, normal angiotensin-converting enzyme level, and PET scan). Final diagnosis was based on the consistent clinical presentation, EUS-FNA/FNB findings, and response to therapy (clinical improvement and regression of lymph node size on imaging). Nondiagnostic EUS-FNA/FNB results were treated as false negative for calculation of sensitivity, specificity, and positive and negative predictive value [Tables 1 and 2].[25]
Table 1.
Endoscopic ultrasound echo features in various etiologic conditions
| Variables | Final diagnosis |
Total (n=72), n (%) | P | ||||
|---|---|---|---|---|---|---|---|
| Reactive lymphadenitis (n=6), n (%) | TB lymphadenitis (n=48), n (%) | Sarcoidosis (n=4), n (%) | Lymphoma (n=2), n (%) | Malignant LN (n=12), n (%) | |||
| LNLA (mm) | 24.33±3.61 | 35.42±9.08 | 36.50±2.89 | 36.00±0.00 | 32.00±7.98 | 34.00±8.72 | 0.041 |
| LNSA (mm) | 11.67±1.37 | 15.92±7.23 | 22.5±10.97 | 32.0±0.00 | 24.5±7.57 | 17.81±8.30 | <0.001 |
| LNSA/LNLA ratio | 0.48±0.02 | 0.45±0.17 | 0.64±0.35 | 0.88±0.00 | 0.76±0.16 | 0.53±0.21 | <0.001 |
| <0.5 | 4(66.7) | 38 (79.2) | 2 (50) | 0 | 2 (16.7) | 46 (63.9) | <0.001 |
| ≥0.5 | 2 (33.3) | 10 (20.8) | 2 (50) | 2(100) | 10 (83.3) | 26 (36.1) | |
| LN shape | |||||||
| Irregular | 0 | 32 (66.7) | 0 | 0 | 0 | 32 (44.4) | <0.001 |
| Oval | 6(100) | 10 (20.8) | 2 (50) | 0 | 4 (33.3) | 22 (30.6) | |
| Round | 0 | 6 (12.5) | 2 (50) | 2(100) | 8 (66.7) | 18 (25) | |
| Margin | |||||||
| Indistinct | 4 (66.7) | 42 (87.5) | 0 | 0 | 2 (16.7) | 48 (66.7) | <0.001 |
| Sharp | 2 (33.3) | 6 (12.5) | 4 (100) | 2 (100) | 10 (83.3) | 24 (33.3) | |
| Hypoechoic | 0 | 16 (33.3) | 4 (100) | 2 (100) | 10 (83.3) | 32 (44.4) | 0.002 |
| Hyperechoic | 6 (100) | 32 (66.7) | 0 | 0 | 2 (16.7) | 40 (55.6) | 0.002 |
| Homogenous | 6 (100) | 16 (33.3) | 2 (50) | 2 (100) | 8 (66.7) | 34 (47.2) | 0.002 |
| Heterogenous | 0 | 32 (66.7) | 2 (50) | 0 | 4 (33.3) | 38 (52.8) | 0.002 |
| Calcification | 0 | 26 (54.2) | 2 (50) | 0 | 6 (50) | 34 (47.2) | 0.063 |
| GXP result | 0 | 26 (54.2) | 0 | 0 | 0 | 26 (36.1) | <0.001 |
LN: Lymph node, LNLA: LN long axis, LNSA: LN short axis, GXP: GeneXpert
Figure 2.
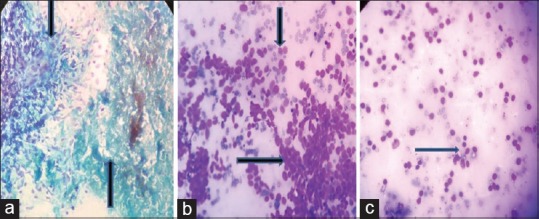
Photomicrograph showing cellular smear with abundant caseous necrosis (a, upward arrow) with granulomatous inflammation (a, downward arrow) suggestive of tuberculous lymphadenitis (PAP, ×400). Monotonous small-to-medium-sized cells with high N:C ratio with scant cytoplasm (b, rightward arrow) also nuclear molding seen (b, downward arrow) suggestive of metastatic small cell carcinoma. Mature and transformed lymphocytes admixed with histiocytes (c) suggestive of reactive lymphadenitis (Giemsa, ×400)
Table 2.
Sensitivity, specificity, and positive predictive and negative predictive value of endoscopic ultrasound-guided-fine needle aspiration/fine needle biopsy
| Findings | Observation |
Correlation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | Total | Sensitivity | Specificity | PPV | NPV | Accuracy | P | |
| Reactive lymphadenitis | 6 | 0 | 0 | 66 | 72 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | <0.001 |
| Tuberculous lymphadenitis | 45 | 0 | 3 | 27 | 72 | 93.75 | 100.0 | 100.0 | 90.00 | 96.00 | <0.001 |
| Sarcoidosis | 4 | 0 | 0 | 68 | 72 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | <0.001 |
| Lymphoma | 2 | 0 | 0 | 70 | 72 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | <0.001 |
| Metastatic LN | 12 | 0 | 0 | 60 | 72 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | <0.001 |
TP: True positive, FP: False positive, FN: False negative, TN: True negative, PPV: Positive predictive value, NPV: Negative predictive value, LN: Lymph node
DISCUSSION
Considering the wide range of etiologies, MLA poses a diagnostic challenge. As it can readily identify the periesophageal mediastinal masses or lymph nodes, EUS is the procedure of choice for their evaluation.[26] With its increasing acceptance, endosonologists are frequently consulted for EUS-FNA/FNB evaluation of the periesophageal mediastinal lesions.
In the present study, we evaluated echo features to predict the nature of a LN. EUS echo features may not always differentiate between malignant and benign LNs. Catalano et al.[27] first studied EUS echo features of malignant LNs in cohort of patients with esophageal carcinoma. Hypoechoic pattern, sharp border, rounded contour, and size more than 10 mm were associated malignancy when all four features were present with 100% accuracy.[27] Later on, Bhutani et al.[28] reported endosonographic features and FNA results of the mediastinal, celiac axis, and peripancreatic LNs in 25 patients with malignancies (esophageal, lung, and pancreatic cancers) with accuracy of 80% for predicting malignancy when all four echo features were present.[28] In 378 cases Faige[29] assessed the EUS characteristics to differentiate benign from malignant LNs. LN size of 1 cm or more within 1 cm of the tumor and with a morphology score (sum of roundness, homogeneity, and echogenicity) of 14 had a positive predictive value of 81% and a negative predictive value of 92% for differentiating malignant from benign lymph nodes.[29] While Jamil et al.[25] did not find EUS echo features reliable for determining etiology in MLA in 162 cases and suggested that FNA should be carried out whenever feasible. In a prospective study of 142 patients with intra-abdominal and intrathoracic lymphadenopathy, Okasha et al.[30] found that EUS diagnosis and shortest diameter of the LN were useful in predicting malignancy and LN elasticity score is complimentary with a sensitivity of 81.1% and specificity of 82.6% for diagnosis of malignancy. However, in case of MLA, size may not be reliable criteria due to normal variation in size of LNs at different locations within mediastinum. Ziyade et al.[31] carried out postpartum analysis of mediastinal LNs in otherwise normal individuals died of noninfectious and nononcological causes and made an observation of normal LN diameter of 1.5 cm and 2 cm, for stations 4R and 7, respectively, while for other stations normal LN diameter was 1 cm. Another limitation of echo features is inter- and intra-observer agreement for mediastinal or hilar LNs. Garcia-Olivé et al.[32] found good inter- and intra-observer agreement for shape or size but not good enough for the other ultrasonographic features such as coagulation necrosis, heterogenicity, and margins. These normal variations need consideration while evaluating MLA. In the present study, we found sensitivity and specificity of 90.90% and 100% for predicting malignant nature of LN when all four echo features were used in combination. Results of our study are in agreement with the previous studies. However, most of the participants in the previously mention studies were of suspected malignancy, while we have studied MLA of unknown etiology. Results of our study may not reflect the true utility of EUS echo features for predicting malignant LN as number patients with malignant nodes were small. As EUS echo features cannot reliably predict the nature of LN and whenever feasible EUS-FNA/FNB needs to be carried out if there is pathologic suspicion. In this regard, EUS echo features are particularly useful for selecting LN to be FNA/FNB.
Patients with benign lymphadenopathy were categorized according to their etiology and their EUS characteristics were studied. This has been done in only few of the previous studies.[21,25] Calcification of LNs was not studied on EUS in the past. We found that calcification is uncommon in reactive lymphadenitis and lymphoma and seen with equal frequency in tuberculous lymphadenitis, sarcoidosis, and metastatic malignant LNs.
Tuberculous lymphadenitis was the main etiology of MLA in our patients. This is due to the endemicity of tuberculosis (TB) in our region. However, referral bias cannot be excluded as majority of the patients were referred by chest physicians. Sharma et al.[33] studied 266 patients with mediastinal LNs, out of which 134 diagnosed as TB. In this study, the most common LN location was station 7, while median long-axis size of LN was 12 mm (5–25 mm). Shape of LN was oval or round in 55 cases and elliptical or crescent in 25 cases and shape could not be defined in 44 cases of confluent nodes. LNs were discrete in 59% and confluent in 41% participants, among confluent group, 82% had preserved outer borders and 18% had absent borders. Hypoechoeic and hyperechoic areas were noted in 88% and 63/134 (47%) cases, respectively.[33] In the present study, we evaluated LN shape as irregular versus round or oval, margins as distinct versus indistinct, and echo features as predominantly homogenous versus heterogeneous and hypoechoic versus hyperechoic [Figures 3 and 4]. In our study, among the patients with tubercular lymphadenitis, mean long-axis and short-axis size of LN was 35.42 ± 9.08 mm and 15.92 ± 7.23 mm, respectively, and most common location of LN was station 7 which is consistent, with the finding of previous study [Table 1]. LN was irregular shape in 32/48 (66.7%) and borders were indistinct in 42/48 (87.5%) cases [Table 1] which represent tendency of tubercular LN to fuse with their adjacent borders.[34,35,36] We found heterogeneous LN in 32 (66.7%), hypoechoic LN in 16 (33.3%), and hyperechoic LN in 32 (66.7%) cases [Table 1]. Heterogeneous appearance was due to the presence of hyperechoic and hypoechoic areas.[29] Hypoechoic area in tubercular LN is due to the presence of necrotic process and loss of central vascularity, while hyperechoic areas represent necrotic debris, calcification, or air foci.[21,33,34,35,36] Distinction of whether hypoechoic and or hyperechoic areas were located centrally or peripherally was not done at the time of initial reporting, so we could not study these features in this retrospective analysis. Furthermore, definition and utility of these features are controversial not uniform, and in addition, there is no good intraobserver and interobserver variability for these features.[32,37,38,39]
Figure 3.
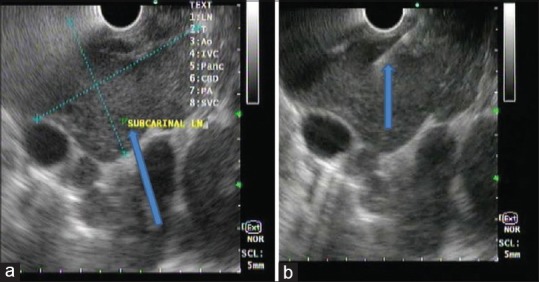
Endoscopic ultrasound examination of the mediastinum using linear echoendoscope at 7.5 mHz showing predominantly hypoechoic heterogeneous mass at subcarina with indistinct margin (a), lesion was punctured using 22 G fine-needle aspiration needle, 2–3 passes taken (b)
Figure 4.
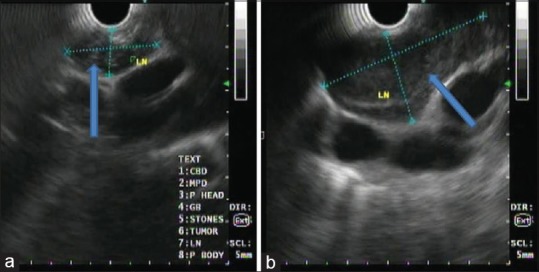
Endoscopic ultrasound examination of the mediastinum using linear echoendoscope at 7.5 mHz showing predominantly hypoechoic homogenous mass at the aortopulmonary window with well-defined margin (a), another predominantly hypoechoic homogenous lesion at subcarina (b)
We performed GXP and AFB culture in all patients. GXP is an automated diagnostic test for the detection of MTB. It is a DNA-based test that detects MTB rpoB gene. GXP also detects mutations in rpoB that may cause rifampicin resistance. Results are available after 2 h with minimal hands-on technical time. AFB culture was inoculated on the mycobacterial growth indicator tube a gold standard liquid culture method. A Cochrane review found that GXP accurately detects MTB and rifampicin resistance when used on sputum specimens.[40] The WHO published updated guidance on use of GXP in 2013. This updated policy statement expanded recommendations for the use of GXP for extrapulmonary TB.[41] A systemic review on 6026 nonrespiratory samples established the efficacy of GXP; however, none of the studies mention EUS-FNA/FNB.[42] To our knowledge, we are the first to perform GXP with an EUS-FNA sample. In a study by Puri et al.,[21] out of the 46 patients with TB, EUS-FNA provided a diagnosis of definitive TB or cytomorphic TB in 32 patients and the remaining 14 patients were treated for TB based on cytomorphologic and clinical findings. For diagnosing TB, EUS-FNA had a sensitivity of 70%, specificity of 100%, PPV of 100%, and NPV of 50%. In this study, AFB positivity (bacteriologically confirmed TB) or granuloma with necrosis (cytomorphic TB) was the criteria for diagnosis of tuberculous lymphadenitis. We used the same criteria for diagnosing tuberculous lymphadenitis, and in addition, we also performed GXP/AFB culture which is very useful in early diagnosis and detection of rifampicin resistance. Dhasmana et al.[20] studied the performance of GXP in the diagnosis of tuberculous MLA by endoscopic bronchial ultrasound in 88 patients with bacteriologically confirmed TB (AFB culture positive). Sensitivity, specificity, and positive and negative predictive values were compared between cytology and GXP. Sensitivity and specificity for GXP were 66.7% and 96.3%. Dhooria et al.[19] studied 147 patients with MLA and found the sensitivity and specificity of GXP of 49.1% and 97.9%, respectively, for diagnosis of TB. The present study found the sensitivity of 54.2% and specificity of 100% for tuberculous lymphadenitis. We could obtain tissue for bacteriological confirmation and avoid empiricism in these difficult extrapulmonary cases which was in consensus with the current WHO guidelines for TB diagnosis. There are very few studies on GXP/AFB culture with EUS-FNA, and we suggest incorporation of GXP/AFB culture in evaluation of MLA in TB endemic regions. Sarcoidosis, though uncommon in this region of our country, is a close differential diagnosis of TB. Wildi et al.[43] reported a sensitivity and specificity of 89% (95% confidence interval [CI] 82–94) and 96% (95% CI 91–98), respectively, for EUS-FNA in detecting sarcoidosis. Noncaseating granulomas without necrosis on EUS-FNA was reported by Annema et al. in 41 of 50 (82%) patients with the final diagnosis of sarcoidosis.[44] Specific ultrasound features of clustered, well-demarcated isoechoic LNs were observed in 64% of patients with sarcoidosis in this study. Fritscher-Ravens et al.[45] studied 19 patients with suspected sarcoidosis, the final diagnosis was considered as sarcoidosis in 18 patients, based on the cytologic evidence of noncaseating granulomatous inflammation along with clinical follow-up and negative mycobacterial cultures. The overall diagnostic accuracy and sensitivity of EUS-FNA in the diagnosis of sarcoidosis were 94% and 100%, respectively. Although we had few patients with sarcoidosis, the results are similar to previous studies with sensitivity and specificity of 100%. The prevalence of sarcoidosis in this region of India (i.e., Western India) is much lesser than in the rest of India and world.
Yasuda et al.[46] found an overall accuracy of 98% for the diagnosis of mediastinal and abdominal lymphoma by EUS-FNA. It was possible to classify the lymphomas in accordance with the World Health Organization classifications in 88% of the 104 cases in this study. Ribeiro et al.[47] reviewed records of 38 suspected lymphoma patients who underwent EUS-FNA of LNs or the gut wall. The overall sensitivity, specificity, and accuracy of EUS-FNA cytology with flow cytometry/immunocytochemistry (FC/IC) for the diagnosis of lymphoma were, respectively, 74%, 93%, and 81%. When comparing patients who had EUS-FNA with FC/IC versus those who had EUS-FNA without FC/IC, sensitivity was 86% versus 44% (P = 0.04) and specificity was 100% versus 90%. In study by Stacchini et al.,[48] 56 patients were evaluated by cytology with FC after EUS-FNA, material was inadequate for FC analysis in only one patient with diagnosis of lymphoma in 11 patients, specific histologic type was defined by FC alone in eight patients. We found a 100% sensitivity and specificity for diagnosis lymphoma by EUS-FNA/FNB. All the lymphoma patients responded to standard therapy. Hence, EUS-FNA/FNB decreased the need for surgical biopsies in our patients and treatment could be started on the basis of EUS-FNA/FNB alone.
Limitation of the present study
We could not validate the results of EUS-FNA/FNB with surgical biopsy samples. Hence, there was no gold standard to compare our results. However, this drawback has possibly been overcome by long-term follow-up and clinicoradiological response to treatment. Most of the literature on EUS-FNA/FNB applied the same criteria for defining the gold standard. Furthermore, TB is the most common etiology, the samples were subjected to both GXP and AFB culture testing which is the gold standard for diagnosis of TB
Another limitation was the absence of on-site pathologist for rapid on-site evaluation (ROSE) of EUS-FNA/FNB samples. However, recent studies have suggested comparable results with ROSE in the absence of on-site pathologist. Moreover, our endosonographer has been trained in the evaluation of EUS-FNA/FNB specimens. Also with TB being the most common diagnosis in our setup, ROSE was of limited utility
Since most of the patients in the present study were of tuberculous lymphadenitis, the results are possibly not relevant for other diseases, as the number of patients in these groups was small. There is an inherent selection bias in the present study due to the endemic nature of TB in this country
We did not perform EUS elastography for differentiating benign from malignant LNs. Recent studies have shown it to be a complementary feature with this respect,[31] we tried to overcome this by using strict criteria with all available echo features for predicting the nature of the LNs
Another limitation is the inability of EUS-FNA to completely assess and stage lung cancer since for that bronchoscopic examination is still warranted and that ideally pulmonary lesions and mediastinal enlargements should be assessed by one examination and that can only be bronchoscopy + linear EBUS. Having said that it is fair to note that if these facilities are not available, EUS approach can solve many diagnotic challenges.
An important note to make here is; apart from EUS-FNA/FNB, EUS-TBNA, and EUS-B-FNA are safe efficacious and useful modalities in the evaluation of MLA.[49] Anatomic factors, LN accessibility, and availability of trained operators and modality should dictate which particular modality to be used first during evaluation of mediastinal lesions.[49,50,51] All three modalities serve as complementary to each other and none of them can be ignored.
CONCLUSION
For evaluation of MLA, EUS is a useful modality. Echo features on EUS along with EUS-FNA/FNB can diagnose MLA of unknown origin based on which treatment can be initiated and surgical biopsy can be avoided.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to thank Dr. Unnati Desai, associate professor and head in charge, Department of Respiratory Medicine, Topiwala National Medical College and B.Y.L. Nair Charitable Hospital, Mumbai, for recruitment of patients and final review of article.
REFERENCES
- 1.Prenzel KL, Mönig SP, Sinning JM, Baldus SE, Brochhagen HG, Schneider PM, et al. Lymph node size and metastatic infiltration in non-small cell lung cancer. Chest. 2003;123:463–7. doi: 10.1378/chest.123.2.463. [DOI] [PubMed] [Google Scholar]
- 2.Medina Gallardo JF, Borderas Naranjo F, Torres Cansino M, Rodriguez-Panadero F. Validity of enlarged mediastinal nodes as markers of involvement by non-small cell lung cancer. Am Rev Respir Dis. 1992;146:1210–2. doi: 10.1164/ajrccm/146.5_Pt_1.1210. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JW, Jr, Pearlberg JL, Beute GH, Alpern M, Kvale PA, Gross BH, et al. Can computed tomography of the chest stage lung cancer? Yes and no. Ann Thorac Surg. 1990;49:591–5. doi: 10.1016/0003-4975(90)90306-q. [DOI] [PubMed] [Google Scholar]
- 4.Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: A review of the current evidence. Chest. 2003;123:137S–46S. doi: 10.1378/chest.123.1_suppl.137s. [DOI] [PubMed] [Google Scholar]
- 5.Dunagan D, Chin R, Jr, McCain T, Case L, Harkness B, Oaks T, et al. Staging by positron emission tomography predicts survival in patients with non-small cell lung cancer. Chest. 2001;119:333–9. doi: 10.1378/chest.119.2.333. [DOI] [PubMed] [Google Scholar]
- 6.Gupta NC, Tamim WJ, Graeber GG, Bishop HA, Hobbs GR. Mediastinal lymph node sampling following positron emission tomography with fluorodeoxyglucose imaging in lung cancer staging. Chest. 2001;120:521–7. doi: 10.1378/chest.120.2.521. [DOI] [PubMed] [Google Scholar]
- 7.Roberts PF, Follette DM, von Haag D, Park JA, Valk PE, Pounds TR, et al. Factors associated with false-positive staging of lung cancer by positron emission tomography. Ann Thorac Surg. 2000;70:1154–9. doi: 10.1016/s0003-4975(00)01769-0. [DOI] [PubMed] [Google Scholar]
- 8.Graeter TP, Hellwig D, Hoffmann K, Ukena D, Kirsch CM, Schäfers HJ. Mediastinal lymph node staging in suspected lung cancer: Comparison of positron emission tomography with F-18-fluorodeoxyglucose and mediastinoscopy. Ann Thorac Surg. 2003;75:231–5. doi: 10.1016/s0003-4975(02)04350-3. [DOI] [PubMed] [Google Scholar]
- 9.Silvestri GA, Tanoue LT, Margolis ML, Barker J, Detterbeck F. American College of Chest Physicians. The noninvasive staging of non-small cell lung cancer: The guidelines. Chest. 2003;123:147S–56S. doi: 10.1378/chest.123.1_suppl.147s. [DOI] [PubMed] [Google Scholar]
- 10.Kazerooni EA, Lim FT, Mikhail A, Martinez FJ. Risk of pneumothorax in CT-guided transthoracic needle aspiration biopsy of the lung. Radiology. 1996;198:371–5. doi: 10.1148/radiology.198.2.8596834. [DOI] [PubMed] [Google Scholar]
- 11.Harrow EM, Abi-Saleh W, Blum J, Harkin T, Gasparini S, Addrizzo-Harris DJ, et al. The utility of transbronchial needle aspiration in the staging of bronchogenic carcinoma. Am J Respir Crit Care Med. 2000;161:601–7. doi: 10.1164/ajrccm.161.2.9902040. [DOI] [PubMed] [Google Scholar]
- 12.Coughlin M, Deslauriers J, Beaulieu M, Fournier B, Piraux M, Rouleau J, et al. Role of mediastinoscopy in pretreatment staging of patients with primary lung cancer. Ann Thorac Surg. 1985;40:556–60. doi: 10.1016/s0003-4975(10)60348-7. [DOI] [PubMed] [Google Scholar]
- 13.Akahoshi K, Misawa T, Fujishima M. Pre-operative evaluation of gastric cancer by endoscopic ultrasound. Gut. 1991;32:479–82. doi: 10.1136/gut.32.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyce GA, Sivak MV, Jr, Lavery IC, Fazio VW, Church JM, Milsom J, et al. Endoscopic ultrasound in the pre-operative staging of rectal carcinoma. Gastrointest Endosc. 1992;38:468–71. doi: 10.1016/s0016-5107(92)70478-7. [DOI] [PubMed] [Google Scholar]
- 15.Rice TW, Boyce GA, Sivak MV, Adelstein DJ, Kirby TJ. Esophageal carcinoma: Esophageal ultrasound assessment of preoperative chemotherapy. Ann Thorac Surg. 1992;53:972–7. doi: 10.1016/0003-4975(92)90369-f. [DOI] [PubMed] [Google Scholar]
- 16.Rösch T, Braig C, Gain T, Feuerbach S, Siewert JR, Schusdziarra V, et al. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology. 1992;102:188–99. doi: 10.1016/0016-5085(92)91800-j. [DOI] [PubMed] [Google Scholar]
- 17.Rösch T, Lorenz R, Braig C, Feuerbach S, Siewert JR, Schusdziarra V, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37:347–52. doi: 10.1016/s0016-5107(91)70729-3. [DOI] [PubMed] [Google Scholar]
- 18.Puli SR, Batapati Krishna Reddy J, Bechtold ML, Ibdah JA, Antillon D, Singh S, et al. Endoscopic ultrasound: It's accuracy in evaluating mediastinal lymphadenopathy? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:3028–37. doi: 10.3748/wjg.14.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhooria S, Gupta N, Bal A, Sehgal IS, Aggarwal AN, Sethi S, et al. Role of xpert MTB/RIF in differentiating tuberculosis from sarcoidosis in patients with mediastinal lymphadenopathy undergoing EBUS-TBNA: A study of 147 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:258–66. [PubMed] [Google Scholar]
- 20.Dhasmana DJ, Ross C, Bradley CJ, Connell DW, George PM, Singanayagam A, et al. Performance of xpert MTB/RIF in the diagnosis of tuberculous mediastinal lymphadenopathy by endobronchial ultrasound. Ann Am Thorac Soc. 2014;11:392–6. doi: 10.1513/AnnalsATS.201308-250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puri R, Vilmann P, Sud R, Kumar M, Taneja S, Verma K, et al. Endoscopic ultrasound-guided fine-needle aspiration cytology in the evaluation of suspected tuberculosis in patients with isolated mediastinal lymphadenopathy. Endoscopy. 2010;42:462–7. doi: 10.1055/s-0029-1244133. [DOI] [PubMed] [Google Scholar]
- 22.Chin YK, Iglesias-Garcia J, de la Iglesia D, Lariño-Noia J, Abdulkader-Nallib I, Lázare H, et al. Accuracy of endoscopic ultrasound-guided tissue acquisition in the evaluation of lymph nodes enlargement in the absence of on-site pathologist. World J Gastroenterol. 2017;23:5755–63. doi: 10.3748/wjg.v23.i31.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma SS, Jain M, Maharshi S. High diagnostic yield of endoscopic ultrasound-guided fine needle aspiration without an on-site cytopathologist. Indian J Gastroenterol. 2017;36:88–91. doi: 10.1007/s12664-017-0730-z. [DOI] [PubMed] [Google Scholar]
- 24.Ramakrishna K. Rapid on-site evaluation of cytology for EUS-and EBUS-guided fine-needle aspiration. Indian J Gastroenterol. 2017;36:75–6. doi: 10.1007/s12664-017-0739-3. [DOI] [PubMed] [Google Scholar]
- 25.Jamil LH, Kashani A, Scimeca D, Ghabril M, Gross SA, Gill KR, et al. Can endoscopic ultrasound distinguish between mediastinal benign lymph nodes and those involved by sarcoidosis, lymphoma, or metastasis? Dig Dis Sci. 2014;59:2191–8. doi: 10.1007/s10620-014-3164-9. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson BC, Hirota WK, Goldstein JL, Leighton JA, Mallery JS, Waring JP, et al. The role of EUS for evaluation of mediastinal adenopathy. Gastrointest Endosc. 2003;58:819–21. doi: 10.1016/s0016-5107(03)01996-5. [DOI] [PubMed] [Google Scholar]
- 27.Catalano MF, Sivak MV, Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442–6. doi: 10.1016/s0016-5107(94)70206-3. [DOI] [PubMed] [Google Scholar]
- 28.Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474–9. doi: 10.1016/s0016-5107(97)70176-7. [DOI] [PubMed] [Google Scholar]
- 29.Faige DO. EUS in patients with benign and malignant lymphadenopathy. Gastrointest Endosc. 2001;53:593–8. doi: 10.1067/mge.2001.114060. [DOI] [PubMed] [Google Scholar]
- 30.Okasha H, Elkholy S, Sayed M, Salman A, Elsherif Y, El-Gemeie E. Endoscopic ultrasound-guided fine-needle aspiration and cytology for differentiating benign from malignant lymph nodes. Arab J Gastroenterol. 2017;18:74–9. doi: 10.1016/j.ajg.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Ziyade S, Pinarbasili NB, Ziyade N, Akdemir OC, Sahin F, Soysal Ö, et al. Determination of standard number, size and weight of mediastinal lymph nodes in postmortem examinations: Reflection on lung cancer surgery. J Cardiothorac Surg. 2013;8:94. doi: 10.1186/1749-8090-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Olivé I, Radua J, Serra P, Andreo F, Sanz-Santos J, Monsó E, et al. Intra- and interobserver agreement among bronchial endosonographers for the description of intrathoracic lymph nodes. Ultrasound Med Biol. 2012;38:1163–8. doi: 10.1016/j.ultrasmedbio.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Sharma M, Ecka RS, Somasundaram A, Shoukat A, Kirnake V. Endoscopic ultrasound in mediastinal tuberculosis. Lung India. 2016;33:129–34. doi: 10.4103/0970-2113.177451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fritscher-Ravens A, Ghanbari A, Topalidis T, Pelling M, Kon OM, Patel K, et al. Granulomatous mediastinal adenopathy: Can endoscopic ultrasound-guided fine-needle aspiration differentiate between tuberculosis and sarcoidosis? Endoscopy. 2011;43:955–61. doi: 10.1055/s-0031-1271110. [DOI] [PubMed] [Google Scholar]
- 35.Hall JD, Kahaleh M, White GE, Talreja J, Northup PG, Shami VM. Presence of lymph node vasculature: A new EUS criterion for benign nodes? Dig Dis Sci. 2009;54:118–21. doi: 10.1007/s10620-008-0314-y. [DOI] [PubMed] [Google Scholar]
- 36.Rana SS, Bhasin DK, Srinivasan R, Singh K. Endoscopic ultrasound (EUS) features of mediastinal tubercular lymphadenopathy. Hepatogastroenterology. 2011;58:819–23. [PubMed] [Google Scholar]
- 37.Dhooria S, Agarwal R, Aggarwal AN, Bal A, Gupta N, Gupta D. Differentiating tuberculosis from sarcoidosis by sonographic characteristics of lymph nodes on endobronchial ultrasonography: A study of 165 patients. J Thorac Cardiovasc Surg. 2014;148:662–7. doi: 10.1016/j.jtcvs.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 38.Bhutani MS, Saftoiu A, Chaya C, Gupta P, Markowitz AB, Willis M, et al. Irregular echogenic foci representing coagulation necrosis: A useful but perhaps under-recognized EUS echo feature of malignant lymph node invasion. J Gastrointestin Liver Dis. 2009;18:181–4. [PubMed] [Google Scholar]
- 39.Roberts SA, Mahon BS, Evans R. Coagulation necrosis in malignant mediastinal nodes on endoscopic ultrasound: A new endosonographic sign. Clin Radiol. 2005;60:587–91. doi: 10.1016/j.crad.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;21(1):CD009593. doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization. Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children. Geneva: World Health Organization; 2013. [Last accessed on 2019 Jan 23]. Available from: http://apps.who.int/iris/handle/10665/112472 . [PubMed] [Google Scholar]
- 42.Maynard-Smith L, Larke N, Peters JA, Lawn SD. Diagnostic accuracy of the xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: A systematic review. BMC Infect Dis. 2014;14:709. doi: 10.1186/s12879-014-0709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wildi SM, Judson MA, Fraig M, Fickling WE, Schmulewitz N, Varadarajulu S, et al. Is endosonography guided fine needle aspiration (EUS-FNA) for sarcoidosis as good as we think? Thora×. 2004;59:794–9. doi: 10.1136/thx.2003.009472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Annema JT, Veseliç M, Rabe KF. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of sarcoidosis. Eur Respir J. 2005;25:405–9. doi: 10.1183/09031936.05.00098404. [DOI] [PubMed] [Google Scholar]
- 45.Fritscher-Ravens A, Sriram PV, Topalidis T, Hauber HP, Meyer A, Soehendra N, et al. Diagnosing sarcoidosis using endosonography-guided fine-needle aspiration. Chest. 2000;118:928–35. doi: 10.1378/chest.118.4.928. [DOI] [PubMed] [Google Scholar]
- 46.Yasuda I, Tsurumi H, Omar S, Iwashita T, Kojima Y, Yamada T, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy. 2006;38:919–24. doi: 10.1055/s-2006-944665. [DOI] [PubMed] [Google Scholar]
- 47.Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, Wang KK, Clain JE, Wiersema MJ. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485–91. doi: 10.1067/mge.2001.112841. [DOI] [PubMed] [Google Scholar]
- 48.Stacchini A, Carucci P, Pacchioni D, Accinelli G, Demurtas A, Aliberti S, et al. Diagnosis of deep-seated lymphomas by endoscopic ultrasound-guided fine needle aspiration combined with flow cytometry. Cytopathology. 2012;23:50–6. doi: 10.1111/j.1365-2303.2010.00842.x. [DOI] [PubMed] [Google Scholar]
- 49.Medford AR, Agrawal S. Single bronchoscope combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration for tuberculous mediastinal nodes. Chest. 2010;138:1274. doi: 10.1378/chest.10-0617. [DOI] [PubMed] [Google Scholar]
- 50.Sharma M, Arya CL, Somasundaram A, Rameshbabu CS. Techniques of linear endobronchial ultrasound imaging. J Bronchology Interv Pulmonol. 2010;17:177–87. doi: 10.1097/LBR.0b013e3181dca122. [DOI] [PubMed] [Google Scholar]
- 51.ASGE Standards of Practice Committee. Jue TL, Sharaf RN, Appalaneni V, Anderson MA, Ben-Menachem T, et al. Role of EUS for the evaluation of mediastinal adenopathy. Gastrointest Endosc. 2011;74:239–45. doi: 10.1016/j.gie.2011.03.1255. [DOI] [PubMed] [Google Scholar]


