Abstract
Background:
Central airway obstruction (CAO) is defined as obstruction of the airway lumen in the trachea or mainstem bronchi, most commonly due to primary or metastatic malignancy; and is classified as extraluminal, endoluminal or mixed. The majority of malignant CAO are advanced stage and require a multimodality palliative approach, including stent placement. We describe a retrospective review of a novel self-expandable metallic stent (SEMS), the Bonastent® (Thoracent, Inc. Alpharetta, GA); a fully covered, nitinol braided airway stent which conforms to airway tortuosity without loss of diameter in the management of CAO.
Methods:
We performed a retrospective chart review of patients with CAO who underwent Bonastent® placement at a single center between February 2017 and March 2018. Ease of stent placement, short term complications (within 24 hours of stent placement) and long-term complications (within 3 months of stent placement) were recorded.
Results:
Eleven patients were identified, reviewed and included in the study. Thirteen stents in 11 patients were placed for predominantly malignant CAO. One patient had a short-term complication of stent migration. Four patients had long term complications; of which three patients had in stent mucus impaction requiring bronchoscopy. In our study, the stent related complication rates were comparable to the reported literature.
Conclusion:
In our experience, Bonastent® is an easy to use option which adds to the armamentarium of SEMS to treat malignant CAO.
Keywords: Airway stent, Malignant central airway obstruction, Rigid bronchoscopy, Self-Expanding metallic airway stent
INTRODUCTION:
Central airway obstruction can be malignant or non-malignant in origin. Malignant central airway obstruction (CAO) is defined as obstruction of the airway lumen in the trachea or mainstem bronchi secondary to primary or metastatic malignancy1. The typical scenario of malignant CAO is direct extension of a tumor, more commonly bronchogenic carcinoma. About 20–30% of patients who have lung cancer will develop airway complications and nearly 40% of lung cancers deaths are secondary to regional disease5, 6. Primary tumors of the airway are less common; however squamous cell carcinoma and adenoid cystic carcinoma comprise the majority of those arising in the trachea. Distant tumors can metastasize to the airway, including renal cell carcinoma, thyroid carcinoma, carcinoid, colon, and others7.
CAO is classified as extra luminal (extrinsic), endoluminal (intrinsic) or mixed2. The majority of malignant CAO are advanced stage and require a multimodality palliative approach3. There is a proposed algorithm for the management of CAO1, however, an individualized patient-centered approach is paramount. Therapeutic approaches available to palliate symptoms due to CAO include but are not limited to mechanical dilatation (bronchoplasty), mechanical debridement, use of thermal therapy, photodynamic therapy, brachytherapy, and airway stenting8.
An airway stent is an endobronchial prosthesis used to maintain airway patency. Multiple types of airway stents are available including self-expanding metallic stents (SEMS). We describe a retrospective review of a novel SEMS, Bonastent® (Thoracent, Inc. Alpharetta, GA) (Figure 1); a fully covered, nitinol braided airway stent with an ultra-thin delivery system (with the diameter of the deployment catheter ranging from 2.66mm (8fr) to 5.20mm(15.6Fr))4, with flare-ends to reduce migration, which can be re-captured up to 70% deployment4 and conforms to airway tortuosity without loss of diameter.
Figure 1.

Bonastent. Fully covered, nitinol braided, self-expanding metallic stent. (Image courtesy of Thoracent, Inc)
MATERIALS AND METHODS:
We performed a retrospective chart review of patients with CAO who underwent Bonastent® (Thoracent, Inc. Alpharetta, GA) placement at a single tertiary care center between February 2017 and March 2018. Institutional databases were queried for airway stent placements performed by Interventional Pulmonology.
This study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board (IRB# 18–355) with a waiver to informed consent provided. This study was supported by the NIH/NCI Cancer Support GrantP30 CA008748. Patient data was collected from the electronic medical record using a standardized form. All definitions were constructed prior to chart review to ensure consistency. Demographic data (age, sex), stent data (date of placement, type of stent, size of stent, post stent airway patency, stent related complications, and date of removal), and bronchoscopic data (type of bronchoscopy, therapeutic interventions used) were collected.
The objectives of this study were to look at safety, intra-operative feasibility, and early (within 24 hours) and late (within 90 days) stent-related complications at a single, tertiary care center.
RESULTS:
Patient Characteristics:
In the period between February 2017 and March 2018, 38 stents were placed at our institution for CAO; out of which 11 patients had 13 Bonastent® placed. Six patients (54.5%) were women (Table 1). Mean patient age was 52.54 years. Ten patients (91%) underwent stent placement for malignant airway obstruction and one patient (patient number 4) underwent stent placement for bronchial stenosis secondary to sequelae of prior tuberculosis. All patients subjectively achieved symptomatic palliation post stent placement.
Table 1.
Baseline Patient, and Stent Characteristics
| Pt. No. | Age | Sex | Type of Obstruction | Stent Location | Stent Size | Other Modalities Used |
|---|---|---|---|---|---|---|
| 1a | 53 | Male | Intrinsic | LMB | 14×40 | None |
| 1b | 53 | Male | Intrinsic | LMB | 12×40 | Balloon Dilatation |
| 2a | 62 | Male | Mixed | BI | 10×30 | Electrocautery Snare |
| 2b | 62 | Male | Mixed | BI | 10×20 | None |
| 3 | 81 | Female | Intrinsic | RMB/BI | 12×30 | Balloon Dilatation |
| 4 | 54 | Female | Mixed | RMB/BI | 10×30 | Balloon Dilatation |
| 5 | 54 | Female | Extrinsic | RMB | 10×20 | Balloon Dilatation |
| 6 | 50 | Female | Extrinsic | Distal RMB/BI | 10×30 | None |
| 7 | 50 | Male | Intrinsic | LMB | 12×40 | Mechanical debulking |
| 8 | 43 | Female | Intrinsic | Trachea | 12×40 | None |
| 9 | 41 | Female | Extrinsic | LMB | 10×30 | Balloon Dilatation |
| 10 | 26 | Male | Extrinsic | BI | 10×30 | None |
| 11 | 54 | Male | Mixed | LMB | 12×40 | Balloon Dilatation |
RMB = Right mainstem bronchus; LMB = Left mainstem bronchus; BI = Bronchus intermedius
Stent Characteristics:
The size and location of the stents are outlined in Table 1. Eleven stents were deployed under fluoroscopy using external markers and over a guidewire. Two stents were deployed under direct airway visualization with the use of an ultrathin bronchoscope parallel to the deployment catheter within a rigid bronchoscope. Four stents were placed using a flexible bronchoscope alone and 9 using a rigid bronchoscope based upon patient characteristics and operative findings.
All stents conformed well in the abnormal airway per operator report. Balloon dilatation was performed along with stent placement in six cases. Four of these were post stent placement, to achieve immediate improvement of airway patency in those with high grade, severe obstruction (Figure 2). 45.4% of patients did not require any additional interventions to achieve acceptable airway patency.
Figure 2.
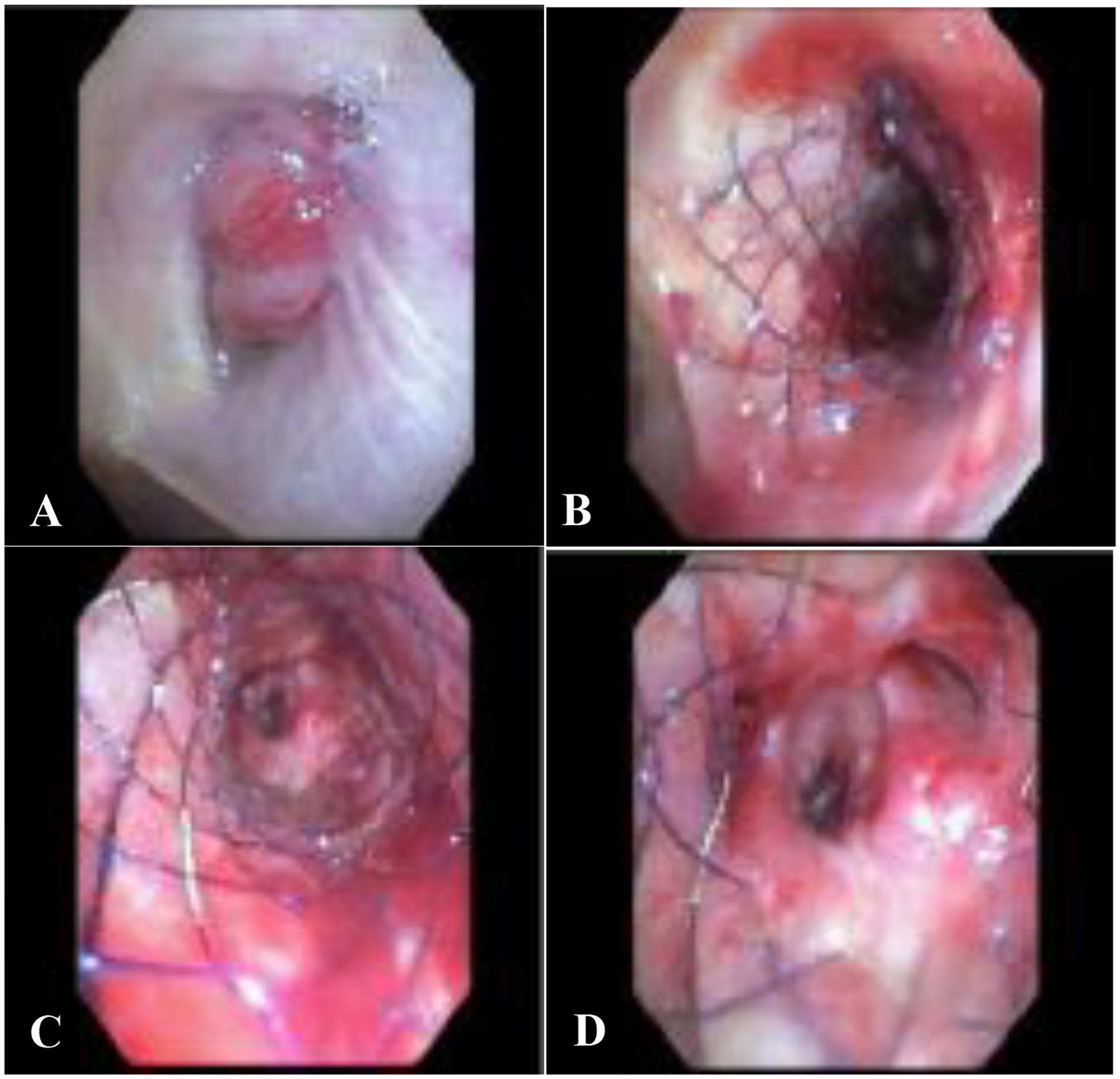
A) Right main stem endobronchial tumor arising from the right upper lobe bronchus and occluding the airway lumen >90%. This tumor extended down to mid bronchus intermedius. B) Post stent placement and balloon dilatation. The stent conforms well to the obstructed airway keeping an adequate radial force throughout at every level, maintaining airway patency C) view inside of the stent at mid portion, and D) distal aspect of the stent with patent right middle lobe and lower lobe airways.
Analysis of Stent Complications:
There was one early complication. In patient 2, a stent placed in the bronchus intermedius for mixed CAO migrated proximally and partially obstructed the right upper lobe bronchus leading to progressive atelectasis. This stent was replaced the following day. We believe this complication was secondary to extrinsic compression of an incorrectly sized stent. Four patients had late stent-related complications (Table 2). The time to stent related complication ranged from as early as 1 day to 63 days from stent placement. The most frequently occurring complication was mucostasis requiring toilet bronchoscopy.
Table 2.
Stent related “Late Complications”
| Pt. No. | Time from stent placement to complication (Days) | Type of Complication | Management Of Complication |
|---|---|---|---|
| 1a | 51 | Obstruction - mucus | Toilet bronchoscopy* |
| 4 | 35 | Obstruction – Mucus +Granulation | Toilet bronchoscopy |
| 5 | 33 | Stent Migration; Vocal cord edema | Stent removal; Oral Steroids |
| 6 | 3 | Mucus plug | Toilet bronchoscopy |
Managed with toilet bronchoscopy at an outside institution.
Out of the 13 stents that were placed, 8 were removed. Two stents were removed because of stent migration, one stent was removed because of tumor growth leading to stent migration, and two patients underwent pneumonectomies where the stents were removed as part of the surgery. Two patients had improved airway obstruction post radiation resulting in no further need of the airway stents. One stent was placed for a tracheo-esophageal (TE) fistula, the stent required to be changed 7 months later due to progression of the TE fistula. It was changed for an alternate SEMS because of in-house unavailability of the Bonastent® in the size required. Four out of the eleven patients died within three months of stent placement.
No procedural related complications were noted at the time of stent removal. In two patients, stent removal was deemed moderately difficult by the bronchoscopist because of airway tumor growth and bleeding.
DISCUSSION:
CAO can occur by a variety of malignant and non-malignant causes. Malignant central airway obstruction can occur as a result of primary or metastatic intrathoracic disease. Malignancies adjacent to the airways or primary mediastinal malignancies can cause airway obstruction by direct extension into the airways or by external compression while extrathoracic malignancies can metastasize to the airways2. Less commonly, primary airway malignancies such as tracheal tumors, pulmonary neuroendocrine tumors, and bronchial carcinoid can also cause malignant CAO7,9,10. Therapeutic approaches available to palliate symptoms due to CAO include but are not limited to mechanical dilatation (bronchoplasty), thermal therapy, photodynamic therapy, radiation therapy and airway stents.
An airway stent is an endobronchial prosthesis used to maintain airway patency. These can provide immediate relief and improve the quality of life of patients14. Indications for stent placement include, severe symptomatic CAO, persistent tracheobronchial obstruction despite bronchoscopic interventions, and excessive dynamic airway collapse11,12. Airway stenting is more suitable if there is evidence of extrinsic compression or inability to otherwise gain airway patency bronchoscopically, usually as a bridge to a more definitive treatment that may include radiation and/or systemic therapy in cases of malignant CAO. In cases of endoluminal disease, efforts are made to perform tumor or tissue destruction, commonly with the use of thermal therapy or by mechanical means to regain an airway lumen. If this cannot be accomplished, then airway stenting may still be indicated. Multiple types of airway stents are available including self-expanding metallic stents (SEMS).
In this study, we performed a retrospective analysis of patients with CAO who underwent placement of a novel SEMS, Bonastent® (Thoracent, Inc. Alpharetta, GA). It was FDA approved in October 2014 and was officially launched in the US by Endochoice in 201615. It has been used outside of the US, mainly in Europe (Germany, Italy) and Asia (Korea) since 201216. We chose to use this stent in the reported cases due to its purported low-profile and ability to conform to the airway without loss of diameter. General anesthesia was employed in all cases, and jet ventilation was used for all rigid bronchoscopies. This has been described to be associated with lower rates of complication13. Twelve stents were placed for malignant CAO and one stent was placed for benign airway obstruction. The use of SEMS for benign airway obstruction has a United States Food and Drug Association a black box warning, however, studies by Fortin et al. have shown that third generation SEMS are a safe option for complex benign airway stenosis17. By being fully covered metal stents, the 3rd generation SEMS result in less granulation tissue formation, aid in easier removal and thus can be used in benign airway stenosis. Our patient had complex airway stenosis with no possible rigid bronchoscopic approach for possible silicon stent delivery, secondary to a history of pulmonary tuberculosis.
We placed 62% of stents using rigid bronchoscopy and in 84.6% of the cases stents were deployed under fluoroscopy using external markers and over a guidewire. The choice of rigid bronchoscopy was based upon patient and procedural characteristics, operator preference and in some cases airway bleeding. The deployment system of the Bonastent® is thin, which allows delivery of a 10mm diameter stent (8F delivery device) via a 3mm working channel. We choose to use fluoroscopy in certain cases to better visualize stent delivery and its conformation to the airway. Additionally, in certain cases the high grade of obstruction did not allow room for both a flexible bronchoscope and the stent delivery device. The stents were placed without difficulty as per the operator in a majority of the cases however, in two instances the placement was considered “moderately difficult” because of bleeding and difficult airway anatomy. A complication rate of 36.4% was noted in a 3-month follow-up period with obstruction secondary to mucostasis being the most frequent complication. This was comparable to the existing literature which shows that stent related complications vary between 40% to 60%, and most often occur within 2–3 months following stent placement1,10,18,19.
In summary, in our experience, like other SEMS, the Bonastent® is easy to deploy and can be inserted using flexible or rigid bronchoscopy; however rigid bronchoscopy may be preferred for their removal due to the inherent inability to purse-string the proximal stent orifice. Other advantages of SEMS include lesser rates of migration, less need for balloon dilatation prior to the stent placement because of the controlled radial force generated upon deployment, favorable inner to outer diameter ratio, and the ability to deploy across a high-grade obstruction14,15. Additionally, the Bonastent® is re-sheathable at 70% deployment; which is a property not found in other SEMS. The operators leveraged this property as during deployment one may inadvertently advance or retract the device from the area to cover, resulting in final stent malposition. We believe that the stent has a limitation in that it lacks a proximal string to pull for repositioning or removal. Of note, relative to other SEMS, this stent is difficult to visualize under fluoroscopy at the time of deployment and on subsequent chest radiographs once placed. This limitation was also noted by Holden and colleagues in their prospective analysis of safety and efficacy of the Bonastent®16. We gained increasing comfort with identifying the stent during deployment under fluoroscopy. The proximal and distal radiopaque sheath marks (Figure 3) can aid in identification at the time of bronchoscopy.
Figure 3.
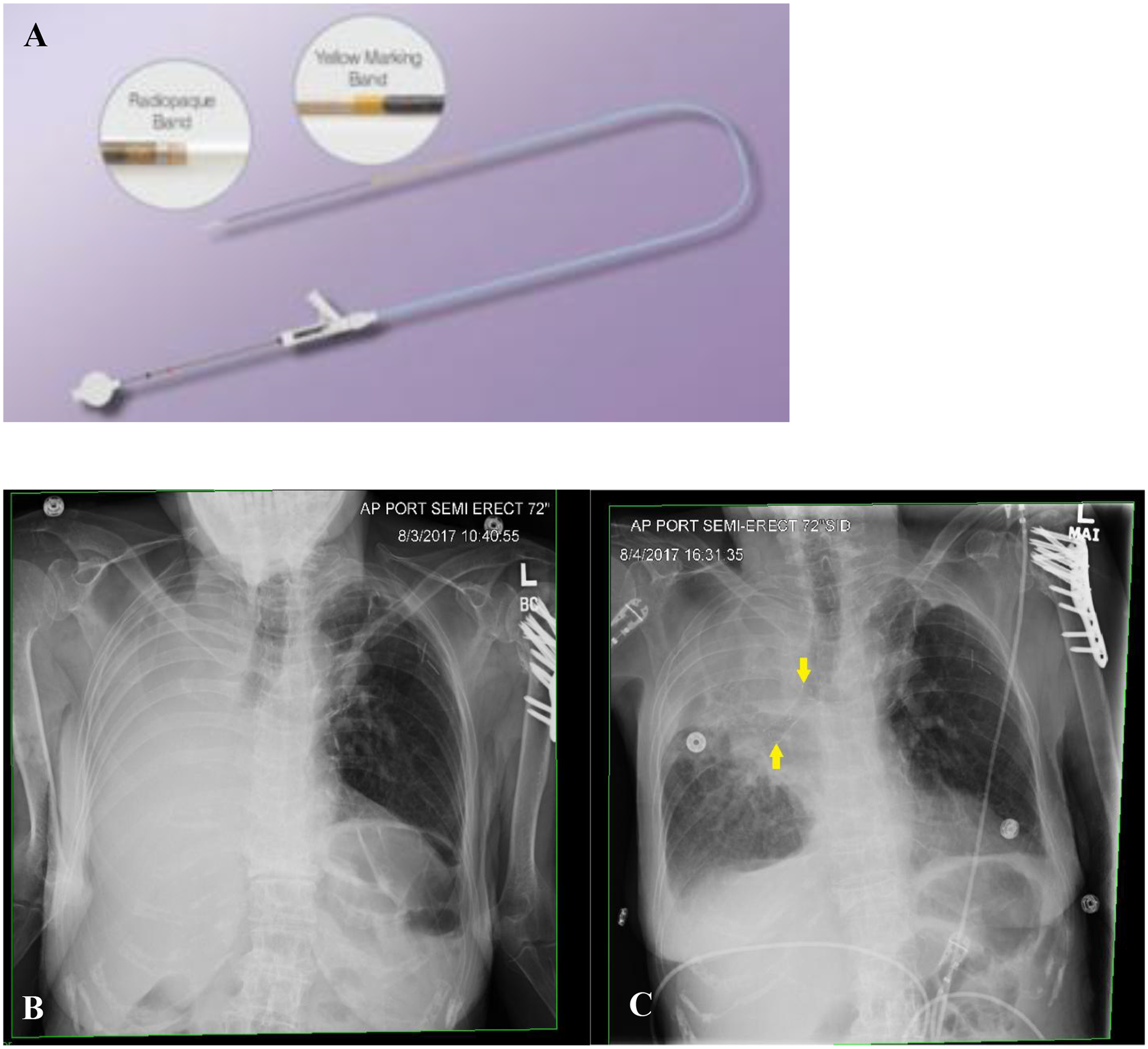
A. Deployment catheter showing its radiopaque marks on its distal aspect (Image courtesy of Thoracent, Inc). B. Portable chest x-ray pre (B) and post stent deployment (C) to the right main-stem bronchus and bronchus intermedius showing the stent in satisfactory position. The stent is easily visible on chest x-ray further enhanced by its radiopaque markers on its proximal, mid and distal portions (yellow arrows).
The limitation of our study is its small retrospective nature. Furthermore, the positive results of the study may not be reproducible at centers with lower volumes of airway interventions. Finally, large prospective studies are necessary to better investigate outcomes, provide additional data regarding the safety and long-term outcomes of Bonastent®. In conclusion, in our experience, the Bonastent® is an easy to use option which adds to the armamentarium of SEMS to treat malignant CAO.
Footnotes
Conflicts of Interest
The authors do not have any financial, institutional, or consultant conflict of interest to declare.
Disclosures: None
REFERENCES
- 1.Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med 2004;169:178. [DOI] [PubMed] [Google Scholar]
- 2.Mudambi L, Miller R, Eapen GA. Malignant central airway obstruction. Journal of Thoracic Disease. 2017;S1087–S1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folch E, Keyes C. Airway stents. Annals of Cardiothoracic Surgery. 2018;7(2):273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonastent® [Package insert]. Thoracent® 2018.
- 5.Venuta F, Rendina EA, De Giacomo T, et al. Nd:YAG laser resection of lung cancer invading the airway as a bridge to surgery and palliative treatment. Ann Thorac Surg. 2002;74:995–998. [DOI] [PubMed] [Google Scholar]
- 6.Chin CS, Litle V, Yun J, Weiser T, Swanson SJ. Airway stents, Ann Thorac Surg, 2008;85 :S792–S796 [DOI] [PubMed] [Google Scholar]
- 7.Macchiarini P Primary tracheal tumours. Lancet Oncol. 2006;7:83–91. [DOI] [PubMed] [Google Scholar]
- 8.Simoff Michael J., et al. “Symptom Management in Patients With Lung Cancer.” Chest. 2013;143(5):12–2366. [DOI] [PubMed] [Google Scholar]
- 9.Harpole DH, Feldman JM, Buchanan S, et al. Bronchial carcinoid tumors: a retrospective analysis of 126 patients. Ann Thorac Surg.1992;54:50–4. [DOI] [PubMed] [Google Scholar]
- 10.Skuladottir H, Hirsch FR, Hansen HH, et al. Pulmonary neuroendocrine tumors: incidence and prognosis of histological subtypes. A population-based study in Denmark. Lung Cancer.2002;37:127–35. [DOI] [PubMed] [Google Scholar]
- 11.Jordhoy MS, Fayers P, Loge JH, Saltnes T, Ahlner-Elmqvist M, Kaasa S. Quality of life in advanced cancer patients: the impact of sociodemographic and medical characteristics. British Journal of cancer.2001;85(10): 1478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- 13.Ost David E., et al. “Complications Following Therapeutic Bronchoscopy for Malignant Central Airway Obstruction.” Chest. 2015;148 (2):450–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saad CP, Ghamande SA, Minai OA, et al. The role of self-expandable metallic stents for the treatment of airway complications after lung transplantation. Transplantation. 2003; 75:1532. [DOI] [PubMed] [Google Scholar]
- 15.Accessdata FDA, 2014, www.accessdata.fda.gov/cdrh_docs/pdf14/K140472.pdf.
- 16.Holden VK, et al. “Safety and Efficacy of a New Fully Covered Self-Expandable Metallic Airway Stent [Abstract]. AJRCCM. 2018:197. [Google Scholar]
- 17.Fortin M, Lacasse Y, Elharrar X, Tazi-Mezalek R, Laroumagne S, Guinde J, Astoul P, Dutau H: Safety and Efficacy of a Fully Covered Self-Expandable Metallic Stent in Benign Airway Stenosis. Respiration 2017;93:430–435. [DOI] [PubMed] [Google Scholar]
- 18.Chung FT, Chen HC, Chou CL, et al. An outcome analysis of self-expandable metallic stents in central airway obstruction: a cohort study. J Cardiothorac Surg.2011;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dooms C, De Keukeleire T, Janssens A, et al. Performance of fully covered self-expanding metallic stents in benign airway strictures. Respiration. 2009;77:420. [DOI] [PubMed] [Google Scholar]


