Short abstract
Introduction
The endoscopic endonasal approach to management of orbital pathology has expanded. Due to the rarity of these conditions, most reports in the literature consist of small case reports. We report a series from a single institution with a focus on outcomes.
Methods
A retrospective chart review was carried out between 2010 and 2018.
Results
Twenty-four patients were identified (average age 58 years, 15 males, 9 females). Average follow-up was 14.9 months. Most common etiologies included cavernous hemangioma (7), metastases (6), idiopathic orbital inflammatory syndrome (6), orbital hematoma/clot (2), and schwannoma (1). Most common presenting symptoms were decreased visual acuity (8), proptosis (8), diplopia (7), and incidental findings (2). All patients underwent endoscopic medial wall orbital decompressions. Sixteen involved a combined open approach by an ophthalmologist. Pathology was either biopsied (15), resected (6), or could not be identified (3). No intraoperative complications were noted. No patients underwent orbital reconstruction of the medial wall. Six patients developed postoperative sinusitis successfully managed with antibiotics. One patient developed epistaxis managed conservatively. In 5 patients, Sino-Nasal Outcome Test-22 scores increased immediately postop and then decreased, whereas scores only decreased in 6 patients. Six patients noted reduced proptosis. There were no new cases of diplopia or worsening visual acuity.
Conclusions
A combined endoscopic endonasal and external approach can be useful for managing orbital lesions. Patients tolerated the procedure well with improvement in ocular symptoms and minimal sinonasal complications. Reconstruction of the medial wall may not be warranted to prevent postoperative diplopia.
Keywords: orbital mass, endoscopic orbital surgery, orbit, cryoprobe, cavernous hemangioma, endoscopic sinus surgery, idiopathic orbital inflammatory syndrome
Introduction
Orbital masses are rare lesions with a reported incidence of only 3 to 5 tumors occurring per 1 million people per year. Etiologies include an array of benign and malignant pathology such as vascular lesions (eg, cavernous hemangioma), cystic lesions, inflammatory conditions, schwannomas, meningiomas, and malignancies (eg, lymphoma, metastases).1–4
The orbit represents a unique anatomical region at the confluence of the paranasal sinus and anterior cranial base. Moreover, it contains a variety of critical structures in close proximity including the globe, extraocular muscles, branches of the ophthalmic artery, and optic nerve. Based on the anatomic location of orbital pathology in relation to these critical structures, multiple routes of surgical access have been proposed.5 Traditionally, external approaches to the orbit were utilized; however, the endoscopic endonasal approach has more recently evolved into a useful technique for select lesions.
The endoscopic endonasal approach to the orbit provides outstanding visualization and access to lesions located within the medial and inferior orbit. The endonasal corridor may be particularly useful for more posteriorly located lesions near the orbital apex, which are exceedingly difficult to visualize via external approaches.1,6 In particular, a corridor between the medial rectus and inferior rectus muscles is typically used for access to the inferomedial orbit. For more posteriorly based lesions that lie superomedially, a corridor between the medial rectus and superior oblique muscles can be employed with care taken to identify and manage the anterior ethmoid artery.7
At our institution, we enjoy a close working relationship with our ophthalmology colleagues and typically utilize a combined surgical approach to orbital masses where needed. The ophthalmologist will typically perform an external orbitotomy to help deliver orbital lesions more medially while we simultaneously perform endoscopic dissection, in cases where the lesion cannot easily be dissected out through solely endoscopic means. We feel that this technique reduces the extent of endoscopic dissection and helps to protect critical structures. Due to the rarity of these conditions, most reports in the literature consist of small case reports or systematic reviews. We report a relatively large series from a single institution with a focus on outcomes.
Methods
We carried out an institutional review board (IRB)-approved retrospective chart review of cases performed at our institution for the management of orbital masses between 2010 and 2018. Our electronic health record system was queried for the CPT code 31292 corresponding to endoscopic orbital decompression. Patients with thyroid eye disease, nasolacrimal system pathologies, or infectious lesions (eg, mucocele, abscess, invasive fungal disease) were excluded to identify the desired list of patients with orbital masses. Patient demographics, medical histories, imaging, operative details, and follow-up clinic notes were reviewed as available in accordance with IRB policies.
Results
Demographics and Presentation
Twenty-four patients were identified who underwent endoscopic or combined (orbitotomy + endonasal endoscopic) orbital surgery for orbital masses in the defined study period. Fifteen males and 9 females were included. The average age was 58 years (range 36–89 years). The right orbit was affected more commonly (17/24). Seven patients reported a prior history of sinusitis. Average follow-up period with otolaryngology or ophthalmology was 14.9 months (range 2 weeks–84 months).
Most common etiologies included cavernous hemangioma (7), malignancy (6), idiopathic orbital inflammatory syndrome (6), orbital hematoma/clot (2), and schwannoma (1). Most common presenting symptoms reported by patients were decreased visual acuity (8), proptosis (8), and diplopia (7). Breakdown of pathologies and presentations are noted in Table 1.
Table 1.
Orbital Pathology and Clinical Presentations.
| Pathology (Count) | Diplopia | Vision Loss | Proptosis | Edema | Pain | Incidental |
|---|---|---|---|---|---|---|
| Cavernous hemangioma (7) | 1 | 3 | 2 | |||
| Malignancy (metastases 2, lymphoma 3, lacrimal carcinoma 1) | 2 | 1 | 3 | 2 | 2 | |
| Schwannoma (1) | 1 | |||||
| Idiopathic orbital inflammatory syndrome (6) | 3 | 2 | 2 | |||
| Blood clot/hematoma (2) | 1 | 1 | ||||
| Granulomatosis with polyangiitis (2) | 1 | 2 |
Six patients had undergone previous endoscopic sinus surgery. Three of these patients carried a diagnosis of orbital malignancy (adenocarcinoma, metastatic renal cell carcinoma, and mucosa-associated lymphoid tissue [MALT] lymphoma), 2 patients had a history of orbital granulomatosis with polyangiitis, and 1 patient had recurrent idiopathic orbital inflammatory syndrome.
Anatomical Location
Most pathology was determined to be intraconal (75%) based on preoperative imaging. Six lesions were noted to have both intraconal and extraconal extension. Excluding cavernous hemangiomas, which are classified separately, 6 lesions were primarily located inferomedially within intraconal orbit, 1 lesion was solely within the superomedial intraconal orbit, 5 lesions were located at the orbital apex, and 2 lesions were diffusely located within the medial orbit. The cavernous hemangiomas were classified according to the recently published Cavernous Hemangioma Exclusively Endonasal Resection (CHEER) staging system noted in Table 2.6 Cavernous hemangiomas in this series consisted of a stages I, III, IVA, and IVB.
Table 2.
Features and CHEER Staging of Cavernous Hemangiomas.
| Patient Number | Size (cm) | CHEER Stage | Surgery | Technique |
|---|---|---|---|---|
| 1 | 1.5 × 1 × 1.4 | Ia | Unable to locate | Combined |
| 2 | 5 × 1.9 × 1.9 | I | Resection | Combined, cryoprobe |
| 3 | 1.3 × 1.1 × 1.5 | IIIb | Unable to locate | Combined |
| 4 | 1.1 × 1 × 1.8 | IVbc | Resection | Combined, cryoprobe |
| 5 | 1.4 × 1.5 × 1.9 | IVad | Resection | Combined, cryoprobe |
| 6 | 1.6 × 1.4 × 1.2 | III | Unable to locate | Combined |
| 7 | 1.4 × 2 × 2.2 | IVa | Biopsy | Combined |
Abbreviation: CHEER, Cavernous Hemangioma Exclusively Endonasal Resection.
aStage I = extraconal.
bStage III = Intraconal, anterior to inferomedial muscular trunk of ophthalmic artery, superior to horizontal access of medial rectus.
cStage IVb = Intraconal, posterior to inferomedial muscular trunk of ophthalmic artery with extension into optic canal.
dStage IVa = Intraconal, posterior to inferomedial muscular trunk of ophthalmic artery without extension into optic canal.
Surgery
All patients underwent unilateral endoscopic sinus surgery (maxillary antrostomy, anterior and posterior ethmoidectomy, and sphenoidotomy) unless previously performed, in order to identify surgical landmarks, expose the lamina papyracea, and limit the risk of postobstructive sinusitis following any medial herniation of orbital fat. The lamina papyracea was then fractured and removed, followed by opening of the periorbita to begin endoscopic dissection. For a combined approach, an eyelid, tranconjunctival, or transcaruncular orbitotomy was simultaneously performed and the lesion was identified and dissected from the surrounding extra- and intraconal contents.8
The decision to pursue an endoscopic endonasal only versus combined approach was individualized based on goals of surgery, location of lesion, and surgeon availability. One patient also simultaneously underwent optic nerve decompression. Image-guided navigation was used in all cases to assist with endoscopic localization of lesions. A summary of surgical approaches for each lesion is noted in Table 3.
Table 3.
Summary of Surgical Approaches (Excluding Cavernous Hemangiomas).
| Pathology (count) | Biopsy vs Resection | Technique |
|---|---|---|
| Malignancy (metastases 2, lymphoma 3, lacrimal carcinoma 1) | Biopsy (6) | Combined (1), endoscopic only (5) |
| Schwannoma (1) | Debulking | Combined (decompression not resection) |
| Idiopathic orbital inflammatory syndrome (6) | Biopsy (6) | Combined (5), endoscopic only (1) |
| Blood clot/hematoma (2) | Resection (2) | Combined (1), endoscopic only (1) |
| Granulomatosis with polyangiitis (2) | Biopsy (2) | Combined (2) |
Sixteen cases involved a combined (endoscopic endonasal + external orbitotomy) approach. The majority (88%) involved a transconjunctival external approach. One patient underwent a transcaruncular approach and 1 patient underwent a transcutaneous approach via a Lynch incision for better access to the orbital apex.
Pathology was either biopsied (15), resected (6), or could not be readily identified on both endoscopic and external orbital evaluation (3). For patients in which the procedure did not reveal obvious pathology on the surgical field, 1 patient had a suspected CHEER stage I cavernous hemangioma incidentally found on imaging for vertigo who presented for confirmatory biopsy versus resection. Given his lack of symptoms, when the lesion could not be readily identified with a limited approach, further dissection was deferred in lieu of surveillance imaging, rather than risk causing diplopia or other visual complications. The other 2 patients had symptomatic suspected CHEER stage III cavernous hemangiomas based on imaging that could not be located intraoperatively. Both patients received maximal orbital decompressions to alleviate orbital mass effect.
Of the 8 patients who underwent an endoscopic endonasal only approach for biopsy, 5 were for diagnosis of malignancy, 2 were for idiopathic orbital inflammatory syndrome, and 1 was for management of an orbital clot/hematoma.
Three patients with cavernous hemangiomas were resected with a combined approach utilizing an endoscopic cryoprobe device (Cooper Surgical Inc., Trumbull, Connecticut). The cryoprobe was developed for ophthalmic use and modified with a long, insulated shaft for endoscopic use. A Frigitronics CE-2000 control console and foot pedal control was used to allow hands-free operation. Pressurized nitrous oxide (N2O) gas, in the liquid phase at −127°F, is applied onto the surface of the lesion through an applicator. The rapid drop in probe temperature makes it capable of tissue adherence, best suited to tumors with a high fluid composition. We have found that the cryoprobe is well suited to “grasp” these lesions, as the soft consistency of cavernous hemangiomas combined with their immersion in orbital fat makes dissection with traditional instrumentation more challenging.
No patients underwent orbital reconstruction of the medial wall. A small sheet of absorbable gelatin film (Gelfilm®, Pfizer, Inc.) was typically placed in the middle meatus at the end of the procedure to prevent adhesion formation and removed at follow-up if still present. No intraoperative complications were noted.
Postoperative Outcomes
No postoperative complications required surgical control. Three patients developed postoperative acute sinusitis diagnosed as symptoms of sinusitis (eg, congestion, facial pressure/pain, nasal drainage) in combination with endoscopic evidence of inflammation and/or mucopus. One of these 3 patients had a preoperative history of chronic sinusitis. Each of the 3 patients was successfully managed with a course of antibiotics. Of note, 1 patient with sinusitis receiving chemotherapy for orbital B-cell lymphoma was noted to have adhesions of the middle meatus and lateralization of the middle turbinate contributing to obstruction. The adhesions were divided in the office with local anesthesia and the patient’s symptoms resolved. While there is some medial prolapse of orbital contents following endoscopic orbital surgery, the sinuses remained patent and healthy in the vast majority of patients.
One patient developed epistaxis while on therapeutic anticoagulation, which was successfully managed with conservative measures.
Sino-Nasal Outcome Test-22 (SNOT-22) scores were used to assess sinonasal symptoms. Due to variability in presentation and preoperative work up, only 8 patients (33%) had preoperative SNOT-22 scores available. The average preoperative SNOT-22 score was 21.9. Of these patients, at the initial postoperative visit, 3 patients reported increased scores (average increase 9.7 points, range 5–13 points), 3 reported decreased scores (average decrease 22 points, range 2–34 points), and 1 patient reported the same as the preoperative score (and 1 patient was lost to follow-up). In extended postoperative follow-up for all patients, 5 patients reported SNOT-22 scores that increased immediately postop and then decreased to below initial value, whereas scores only decreased in 6 patients. The average first postoperative SNOT-22 score was 24.2 (range 0–90). The average SNOT-22 score at last follow-up was 18.3 (range 0–86).
Of 7 patients with documented preoperative diplopia, symptoms were noted to be stable (5) or improved (2) at the time of ophthalmology follow-up. There were no new or worsening cases of diplopia. Two patients noted improved visual acuity postoperatively. There were no new cases of worsening visual acuity. Eleven patients had some degree of documented preoperative proptosis, and 6 patients had reduced proptosis postoperatively. Pre- and postoperative Hertel exophthalmometer measurements were obtained for 5 patients—1 CHEER stage 1 cavernous hemangioma completely resected using a cryoprobe, 1 CHEER stage 3 cavernous hemangioma that was not located, 2 patients with biopsied idiopathic orbital inflammatory syndrome, and 1 patient with biopsied MALT lymphoma. The mean change in Hertel measurement of the affected eye was −1 mm (range −4 to +3). The positive postoperative change in Hertel measurement for 1 patient was for a patient with idiopathic orbital inflammatory syndrome who underwent a biopsy. In no cases did the affected eye demonstrate more postoperative enophthalmos than the unaffected eye.
Discussion
Mass lesions of the orbit are rare entities consisting of a variety of different pathologies.3 Surgical management of these conditions may involve resection, biopsy to guide medical therapy, or orbital decompression to reduce pressure from mass effect. The surgical approach to the orbit has evolved to include the endonasal endoscopic technique as an option for management of lesions of the medial orbit and orbital apex. It affords a minimally invasive approach with excellent visualization and more direct access to the orbital apex than other external approaches.
We believe that a multidisciplinary combined approach consisting of external orbitotomy, along with a simultaneous endoscopic endonasal approach, harnesses the advantages of both procedures while leading to low complication rates. Similarly, by aiding the orbital exposure via wide endoscopic view, oftentimes smaller and more easily hidden orbitotomy incisions are possible leading to better cosmesis with less postoperative scarring. The majority of cases in our series underwent a transconjunctival approach as it provides wide access to the inferomedial orbit with a hidden conjunctival incision. The transcaruncular approach may also be considered as it provides direct external access to the medial orbit; however, this is through a more narrow corridor.8 The ultimate decision on external orbitotomy approach rests on the location of the lesion and comfort of the surgeon.
The endoscopic technique provides excellent visualization and access to the medial orbit, while the external orbitotomy allows for simultaneous protection of orbital structures during dissection. In addition, orbital masses can be challenging to localize within the orbital fat. The orbitotomy provides additional assistance delivering the mass medially into the sinonasal cavity and facilitates the endoscopic dissection through the inferomedial (between the medial and inferior rectus muscles) or superomedial (between the medial rectus and superior oblique muscles) corridors.7 Furthermore, the vascular anatomy of the intraconal orbit is complex and challenging bleeding may be encountered. After passing medially over (most commonly) or under the optic nerve, the ophthalmic artery then turns anteriorly near the common border of the superior oblique and medial rectus muscles at the orbital apex. Branches from the inferomedial muscular trunk of the ophthalmic artery have been identified as supplying the inferior and medial rectus muscles.9 In addition, the anterior ethmoid artery may be identified through the superomedial corridor.7 Such complex vascular anatomy immersed in orbital fat requires delicate gentle dissection. We feel that our combined approach limits the extent of required endoscopic dissection while simultaneously providing additional means of accessing and controlling arterial bleeding should it be encountered.
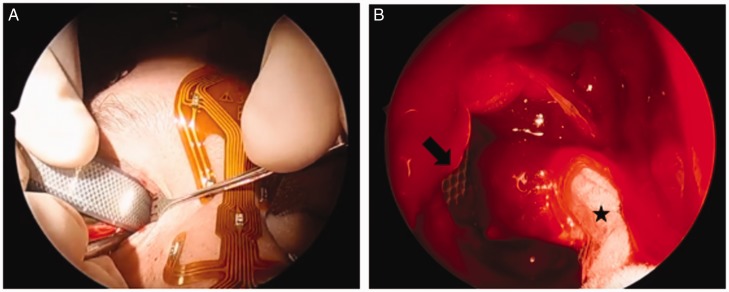
Intraconal orbital masses are often small, soft lesions embedded within orbital fat with multiple critical structures in close proximity, including extraocular muscles, optic nerve, and branches of the ophthalmic artery. Safe retraction of the medial rectus to provide access and careful dissection through orbital fat to avoid hemorrhage are keys to effective endoscopic orbital surgery. Significant recent research has focused on delineating endoscopic orbital anatomy to facilitate the expansion of solely endoscopic endonasal approaches for management of these lesions.6,9 Binarial transseptal approaches have been described to augment endoscopic dissection and retraction using a 4-handed technique.5,10–12 In 3 of our reported cavernous hemangioma resections, we utilized a cryoprobe device to securely grasp the lesion and facilitate the dissection. We have found that the texture of vasculogenic lesions can be difficult to grasp and dissect from surrounding fat using standard sinus instruments, even with assistance from ophthalmology delivering the lesion medially. The cryoprobe device assists with obtaining a secure hold on the lesion once it is identified (Figure 1).13 The cryoprobe may help to obviate the need for a transseptal approach and additional instrumentation, thereby decreasing sinonasal morbidity.
Figure 1.
Combined approach to orbital cavernous hemangioma resection using cryoprobe. A, Orbitotomy approach. B, Endoscopic view of cryoprobe device attached to cavernous hemangioma (star) and retractor (arrow) through orbitotomy helping to deliver hemangioma into nasal cavity.
None of the patients in our series underwent resection of orbital fat or reconstruction of the medial orbital wall. Advocates of reconstruction feel that it may prevent excessive enophthalmos and diplopia which may occur after unbalanced removal of the medial orbital wall and opening of the periorbita. This is most significant for instances requiring significant intraconal dissection with disruption of orbital septa resulting in substantial intranasal fat herniation. Multiple methods of medial orbital wall reconstruction have been proposed, from rigid materials such as septal cartilage or porous polyethylene implants (Medpor®, Stryker, Inc.) to nonrigid techniques such as free mucosal grafts or nasoseptal flaps.1,14,15 Nonrigid methods of reconstruction are preferable, as they will accommodate postoperative swelling and provide an outlet for blood to reduce the risk of increased intraorbital pressure and ischemia.1
Despite no reconstruction, our outcomes were favorable with no intraoperative complications and minimal postoperative complications. No patients were noted to have developed new postoperative diplopia and no patients developed worsening visual acuity. Several patients with preoperative proptosis noted a reduction in proptosis without diplopia. However, in our series, it should be noted that few patients underwent complete resection (3/7 cavernous hemangiomas and 2 orbital clots), which may have implications on the extent of dissection and resultant loss of orbital volume without reconstruction. No completely resected cavernous hemangiomas in our series reached CHEER stages VA or VB (intracranial extension through the superior orbital fissure), and these were stages for which most panelists of the CHEER staging system would “always” or “almost always” perform reconstruction. For stage IVB, 41% of panelists “never” or “infrequently” perform reconstruction. For lesser stages with reduced intraconal dissection, an increasing majority of panelists do not reconstruct the medial orbital wall. Of note, the CHEER staging system was developed with the intent of surgeons performing a purely endoscopic approach to complete resection, which is not consistent with our combined technique.
The management decision to perform full resection must be balanced with the patient’s preoperative symptoms and risk of postoperative complications. In multiple instances, we avoided extensive dissection in favor of a conservative approach given a lack of preoperative symptoms. Furthermore, often times a biopsy is needed first to rule out other pathology and guide appropriate management.
We find no similar study examining outcomes from a combined approach. A systematic review examined 39 studies covering short-term postoperative complications across 71 cases of exclusively endoscopic resection of orbital masses.16 In the review, most postoperative complications were transient in nature and did not vary between intraconal and extraconal tumors. The most common complication was diplopia (15%); however, the specific cases involved and if the symptom was present preoperatively are not clear. Sinusitis (our most common postoperative complication) was not assessed in the systematic review. Furthermore, it may be difficult to compare results from exclusively endoscopic approaches to our combined approach.
Sinonasal outcomes in our series were also encouraging. Postoperative SNOT-22 scores generally decreased from an average of 24.2 to 18.3 at last follow-up. Six patients developed postoperative sinusitis treated successfully with antibiotics, and 1 patient developed isolated epistaxis managed conservatively. Antisdel et al. examined sinonasal outcomes following orbital decompression and similarly found the incidence of postoperative complications to be low—involving sinusitis, epistaxis, and adhesions.17
We acknowledge the limitations of this retrospective study. Due to the tertiary nature of our department and rarity of patients with orbital pathology, long-term follow-up was not possible for all patients and some downstream complications may not be captured in this analysis. In addition, there may be a lack of consistency among reported SNOT-22 scores if they are obtained at different postoperative intervals. Regardless, all available SNOT-22 scores ultimately decreased from a previous value. Hertel exophthalmometer measurements were only obtained for a minority of patients, although no patients become enophthalmic in the affected eye relative to their normal eye. There is inherent error in Hertel measurements.18 As such, some ophthalmologists involved in our study have found that subjective evaluation of outcomes in terms of patient-reported diplopia and perceived proptosis may be more clinically relevant. Finally, our series consists of patients who underwent orbital decompression, orbital biopsy, and complete resection of the orbital mass. While no patients underwent reconstruction of the medial orbital wall, it may be difficult to consistently extrapolate our results to complete resections performed exclusively via the endoscopic route.
Conclusions
A combined endoscopic endonasal and orbitotomy can be useful for the surgical management of orbital lesions. While it is important to continue expanding the endoscopic endonasal approach, patients undergoing our combined approach tolerated the procedure well with improvement in ocular symptoms and minimal sinonasal complications. Reconstruction of the medial wall may not be warranted to prevent postoperative diplopia after biopsy or resection of smaller lesions. We believe that a multidisciplinary approach most effectively positions our team to effectively perform these operations and manage potential complications.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
This study was approved by our institutional review board.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Ryan A. Rimmer https://orcid.org/0000-0002-9010-8183
Statement of Human and Animal Rights
This article does not contain any studies with human or animal subjects.
Statement of Informed Consent
There are no human subjects in this article and informed consent is not applicable.
References
- 1.Yao C, Bleier BS. Endoscopic management of orbital tumors. Curr Opin Otolaryngol Head Neck Surg. 2016; 24:57–62. [DOI] [PubMed] [Google Scholar]
- 2.Tailor TD, Gupta D, Dalley RW, Keene CD, Anzai Y. Orbital neoplasms in adults: clinical, pathologic, and radiologic review. RadioGraphics. 2013; 33:1739–1758. [DOI] [PubMed] [Google Scholar]
- 3.Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions. Ophthalmology. 2004; 111:997–1008 [DOI] [PubMed] [Google Scholar]
- 4.Neems L, Echalier EL, Subramanian PS. Orbital tumors and inflammatory disorders: diagnosis and management. Int Ophthalmol Clin. 2018; 58(2):181–195. [DOI] [PubMed] [Google Scholar]
- 5.Paluzzi A, Gardner PA, Fernandez-Miranda JCet al. “Round-the-clock” surgical access to the orbit. J Neurol Surg B. 2015; 76:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Rassi EE, Adappa ND, Battaglia Pet al. Development of the international orbital Cavernous Hemangioma Exclusively Endonasal Resection (CHEER) staging system. Int Forum Allergy Rhinol. 2019; 9(7):804–812. [DOI] [PubMed] [Google Scholar]
- 7.Castelnuovo P, Turri-Zanoni M, Battaglia P, Locatelli D, Dallan I. Endoscopic endonasal management of orbital pathologies. Neurosurg Clin N Am. 2015; 26(3):463–472. [DOI] [PubMed] [Google Scholar]
- 8.Shorr N, Baylis HI, Goldberg RA, Perry JD. Transcaruncular approach to the medial orbit and orbital apex. Ophthalmology. 2000; 107:1459–1463. [DOI] [PubMed] [Google Scholar]
- 9.Bleier BS, Healy DY, Jr, Chhabra N, Freitag S. Compartmental endoscopic surgical anatomy of the medial intraconal orbital space. Int Forum Allergy Rhinol. 2014; 4:587–591. [DOI] [PubMed] [Google Scholar]
- 10.Bleier BS, Castelnuovo P, Battaglia Pet al. Endoscopic endonasal orbital cavernous hemangioma resection: global experience in techniques and outcomes. Int Forum Allergy Rhinol. 2016; 6(2):156–161. [DOI] [PubMed] [Google Scholar]
- 11.Murchison AP, Rosen MR, Evans JJ, Bilyk JR. Posterior nasal septectomy in endoscopic orbital apex surgery. Ophthal Plast Reconstr Surg. 2009; 25(6):458–463. [DOI] [PubMed] [Google Scholar]
- 12.Stokken J, Gumber D, Antisdel J, Sindwani R. Endoscopic surgery of the orbital apex: outcomes and emerging techniques. Laryngoscope. 2016; 126:20–24. [DOI] [PubMed] [Google Scholar]
- 13.Campbell PG, Yadla S, Rosen MR, Bilyk JR, Murchison AP, Evans JJ. Endoscopic transnasal cryo-assisted removal of an orbital cavernous hemangioma: a technical note. Minim Invas Neurosurg. 2011; 54:41–43. [DOI] [PubMed] [Google Scholar]
- 14.Colletti G, Saibene AM, Pessina Fet al. A shift in the orbit: immediate endoscopic reconstruction after transnasal orbital tumors resection. J Craniofac Surg. 2017; 28(8):2027–2029. [DOI] [PubMed] [Google Scholar]
- 15.Bleier BS. A shift in the orbit: immediate endoscopic reconstruction after transnasal orbital tumors resection: response. J Craniofac Surg. 2018; 29(6):1674–1675. [DOI] [PubMed] [Google Scholar]
- 16.Dubal PM, Svider PF, Denis D, Folbe AJ, Eloy JA. Short-term outcomes of purely endoscopic endonasal resection of orbital tumors: a systematic review. Int Forum Allergy Rhinol. 2014; 4:1008–1015. [DOI] [PubMed] [Google Scholar]
- 17.Antisdel JL, Gumber D, Holmes J, Sindwani R. Management of sinonasal complications after endoscopic orbital decompression for Graves’ orbitopathy. Laryngoscope. 2013; 123:2094–2098. [DOI] [PubMed] [Google Scholar]
- 18.Sleep TJ, Manners RM. Interinstrument variability in Hertel-type exophthalmometers. Ophthal Plast Reconstr Surg. 2002; 18(4):254–257. [DOI] [PubMed] [Google Scholar]