Short abstract
Background
Monocyte-mediated inflammation increases the risk of developing type 2 diabetes mellitus and cardiovascular disease. This is the first systematic review and meta-analysis of studies reporting on monocyte-mediated inflammation in type 2 diabetes mellitus.
Methods
This systematic review and meta-analysis was registered in the international prospective register of a systematic review: CRD42019132902. The MEDLINE, EMBASE and Google scholar electronic databases were searched, and a random-effects model was used to generate pooled standardised mean differences between patients with type 2 diabetes mellitus and healthy controls.
Results
The clinical studies (n = 20) comprised of 1065 patients with type 2 diabetes mellitus and 1103 healthy controls. Notably, the levels of monocyte activation were higher in patients with type 2 diabetes mellitus compared to healthy controls (standardised mean difference = 0.47, 95% confidence interval (0.10, 0.84), p = 0.01) (χ2 = 65.72, I2 = 83%, p < 0.00001). Patients with type 2 diabetes mellitus had an increased risk of cardiovascular disease compared to healthy controls (standardised mean difference = 0.37, 95% confidence interval (0.13, 0.61), p = 0.003) (χ2 = 958.77, I2 = 95%, p < 0.00001). All included pre-clinical studies reported on the C57BL/6 mice strain, with a majority of the studies 57% of reporting on high fat diet-induced C57BL/6 mice model. The overall quality of the studies was good with a median score and range of 16 (13–19).
Conclusion
Our meta-analysis suggests that there is increased monocyte activation in patients with type 2 diabetes mellitus.
Keywords: Monocytes, inflammation, type 2 diabetes, cardiovascular diseases
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder that is characterised by hyperglycaemia, insulin resistance and chronic immune activation.1,2 Obesity remains a leading modifiable risk factor for the development of T2DM and is characterised by excessive adiposity resulting from an imbalance between caloric intake and expenditure.3 Notably, obesity-induced inflammation has been shown to promote chronic immune activation in individuals living with T2DM.1,4–6 This has been characterised by the elevated release of pro-inflammatory cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β.7,8 In addition, these cytokines have been reported to directly interfere with insulin signal transduction leading to the development of insulin resistance in T2DM.1,5,9,10 Moreover, the persistent dysregulation of the immune system in T2DM has been implicated in the development of inflammation-associated complications such as cardiovascular disease (CVD).5,7,9,11–14 As a consequence, individuals with T2DM are at a four-fold increased risk of developing CVD when compared to normoglycaemic individuals.9,14,15
Recent studies have described the involvement of T-cells, innate lymphoid cells, macrophages and natural killer T-cells in the pathogenesis of T2DM and its associated complications such as CVD.2,4,5,10,16 However, the exact role that monocytes play in T2DM is poorly understood. Activated monocytes are known to secrete pro-inflammatory cytokines such as TNF-α and IL-6, which activate other leucocytes and exacerbate inflammation.4,15,17–20 Several studies have reported on increased monocyte activation in patients living with T2DM when compared to healthy controls.4,15,18,19 In contrast, others have reported no significant differences in monocytes levels between T2DM and control groups.6,11 The exact role of monocytes in the pathogenesis of T2DM remains elusive. We therefore conducted a systematic review and meta-analysis of available clinical and pre-clinical studies, to assess monocyte function in patients with T2DM as well as their cardiovascular risk profiles.
Methods
This systematic review and meta-analysis was prepared following the Preferred Reporting Items for Systematic Review and Meta-Analysis 2009 guidelines.21 The protocol was registered with the International Prospective Register of a Systematic Review, registration number: CRD42019132902.
Search strategy
A comprehensive search was conducted on MEDLINE and EMBASE databases as well as grey literature from inception until 8 August 2019 by two independent reviewers (KM and BBN). In case of disagreements, a third reviewer (PVD) was consulted for arbitration. The search strategy syntax was adapted for each electronic database using Medical Subject-Heading terms such as ‘myeloid cell’, ‘inflammation’, ‘type 2 diabetes mellitus’, ‘monocytes’, ‘insulin resistance’ and ‘hyperglycaemia’ and their respective synonyms and associated words/phrases. The detailed search strategy is provided in Supplementary File 2. No language restrictions were applied in the search strategy.
Inclusion and exclusion criteria
This systematic review and meta-analysis included human studies reporting on monocyte function in T2DM. In addition, the qualitative synthesis of this review also included animal studies reporting on monocyte function in animal models of T2DM. However, reviews, editorials, books and letters were excluded. Moreover, studies without a suitable comparator group were excluded.
Data items and extraction
A predefined data extraction sheet was used by two independent investigators (KM and BBN) to extract data items which included names of the authors, year of publication, experimental models used, study size and main findings of each study. In addition, the Mendeley Reference Manager version (1.19.4) software (Elsevier, Amsterdam, the Netherlands) was used to manage extracted information including identifying and removal of study duplicates.
Quality assessment and risk of bias
Two independent reviewers (KM and SHY) assessed the quality and risk of bias of included studies. In cases of disagreements, BBN was consulted for arbitration. The modified Downs and Black checklist was used for human studies.22 In addition, the Joanna Briggs Institute (JBI) checklist and Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines were used for animal studies.23
Data analysis
Data analysis was performed using the Review Manager (RevMan) version 5.3. The chi-squared (χ2) test and Higgin’s I2 statistics were used to test for statistical heterogeneity. A random-effects model was used to generate pooled effect estimates in cases where substantial heterogeneity existed (I2 > 50%). The standardised mean difference (SMD) and 95% confidence interval (CI) of various effect measures reporting on the same outcome were pooled and reported. Funnel plots were used to assess the publication bias of the included studies. Moreover, inter-rater reliability was assessed using Cohen’s Kappa (ĸ) and a score of < 0 was considered poor, 0.01–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial and 0.81–1.00 as perfect. A p value of less than 0.05 was considered statistically significant.
Results
Selected studies
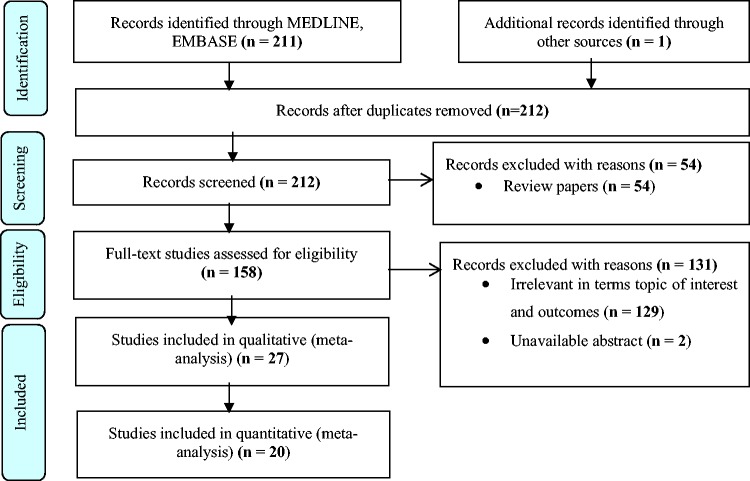
A total of 212 studies were retrieved using the search strategy and only 27 studies met the inclusion criteria, while 185 studies were excluded. Amongst the excluded studies, 54 were reviews, 129 were not relevant to the topic of interest and 2 had no available full text. The 27 included studies included 19 human studies, 7 animal studies and 1 study that reported on both animals and humans (Figure 1). Only 20 human studies had sufficient study-level data and these were included in the meta-analysis.
Figure 1.
Preferred Reporting Items for Systematic Review and Meta-Analysis flow diagram of included studies.
Characteristics of included human studies
All included studies were published in peer-review journals between 2001 and 2019, and their characteristics are shown in Tables 1S and 2S. In total, the included studies comprised of 2168 participants of which 1065 (49.8%) were living with T2DM and 1103 (50.6%) were normal individuals. Of the included 20 human studies, 18 were cross-sectional5–9,11–20,24,25 and 2 were cohort studies.4,26 The mean age of the study population was 52.28 ± 8.07 with a male to female ratio of 1.39 (Table 1S). The publication trends on monocyte function in T2DM in both human and animal studies are presented in Figure 1S. Briefly, 2 studies were published between 2001 and 2005,9,12 5 studies between 2006 and 2010,7,11,13,20,27 10 studies between 2011 and 20151,4,6,10,14–16,18,19,24 and another 10 studies from 2016 to 2019.3,5,8,14,17,25,26,28–30 The included studies were spread across Europe, Asia and United States with most of the studies from the United States (Figure 1S).
Study quality and risk of bias
The quality of human studies (n = 20) was assessed using the modified Downs and the Blacks checklist.22 One study was scored as good (19 points), while seven studies were scored as fair (13–18 points) and the rest as poor (8–12 points). The overall median range for all included studies was 12 (8–19) out of a possible score of 20. Here, most studies had low reporting bias, with a median of 7 (5–10) out of a possible score of 10 (ĸ = 0.6) and internal validity with a median of 2 (2–4) out of the possible 7 scores (ĸ = 0.71). The included studies had poor external validity and selection bias with a median score of 1 (0–3) out of possible 3 scores (ĸ = 0.33) and a median of 1 (0–4) out of the possible score of 4 (ĸ = 0.33), respectively (Table 6Sa).
On the other hand, the quality of included animal studies (n = 8) was assessed and three studies10,27,28 were scored as fair 13 (13–14) and the rest as high 17 (16–19) using ARRIVE guidelines (Table 6Sb). In addition, all included studies were of good quality with a median score of 7.5 (4–8) out of a possible score of 9 when using JBI critical appraisal tool, except for one study that was scored as poor28 (Table 6Sc). The use of funnel plots revealed that all included human studies had no publication bias on the outcomes reported (Figure 4S).
Data synthesis
Reported glucose metabolic profiles
Fasting blood glucose levels
The pooled effect estimates of fasting blood glucose were significantly increased in individuals with T2DM when compared to controls (SMD = 3.26, 95% CI (2.47, 4.04), p < 0.00001). The included studies showed a substantial level of heterogeneity (χ2 = 429.39, I2 = 97%, p < 0.00001) (Figure 2S and Table 3S).
Insulin levels
The pooled effect estimates showed no significant effect differences in the insulin levels of individuals with T2DM compared to controls (SMD = 0.84, 95%CI (−0.31, 2.00), p = 0.15). However, the level of heterogeneity in these studies was substantial (χ2 = 40.63, I2 = 90%, p < 0.00001) (Figure 2S and Table 3S).
Glycated haemoglobin levels
The pooled effect estimates showed significantly increased levels of HbA1c in individuals with T2DM when compared to controls (SMD = 2.48, 95% CI (1.63, 3.34), p < 0.00001). However, substantial levels of heterogeneity were also observed in these studies (χ2 = 162.47, I2 = 90%, p < 0.00001) (Figure 2S and Table 4S).
As expected, pooled estimates of glycaemic profiles of the patients with T2DM were elevated when compared to healthy controls (SMD = 2.33, 95% CI (1.91, 2.76), p < 0.00001). Notably, the levels of statistical heterogeneity in these studies were substantial (χ2 = 666.61, I2 = 95%, p < 0.00001) (Figure 2S).
Reported markers of monocyte activation
The overall pooled estimate for monocytes activation
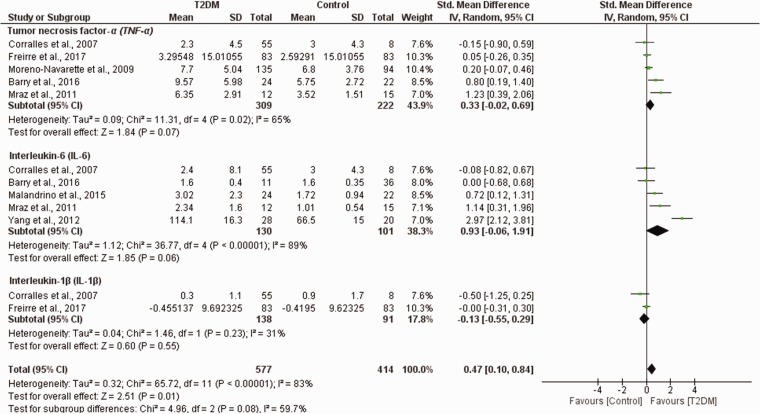
The pooled levels of monocyte activation were higher in patients with T2DM compared to control (SMD = 0.47, 95% CI (0.10, 0.84), p = 0.01, I2 = 83%). However, these studies showed a substantial level of statistical heterogeneity (χ2 = 65.72, I2 = 83%, p < 0.00001) (Figure 2) and subgroup analysis based on the reported effect measure of monocyte activation was conducted (Figure 2).
Figure 2.
Pooled estimates of monocyte activation in patients with T2DM compared to healthy controls. SD: standard deviation; CI: confidence interval; T2DM: type 2 diabetes mellitus.
Monocyte-secreted IL-6
Interestingly, the levels of monocyte-secreted IL-6 were comparable between patients with T2DM and healthy controls (SMD = 0.93, 95% CI (−0.06, 1.91), p = 0.06). However, the studies showed substantial level of heterogeneity (χ2 = 36.77, I2 = 89%, p < 0.00001) (Figure 2 and Table 4S).
Monocyte-secreted TNF-α
The levels of TNF-α were similar between patients with T2DM and healthy controls (SMD = 0.33, 95% CI (−0.02, 0.69), p = 0.07). In addition, a substantial level of statistical heterogeneity was observed in these studies (χ2 = 11.31, I2 = 65%, p = 0.02) (Figure 2 and Table 4S).
Monocyte-secreted-IL-1β
The pooled effect estimates showed insignificant differences in IL-1β levels in individuals with T2DM and controls (SMD = −0.13, 95% CI (−0.55, 0.29), p = 0.55). In addition, these studies had substantial level of heterogeneity (χ2 = 1.46, I2 = 31%, p = 0.23) (Figure 2 and Table 4S).
Inflammatory profile of patients with T2DM compared to healthy controls
The overall pooled effect estimates showed a significant increase in the inflammatory markers in individuals with T2DM and controls (SMD = 0.56, 95% CI (0.21, 0.91), p = 0.67, I2 = 89%). However, the heterogeneity in these studies was substantial (χ2 = 71.07, I2 = 89%, p < 0.00001) (Figure 3S).
C-reactive protein
The pooled effect estimates showed a significant increase in C-reactive protein (CRP) in individuals with T2DM compared to controls (SMD = 0.63, 95% CI (0.21, 1.06), p = 0.003) (χ2 = 13.15, I2 = 70%, p = 0.01) (Figure 3S and Table 4S).
Neutrophil–lymphocyte ratio
The pooled effect estimates showed an insignificant increase in neutrophil–lymphocyte ratio (NLR) in individual with T2DM compared to healthy controls (SMD = 0.47, 95% CI (−0.13, 1.08), p = 0.12) (χ2 = 57.88, I2 = 95%, p < 0.00001) (Figure 3S and Table 4S).
Prevalence of cardiovascular risk factors in patients with T2DM compared to healthy controls
Pooled estimates showed an increased risk of CVDs in patients with T2DM compared to healthy controls (SMD = 0.37, 95% CI (0.13, 0.61), p = 0.003) (χ2 = 958.77, I2 = 95%, p < 0.00001) (Table 5S). A subgroup analysis of the reported effect measures was conducted due to a significant level of heterogeneity.
Diastolic and systolic blood pressure measurements
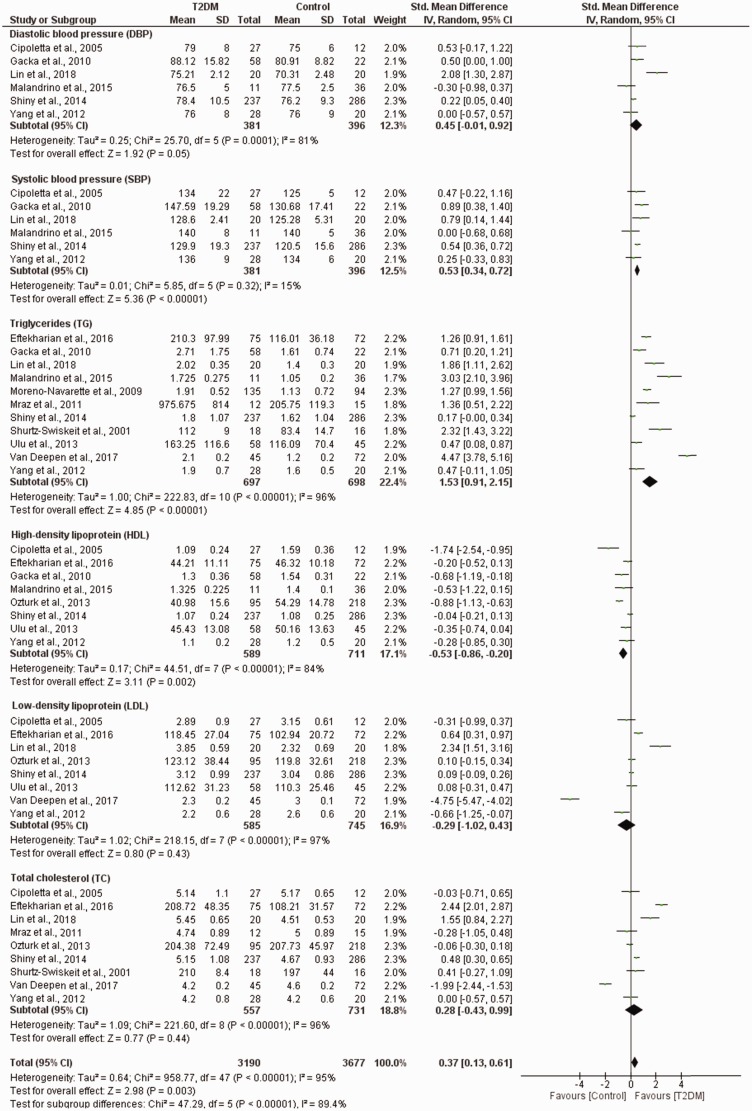
Patients with T2DM had higher diastolic blood pressure (DBP) levels compared to healthy controls (SMD = 0.45, 95% CI (−0.01, 0.92), p = 0.0001) (χ2 = 25.70, I2 = 81%, p = 0.0001) (Figure 3). In addition, the systolic blood pressure (SBP) levels were also elevated in patients with T2DM (SMD = 0.53, 95% CI (0.34, 0.72), p = 0.32) compared to healthy controls (χ2 = 5.85, I2 = 15%, p < 0.00001) (Figure 3 and Table 3S).
Figure 3.
The prevalence of CVD risk factors in T2DM compared to healthy controls. SD: standard deviation; CI: confidence interval; T2DM: type 2 diabetes mellitus.
Triglyceride levels
The subgroup analysis showed increased triglycerides levels in patients with T2DM compared to healthy controls (SMD = 1.53, 95% CI (0.91, 2.15), p < 0.00001). However, substantial levels of heterogeneity were observed in these studies (χ2 = 222.83, I2 = 96%, p < 0.00001) (Figure 3 and Table 4S).
Cholesterol levels
The pooled effect estimates showed an insignificant increase in total cholesterol levels in individuals with T2DM compared to controls (SMD = 0.28, 95% CI (−0.40, 0.99), p = 0.44). However, substantial levels of heterogeneity were observed in these studies (χ2 = 221.60, I2 = 96%, p < 0.00001) (Figure 3 and Table 4S).
Low-density lipoprotein
The pooled effect estimates showed an insignificant decrease in the levels of low-density lipoprotein (LDL) in individuals with T2DM when compared to controls (SMD = −0.29, 95% CI (−1.02, 0.43), p = 0.43). However, the level of heterogeneity in these studies was substantial (χ2 = 218.15, I2 = 97%, p < 0.00001) (Figure 3 and Table 4S).
High-density lipoprotein
The pooled effect estimates showed decreased levels of high-density lipoprotein (HDL) in individuals with T2DM when compared to controls (SMD = –0.53, 95% CI (−0.86, −0.20), p = 0.002). However, these studies showed substantial level of heterogeneity (χ2 = 44.51, I2 = 84%, p < 0.00001) (Figure 3 and Table 4S).
A narrative synthesis of pre-clinical studies
Monocyte activation in a diet-induced model of obesity
Fifty-seven percent of the studies reported on the C57BL/6 mice strain on a high-fat diet (HFD). The overall quality of the studies was scored as good with a median score and range of 16 (13–19). However, the included studies reported on contradictory findings regarding monocyte secreted IL-1β in HFD mice. Two studies showed an upregulation in IL-1β,3,30 while in one study the levels remained the same.10 The levels of macrophage activation markers including F4/80,27 CD685,27 and CD11c1 were decreased in HFD-fed mice while IL-8 increased29 (study-level outcomes are reported in Table 2S).
Discussion
This systematic review and meta-analysis aimed at assessing available literature on the role of monocytes activation in the pathogenesis of T2DM and the development of CVDs as its complications. Data synthesised in this study revealed increased monocytes activation in individuals with T2DM coupled with increased risk of developing CVD when compared to the control group. The monocyte-derived TNF-α and IL-6 were comparable between patients with T2DM and healthy controls. Changes in TNF-α metabolism may initiate the onset of T2DM and the progression of the disease.17,19,20 IL-6 is an active cytokine, which predicts and induces T2DM independently and is involved in inflammation, insulin resistance and β cell-associated dysfunction.4,15,19 In addition, IL-1β, the pro-inflammatory cytokine that is essential for host-defence responses to injury, was reduced in T2DM individuals; however, this was not statistically significant.11,25 Interestingly these findings were also observed in animal studies, showing that monocytes/macrophage markers such as TNF-α, IL-6 and IL-1β are elevated in HFD-fed C57BL/6 mice resembling T2DM in humans.
Consistently, this study showed an increase in inflammatory markers of monocyte activation including CRP and NLR with T2DM,2,6,14,15,19,24 thus highlighting the impact of these inflammatory molecules in aggravating the development of T2DM. In agreement, existing evidence shows that inflammation and monocyte activation promotes the development of T2DM and its associated complications.11 These complications can influence numerous organs and tissues causing retinopathy, nephropathy, atherosclerosis and CVD.14 The role of inflammation and monocytes in the development of insulin resistance and progression of T2DM is widely recognised; however, precisely implicated mechanisms are still complex. It is hypothesised that the accumulation of M1 macrophage in an obese state can induce insulin resistance by producing IL-1β which are known to stimulate TNF-α and IL-6, rendering the recruitment of other immune cells and further exacerbate inflammation through negative feedback mechanisms.11,25 Nevertheless, enhanced levels of TNF-α and IL-6 are consistent with the development of chronic inflammatory disease and atherosclerosis11,25 which could explain the increased risk of CVD in T2DM patients.7,12,14,15
Dyslipidaemia is associated T2DM and promotes the development of CVD. Interestingly in our meta-analysis the lipid profiles (HDL and LDL) were significantly reduced in individuals living with T2DM. In fact previous studies have suggested that LDLs are not always elevated in T2DM individuals.5,9,15 Notably, our meta-analysis showed that the levels of total cholesterol and triglycerides were significantly elevated in T2DM compared to healthy individuals. Thus, the altered blood lipid status as supported by evidence synthesised in this study shows that T2DM individuals indeed have an increased risk of developing CVD. The evidence of enhanced blood lipid profiles was consistent with that of DBP and SBP measurements, showing that these parameters were significantly elevated in individuals with T2DM when compared to control subjects, further demonstrating that age was directly proportional to individual blood pressure. This was further supported by studies7,26 which indicated that individuals above 30 years have an increased risk of developing hypertension and further into CVD. With the evidence provided in this study, individuals with T2DM have a substantial increase in monocyte activation and risk of developing CVD.
Study limitations
The overall quality of evidence synthesised in this study was low, mainly due to the study design of the included studies and the lack of randomised trials. In addition, the included studies showed a high level of statistical heterogeneity. Lastly, the potential role of disease duration in influencing the degree of monocyte activation could not be assessed as most of the included studies were cross-sectional, and no longitudinal studies with participant follow-up were available. Nonetheless, to our knowledge, this is the first systematic review and meta-analysis to critically analyse and evaluate the role of monocyte activation in T2DM and the development of cardiovascular risk factors. Moreover, these findings highlight the potential value of assessing monocyte activation in thrombotic risk stratification of individuals living with T2DM.
Conclusion
This meta-analysis suggests that patients with T2DM have increased levels of activated circulating monocytes and are at a higher risk of developing CVD. The evaluation of monocyte activation and the inflammatory profile of patients with T2DM may be useful in the thrombotic risk stratification of patients with T2DM. There is a need for well-designed clinical trials aimed at evaluating monocyte activation and the development of CVD in T2DM, as these would provide conclusive clinical relevance of the role of activated monocytes in the pathogenesis of T2DM and CVD.
Supplemental Material
Supplemental material, CVD900748 Supplemental Material1 for Monocyte-mediated inflammation and cardiovascular risk factors in type 2 diabetes mellitus: A systematic review and meta-analysis of pre-clinical and clinical studies by Kabelo Mokgalaboni, Phiwayinkosi V Dludla, Tawanda M Nyambuya, Sinethemba H Yakobi, Vuyolwethu Mxinwa and Bongani B Nkambule in JRSM Cardiovascular Disease
Supplemental material, CVD900748 Supplemental Material2 for Monocyte-mediated inflammation and cardiovascular risk factors in type 2 diabetes mellitus: A systematic review and meta-analysis of pre-clinical and clinical studies by Kabelo Mokgalaboni, Phiwayinkosi V Dludla, Tawanda M Nyambuya, Sinethemba H Yakobi, Vuyolwethu Mxinwa and Bongani B Nkambule in JRSM Cardiovascular Disease
Acknowledgements
PV Dludla was partially supported as a Post-Doctoral Fellow by funding from the SAMRC through its division of Research Capacity Development under the Intra-Mural Postdoctoral Fellowship Programme from funding received from the South African Treasury. The content hereof is the sole responsibility of the authors and do not necessary represent the official views of the SAMRC or the funders.
Contributorship
KM, PVD, TMN and BBN conceptualised, designed and drafted the review. All authors including SHY and VM wrote and approved the final manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
None.
Funding
This study is partially funded by the National Research Foundation of South Africa (Grant Number: 107519 awarded to BBN) and the South African Medical Research Council (Self-Initiated Research Grant 9894 awarded to BBN). BBN is also a University of KwaZulu-Natal Developing Research Innovation, Localisation and Leadership in South Africa (DRILL) fellow. DRILL is an NIH D43 Grant (D43TW010131) awarded to University of KwaZulu-Natal in 2015 to support a research training and induction programme for early-career academics.
Guarantor
BBN.
ORCID iDs
Phiwayinkosi V Dludla https://orcid.org/0000-0001-5965-3610
Bongani B Nkambule https://orcid.org/0000-0001-8846-1992
Supplemental material
Supplemental material for this article is available online.
References
- 1.Jia L, Vianna CR, Fukuda Met al. Hepatocyte toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat Commun 2014; 5: 3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eftekharian MM, Karimi J, Safe Met al. Investigation of the correlation between some immune system and biochemical indicators in patients with type 2 diabetes. Hum Antibodies 2016; 24: 25–31. [DOI] [PubMed] [Google Scholar]
- 3.Lee HY, Kim J, Quan Wet al. Autophagy deficiency in myeloid cells increases susceptibility to obesity-induced diabetes and experimental colitis. Autophagy 2016; 12: 1390–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malandrino MI, Fucho R, Weber Met al. Enhanced fatty acid oxidation in adipocytes and macrophages reduces lipid-induced triglyceride accumulation and inflammation. Am J Physiol Endocrinol Metab 2015; 308: E756–E769. [DOI] [PubMed] [Google Scholar]
- 5.Van Diepen JA, Robben JH, Hooiveld GJet al. SUCNR1-mediated chemotaxis of macrophages aggravates obesity-induced inflammation and diabetes. Diabetologia 2017; 60: 1304–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiny A, Bibin YS, Shanthirani CSet al. Association of neutrophil-lymphocyte ratio with glucose intolerance: an indicator of systemic inflammation in patients with type 2 diabetes. Diabetes Technol Ther 2014; 16: 524–530. [DOI] [PubMed] [Google Scholar]
- 7.Gacka M, Dobosz T, Szymaniec Set al. Proinflammatory and atherogenic activity of monocytes in type 2 diabetes. J Diabetes Complications 2010; 24: 1–8. [DOI] [PubMed] [Google Scholar]
- 8.Ip B, Cilfone NA, Belkina ACet al. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNFalpha production. Obesity (Silver Spring) 2016; 24: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipolletta C, Ryan KE, Hanna EVet al. Activation of peripheral blood CD14+ monocytes occurs in diabetes. Diabetes 2005; 54: 2779–2786. [DOI] [PubMed] [Google Scholar]
- 10.Dale Buras E, Yang L, Saha Pet al. Proinsulin-producing, hyperglycemia-induced adipose tissue macrophages underlie insulin resistance in high fat-fed diabetic mice. FASEB J 2015; 29: 3537–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corrales JJ, Almeida M, Burgo RMet al. Decreased production of inflammatory cytokines by circulating monocytes and dendritic cells in type 2 diabetic men with atherosclerotic complications. J Diabetes Complications 2007; 21: 41–49. [DOI] [PubMed] [Google Scholar]
- 12.Shurtz-Swirski R, Sela S, Herskovits ATet al. Involvement of peripheral polymorphonuclear leukocytes in oxidative stress and inflammation in type 2 diabetic patients. Diabetes Care 2001; 24: 104–110. [DOI] [PubMed] [Google Scholar]
- 13.Vaidyula VR, Boden G, Rao AK. Platelet and monocyte activation by hyperglycemia and hyperinsulinemia in healthy subjects. Platelets 2006; 17: 577–585. [DOI] [PubMed] [Google Scholar]
- 14.Öztürk ZA, Kuyumcu ME, Yesil Yet al. Is there a link between neutrophil-lymphocyte ratio and microvascular complications in geriatric diabetic patients? J Endocrinol Invest 2013; 36: 593–599. [DOI] [PubMed] [Google Scholar]
- 15.Yang M, Gan H, Shen Qet al. Proinflammatory CD14 +CD16 + monocytes are associated with microinflammation in patients with type 2 diabetes mellitus and diabetic nephropathy uremia. Inflammation 2012; 35: 388–396. [DOI] [PubMed] [Google Scholar]
- 16.Jagannathan-Bogdan M, McDonnell ME, Shin Het al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol 2011; 186: 1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry JC, Shakibakho S, Durrer Cet al. Hyporesponsiveness to the anti-inflammatory action of interleukin-10 in type 2 diabetes. Sci Rep 2016; 6: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai X, Zhan J, Demmy TAet al. Monocytes play different roles in stimulating T cells in obese diabetic individuals. Int J Immunopathol Pharmacol 2015; 28: 374–383. [DOI] [PubMed] [Google Scholar]
- 19.Mraz M, Lacinova Z, Drapalova Jet al. The effect of very-low-calorie diet on mRNA expression of inflammation-related genes in subcutaneous adipose tissue and peripheral monocytes of obese patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2011; 96: E606–E613. [DOI] [PubMed] [Google Scholar]
- 20.Moreno-Navarrete JM, Ortega FJ, Bassols Jet al. Decreased circulating lactoferrin in insulin resistance and altered glucose tolerance as a possible marker of neutrophil dysfunction in type 2 diabetes. J Clin Endocrinol Metab 2009; 94: 4036–4044. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff Jet al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor SR, Tully MA, Ryan Bet al. Failure of a numerical quality assessment scale to identify potential risk of bias in a systematic review: a comparison study. BMC Res Notes 2015; 8: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilkenny C, Browne WJ, Cuthill ICet al. Improving bioscience research reporting: the arrive guidelines for reporting animal research. Animals 2013; 4: 35–44. [DOI] [PubMed] [Google Scholar]
- 24.Ulu SM, Dogan M, Ahsen Aet al. Neutrophil-to-lymphocyte ratio as a quick and reliable predictive marker to diagnose the severity of diabetic retinopathy. Diabetes Technol Ther 2013; 15: 942–947. [DOI] [PubMed] [Google Scholar]
- 25.Freire MO, Dalli J, Serhan CNet al. Neutrophil resolvin E1 receptor expression and function in type 2 diabetes. J Immunol 2017; 198: 718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Y, Ye S, He Yet al. Short-term insulin intensive therapy decreases MCP-1 and NF-κB expression of peripheral blood monocyte and the serum MCP-1 concentration in newly diagnosed type 2 diabetics. Arch Endocrinol Metab 2018; 62: 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong E, Ko HJ, Cho Yet al. Interleukin-10 prevents diet-induced insulin resistance skeletal muscle. Diabetes 2009; 58: 2525–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimball AS, Joshi A, Carson WFet al. The histone methyltransferase mll1 directs macrophage-mediated inflammation in wound healing and is altered in a murine model of obesity and type 2 diabetes. Diabetes 2017; 66: 2459–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prattichizzo F, De Nigris V, Mancuso Eet al. Short-term sustained hyperglycaemia fosters an archetypal senescence-associated secretory phenotype in endothelial cells and macrophages. Redox Biol 2018; 15: 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dror E, Dalmas E, Meier DTet al. Postprandial macrophage-derived IL-1β stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat Immunol 2017; 18: 283–292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CVD900748 Supplemental Material1 for Monocyte-mediated inflammation and cardiovascular risk factors in type 2 diabetes mellitus: A systematic review and meta-analysis of pre-clinical and clinical studies by Kabelo Mokgalaboni, Phiwayinkosi V Dludla, Tawanda M Nyambuya, Sinethemba H Yakobi, Vuyolwethu Mxinwa and Bongani B Nkambule in JRSM Cardiovascular Disease
Supplemental material, CVD900748 Supplemental Material2 for Monocyte-mediated inflammation and cardiovascular risk factors in type 2 diabetes mellitus: A systematic review and meta-analysis of pre-clinical and clinical studies by Kabelo Mokgalaboni, Phiwayinkosi V Dludla, Tawanda M Nyambuya, Sinethemba H Yakobi, Vuyolwethu Mxinwa and Bongani B Nkambule in JRSM Cardiovascular Disease