Abstract
Background: Peritonitis remains a complication of peritoneal dialysis (PD) and contributes to morbidity. Adherence to evidence-based recommendations should resolve peritonitis within 5 days; however, hospital length of stay (LOS) for patients with PD-associated peritonitis (PDAP) varies. Factors contributing to increased LOS and vigilance with antimicrobial stewardship (ASP) in this population are not well described. Methods: This was a system-wide, retrospective cohort of adult patients presenting with PDAP from August 2012 to August 2017. Patients were divided into 2 groups based on LOS: <7 days (reduced LOS) versus ≥7 days (prolonged LOS). Patient demographics, resolution of peritonitis by day 5, intensive care unit (ICU) admission, infectious diseases (ID) consultation, changes in dialysis modality, blood glucose, and pathogen/antimicrobial characteristics were compared. In-hospital mortality and 30-day readmissions were also evaluated. Results: Of the 401 patients screened, 90 were included: 53% women, 88% African American, age 52 ± 2 years (reduced LOS: 46 patients; prolonged LOS: 44 patients). Increased LOS was associated with ICU admission (P = .014), ID consultation (P = .015), PD catheter removal (P = .001), hemodialysis conversion (P < .001), antifungal therapy (P = .021), and days with blood glucose >180 mg/dL (P = .028). Opportunities for antimicrobial de-escalation were identified in 24 (52%) and 22 (50%) patients in the reduced and prolonged LOS groups, respectively; however, de-escalation occurred in only 5 (21%) and 6 (27%) of these patients. There were no differences in mortality or 30-day readmissions. Conclusions: Longer LOS was influenced by acuity of illness and possibly lack of enforced ASP. Improvement of ASP within the PDAP population is necessary.
Keywords: anti-infectives, infectious diseases, nephrology
Introduction
Of the more than 480,000 patients requiring renal replacement therapy (RRT) for the treatment of end-stage renal disease (ESRD) in the United States, nearly 10% of patients receive peritoneal dialysis (PD). While the number of patients receiving PD is relatively low compared with hemodialysis (HD), PD use has continued to rise over the past decade to a rate that is over 70% higher than in 2007.1 Unfortunately, the number of patients maintained on PD is declining, in part, due to the high incidence of peritonitis.2-3 Although hospital admission rates for patients with PD have decreased 24% in the past decade, peritonitis remains the most common reason for infection-related hospitalizations in patients with PD and contributes to increased health care costs and mortality.1,4-5
The International Society of Peritoneal Dialysis (ISPD) Peritonitis Guidelines provide recommendations on empiric treatment of suspected/confirmed peritonitis, duration of treatment, and antimicrobial de-escalation while supporting the intraperitoneal (IP) route as the preferred route for antibiotic administration.3 In the outpatient setting, dialysis facilities commonly rely on these recommendations to design treatment algorithms; however, peritonitis treatment regimens are inconsistent in the inpatient setting. Lack of inpatient peritoneal dialysis–associated peritonitis (PDAP) protocols can lead to variations in antibiotic selection, dosing regimens, route of administration, and the prescribed duration of therapy, which have been observed.6 This may be due, in part, to the fact that most providers are likely less familiar with the dosing regimens and logistics of IP administration. This may lead to frequent prescribing of antibiotics by the intravenous (IV) route and failure to de-escalate therapy once an organism is identified. These inconsistencies in the treatment of PDAP, coupled with numerous factors that occur throughout hospitalization, may contribute to an increased hospital length of stay (LOS), as adherence to evidence-based recommendations is expected to result in peritonitis resolution within a 5-day period; however, hospital LOS for patients with PDAP varies.3
The primary objective of this study was to identify factors associated with an increased hospital LOS in patients presenting with PDAP. Secondary objectives were to evaluate the appropriateness of antimicrobial route of administration and whether antimicrobial de-escalation occurred, with the goal of providing guidance to clinicians to improve antimicrobial stewardship (ASP) in this patient population.
Materials and Methods
Patient Selection and Evaluation
This was a system-wide, retrospective cohort of adult patients with ESRD admitted to Methodist Le Bonheur Healthcare from August 2012 to August 2017. Methodist Le Bonheur Healthcare is a 5-hospital system containing nearly 1,500 inpatient beds. The principal teaching hospital, Methodist University Hospital, administered 780 PD treatments in 2017. At the time of this analysis, our system lacked an inpatient PDAP treatment protocol, with treatment and antifungal prophylaxis decisions based mainly on internal medicine, nephrology, and infectious diseases provider discretion. Patients with ESRD were included if they were ≥18 years of age, received PD as their primary mode of RRT, and presented with a diagnosis of PDAP. Patients were excluded if they had incomplete medical records, lacked initial/repeat effluent culture(s), or had documented positive effluent culture(s) at an outside facility and were transferred to our system without medical records. Patients were identified through cross-referencing of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes for ESRD and peritonitis.
Peritonitis diagnosis was based on established criteria defined in the 2016 ISPD Peritonitis Guidelines. At least 2 of the following were considered to be diagnostic of peritonitis: clinical features consistent with peritonitis (ie, cloudy effluent and/or abdominal pain); dialysis effluent white blood cell >100/μL or >0.1 × 109/L (after dwell time of ≥2 hours), with >50% polymorphonuclear cells; and/or positive dialysis effluent culture(s).3
Data extracted from the electronic medical record included the following: patient demographics; comorbidities; medication allergies; laboratory parameters for systemic inflammatory response syndrome (SIRS); previous episode(s) of peritonitis; previous hospitalization(s) within 30 days; hospital LOS; intensive care unit (ICU) admission; attainment of ID consultation; peritoneal effluent results: date/time positive effluent was collected, white blood cell count, neutrophil count, organism(s), minimum inhibitory concentrations, and date/time of subsequent effluent culture(s); other positive culture(s); number of days with majority (>50%) of blood glucose readings >180 mg/dL; antimicrobial data: medication name, dosing regimen, date/time of administration/discontinuation, route of administration (oral, IV, or IP), and missed doses; serum drug levels (if applicable); infection outcome (relapse, recurrence, refractory); dialysis modality changes; 30-day readmission(s); and in-hospital mortality.
Patients were divided into 2 groups based on LOS: <7 days (reduced LOS) versus ≥7 days (prolonged LOS). Patient demographics, adherence to guideline-based recommendations for antimicrobial therapy, antimicrobial route of administration, appropriate de-escalation of antimicrobials, antimicrobial allergies, therapeutic drug monitoring, resolution of peritonitis by day 5, blood glucose, admission to the ICU, ID consultation, dialysis modality changes, positive cultures, and pathogen characteristics were compared. In-hospital mortality, 30-day readmissions, and relapsed, recurrent, and refractory episodes were also compared.
Definitions
Empiric antimicrobials were defined as those administered within the first 48 hours of suspected peritonitis, whereas missed doses were defined as an ordered antimicrobial without documentation in the electronic medical record. Multidrug-resistant organisms were defined as documented resistance to at least 1 agent in 3 or more antibiotic classes. Relapse was defined as peritonitis occurring within 30 days of a prior appropriately treated episode of peritonitis in which the culture of the dialysate grew the same microorganism that caused the original episode. Recurrence was defined as peritonitis occurring within 30 days of a prior appropriately treated episode of peritonitis in which the culture of the dialysate grew a different microorganism than that which caused the original episode. Refractory episodes were defined as failure to clear the effluent after 5 days of appropriate antimicrobial therapy.
ISPD Peritonitis Guideline–based therapy was defined as empiric therapy that included both Gram-positive and Gram-negative coverage. Appropriate Gram-positive coverage for peritonitis was defined as the use of cefazolin, vancomycin, clindamycin, or daptomycin. Appropriate Gram-negative coverage for peritonitis was defined as the use of ceftriaxone, cefepime, ceftazidime, gentamicin, tobramycin, amikacin, aztreonam, amoxicillin, ampicillin, piperacillin/tazobactam, ampicillin/sulbactam, meropenem, doripenem, ciprofloxacin, or levofloxacin. Appropriate de-escalation was interpreted by the authors and defined as discontinuing broad-spectrum antimicrobials and initiating a more narrow-spectrum agent once in vitro susceptibility results returned or discontinuing either the empiric Gram-positive or Gram-negative agent once the effluent culture grew a specific Gram-positive or Gram-negative microorganism.
Statistical Analysis
The association between nominal variables was evaluated using χ2 test or Fisher exact test. For continuous variables, the Student t-test was used to compare parametric variables and the Mann-Whitney test was used to compare nonparametric variables. All analyses were conducted using SPSS version 23.0 (SPSS Inc., Armonk, NY, USA). A value of P < .05 was considered statistically significant.
Ethical Approval
This study was approved by the University of Tennessee Institutional Review Board prior to initiation.
Results
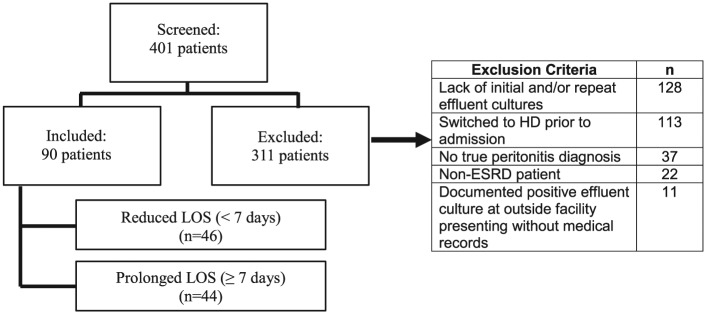
Of the 401 patients screened, 90 patients met inclusion criteria. Reasons for exclusion are highlighted in Figure 1. The mean age of the total population was 52 ± 2 years and median weight 87 kg (25%-75% interquartile range [IQR], 70-102 kg), with the majority women (53%) and African American (88%). There were 46 patients in the reduced LOS group and 44 patients in the prolonged LOS group. There were no statistically significant differences noted in baseline characteristics between groups (Table 1).
Figure 1.
Patient selection.
Note. LOS = length of stay; HD = hemodialysis; ESRD = end-stage renal disease.
Table 1.
Baseline Characteristics.
| Characteristics | LOS < 7 days (n = 46) | LOS ≥ 7 days (n = 44) | P value |
|---|---|---|---|
| Age, y, mean (±SD) | 51 (±11) | 58 (±29) | .090 |
| African American | 41 (89) | 38 (86) | .689 |
| Male | 24 (52) | 18 (41) | .284 |
| Weight, kg, median (25%-75% IQR) | 86 (71-101) | 91 (69-104) | .949 |
| Hypertension | 44 (96) | 40 (91) | .429 |
| Type 2 diabetes mellitus | 21 (46) | 22 (50) | .680 |
| Coronary artery disease | 10 (22) | 10 (23) | .910 |
| Peripheral vascular disease | 3 (7) | 3 (7) | > .999 |
| Human immunodeficiency virus | 4 (9) | 0 | .117 |
| History of transplant | 4 (9) | 3 (7) | > .999 |
| Systemic lupus erythematosus | 5 (11) | 2 (5) | .435 |
| History of cancer | 1 (2) | 2 (5) | .612 |
| History of peritonitis within 12 months | 15 (33) | 12 (27) | .581 |
| Immunosuppression | 6 (13) | 2 (5) | .267 |
| SIRS on admission | 16 (35) | 11 (25) | .311 |
| White blood cell count on admission, mean (±SD) | 11 (±7) | 10 (±7) | .897 |
| Heart rate on admission, mean (±SD) | 92 (±24) | 92 (±20) | .840 |
| Temperature on admission, mean (±SD) | 36.9 (±0.6) | 36.8 (±0.5) | .740 |
| Respiratory rate on admission, mean (±SD) | 18 (±2) | 18 (±3) | .761 |
Note. All data reported as n (%) unless otherwise noted. LOS = length of stay; IQR = interquartile range; SIRS = systemic inflammatory response syndrome.
Factors associated with an increased LOS included ICU admission (P = .014), ID consultation attainment (P = .015), PD catheter removal (P = .001), HD conversion (P < .001), concomitant antifungal therapy (P = .021), and mean number of days with blood glucose >180 mg/dL (0.72 (±1.21) vs 1.61 (±2.43) days; P = .028). However, no differences were noted in LOS between patients with a penicillin allergy, patients who grew a pan-susceptible or multidrug-resistant organism in the dialysis effluent, patients with another positive culture throughout hospitalization, or patients receiving an antimicrobial that required serum drug monitoring who did not have levels ordered (Table 2).
Table 2.
Potential Risk Factors Associated With Increased Hospital Length of Stay.
| Risk factors | LOS < 7 days (n = 46) | LOS ≥ 7 days (n = 44) | P value |
|---|---|---|---|
| ICU admission | 1 (2) | 8 (18) | .014 |
| ID consult | 31 (67) | 39 (89) | .015 |
| Antifungal usage | 4 (9) | 12 (27) | .021 |
| Days with blood glucose >180 mg/dL, mean (±SD) | 0.72 (±1.21) | 1.61 (±2.43) | .028 |
| PD catheter pulled | 7 (15) | 21 (48) | .001 |
| Switched to HD | 8 (17) | 23 (52) | <.001 |
| Penicillin allergy | 7 (15) | 8 (18) | .706 |
| Other positive culture(s) | 9 (20) | 8 (18) | .867 |
| Antimicrobial therapeutic drug monitoring | 30 (65) | 34 (77) | .213 |
| Pan-susceptible organism | 11 (24) | 11 (25) | .905 |
| MDR organism | 4 (9) | 6 (14) | .518 |
Note. All data reported as n (%) unless otherwise noted. LOS = length of stay; ICU = intensive care unit; ID = infectious diseases; PD = peritoneal dialysis; HD = hemodialysis; MDR = multidrug-resistant.
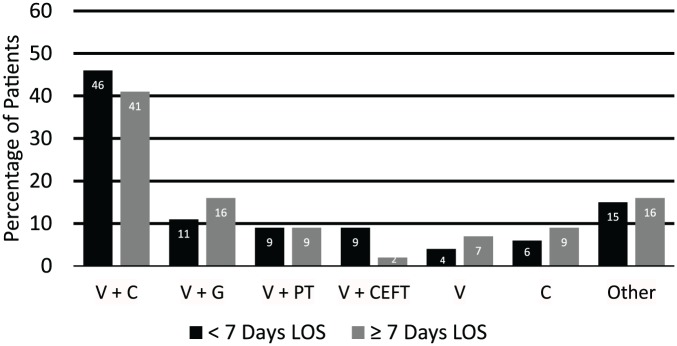
In terms of antimicrobial selection, the majority of patients in the reduced and prolonged LOS groups received ISPD Peritonitis Guideline–based empiric antimicrobial therapy in regard to appropriate organism coverage (87% and 90%, respectively) and had resolution of peritonitis by day 5 (98% in each group). There were no statistically significant differences between initial empiric antimicrobial regimens between the 2 groups (Figure 2).
Figure 2.
Empiric antimicrobial regimens.
Note. No statistically significant differences between the 2 groups. V = vancomycin; C = cefepime; G = gentamicin; PT = piperacillin/tazobactam; CEFT = ceftriaxone.
Effluent microbiology characteristics and causative pathogens are listed in Table 3. The causative pathogens were Gram-positive in 47 patients (52%) and Gram-negative in 18 patients (20%). There were 25 patients (28%) with culture-negative peritonitis and no patients with fungal peritonitis. Although there were no statistically significant differences in baseline pathogens between the groups, there was a trend toward decreased LOS when the baseline pathogen was methicillin-resistant Staphylococcus aureus (MRSA) (15% vs 2%; P = .059) and a trend toward increased LOS when the baseline pathogen was Acinetobacter spp. (0% vs 9%; P = .053).
Table 3.
Effluent Microbiology Characteristics.
| Effluent characteristics | LOS < 7 days (n = 46) | LOS ≥ 7 days (n = 44) | P value |
|---|---|---|---|
| Patients with effluent microorganism growth | 34 (74) | 31 (71) | .714 |
| Gram-positive Organisms | 27 (59) | 20 (46) | .180 |
| MRSA | 7 (15) | 1 (2) | .059 |
| MSSA | 4 (9) | 4 (9) | >.999 |
| MRSE | 6 (13) | 7 (16) | .699 |
| MSSE | 9 (20) | 9 (21) | .916 |
| Streptococcus spp. | 2 (4) | 4 (9) | .429 |
| Enterococcus spp. | 2 (4) | 1 (2) | > .999 |
| Gram-negative Organisms | 7 (15) | 11 (25) | .180 |
| Acinetobacter spp. | 0 | 4 (9) | .053 |
| Citrobacter spp. | 2 (4) | 0 | .495 |
| Other | 9 (20) | 8 (18) | .922 |
Note. All data reported as n (%). LOS = length of stay; MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-susceptible Staphylococcus aureus; MRSE = methicillin-resistant Staphylococcus epidermidis; MSSE = methicillin-susceptible Staphylococcus epidermidis.
The majority of patients in the reduced and prolonged LOS groups initially received antibiotics by the IV route and were subsequently switched to IP antibiotics (54% vs 64%, respectively; P = .371). Of the total population who received IV antibiotics and switched to IP administration, 8 patients (17%) in the reduced LOS group and 6 patients (14%) in the prolonged LOS received only one dose of IV antibiotics (most commonly in the emergency department) prior to their switch to IP administration. Most other patients in our analysis received strictly IV antibiotics (31% vs 36%, respectively; P = .551). A total of 13% of patients in the reduced LOS group and 0% of patients in the prolonged LOS group received strictly IP antibiotics (P = .026) despite the fact that, according to ISPD guidelines, 30 patients (65%) in the reduced LOS group and 33 patients (75%) in the prolonged LOS group were eligible for solely IP antibiotics. In the reduced LOS group, 2% of patients received no antibiotics throughout their hospitalization. Of the available documented information in the electronic medical record, 59% of patients who did not finish their PDAP treatment throughout hospitalization were discharged on IP antibiotics.
Opportunities for antimicrobial de-escalation were identified in 24 (52%) and 22 (50%) patients in the reduced and prolonged LOS groups, respectively; however, de-escalation occurred in only 5 (21%) and 6 (27%) patients throughout hospitalization/discharge. The most common reasons patients were not included in the antimicrobial de-escalation analysis were due to lack of effluent growth or appropriate continuation of antimicrobials.
There were no differences in recurrence, relapse, or refractory episodes; 30-day readmission rates; or in-hospital mortality between the 2 groups.
Discussion
In this 5-year analysis of PD patients admitted for peritonitis, numerous factors correlated with acuity of illness were associated with an increased hospital LOS. There were also opportunities to improve ASP throughout the PDAP population within our health system. These findings are particularly robust, as there is a lack of literature of ASP trends within the PDAP population.
It is expected that patients admitted to the hospital with PDAP should clear their effluent within 5 days, have a timely hospital discharge, and not be admitted to the ICU. It is known that patients who are admitted to the ICU are typically more acutely ill, and numerous studies have shown that increased ICU LOS is linked with higher mortality.7,8 Although not surprising, our study showed that PDAP patients with ICU admission had an overall increased hospital LOS (2% vs 18%; P = .014).
There are several studies that have shown that ID consultation in certain infectious pathologies improves patient outcomes.9-11 In contrast, Hamandi et al showed that ID consultation was associated with an increased LOS; however, this was in solid organ transplant patients.12 In our study, hospital LOS was not decreased in patients with ID consultation (67% vs 89%; P = .015), a finding not previously reported for patients with PDAP.
The 2016 ISPD Peritonitis Guidelines recommend antifungal prophylaxis to prevent fungal peritonitis when patients with PD receive antibiotic courses, as it has been shown that preceding bacterial peritonitis and antibiotic therapy can be associated with fungal peritonitis episodes.3,13-15 In our study, those patients who received antifungal therapy for prophylaxis/treatment of fungal infections throughout their hospitalization had a statistically significant increased LOS (9% vs 27%; P = 0.021). However, it is quite possible that patients with ICU admission, ID consultation, or who received antifungal therapy had a higher acuity of illness and other confounding variables that could have led to their increased LOS.
For patients presenting with PDAP, PD catheter removal and HD conversion throughout their hospitalization and/or as their primary mode of RRT may be clinically indicated. Our study showed that there was an increased LOS in patients who had their PD catheter removed (15% vs 48%; P = .001) and/or switched to HD (17% vs 52%; P < .001). Troidle et al conducted a retrospective analysis evaluating the outcomes of patients on chronic PD who had their catheter removed due to peritonitis and showed that only 20% of patients who had their catheter removed remained on PD 1 year after catheter removal.16 Nearly all of the patients in our study who had their PD catheter removed were switched to HD and had a prolonged LOS. It is likely that many of these patients will remain on HD as suggested by previous studies.
Hyperglycemia can be a common complication of PD due to the utilization of dextrose-containing dialysis effluents.17,18 Extensive data indicate that hyperglycemia throughout hospitalization can lead to increased complications.19-22 Szeto et al showed that new-onset hyperglycemia is common in patients started on PD and that even mild hyperglycemia is associated with increased mortality.18 Our study supports an associated increased LOS with more frequent episodes of hyperglycemia.
Staphylococcus aureus and Staphylococcus epidermidis are the most causative pathogens of PDAP, as PDAP is most often caused by skin flora contamination.4,23 Kofteridis et al found no differences in peritonitis course due to the type of infecting organism, while Bunke et al found that outcomes in non–Pseudomonas spp. Gram-negative pathogens were significantly worse than those compared with Staphylococcal species.2,24 Although there were no differences in LOS between baseline causative pathogens in the present study, the trend toward decreased LOS with MRSA and increased LOS with Acinetobacter spp. should be further evaluated, as earlier studies have shown conflicting results in peritonitis course due to baseline pathogen.2,24,25
Although there were no statistically significant differences between the 2 groups in number of patients with appropriate antimicrobial de-escalation, this study highlights the lack of ASP within the PDAP population. One area for improvement relates to route of antibiotic administration. The 2016 ISPD Peritonitis Guidelines recommend that the preferred route of antibiotic administration is IP in patients who lack systemic signs of sepsis.3 It was hypothesized that most inpatient providers likely lack comfort with IP administration and may be prone to administer antimicrobials by the IV route. It was also hypothesized that if providers did give antibiotics via the IP route, they may be less likely to de-escalate. Based on lack of SIRS criteria on presentation, 65% of patients in the reduced LOS group and 75% of patients in the prolonged LOS group were eligible for IP antibiotics only. Only 13% of patients received antibiotics strictly via the IP route, and these patients were all in the reduced LOS group (P = .026). It is common for patients with PDAP to present to the emergency department, and they will likely receive an initial dose of antibiotics via the IV route. Important to note, however, is that of the 54 patients (60%) in the total population who received IV and IP administration, only 14 patients (16%) received just one dose of IV antibiotics prior to their switch to IP antibiotics. Although some patients did have other suspected/confirmed infection present, the majority of included patients in our study solely had PDAP as their infectious pathology, which indicates that IV therapy was inappropriately continued. This could be due, in part, to the lack of comfort by providers with IP antimicrobial administration, further emphasized by the fact that only 59% of patients were prescribed IP antibiotics on discharge to complete their treatment. It is crucial to re-emphasize that patients who lack systemic signs of infection who are admitted to the hospital should be switched to the IP route, as this approach promotes maximal antimicrobial concentrations at the infection site and could help facilitate discharge. Furthermore, opportunities for antimicrobial de-escalation were missed in nearly 40% of our total patient population, emphasizing opportunities to improve ASP efforts in the PDAP population. Numerous studies have shown hospital LOS can be reduced through ASP efforts.26-28 Promoting IP administration for PDAP and antimicrobial de-escalation are important ASP principles that could be of benefit to patients and health care systems. Antimicrobial stewardship efforts could be improved through development of inpatient PDAP treatment protocols.
Limitations of our study included the retrospective nature of our analysis, that we lacked the ability to account for readmissions to other facilities, and that we did not determine the obtainment of nephrology consultations between the 2 groups. Also, given that hospital LOS defined our 2 groups for comparison, there are confounding variables that could have led to an increased hospital LOS. For example, one confounding variable could have been reason(s) for ICU admission (which was not collected). A standardized acuity or mortality indicator (other than SIRS) could have further classified severity of disease in each group, but this was not collected. With regard to our patient population, it is important to note that patients in our study were included prior to the 2016 ISPD Peritonitis Guideline update, and the definition of PDAP did vary slightly in the previous guidelines; however, we considered the updated definition to be stricter and we included patients who met these criteria, irrespective of the time of diagnosis. It is also possible that antimicrobials were administered in outpatient PD clinics prior to hospitalization, which could have contributed to the culture-negative peritonitis seen in nearly 30% of our sample. Finally, bias could be present in our antimicrobial de-escalation analysis, as antimicrobial de-escalation opportunities were interpreted by the authors.
Despite these limitations, this PDAP study is the only study to our knowledge to more specifically evaluate factors associated with LOS in the inpatient setting, which can add to the paucity of information that is currently available. This study also emphasizes the lack of ASP that occurred in our patient population. Whether this is a more global problem remains to be determined.
Conclusions
Increased hospital LOS was correlated with acuity of illness, but did not seem to be influenced by the choice of empiric antimicrobial therapy or relapse, recurrent, or refractory episodes of peritonitis. This study highlights the fact that ASP efforts to address PDAP in the inpatient setting need to be improved, which could potentially help facilitate discharge.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. United States Renal Data System. 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016. [Google Scholar]
- 2. Kofteridis DP, Valachis A, Perakis K, Maraki S, Daphnis E, Samonis G. Peritoneal dialysis-associated peritonitis: clinical features and predictors of outcome. Int J Infect Dis. 2010;14:e489-e493. [DOI] [PubMed] [Google Scholar]
- 3. Li PK-T, Szeto CC, Piraino B, et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36:481-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akoh JA. Peritoneal dialysis associated infections: an update on diagnosis and management. World J Nephrol. 2012;2:106-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pérez Fontan M, Rodríguez-Carmona A, García-Naveiro R, Rosales M, Villaverde P, Valdés F. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int. 2005;25:274-284. [PubMed] [Google Scholar]
- 6. Boudville N, Johnson DW, Zhao J, et al. Regional variation in the treatment and prevention of peritoneal dialysis-related infections in the Peritoneal Dialysis Outcomes and Practice Patterns Study [published online ahead of print July 23, 2018]. Nephrol Dial Transplant. doi: 10.1093/ndt/gfy204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williams TA, Ho KM, Dobb GJ, et al. Effect of length of stay in intensive care unit on hospital and long-term mortality of critically ill adult patients. Br J Anaesth. 2010;104:459-464. [DOI] [PubMed] [Google Scholar]
- 8. Iwashyna TJ, Kramer AA, Kahn JM. Intensive care unit occupancy and patient outcomes. Crit Care Med. 2009;37:1545-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burnham JP, Olsen MA, Stwalley D, Kwon JH, Babcock HM, Kollef MH. Infectious diseases consultation reduces 30-day and 1-year all-cause mortality for multidrug-resistant organism infections. Open Forum Infect Dis. 2018;5(3):ofy026. doi: 10.1093/ofid/ofy026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bai AD, Showler A, Burry L, et al. Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis. 2015;60:1451-1461. [DOI] [PubMed] [Google Scholar]
- 11. Spec A, Olsen MA, Raval K, Powderly WG. Impact of infectious diseases consultation on mortality of Cryptococcal infection in patients without HIV. Clin Infect Dis. 2017;64:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamandi B, Husain S, Humar A, Papadimitropoulos EA. Impact of infectious disease consultation on the clinical and economic outcomes of solid organ transplant recipients admitted for infectious complications. Clin Infect Dis. 2014;59:1074-1082. [DOI] [PubMed] [Google Scholar]
- 13. Prasad KN, Prasad N, Gupta A, Sharma RK, Verma AK, Ayyagari A. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis: a single centre Indian experience. J Infect. 2004;48:96-101. [DOI] [PubMed] [Google Scholar]
- 14. Wang AY, Yu AW, Li PK, et al. Factors predicting outcome of fungal peritonitis in peritoneal dialysis: analysis of a 9-year experience of fungal peritonitis in a single center. Am J Kidney Dis. 2000;36:1183-1192. [DOI] [PubMed] [Google Scholar]
- 15. Goldie SJ, Kiernan-Troidle L, Torres C, et al. Fungal peritonitis in a large chronic peritoneal dialysis population: a report of 55 episodes. Am J Kidney Dis. 1996;28:86-91. [DOI] [PubMed] [Google Scholar]
- 16. Troidle L, Gorban-Brennan N, Finkelstein FO. Outcome of patients on chronic peritoneal dialysis undergoing peritoneal catheter removal because of peritonitis. Adv Perit Dial. 2005;21:98-101. [PubMed] [Google Scholar]
- 17. McCormick BB, Bargman JM. Noninfectious complications of peritoneal dialysis: implications for patient and technique survival. J Am Soc Nephrol. 2007;18:3023-3025. [DOI] [PubMed] [Google Scholar]
- 18. Szeto CC, Chow KM, Kwan BC, Chung KY, Leung CB, Li PK. New-onset hyperglycemia in nondiabetic Chinese patients started on peritoneal dialysis. Am J Kidney Dis. 2007;49:524-532. [DOI] [PubMed] [Google Scholar]
- 19. The NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283-1297. [DOI] [PubMed] [Google Scholar]
- 20. Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471-1478. [DOI] [PubMed] [Google Scholar]
- 21. Montori VM, Bistrian BR, McMahon MM. Hyperglycemia in acutely ill patients. JAMA. 2002;288:2167-2169. [DOI] [PubMed] [Google Scholar]
- 22. Kotagal M, Symons RG, Hirsch IB, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015;261:97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montelli AC, Sadatsune T, Mondelli AL, et al. Frequency and antimicrobial susceptibility of bacterial agents causing peritoneal dialysis-peritonitis in a Brazilian single center over 20 years. Cogent Med. 2016;3:1242246. doi: 10.1080/2331205X.2016.1242246. [DOI] [Google Scholar]
- 24. Bunke CM, Brier ME, Golper TA. Outcomes of single organism peritonitis in peritoneal dialysis: gram negatives versus gram positives in the Network 9 Peritonitis Study. Kidney Int. 1997;52:524-529. [DOI] [PubMed] [Google Scholar]
- 25. Troidle L, Gorban-Brennan N, Kliger A, Finkelstein F. Differing outcomes of gram-positive and gram-negative peritonitis. Am J Kidney Dis. 1998;32:623-628. [DOI] [PubMed] [Google Scholar]
- 26. Karanika S, Paudel S, Grigoras C, Kalbasi A, Mylonakis E. Systematic review and meta-analysis of clinical and economic outcomes from the implementation of hospital-based antimicrobial stewardship programs. Antimicrob Agents Chemother. 2016;60:4840-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Güerri-Fernández R, Villar-García J, Herrera-Fernández S, et al. An antimicrobial stewardship program reduces antimicrobial therapy duration and hospital stay in surgical wards. Rev Esp Quimioter. 2016;29:119-121. [PubMed] [Google Scholar]
- 28. Pogue JM, Mynatt RP, Marchaim D, et al. Automated alerts coupled with antimicrobial stewardship intervention lead to decreases in length of stay in patients with gram-negative bacteremia. Infect Control Hosp Epidemiol. 2014;35:132-138. [DOI] [PubMed] [Google Scholar]