Abstract
Introduction: Due to the renal handling mechanism of magnesium, prolonging the time for infusion of intravenous (IV) magnesium has been postulated to decrease magnesium requirements; however, a paucity of clinical evidence exists to support prolonging infusion rates. Objective: To assess if there is a difference in magnesium replacement required in the medicine population at an academic medical center when prolonged infusion rates (0.5 g/h) are compared to short infusion rates of > 0.5 g/h. Methods: A retrospective chart review was performed before and after implementation of the hypomagnesemia protocol (November 2015). Patients who received at least one dose of IV magnesium during hospitalization were selected from general medicine units. Primary aim was to determine if a difference exists in percent of days IV magnesium repletion required between patients receiving prolonged versus short infusion rates. Secondary objectives were to determine if a difference exists in total grams of magnesium received, percent of days magnesium levels were maintained in the optimal (1.4-2.7) and desired (2-2.7) therapeutic ranges, and incidence of hypomagnesemia (< 1.4 g/dL) and hypermagnesemia (> 2.7 g/dL). For safety, incidence of hypotension (systolic blood pressure < 90/60 mm Hg) during the magnesium infusion was recorded. Results: Totally, 45 patients were included in each cohort for a total of 90 patients to meet power. No differences existed between protocol groups for any demographic variables (all P > .05). Median infusion rate for the short infusion cohort was 1.8 g/h (range 1-2 g/h). Percent of days IV magnesium was replaced was 34.8% versus 37.8% (P = .39) in the short and prolonged infusion groups, respectively. No difference existed between groups for secondary outcomes (all P > .05). Conclusion: Prolonged magnesium infusion rates did not decrease magnesium replacement requirements. These results have been used to propose revision of our current magnesium infusion protocol to reduce infusion length.
Keywords: intravenous therapy, fluid and electrolyte disorders, residency training, programs, formulary management, P & T
Introduction
Magnesium is an intracellular cation important for the regulation of bodily functions. It is involved in multiple enzymatic reactions, energy production, bone mineralization, muscular relaxation, and neurotransmission.1 Hypomagnesemia (magnesium level < 1.4 mg/dL) has been noted in up to 12% of hospitalized patients and the incidence may rise above 60% in patients in the intensive care settings (ICU).2 Magnesium deficiency can produce a variety of clinical manifestations, including positive Chvostek’s and Trousseau’s sign, seizures, muscle cramps, vertigo, nystagmus, and/or psychiatric manifestations. In addition, cardiac arrhythmias, such as supraventricular tachycardia and torsades, can occur.3
Magnesium balance in the body mainly takes places through the gastrointestinal tract and the kidneys. On average 25 mEq of magnesium is ingested in a daily balanced diet, of which one-third is eliminated in the urine.1 Gastrointestinal losses of magnesium mainly occur due to diarrheal malabsorption states, malnutrition, and inflammatory bowel diseases.4 Kidneys also play a vital role in magnesium balance in the body. Approximately 75% of the total plasma magnesium is filtered through the glomerular membrane.5 In contrast to Na+ and Ca2+, only 15% of the filtered magnesium is reabsorbed in the proximal tubules, most in the thick ascending loop of Henle.5 In patients with normal renal function, approximately 50% of the administered dose is retained by the body. Several drugs have also been associated with urinary magnesium wasting, including aminoglycosides, amphotericin B, foscarnet, cisplatin, cyclosporine A, tacrolimus, digoxin, loop diuretics, and pentamidine.3
Administering magnesium at a prolonged infusion rate has been postulated to decrease magnesium excretion because of the Ca2+/Mg2+ sensing receptor, located on the capillary side of the thick-ascending-limb cells, sense changes in magnesium levels. In hypermagnesemic states, achieved due to rapid infusion, the regulator receptors can inhibit magnesium reabsorption from the loop transport.4 Therefore, some institutions like ours implemented protocols to prolong intravenous magnesium infusion rates. However, a paucity of data exists to support the benefits of this practice on clinically relevant patient outcomes.
The historical standard of practice at our institution was to infuse IV magnesium at a rate of 1-2 g/h. In November 2015, the hospital-wide protocol was changed to administer IV magnesium at a prolonged rate of 0.5 g/h regardless of total dose (i.e. 2 g over 4 hours, 8 g over 16 hours, etc.). The dose and level at which to replete is based on the providers’ discretion. The basis for this change was the hypothesis that infusing magnesium at a slower rate may increase magnesium retention as described above.
The purpose of this study was to determine if our current protocol of prolonged infusion of IV magnesium shows a difference in maintaining therapeutic magnesium levels safely in our general medicine population as compared to short infusion rates.
Materials and Methods
Study Design
A single center, retrospective, local institutional review board approved observational cohort study was conducted on patients in the general medicine units who received intravenous magnesium for repletion during their hospitalization. A report of hospitalized patients who received intravenous magnesium before and after protocol implementation (November 2015) was generated and divided into two cohorts based on the time period patients received IV magnesium. Patients in the short cohort (October 2014—October 2015) received magnesium up to a year before protocol implementation at an infusion rate of > 0.5 g/h (~1-2 g/h) while those in the prolonged cohort (December 2015—December 2017) received magnesium after protocol implementation at a standard rate of 0.5 g/h.
Patients in each cohort were screened sequentially for eligibility and excluded if any of the exclusion criteria were met: history of solid organ transplant (SOT), history of hematopoietic cell transplant (HCT), patients receiving total parenteral nutrition (TPN) or magnesium in IV fluids, pregnancy, acute cardiac conditions (atrial fibrillation, acute electrocardiogram (EKG) changes, ST-elevation myocardial infarction (STEMI), Non-ST-elevation myocardial infarction (NSTEMI), acute exacerbation of heart failure), creatinine clearance of < 30 mL/min or receiving dialysis, patients on highly magnesium wasting drugs (tacrolimus, cyclosporine, cisplatin, amphotericin B, or foscarnet), patients without a post-infusion level of magnesium (within 24 hours of magnesium infusion), and patients < 18 years old.
The following demographic data were collected: age, gender, ethnicity, medical service/unit, receipt of oral magnesium and number of days of oral magnesium repletion, magnesium level (within 24 hours) before and after each dose of IV magnesium, total grams of IV magnesium replaced during hospitalization, number of days IV magnesium replacement was needed, total length of stay from first dose of IV magnesium (censored at 30 days length of stay), as well as blood pressure during magnesium infusion to assess for safety.
The primary outcome was percent of days requiring magnesium replacement out of the magnesium length of stay. Magnesium length of stay was defined as number of days from first dose of intravenous magnesium to discharge or day 30, whichever came earlier. Secondary efficacy outcomes included; percent of days magnesium levels were in the optimal and desired therapeutic ranges, total grams of magnesium received, and incidence of hypermagnesemia or hypomagnesemia. Optimal therapeutic range (1.4-2.7 mg/dL) was defined as nonsub/supra therapeutic level while desired therapeutic range was defined as the magnesium level usually in the range of 2-2.7 mg/dL the physician chose to replete to, based on physician discretion since there are no guidelines for magnesium replacement. In order to assess for patient safety, the incidence of hypotension was assessed. Hypotension was defined as blood pressure values less than 90/60 mm Hg, and was assessed as having any hypotensive episode during infusion of magnesium as reported in the vital signs of the electronic medical record.
Statistical Analysis
All statistical analysis was performed using SAS 9.4 and statistical significance was assessed using an alpha level of 0.05. Chi-square or two-sample t tests were used to examine preliminary differences between the short and prolonged infusion groups for nominal and continuous data, respectively. To examine differences in percent of days of magnesium replacement, total grams of magnesium received, or percent of days patients maintained optimal magnesium levels, analysis of covariance was used where the covariate was the percent of days of oral magnesium administration during the patient’s length of stay. Also, t tests were used to examine difference in the percent of days of hypomagnesemia (< 1.4 g/dL) and hypermagnesemia (> 2.7 g/dL) between infusion groups. A Fisher’s exact test was used to examine differences in the incidence of hypotension in the two infusion groups, due to the low frequency of occurrence.
The sample size was determined for the primary outcome of interest, percent of days of magnesium replacement. Due to scarcity of prior literature, the mean event rates in the short infusion group and prolonged infusion group were assumed based on investigators opinions and replacement seen in practice. Using a two-sample t test with a mean in the short infusion group of 30%, an alpha level of 0.05, and power of 80%, the sample size was determined for a mean 15% in the prolonged infusion group and standard deviations of 25%. A sample size of 45 per group (for a total of 90 patients) was calculated to ensure adequate power for the assumed difference in percent days of magnesium replacement.
Results
Demographics
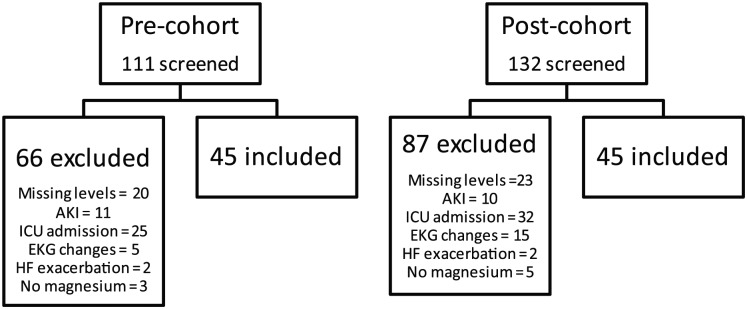
About 45 patients were included in each cohort for a total sample size of 90 patients (Figure 1). Baseline demographic data were similar between the two cohorts (Table 1). Overall the average age of study participants was 57 (±14) and 61 years (±13), 64% and 51% were females, and 49% and 56% were Caucasian in the short and prolonged cohorts, respectively. The magnesium length of stay was similar between the two groups: 6.4 (±6.1) versus 4.4 (±3.2) days in the short versus prolonged cohorts, respectively.
Figure 1.
Inclusion and exclusion.
Note. AKI = acute kidney injury; ICU = intensive care unit; EKG = electrocardiogram; HF = heart failure.
Table 1.
Demographics.
| Variable | Short | Prolonged | P value |
|---|---|---|---|
| Gender, N (%) | |||
| Female | 29 (64.4) | 23 (51.1) | .2 |
| Male | 16 (35.6) | 22 (48.9) | |
| Race, N (%) | |||
| African American | 22 (48.9) | 19 (42.2) | .83 |
| Asian | 1 (2.2) | 1 (2.2) | |
| Caucasian | 22 (48.9) | 25 (55.6) | |
| Service, N (%) | |||
| Surgery | 13 (28.9) | 13 (28.9) | .25 |
| Medicine | 12 (26.6) | 10 (24.4) | |
| Oncology | 11 (24.4) | 11 (24.4) | |
| Cardiology | 4 (8.9) | 2 (4.4) | |
| Hospitalist | 3 (6.7) | 1 (2.2) | |
| Other | 2 (4.4) | 7 (15.6) | |
| Received oral magnesium, N (%) | 11 (24.4) | 9 (20.0) | .61 |
| Magnesium length of stay, days (median, IQ range) | 6.4 (6.1) | 4.4 (3.2) | .05 |
Note. IQ = interquartile range.
Clinical Outcomes
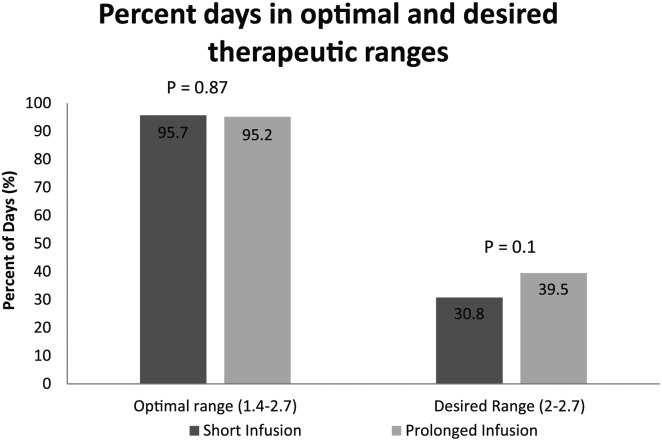
Patients in the short infusion group received intravenous magnesium at an average rate of 1.8 g/h (± 0.4 g/h). No significant difference was seen in the primary outcome of percent days of magnesium replacement in short versus prolonged cohorts. Magnesium infusion was required 34.8% of days in the short cohort compared to 37.5% of days in the prolonged cohort (P = .39). Majority of patients in both the short and prolonged cohorts were able to maintain therapeutic levels in the optimal range (~95% of days each, P = .87). No difference existed in percent of days patients maintained magnesium in optimal or desired therapeutic range (30.8% versus 39.5%, P = .1) (Figure 2).
Figure 2.
Percent days in therapeutic range.
Average total grams of IV magnesium administered during hospitalization was numerically lower for the prolonged versus short infusion groups; however, this difference did not reach statistical significance (3.5 g versus 4.8 g; P = .19). Hypomagnesemia (magnesium level < 1.4 g/dL) was observed in two out of 45 patients (4.4%) in the short infusion group compared to one out of 45 patients (2.2%) in the prolonged infusion group. The two patients in the short infusion group were hypomagnesemic for two out of 21 days and one out of 6 days respectively. The one patient in the in the prolonged infusion group was hypomagnesemic for one out of 6 days from first dose of IV magnesium. With regard to hypermagnesemia, three out of 45 patients (6.6%) in the short infusion group were hypermagnesemic (> 2.7 g/dL). One of these 3 patients was hypermagnesemic for 2 days whereas two patients were hypermagnesemic for one day. One out of 45 patients (2.2%) in the prolonged infusion group was hypermagnesemic for one day (Table 2).
Table 2.
Incidence of Hypomagnesemia and Hypermagnesemia.
| Short infusion (>0.5 g/h) | Prolonged infusion (0.5 g/h) | P value | |
|---|---|---|---|
| Mean percent days hypomagnesemic, (SD) | 0.58 (2.83) | 0.37 (2.48) | .71 |
| Mean percent days hypermagnesemic, (SD) | 0.74 (3.3) | 0.32 (2.13) | .47 |
For safety analysis assessing hypotension, five patients (11%) in the short infusion group had at least one episode of hypotension compared to none (0%) in the prolonged infusion group (P = 0.06). One patient had 3 episodes of hypotension, the first episode was during the 3rd infusion of magnesium given at a rate of 2 g/h, the 2nd episode was during 4th infusion of magnesium at a rate of 2 g/h, and the 3rd episode was during 9th infusion (total 8 g) of magnesium at a rate of 2 g/h. The four other patients had one episode of hypotension, where three out of the four patients received magnesium at a rate of 2 g/h and one received it at 1 g/h. Notably, review of medical records did not report an intervention for hypotension in the instances and two of these patients were hypotensive prior to and/or after magnesium infusion time.
Discussion
As of this time, no published studies can be found in which short infusion rates have been compared to prolonged infusion rates for clinical outcomes in hospitalized medicine patients. However, studies done in the hematopoietic stem cell transplant (HCT) population have revealed no difference in number of days IV magnesium replacement was needed between HCT patients receiving short infusion rates versus prolonged infusion or in grams of magnesium replaced.6,7 Our report represents the first adequately powered study to compare infusions rates of IV magnesium in the general medicine patient population, finding no difference in the number of days requiring magnesium replacement out of the magnesium length of stay. Furthermore, results here in showed no difference in percent of days therapeutic levels were maintained in the optimal (1.4-2.7) or desired therapeutic ranges (2-2.7). Optimal and desired therapeutic ranges were assessed independently due to differing physician repletion practices and goals. Range of 1.4 to 2.7 (optimal) is reported as the normal laboratory value of magnesium; however, often in practice individual goals of 2 to 2.7 (desired) have been targeted. Most if not all patients will have magnesium replaced at the low levels of 1.4 to 1.7; however, only few patients may get magnesium replacement at levels of 1.7 to 1.9, depending on physician practice, thus patients maintaining ranges of 2 to 2.7 in fewer percent of days.
A limitation of this study is the retrospective study design. Accuracy of patient screening for inclusion and exclusion rely on accuracy of documentation in the electronic medical record. Similarly, retrospective methods assume administration of magnesium at the rate recorded in the medication administration record accurately reflects actual administration rate/time. In addition, at what threshold to initiate IV magnesium repletion is not standardized but is physician specific based on patient response to prior doses and goal magnesium level (i.e. > 1.4 mg/dL or > 2 mg/dL). Therefore, this likely affected the number of days of magnesium repletion between patients. However, this variability would be present in both short and prolonged cohorts. Another limitation is differing total lengths of stay; however, this was accounted for and minimized by assessing magnesium length of stay, which was similar between the two groups. With respect to safety analysis, a limitation of retrospective design is the inability to correlate hypotension with magnesium infusion, as the overall clinical status at the time is not taken into account and multiple cofounders exist that could cause hypotension which could not be excluded without affecting the generalizability of the study.
Prolonging magnesium infusion rates to a standard of 0.5 g/h has a major disadvantage of increasing the length of time magnesium is infused. This is a logistical concern as many hospitalized patients have only one IV access. Considering incompatibilities among various IV products which cannot be infused at same time it is conceivable the prolonged rate of IV magnesium infusion leads to interruption of other IV medication schedules, necessity to place additional IV access, or delays in receipt of other IV medications which are incompatible. Based on the results of this study, the hospital-wide protocol to administer IV magnesium at a prolonged rate of 0.5 g/h could have increased logistical burden of IV administration due to limited IV access, without having clinically meaningful impact to patient care like decreasing percent of days of magnesium infusion required.
In conclusion, in the general medicine patient population prolonging the rate of magnesium infusion to 0.5 g/h did not decrease magnesium requirements when compared to short infusion rates.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Shaily Doshi  https://orcid.org/0000-0001-7950-5228
https://orcid.org/0000-0001-7950-5228
References
- 1. Martin KJ, González EA, Slatopolsky E. Clinical consequences and management of hypomagnesemia. J Am Soc Nephrol. 2009;20:2291-2295. [DOI] [PubMed] [Google Scholar]
- 2. Mclean RM. Magnesium and its therapeutic uses: a review. Am J Med. 1994;96:63-76. [DOI] [PubMed] [Google Scholar]
- 3. Topf JM, Murray PT. Hypomagnesemia and hypermagnesemia. Rev Endocr Metab Disord. 2003;4:195-206. [DOI] [PubMed] [Google Scholar]
- 4. Weisinger JR, Bellorín-Font E. Magnesium and phosphorus. Lancet. 1998;352:391-396. [DOI] [PubMed] [Google Scholar]
- 5. Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000;294:1-26. [DOI] [PubMed] [Google Scholar]
- 6. Ku PM, Waller JL, Sportès C, Clemmons AB. Prolonged versus short infusion rates for intravenous magnesium sulfate administration in hematopoietic cell transplant patients. Support Care Cancer. 2018;26:2809-2814. [DOI] [PubMed] [Google Scholar]
- 7. Snyder M, Shillingburg A, Newton M, et al. Impact of intravenous magnesium infusion rate during ambulatory replacements on serum magnesium concentrations after allogeneic stem cell transplant. Support Care Cancer. 2016;24:4237-4240. [DOI] [PubMed] [Google Scholar]