Abstract
Eighteen cases of chikungunya virus infection in travellers returning from Myanmar were reported to the GeoSentinel Surveillance Network, its subnetwork EuroTravNet and TropNet in 2019, reflecting an ongoing local outbreak. This report reinforces the importance of travellers as sentinels of emerging arboviral outbreaks and highlights the importance of vigilance for imported cases, due to the potential for dissemination of the virus into areas with competent local vectors and conducive environmental conditions.
Keywords: Chikungunya virus, Outbreak, travel medicine, surveillance
In early October 2019, a GeoSentinel Surveillance Network site in Madrid, Spain, identified two patients with chikungunya virus (CHIKV) infection who had recently visited Myanmar. Rapid outreach to GeoSentinel sites (including EuroTravNet), TropNet and Laos external collaborators identified 16 additional infected travellers who acquired CHIKV infection in Myanmar during 2019.
According to a newspaper report, the Myanmar Department of Public Health identified an outbreak of CHIKV infection in 2019, mainly in Nay Pyi Taw, Kachin State, and Tanintharyi Region, Myanmar [1]. In the previous 8 years however, no cases of CHIKV infection had been officially reported in the country [2,3].
We describe travellers with imported CHIKV infection from Myanmar who were diagnosed in 2019 (January to November) and identified at GeoSentinel, EuroTravNet and TropNet sites. Some epidemiological and clinical information as well as places visited by the travellers are presented.
Case finding
GeoSentinel is a global surveillance network for emerging infectious diseases that has 68 sites in 28 countries; EuroTravNet is its European subnetwork; TropNet represents a separate European surveillance entity represented by 75 specialised tropical medicine centres in Europe.
For chikungunya surveillance, GeoSentinel follows case definitions proposed by the World Health Organization (WHO) Regional Office for Southeast Asia [4]. Such definitions were used in the current report. A possible case was a patient with acute onset of fever >38.5°C and severe arthralgia/arthritis not explained by other medical conditions. A probable case was a patient meeting both the previously mentioned clinical criteria and the following epidemiological criteria: residing or having visited epidemic areas, having reported transmission within 15 days prior to the onset of symptoms. A confirmed case had to meet one or more of the following laboratory criteria, irrespective of the clinical presentation: (i) virus isolation in cell culture or animal inoculations from acute phase sera, or (ii) presence of viral RNA in acute phase sera as demonstrated by RT-PCR, or (iii) presence of virus-specific IgM antibodies in single serum sample in acute or convalescent stage, or (iv) fourfold increase in virus-specific IgG antibody titre in samples collected at least 3 weeks apart.
For this study, cases were excluded if they were not in Myanmar at the likely time of exposure, which was inferred from their date of symptom onset and the typical incubation period (defined as 3 to 7 days) [5] or if the travellers had more than one potential travel destination exposure based on the incubation period.
Description of cases
Epidemiological and clinical details for each patient were collected from reporting sites.
The 18 cases reported in the current study had a median age of 51 years (range: 19–68 years) and 10 were male (Table 1). Among the overall cases, one had visited friends and relatives in Myanmar at the likely time of exposure, two were there on a business trip and three were expatriates. The rest of the cases, which represented the majority (n = 12), were tourists who had experienced a median length of stay of 13 days in Myanmar (range: 6–65 days).
Table 1. Epidemiological and travel characteristics of confirmed and probable chikungunya cases among travellers returning from Myanmar, 2019 (n = 18).
| Case | Reporting country | Places visited in Myanmar | Pre-travel consultation | Period of stay in Myanmar (length of exposure period) |
Approximate age in yearsa | Underlying medical condition(s) | Month of symptom onset |
|---|---|---|---|---|---|---|---|
| 1 | Japan | Yangon | Yes | Apr 2017 to Jul 2019 (NA) |
60 | Hypertension | Jul 2019 |
| 2 | Japan | Yangon, Naypyidaw | No | Aug 2019 (6 days) |
25 | Atopic dermatitis | Aug 2019 |
| 3 | France | Shan state, Bagan, Yangon | Yes | Jul 2019 (12 days) |
45 | No | Aug 2019 |
| 4 | Italy | Nyaungshwe | Yes | Lived in Myamnar until Aug 2019 (NA) |
60 | No | Aug 2019 |
| 5 | Japan | Yangon | Yes | Aug 2018 to Aug 2019 (NA) |
70 | Hyperlipidaemia, hypertension, diabetes mellitus | Aug 2019 |
| 6 | France | Yangon, Hpa-An, Moulmein, Ye, Mandalay, Bagan, Inle lake, Pindaya | Yes | Jul to Aug 2019 (23 days) |
45 | No | Aug 2019 |
| 7 | United States | Ye, (Mon State), Yangon | Unknown | May to Aug 2019 (65 days) |
20 | No | Jul 2019 |
| 8 | Spain | Yangon, Mandalay, Inle Lake | No | Aug 2019 (7 days) |
30 | No | Aug 2019 |
| 9 | Spain | Yangon Mandalay, Inle Lake, Bagan | No | Aug 2019 (14 days) |
55 | No | Aug 2019 |
| 10 | Italy | Yangon, Mandalay, Inle Lake, | No | Aug to Sep 2019 (12 days) |
50 | No | Sep 2019 |
| 11 | Italy | Yangon, Mandalay, Inle Lake | No | Aug to Sep 2019 (12 days) |
50 | No | Sep 2019 |
| 12 | Laos | Mandalay | No | Sep 2019 (12 days) |
50 | No | Sep 2019 |
| 13 | Germany | Yangon, Inle Lake, Bagan, Mandalay | Yes | Jan 2019 (15 days) |
65 | Mitral valve disease | Feb 2019 |
| 14 | Germany | Yangon, Inle Lake, Bagan, Mandalay | Yes | Jan 2019 (15 days) |
30 | No | Feb 2019 |
| 15 | Italy | Yangon, Bagan, Mandalay | No | Jul to Aug 2019 (12 days) |
65 | Hypertension | Jul 2019 |
| 16 | Italy | Rangoon, Bagan | No | Aug 2019 (10 days) |
55 | No | Aug 2019 |
| 17 | Germany | Yangon, Inle Lake, Mandalay, Bagan, Thandwe/West Coast | No | Oct 2019 (16 days) |
25 | No | Oct 2019 |
| 18 | France | Yangon, Mandalay, Bagan, Inle Lake | Unknown | Oct to Nov 2019 (15 days) |
55 | No | Oct 2019 |
F: female; Ig: immunoglobulin; M: male; NA: not assessed; VFR: visiting friends and relatives.
a Age rounded by five.
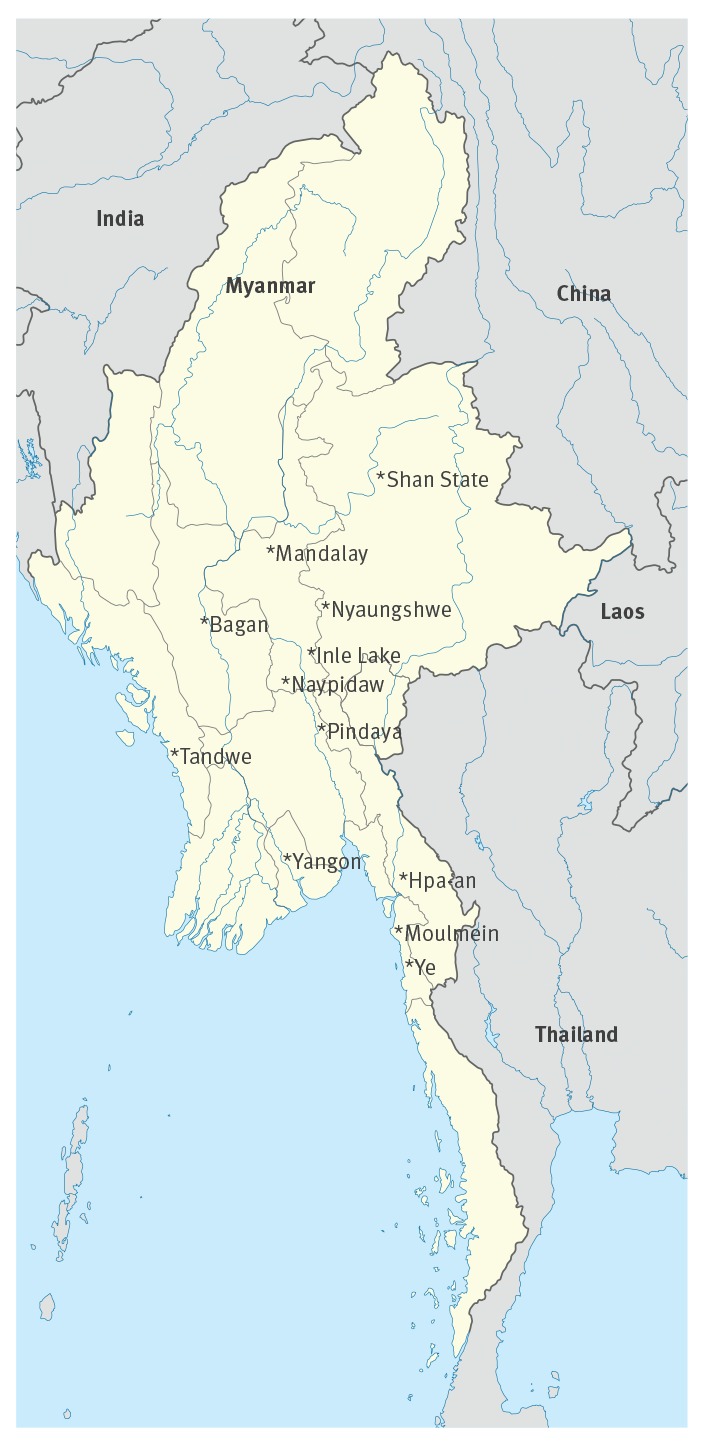
The majority of cases were imported to Europe (n = 13) and Asia (n = 4), with one case in the Americas. Fewer than half (7/16) of travellers with available information, received pre-travel advice. Destinations visited in Myanmar are shown in the Figure.
Figure.
Possible places of exposure: destinations visited by travellers before chikungunya virus infection, Myanmar, 2019 (n = 18)
Asterisks represent the places where travellers in the current study were possibly exposed to chikungunya virus. Some travellers visited the same destinations.
Source: Map adapted from Wikimedia Commons, the free media repository: https://commons.wikimedia.org/wiki/File:Myanmar_location_map.svg
Among the 18 cases, acute symptoms reported included fever (n = 18), arthralgia (n = 16) and rash (n = 14) (Table 2). A median of 9.5 days (range: 1–240 days) elapsed between symptom onset and diagnosis. Most travellers (n = 13) were identified at reporting sites between August and October 2019. Seven cases were confirmed by PCR, including five who were also found IgM positive, and nine cases were confirmed by a positive IgM only; the remaining two patients had probable diagnoses based on a single IgG positive serology. No acute coinfections with other arboviruses were described in the 16 patients tested for additional pathogens.
Table 2. Clinical and diagnostic characteristics of confirmed and probable chikungunya cases among returning travellers from Myanmar, 2019 (n = 18).
| Case | Clinical acute symptoms | Outcome | Need of hospitalisation (country) | Clinical status as at 20 Oct 2019 | Chikungunya diagnostic method | Day of diagnosis in 2019 (time from onset of illness) |
Other arboviral diagnostic test results |
|---|---|---|---|---|---|---|---|
| 1 | Fever, rash, arthralgias (fingers, wrists, ankles, knees) | Persistent arthralgias needing NSAIDs | No | Unknown | IgM+ (N Health Myanmar Co., Ltd, Yangon, Myanmar) | 08 Jul (6 days) |
Dengue: IgM−/IgG−; NS1− Zika: NS1− |
| 2 | Fever, rash, arthralgia (wrists, ankles), retro-orbital pain, conjunctival hyperaemia | Persistent arthralgias needing NSAIDs | No | Improvement of arthralgias | IgM+; PCR+ (NovaLisa, NovaTec, Dietzenbach, Germany) | 13 Aug (6 days) |
Dengue: PCR−; IgM− Zika: PCR−; IgM− |
| 3 | Fever, rash, arthralgia, myalgia, conjunctivitis | Persistent incapacitating arthralgias needing NSAIDs | No | Mild and improving arthralgia, no more NSAIDs needed | RT-PCR+ (RealStar chikungunya RT-PCR, Altona diagnostics, Hamburg, Germany) | 14 Aug (1 day) |
Dengue: PCR− Zika: PCR− |
| 4 | Fever, arthralgia, deep asthenia, profuse sweating | Persistent arthralgias needing NSAIDs and CT (prednisone) | Yes (Italy) | Unknown | IgM+/IgG+ (Euroimmun, Luebeck, Germany) | 14 Aug (9 days) |
Unknown |
| 5 | Fever, rash, arthralgia (fingers, wrists, knees), ankle swelling, conjunctival hyperaemia | Persistent arthralgias needing NSAIDs | Yes (United States) | Improving existing arthritis and arthralgia | IgM+/IgG+ (NovaLisa, NovaTec ) | 21 Aug (20 days) |
Dengue: IgM−/IgG− Zika: IgM− |
| 6 | Fever, ankles oedema | Persistent and recurrent arthralgias needing NSAIDs; fatigue, headache | No | Persisting moderate arthralgia, still needing NSAIDs | IgM+/IgG+ (Euroimmun) | 03 Sep (12 days) |
Dengue: IgM− Zika: IgM− |
| 7 | Fever, rash, chills, myalgias, symmetric arthralgias (knees, ankles, wrists, hands) | Improving with NSAIDs | No | Improving | IgM+/IgG+ (Arup Laboratories, Utah, United States) | 04 Sep (41 days) |
Not tested |
| 8 | Fever, rash, arthralgias (ankles, wrists, feet, interphalangeal joints in hands, bilateral) | Persistent arthralgias needing NSAIDs and CT | No | Improved | IgM+ (Euroimmun) | 09 Sep (10 days) |
Dengue: IgM−/IgG− Zika: IgM ambiguous/IgG− |
| 9 | Fever, rash, arthralgia | Persistent arthralgias needing NSAIDs and CT | No | Improved | IgM+ (Chemiluminescence Virclia UNILABS, Madrid, Spain) | 13 Sep (21 days) |
Dengue: IgM−/ IgG− Zika: IgM−/ IgG− |
| 10 | Fever, rash, arthralgia, diarrhoea, paraesthesia lower limbs, swelling upper and lower limbs, lymphadenopathy | Persistent arthralgias needing NSAIDs | No | Persistent arthralgia | IgM+/IgG−; PCR+ (ChLIA Alifax, Polverada, Italy) | 13 Sep (7 days) |
Dengue: RDT− Zika: PCR−; IgM−/ IgG− |
| 11 | Fever, rash, arthralgia, diarrhoea | Persistent arthralgias needing NSAID | No | Persistent arthralgia | IgM+/IgG-; PCR+ (ChLIA Alifax) | 13 Sep (6 days) |
Dengue: RDT− Zika: PCR−; IgM−/IgG− |
| 12 | Fever, pruritus, rash, head and limbs felt swollen | Persistent arthralgias needing paracetamol | No | Little light headed when exercise | RT-PCR+ (in house RT-PCR: [24]) | 23 Sep (4 days) |
Dengue NS1−; RT−PCR− Zika: not tested |
| 13 | Fever, nausea, arthralgias, ankle oedema | Persistent incapacitating arthralgias and ankle oedema needing NSAIDs and CT | No | Persistent incapacitating arthralgias and ankle oedema needing NSAIDs and corticosteroids | IgG+ (Euroimmun) | 07 Oct (8 months) |
Dengue: NS1−; IgM−, IgG+ (multiple exposures before, IIFT+ with low titre) Zika: not tested |
| 14 | Fever, rash, arthralgia | Not needing any treatment | No | Mild ankles arthralgias | IgG+ (Euroimmun) | 07 Oct (8 months) |
Dengue: NS1−; IgM−/IgG– Zika: not tested |
| 15 | Fever, rash, arthralgias (wrists, right elbow, metacarpophalangeal joints), general malaise and nausea | Persistent arthralgias needing NSAIDs | Yes (Myanmar) | Improve | IgM+/IgG+ (NovaLisa, NovaTec) | 14 Oct (74 days) |
Dengue: NS1− Zika: not tested |
| 16 | Fever, rash, arthralgia | Persistent arthralgias needing NSAIDs | No | Persistent arthralgias | IgM+/IgG+ (Euroimmun) | 16 Oct (60 days) |
Not tested |
| 17 | Fever, rash, arthralgias (hands, ankles) | No drugs needed | No but presented to local outpatient clinic where no testing for CHIK was done. | Mild arthralgias in fingers and ankles | RT-PCR+; IgM+/IgG– (Fast Track Diagnostics. Ltd, Esch-sur-Alzette, Luxembourg) | 21 Oct (7 days) |
Zika: PCR–; IgM–/IgG– Dengue: PCR−; NS1– |
| 18 | Fever, arthralgia | Improving arthralgia, NSAIDs no more needed | Yes (France) | Moderate persisting arthralgias | RT-PCR+ (RealStar chikungunya RT-PCR, Altona diagnostics); IgM+/IgG– (Euroimmun) | 02 Nov (4 days) |
Dengue: PCR–; IgM– Zika: PCR–; IgM– |
CT: corticosteroids; IIFT: indirect immunofluorescence test; Ig: immunoglobulin; NSAID: non-steroidal anti-inflammatory drug; NS1: nonstructural 1 protein antigen; RDT: rapid diagnostic test; RT-PCR: real time PCR.
Four patients were hospitalised, and 15 needed non-steroidal anti-inflammatory drugs; four had corticosteroids added to manage symptoms (Table 2). As at 20 October 2019, fourteen patients still had persistent symptoms, predominantly arthralgia.
Discussion
Chikungunya is often a mild self-limited illness, but severe and life-threatening complications have been described [6]. Persistent polyarthralgia can affect up to 40% of infected individuals and may last for months or years [7,8]. A high proportion of cases in our case series (14 of 18) had persistent sequelae (mainly arthralgia) that interfered with their daily life. Given the potential for long-term morbidity, the absence of a curative treatment or a preventive vaccine, detailed pre-travel counselling should focus on mosquito-bite prevention, particularly for those at high risk of incapacitating complications such as women older than 40 years, people with underlying rheumatic diseases, and professional athletes [9].
Considering the short duration of stay in different parts of Myanmar of many of the currently reported travellers, as well as the usual incubation period of chikungunya, the specific place where they were infected cannot be determined with certainty. Most patients in the case series acquired chikungunya from August to October, likely due to enhanced vector activity at the end or after the monsoon season, which occurs between May and August.
CHIKV has been detected in South and South East Asia since the 1950s [3], but its current epidemiology is poorly understood [10]. In Myanmar, CHIKV infection was first described in 1973 [11]. Since this time, the virus has caused outbreaks in 1998, 2006 and 2008 [3]. The number of reported cases subsequently declined until 2010, when local surveillance identified the last reported case [3]. Thereafter, from 2011 to 2018 no CHIKV cases were officially reported in the country, however a review of travellers who acquired chikungunya in Myanmar in the GeoSentinel database from 2000 to October 2019 revealed two cases in 2015 and 2016, one probable and one confirmed.
In 2019, Myanmar’s National Health Laboratory detected chikungunya cases again [1]. During the same year, other national surveillance systems also identified travellers who acquired chikungunya in Myanmar. For example, the Japanese National Institute of Infectious Diseases, which systematically tracks travellers, detected 28 imported cases of chikungunya from Myanmar to Japan [12]. The Italian Arboviriasis Surveillance System also detected four imported cases in Italy [13]. Our report highlights additional cases of exported CHIKV infection from Myanmar.
Little is known about factors contributing to the multiple detections of CHIKV infections in or from Myanmar in 2019. While aside from this year, no cases within the country had been officially observed since the end of 2010, the detection of exported cases in 2015 and 2016 could suggest that CHIKV might have continued to circulate in Myanmar after 2010. Myanmar’s disease surveillance system includes a Central Epidemiology Unit (CEU) and several vertical control programmes [14]. Certain areas of the country are, however, difficult to access and there could have been underreporting due to limitations of surveillance capacity.
Myanmar has geographical and epidemiological characteristics that put it at risk for CHIKV epidemics: a highly susceptible population [15], a long border with two large neighbouring endemic countries (India and Thailand) with cross-border population movement for trade and travel purposes [16], and competent vectors (both Aedes aegypti and Ae. albopictus) [17]. According to the Thai Ministry of Public Health, CHIKV is currently circulating in Thailand with an increasing incidence: as at 30 October 2019, the country has reported 8,744 cases this year [18]. Coincidentally, GeoSentinel recently documented a rise in the number of travel-associated CHIKV infections from Thailand [19]. Without further epidemiological and phylogenetic evidence, it remains difficult to establish whether any links exist between cases in Thailand and those in Myanmar.
It should be noted that Myanmar is an increasingly popular tourist destination, with more than 3.5 million visitors in 2017 [20]. Hence, although we report more cases exported from Myanmar in 2019 than in previous years, a surge in tourism to this country may have led to an apparent increase of the number of exported cases. GeoSentinel data are moreover not population-based so rates or risk estimates cannot be derived. Also, diagnostics depend on local site interpretation and reporting.
Imported CHIKV infection by viraemic travellers returning to their home countries raises the possibility of virus spread to these countries if competent vectors are present, mainly in hot seasons, as European Ae. albopictus is affected by seasonal temperature and undergoes a winter diapause [21]. There is also a potential risk of transmission by other routes, such as blood donation [22]. In Europe, some countries (Italy in 2007 and 2017, France in 2010, 2014 and 2017) have experienced autochthonous transmission of CHIKV through viraemic travellers [23]. Surveillance and early detection of both imported and autochthonous CHIKV infections is therefore relevant in areas with competent vectors, as well as close vector monitoring and rapid public health response.
This report of imported CHIKV infections reinforces the importance of travellers as sentinels of local outbreaks, particularly in settings with limited public health surveillance and reporting infrastructure.
Acknowledgements
We would like to thank Dr. Chang-Kweng Lim in the Department of Virology, National Institute of Infectious Diseases of Japan, for his kind assistance with chikungunya diagnostics. We thank Pauline Perreau of the Department of Infectious Diseases and Tropical Medicine, CHU de Bordeaux, for her kind assistance with patient data extraction. We would finally like to thank Kristina M. Angelo of the CDC/DDID/NCEZID/DGMQ for her editorial assistance.
Conflict of interest: None declared.
Authors’ contributions: Marta Díaz-Menendez, Elena Trigo Esteban, Mugen Ujiie, Guido Calleri, Camilla Rothe, Denis Malvy, Emanuele Nicastri, Alfred L. Bissinger, Marc Grandadam, Jonathan D. Alpern, Federico Gobbi, Alexandre Duvignaud, Takato Nakamoto, Spinello Antinori provided travel history as well as clinical and biological information on the travellers. Marta Díaz-Menendez, Elena Trigo Esteban and Davidson H. Hamer created the first draft of the manuscript and modified it according to the editorial team’s comments. Emilie Javelle, Patricia Schlagenhauf and Davidson H. Hamer reviewed and edited the manuscript. All authors provided input and reviewed the final version of the manuscript.
References
- 1.Myanmar Times. Chikungunya reappears after 10 years. [Accessed 20 Oct 2019]. Available from: https://www.mmtimes.com/news/chikungunya-reappears-after-10-years.html
- 2. Tun MM, Thant KZ, Inoue S, Nabeshima T, Aoki K, Kyaw AK, et al. Detection of east/central/south African genotype of chikungunya virus in Myanmar, 2010. Emerg Infect Dis. 2014;20(8):1378-81. 10.3201/eid2008.131431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wimalasiri-Yapa BMCR, Stassen L, Huang X, Hafner LM, Hu W, Devine GJ, et al. Chikungunya virus in Asia - Pacific: a systematic review. Emerg Microbes Infect. 2019;8(1):70-9. 10.1080/22221751.2018.1559708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization South-East Asia Regional Office (WHO SEARO). Proposed case definition of Chikungunya Fever (WHO, SEARO). New Delhi: WHO SEARO. [Accessed 19 Dec 2019]. Available from: http://origin.searo.who.int/entity/emerging_diseases/topics/Def_Chikungunya_Fever.pdf
- 5. Silva JVJ, Jr, Ludwig-Begall LF, Oliveira-Filho EF, Oliveira RAS, Durães-Carvalho R, Lopes TRR, et al. A scoping review of Chikungunya virus infection: epidemiology, clinical characteristics, viral co-circulation complications, and control. Acta Trop. 2018;188:213-24. 10.1016/j.actatropica.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Economopoulou A, Dominguez M, Helynck B, Sissoko D, Wichmann O, Quenel P, et al. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005-2006 outbreak on Réunion. Epidemiol Infect. 2009;137(4):534-41. 10.1017/S0950268808001167 [DOI] [PubMed] [Google Scholar]
- 7. Schwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol. 2010;8(7):491-500. 10.1038/nrmicro2368 [DOI] [PubMed] [Google Scholar]
- 8. Consuegra-Rodríguez MP, Hidalgo-Zambrano DM, Vásquez-Serna H, Jimenez-Canizales CE, Parra-Valencia E, Rodriguez-Morales AJ. Post-chikungunya chronic inflammatory rheumatism: Follow-up of cases after 1 year of infection in Tolima, Colombia. Travel Med Infect Dis. 2018;21:62-8. 10.1016/j.tmaid.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 9. van Aalst M, Nelen CM, Goorhuis A, Stijnis C, Grobusch MP. Long-term sequelae of chikungunya virus disease: A systematic review. Travel Med Infect Dis. 2017;15:8-22. 10.1016/j.tmaid.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 10. Pulmanausahakul R, Roytrakul S, Auewarakul P, Smith DR. Chikungunya in Southeast Asia: understanding the emergence and finding solutions. Int J Infect Dis. 2011;15(10):e671-6. 10.1016/j.ijid.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 11. Thaung U, Ming CK, Swe T, Thein S. Epidemiological features of dengue and chikungunya infections in Burma. Southeast Asian J Trop Med Public Health. 1975;6(2):276-83. [PubMed] [Google Scholar]
- 12.National Institute of Infectious Diseases (NIID) Japan; Infectious Diseases Control Division (IDCD), Ministry of Health, Labor and Welfare (MHLW), Japan. IDWR (Infectious Diseases Weekly Report), week 42, as of 6 Oct 2019: NIID; 2019. [Accessed 29 Oct 2019]. Available from: https://www0.nih.go.jp/niid/idsc/idwr/IDWR2019/idwr2019-42.pdf
- 13.Sistema di Sorveglianza delle Arbovirosi Rapporto n.6, 2019. [Accessed 19 Dec 2019]. Available from: https://www.epicentro.iss.it/arbovirosi/pdf/Chik_2019.pdf
- 14. Latt NN, Myat Cho S, Htun NM, Myint MN, Aoki F, Reyer JA, et al. Yu Mon Saw Healthcare in Myanmar. Nagoya J Med Sci. 2016;78(2):123-34. [PMC free article] [PubMed] [Google Scholar]
- 15. Ngwe Tun MM, Inoue S, Thant KZ, Talemaitoga N, Aryati A, Dimaano EM, et al. Retrospective seroepidemiological study of chikungunya infection in South Asia, Southeast Asia and the Pacific region. Epidemiol Infect. 2016;144(11):2268-75. 10.1017/S095026881600056X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pulmanausahakul R, Roytrakul S, Auewarakul P, Smith DR. Chikungunya in Southeast Asia: understanding the emergence and finding solutions. Int J Infect Dis. 2011;15(10):e671-6. 10.1016/j.ijid.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization (WHO). Prevent Dengue & Chikungunya, WHO Myanmar newsletter special; 9 September 2019. Available from: https://www.who.int/docs/default-source/searo/myanmar/prevent-dengue-and-chikungunya.pdf?sfvrsn=9b1b3069_0
- 18.Department of Disease Control. Weekly Disease Forecast No.234_Chikungunya (3-9 November 2019). Department of Disease Control, Ministry of Public Health. [Accessed 08 Jan 2020]. Available from: https://ddc.moph.go.th/uploads/files_en/28420191219085018.pdf
- 19. Javelle E, Florescu S-A, Asgeirsson H, Jmor S, Eperon G, Leshem E, et al. Increased risk of chikungunya infection in travellers to Thailand during ongoing outbreak in tourist areas: cases imported to Europe and the Middle East, early 2019. Euro Surveill. 2019;24(10):1900146. 10.2807/1560-7917.ES.2019.24.10.1900146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Tourism Organization. Myanmar: Country-specific: Basic indicators (Compendium) 2013-2017 (11.2018). [Accessed 24 Oct 2019]. Available from: https://www.e-unwto.org/doi/abs/10.5555/unwtotfb0104010020142018201909
- 21. Medlock JM, Avenell D, Barrass I, Leach S. Analysis of the potential for survival and seasonal activity of Aedes albopictus (Diptera: Culicidae) in the United Kingdom. J Vector Ecol. 2006;31(2):292-304. 10.3376/1081-1710(2006)31[292:AOTPFS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 22. Appassakij H, Khuntikij P, Silpapojakul K, Promwong C, Rujirojindakul P, Suddeaugrai O, et al. Risk of chikungunya virus transmission associated with European travelers returning from southern Thailand (2008-2015). Transfusion. 2019;59(8):2612-21. 10.1111/trf.15401 [DOI] [PubMed] [Google Scholar]
- 23. Díaz-Menéndez M, Crespillo-Andújar C. Literature review of mosquito-borne viral infections in non-tropical European Union territories: A cause of concern? Enferm Infecc Microbiol Clin. 2019;37(9):619-20. 10.1016/j.eimc.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 24. Pastorino B, Bessaud M, Grandadam M, Murri S, Tolou HJ, Peyrefitte CN. Development of a TaqMan RT-PCR assay without RNA extraction step for the detection and quantification of African Chikungunya viruses. J Virol Methods. 2005;124(1-2):65-71. 10.1016/j.jviromet.2004.11.002 [DOI] [PubMed] [Google Scholar]


