Abstract
Introduction
Annual influenza vaccination is recommended for older adults, but evidence regarding the impact of repeated vaccination has been inconclusive.
Aim
We investigated vaccine effectiveness (VE) against laboratory-confirmed influenza and the impact of repeated vaccination over 10 previous seasons on current season VE among older adults.
Methods
We conducted an observational test-negative study in community-dwelling adults aged > 65 years in Ontario, Canada for the 2010/11 to 2015/16 seasons by linking laboratory and health administrative data. We estimated VE using multivariable logistic regression. We assessed the impact of repeated vaccination by stratifying by previous vaccination history.
Results
We included 58,304 testing episodes for respiratory viruses, with 11,496 (20%) testing positive for influenza and 31,004 (53%) vaccinated. Adjusted VE against laboratory-confirmed influenza for the six seasons combined was 21% (95% confidence interval (CI): 18 to 24%). Patients who were vaccinated in the current season, but had received no vaccinations in the previous 10 seasons, had higher current season VE (34%; 95%CI: 9 to 52%) than patients who had received 1–3 (26%; 95%CI: 13 to 37%), 4–6 (24%; 95%CI: 15 to 33%), 7–8 (13%; 95%CI: 2 to 22%), or 9–10 (7%; 95%CI: −4 to 16%) vaccinations (trend test p = 0.001). All estimates were higher after correcting for misclassification of current season vaccination status. For patients who were not vaccinated in the current season, residual protection rose significantly with increasing numbers of vaccinations received previously.
Conclusions
Although VE appeared to decrease with increasing numbers of previous vaccinations, current season vaccination likely provides some protection against influenza regardless of the number of vaccinations received over the previous 10 influenza seasons.
Keywords: Influenza vaccine, vaccine effectiveness, repeated vaccination, older adults
Introduction
Influenza vaccination is the primary strategy to prevent influenza-related morbidity and mortality, especially for older adults, who are at higher risk of severe outcomes [1]. In this age group, influenza vaccines are 24–63% effective in preventing laboratory-confirmed influenza [2-4]. Due to frequent changes in circulating virus strains, annual vaccination is recommended.
However, the impact of repeated vaccination on vaccine effectiveness (VE) is uncertain. A randomised trial (RCT) conducted in the 1970s at a British boarding school found higher influenza incidence among students who had received multiple previous vaccines than among those who received only the current season’s vaccine [5]. Results from a larger RCT among adults in the 1980s did not lead to the same conclusion [6]. Based on the antigenic distance hypothesis put forth by Smith et al., negative or positive interference can result from prior season vaccination depending on differences in the antigenic distances between prior and current vaccine strains and the current epidemic strain [7]. Most studies to date incorporated only a single previous season when examining the impact of repeated vaccination [8-13]. Meta-analyses of these studies found substantial heterogeneity in repeated vaccination effects [14-16].
Two studies examined the impact of repeated vaccination for five previous seasons. Whereas McLean et al. observed current season VE to be higher in those who were not vaccinated in any of the previous five seasons compared with those who were vaccinated in all five previous seasons [17], Örtqvist et al. found no negative effect of repeated vaccination [18]. Thus, the effect of repeated vaccination beyond one previous season also remains unclear. This is of particular interest for older adults because not only do they bear the greatest burden of disease, but they are also recommended to receive the vaccine annually in most countries and therefore may have received many doses.
The objectives of this study were to estimate VE against laboratory-confirmed influenza infection in community-dwelling older adults for the 2010/11 to 2015/16 seasons and to investigate the impact of repeated vaccination for up to 10 previous seasons on current season VE.
Methods
Study population, setting, and design
We studied community-dwelling adults aged > 65 years in Ontario (2016 population aged ≥ 65 years: 2.3 million) who were tested for influenza during inpatient or outpatient healthcare encounters between 1 September 2010 and 31 August 2016. Details regarding these six influenza seasons have been reported previously [19]. We used personal identifiers (health card number, name, date of birth, sex, postal code) and a combination of deterministic and probabilistic methods to link the results of respiratory virus tests performed by a network of 19 public health and academic hospital laboratories to population-based provincial health administrative data (linkage proportion = 97.8%) [19]. These datasets were linked using unique coded identifiers and analysed at ICES (formerly the Institute for Clinical Evaluative Sciences). All patients had universal access to physician services, hospital care, diagnostic testing, prescription medications, and trivalent influenza vaccines during the study.
We estimated VE using the test-negative design, which compares the odds of influenza vaccination among laboratory-confirmed influenza cases and test-negative controls [20].
Ethical statement
Ethics approval for this study was obtained from the participating laboratories (Supplementary Table S1). The planning, conduct, and reporting of this study was in line with the Declaration of Helsinki.
Data sources and definitions
Laboratory data
We included the results of all respiratory virus tests conducted by participating laboratories. The laboratories used monoplex and multiplex PCR assays, viral culture, direct immunofluorescence assay, or enzyme immunoassay tests to test for one or more of the following viruses: adenovirus, bocavirus, coronavirus, enterovirus/rhinovirus, human metapneumovirus, influenza A, influenza B, parainfluenza virus, and respiratory syncytial virus [19]. We combined the results of all specimens for the same individual on the same day into a single testing episode. For participants tested multiple times in the same season, we included their earliest testing episode positive for influenza (or their earliest testing episode if all specimens tested negative for influenza) for analysis. Individuals tested in multiple seasons contributed one testing episode per season, which were treated as separate units in the analysis. Specimens were submitted at the discretion of clinicians as part of routine clinical care. The proportion of patients presenting with acute respiratory illnesses (ARI) who were tested for influenza varied by setting (22.1% for inpatients, 2.5% for patients in emergency departments, and 2.3% for patients in physician offices) [19].
Since only 49% of individuals positive for influenza A had their specimens subtyped, we assessed their generalisability by comparing the characteristics of those with subtyped and unsubtyped influenza A specimens (Supplementary Table S2). Information on lineage for influenza B was not available.
Healthcare encounter data
We identified all healthcare encounters associated with a specimen on the date of collection using the Canadian Institute for Health Information’s Discharge Abstract Database (CIHI-DAD), the National Ambulatory Care Reporting System (NACRS) database, and the Ontario Health Insurance Plan (OHIP) database. The proportion of missing data in each of the healthcare use databases should be very low since healthcare is universally covered for those with provincial health insurance.
Influenza vaccination
We ascertained influenza vaccination status using physician and (starting in 2012, when a policy change permitted pharmacists to administer influenza vaccines) pharmacist billing claims, maintained in the OHIP and Ontario Drug Benefit (ODB) databases, respectively. For VE calculations, participants were considered immunised if a vaccine dose was received ≥ 14 days before the specimen collection date.
Covariates
We obtained demographic information including age, sex, and census area-level neighbourhood income quintile through the Ontario Registered Persons Database. Healthcare use information including the number of hospitalisations in the past 3 years, outpatient visits in the past year, receipt of home care services in the past year, and prescription medications in the past year were determined using CIHI-DAD, OHIP, Home Care Database, and ODB, respectively. We determined the presence of comorbidities that increase the risk of influenza complications (anaemia, cancer, cardiovascular disease, dementia, diabetes, frailty, immunodeficiency due to underlying disease and/or therapy, as well as renal disease and respiratory disease) based on the presence of these diagnoses in various databases before the date of specimen collection [19].
Statistical analysis
Vaccine effectiveness
We used logistic regression to estimate VE, against laboratory-confirmed influenza infection, by comparing the odds of vaccination in the test-positive cases to the odds of vaccination in the test-negative controls through an odds ratio (OR) and using the following formula VE = (1 − OR) × 100%. The models controlled for the demographic characteristics and measures of previous healthcare use listed above, presence of any comorbidity, calendar time (month of test), and influenza season (except when estimating VE by season). These variables were selected a priori and were included because of their clinical importance and conceptualisation as potential confounders. We used a threshold level of 5% test positivity for the province to restrict the analyses to periods when influenza was circulating.
We estimated VE against any influenza and each influenza type/subtype for the 2010/11 to 2015/16 seasons combined and for each season separately. We also performed subgroup analyses by age group, sex, and healthcare setting, and used interaction tests to assess whether VE differed by subgroup.
We conducted a number of sensitivity analyses. First, we restricted the cohort to patients who had a diagnostic code for an ARI [19] for their healthcare encounter, to emulate case definitions used in prospective test-negative studies. Second, we restricted the cohort to patients who were tested by PCR. Third, since Ontario residents may receive influenza vaccines in settings besides physician offices and pharmacies (leading to incorrectly classifying individuals vaccinated outside of these settings as unvaccinated), for each of the above analyses we conducted a quantitative sensitivity analysis using a publicly available macro [21] to correct for misclassification of influenza vaccination status using previously reported parameters for sensitivity (69%) and specificity (90%) of influenza vaccination codes for older adults in Ontario health administrative databases [22]. This macro performs multiple iterations of exposure re-classification for each execution. For each iteration, sensitivity and specificity values within the 95% confidence interval (CI) of the previously reported parameters are selected. Using these values and the observed counts of exposed cases and controls, expected counts are determined to calculate a positive predictive value (PPV) and negative predictive value (NPV) for cases and controls separately. PPV is the probability that the individual was correctly classified as exposed, whereas NPV is the probability that an individual was correctly classified as unexposed. For each individual, a random number is chosen from a uniform distribution between 0 and 1, and compared with the predictive value based on their case and exposure statuses. If the random number is greater than the predictive value, the subject is reclassified [21]. An OR is calculated using the reclassified exposure value for each iteration, and the median OR from the distribution of ORs from all iterations is reported [21]. This macro does not permit incorporation of interaction terms with the main exposure to do an interaction test between subgroups.
Impact of repeated vaccination on current season vaccine effectiveness
Next, we examined the impact of repeated vaccination on current season VE against any influenza and each influenza type/subtype for the 2010/11 to 2015/16 seasons combined. We did this taking into account incrementally longer vaccination history durations (i.e. one, five, and 10 previous influenza seasons). Since influenza vaccination data in Ontario are more accurate among those aged ≥ 65 years [22], we restricted the analysis examining 5-year vaccination history to patients aged ≥ 70 years in the current season to ensure they were ≥ 65 years for all previous seasons. Similarly, we restricted the analysis examining 10-year vaccination history to patients aged ≥ 75 years in the current season. Patients had to be eligible for health insurance in Ontario during the previous seasons investigated.
For each analysis, we stratified the study population based on individuals’ vaccination history (i.e. number of previous vaccinations received) and we estimated current season VE conditioned on vaccination history. Therefore, the reference group for estimating VE is patients who share similar vaccination histories as those who are vaccinated in the current season but are unvaccinated in the current season. For example, we compared patients who had received 9–10 previous vaccinations and who were vaccinated in the current season to those who had received 9–10 previous vaccinations but who were not vaccinated in the current season. The rationale for this approach is that it quantifies the incremental benefit of vaccination in the current season, and acknowledges that since a patient cannot change his/her past vaccination status, comparing to those not vaccinated in the current nor any past season may not be appropriate. Ultimately, this provides more patient-centred results as it aligns with the decision that needs to be made by patients each season regarding the benefit of receiving the current season’s vaccine.
We used interaction tests to assess differences in current season VE estimates between those vaccinated in the prior season and those not vaccinated in the prior season. For previous vaccination histories of five and 10 seasons, we used meta-regression to assess for trends in VE estimates between the vaccination history strata [23].
In sensitivity analyses, we corrected for misclassification of current season vaccination status. For the macro programme to successfully execute, we assumed the same values of sensitivity and specificity for all strata of past vaccination history. We repeated the analyses restricted to patients aged ≥ 75 years in the current season for greater consistency of the VE estimates across the varying vaccination history durations. We also conducted sensitivity analyses in which we manually reclassified past vaccination status for those who were misclassified for the current season based on the macro programme (details in Supplementary Text). In the first scenario, we changed vaccination status from unvaccinated to vaccinated for all previous seasons, effectively moving all misclassified individuals into the most vaccinated category in terms of vaccination history. In the second scenario, we moved individuals ‘up’ a single category (e.g. for the analysis examining 5-year vaccination history, those initially considered vaccinated in none of the previous five seasons were re-categorised to the ‘vaccinated in 1–3 of the previous five seasons’ group).
To facilitate comparisons with previous studies, we repeated these analyses using the conventional approach of estimating VE for all combinations of vaccine exposure in the current and previous seasons against a common reference group of patients who were not vaccinated in the current season and any previous seasons under consideration. To assess for trend with this approach, we included the parameterised vaccination history variable as a continuous variable in the model [24].
Analysis tools and statistical significance
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.4.0 (R Core Team, Vienna, Austria). All tests were two-sided and used p < 0.05 as the level of statistical significance.
Results
We included 58,304 testing episodes (obtained from 54,116 unique patients, including 7% tested during multiple seasons), with 11,496 (20%) testing positive for influenza and 31,004 (53%) vaccinated during the season of testing and before specimen collection. Compared with test-negative controls, test-positive cases were older, were more likely to be female, used fewer health services, had fewer comorbidities, and were less likely to be vaccinated (Supplementary Table S3). Descriptive statistics comparing vaccinated and unvaccinated patients can be found in Supplementary Table S4.
Overall adjusted VE against any influenza for the 2010/11 to 2015/16 seasons combined was 21% (95%CI: 18 to 24%) ( Table 1 ). For the six seasons combined, VE was 38% (95%CI: 28 to 46%) against A(H1N1)pdm09, 22% (95%CI: 16 to 28%) against A(H3N2), and 30% (95%CI: 24 to 36%) against B. VE against unsubtyped influenza A viruses was only 11% (95%CI: 5 to 16%). We observed substantial variability in VE by season (interaction test p < 0.001), by age group (p = 0.01), and by sex (p = 0.03), but not by healthcare setting (p = 0.60). After correcting for misclassification of vaccination status, VE for the six seasons combined increased to 38% (95%CI: 35 to 42%) against any influenza. We observed similar results when restricting the analysis to ARI-coded healthcare encounters and to patients tested by PCR ( Table 1 ). VE estimates stratified by influenza subtype and season are presented in Supplementary Table S5.
Table 1. Influenza vaccine effectiveness estimates for community-dwelling adults aged > 65 years, 2010/11 to 2015/16 influenza seasons in Ontario, Canada (n = 58,304)a .
| Analysis | Test-positive patients No. vaccinated/total |
Test-negative patients No. vaccinated/total |
Unadjusted VE% (95% CI) | Adjusted VE% (95% CI) | Misclassification corrected Adjusted VE% (95% CI) |
|
|---|---|---|---|---|---|---|
| Overallb | 5,575/11,496 | 25,429/46,808 | 21 (18 to 24) | 21 (18 to 24) | 38 (35 to 42) | |
| By influenza type/subtype | ||||||
| Influenza Ac | A(H3N2) | 1,780/3,765 | 25,429/46,808 | 25 (19 to 29) | 22 (16 to 28) | 44 (38 to 49) |
| A(H1N1)pdm09 | 347/830 | 25,429/46,808 | 40 (31 to 47) | 38 (28 to 46) | 61 (53 to 68) | |
| A(unsubtyped) | 2,425/4,772 | 25,429/46,808 | 13 (8 to 18) | 11 (5 to 16) | 25 (18 to 31) | |
| Influenza B | 1,027/2,138 | 25,429/46,808 | 22 (15 to 29) | 30 (24 to 36) | 42 (37 to 49) | |
| By influenza season | ||||||
| 2010/11 | 488/1,204 | 2,561/4,980 | 36 (27 to 43) | 33 (23 to 41) | 54 (45 to 61) | |
| 2011/12 | 195/413 | 1,823/3,216 | 32 (16 to 44) | 32 (16 to 45) | 54 (34 to 66) | |
| 2012/13 | 988/2,253 | 4,339/8,577 | 24 (16 to 31) | 20 (12 to 28) | 38 (29 to 45) | |
| 2013/14 | 711/1,554 | 5,368/9,665 | 32 (25 to 39) | 36 (28 to 42) | 56 (49 to 62) | |
| 2014/15 | 2,416/4,432 | 6,712/12,044 | 5 (−2 to 11) | 6 (−1 to 13) | 12 (2 to 21) | |
| 2015/16 | 777/1,640 | 4,626/8,326 | 28 (20 to 35) | 26 (17 to 34) | 49 (40 to 56) | |
| By age group in years | ||||||
| 66–75 | 1,525/3,601 | 8,387/16,716 | 27 (22 to 32) | 28 (22 to 33) | 42 (34 to 49) | |
| 76–85 | 2,328/4,548 | 10,402/18,181 | 22 (16 to 27) | 20 (14 to 25) | 42 (36 to 48) | |
| ≥ 86 | 1,722/3,347 | 6,640/11,911 | 16 (9 to 22) | 13 (5 to 20) | 31 (22 to 38) | |
| By sex | ||||||
| Male | 2,611/5,348 | 12,470/22,446 | 24 (19 to 28) | 25 (21 to 30) | 44 (38 to 49) | |
| Female | 2,964/6,148 | 12,959/24,362 | 18 (13 to 23) | 17 (12 to 22) | 34 (28 to 40) | |
| By healthcare setting | ||||||
| Inpatient | 4,460/9,224 | 22,424/41,178 | 22 (18 to 25) | 21 (18 to 25) | 40 (35 to 44) | |
| Outpatient | 1,146/2,330 | 3,398/6,368 | 15 (7 to 23) | 18 (10 to 26) | 30 (20 to 39) | |
| ARI-coded encounter | 5,068/10,459 | 16,968/31,185 | 21 (18 to 25) | 21 (18 to 25) | 39 (35 to 43) | |
| Tested by PCRd | 4,741/9,841 | 19,586/35,877 | 23 (19 to 26) | 22 (18 to 26) | 41 (37 to 45) | |
ARI: acute respiratory illness; CI: confidence interval; No.: number; NA: not applicable; VE: vaccine effectiveness.
a The model adjusted for age, sex, census area-level neighbourhood income quintile, number of hospitalisations in the past 3 years, number of outpatient visits in the past year, receipt of home care services in the past year, number of prescription medications in the past year, comorbidities that increase the risk of influenza complications (anaemia, cancer, cardiovascular disease, dementia, diabetes, frailty, immunodeficiency due to underlying disease and/or therapy, as well as renal disease and respiratory disease), calendar time, and influenza season.
b For any influenza, 2010/11 to 2015/16 influenza seasons combined.
c Only 49% of influenza A specimens were subtyped.
d PCR (monoplex or multiplex).
Impact of repeated vaccination on vaccine effectiveness
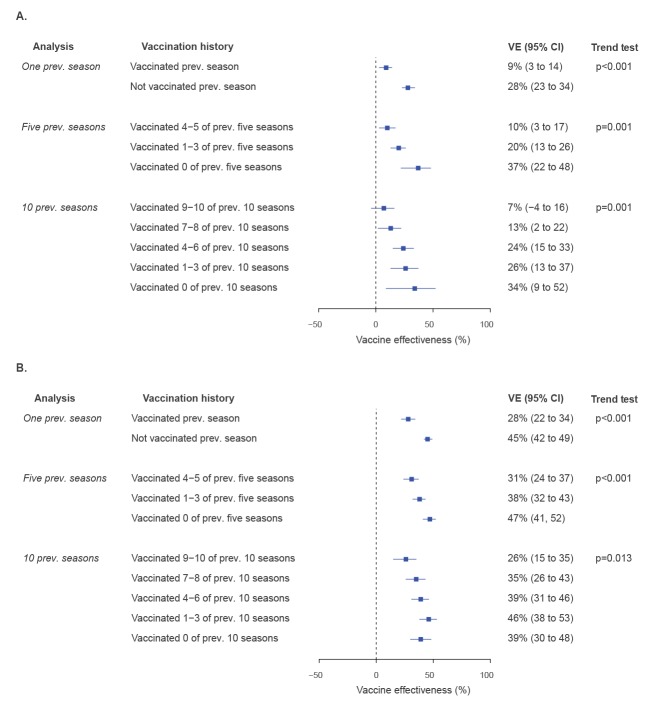
Patients who had received more vaccinations in previous seasons were older and more likely to be male, use health services, and have comorbidities, although the magnitudes of the differences between groups were small ( Table 2 , Table 3 , Table 4 ). Current season adjusted VE was higher for patients not vaccinated in the previous season (28%; 95%CI: 23 to 34%) than for those who were vaccinated in the previous season (9%; 95%CI: 3 to 14%) (interaction test p < 0.001) ( Figure 1a ). In the analysis accounting for 5-year vaccination history, patients who had received no vaccinations in the previous five seasons had the highest VE for current season vaccination (37%; 95%CI: 22 to 48%), with lower but still significant VE estimates for patients who had received 1–3 (20%; 95%CI: 13 to 26%) and 4–5 (10%; 95%CI: 3 to 17%) vaccinations in the previous five seasons (trend test p = 0.001). Similar results were observed when accounting for 10-year vaccination history: patients who had received no vaccinations in the previous 10 seasons had the highest VE for current season vaccination (34%; 95%CI: 9 to 52%), with VE decreasing with more previous vaccinations received over the previous 10 seasons: 26% (95%CI: 13 to 37%) for those vaccinated 1–3 times, 24% (95%CI: 15 to 33%) for those vaccinated 4–6 times, 13% (95%CI: 2 to 22%) for those vaccinated 7–8 times, and 7% (95%CI: −4 to 16%) for those vaccinated 9–10 times (trend test p = 0.001).
Table 2. Descriptive characteristics of community-dwelling adults aged > 65 years for the 2010/11 to 2015/16 influenza seasons, stratified by vaccination history for the previous season, Ontario, Canada (n = 58,021).
| Characteristic | Total (n = 58,021) |
Vaccinated in previous season (n = 33,243) |
Not vaccinated in previous season (n = 24,778) |
p value | |||
|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | ||
| Influenza season | |||||||
| 2010/11 | 6,162 | 10.6 | 3,678 | 11.1 | 2,484 | 10.0 | < 0.001 |
| 2011/12 | 3,607 | 6.2 | 2,081 | 6.3 | 1,526 | 6.2 | |
| 2012/13 | 10,777 | 18.6 | 5,874 | 17.7 | 4,903 | 19.8 | |
| 2013/14 | 11,145 | 19.2 | 6,079 | 18.3 | 5,066 | 20.4 | |
| 2014/15 | 16,411 | 28.3 | 9,743 | 29.3 | 6,668 | 26.9 | |
| 2015/16 | 9,919 | 17.1 | 5,788 | 17.4 | 4,131 | 16.7 | |
| Age (years), mean ± SD | 79.6 ± 8.2 | NA | 80.1 ± 8.0 | NA | 78.8 ± 8.4 | NA | < 0.001 |
| Age group in years | |||||||
| 66–75 | 20,192 | 34.8 | 10,495 | 31.6 | 9,697 | 39.1 | < 0.001 |
| 76–85 | 22,617 | 39.0 | 13,588 | 40.9 | 9,029 | 36.4 | |
| ≥ 86 | 15,212 | 26.2 | 9,160 | 27.6 | 6,052 | 24.4 | |
| Male sex | 27,660 | 47.7 | 16,139 | 48.5 | 11,521 | 46.5 | < 0.001 |
| Neighbourhood income quintile | |||||||
| 1 (lowest) | 13,044 | 22.5 | 7,113 | 21.4 | 5,931 | 23.9 | < 0.001 |
| 2 | 12,321 | 21.2 | 7,112 | 21.4 | 5,209 | 21.0 | |
| 3 | 10,935 | 18.8 | 6,300 | 19.0 | 4,635 | 18.7 | |
| 4 | 10,341 | 17.8 | 6,001 | 18.1 | 4,340 | 17.5 | |
| 5 (highest) | 11,026 | 19.0 | 6,540 | 19.7 | 4,486 | 18.1 | |
| Missing | 354 | 0.6 | 177 | 0.5 | 177 | 0.7 | |
| Medical conditions | |||||||
| Cardiovascular diseasea | 37,212 | 64.1 | 21,778 | 65.5 | 15,434 | 62.3 | < 0.001 |
| Chronic obstructive pulmonary disease | 29,672 | 51.1 | 17,734 | 53.3 | 11,938 | 48.2 | < 0.001 |
| Diabetes | 24,858 | 42.8 | 14,594 | 43.9 | 10,264 | 41.4 | < 0.001 |
| Cancer | 17,082 | 29.4 | 10,167 | 30.6 | 6,915 | 27.9 | < 0.001 |
| Asthma | 16,179 | 27.9 | 9,948 | 29.9 | 6,231 | 25.1 | < 0.001 |
| Anaemia | 13,988 | 24.1 | 8,384 | 25.2 | 5,604 | 22.6 | < 0.001 |
| Chronic kidney disease | 12,853 | 22.2 | 7,456 | 22.4 | 5,397 | 21.8 | 0.063 |
| Dementia/frailty | 11,410 | 19.7 | 6,481 | 19.5 | 4,929 | 19.9 | 0.23 |
| Immunocompromised | 8,185 | 14.1 | 4,983 | 15.0 | 3,202 | 12.9 | < 0.001 |
| Any of the above medical conditions | 55,351 | 95.4 | 32,009 | 96.3 | 23,342 | 94.2 | < 0.001 |
| Received homecare services, past 1y | 28,321 | 48.8 | 16,098 | 48.4 | 12,223 | 49.3 | 0.03 |
| Hospitalisations, past 3y, mean ± SD | 1.6 ± 2.2 | NA | 1.6 ± 2.1 | NA | 1.6 ± 2.3 | NA | < 0.001 |
| Outpatient visits, past 1y, mean ± SD | 14.2 ± 11.0 | NA | 15.5 ± 11.0 | NA | 12.5 ± 10.7 | NA | < 0.001 |
| Prescription medications, past 1y, mean ± SD | 16.5 ± 9.3 | NA | 17.4 ± 9.1 | NA | 15.4 ± 9.5 | NA | < 0.001 |
| Month of influenza testing | |||||||
| November | 1,407 | 2.4 | 785 | 2.4 | 622 | 2.5 | 0.83 |
| December | 9,486 | 16.3 | 5,402 | 16.3 | 4,084 | 16.5 | |
| January | 15,038 | 25.9 | 8,605 | 25.9 | 6,433 | 26.0 | |
| February | 10,304 | 17.8 | 5,904 | 17.8 | 4,400 | 17.8 | |
| March | 10,686 | 18.4 | 6,166 | 18.5 | 4,520 | 18.2 | |
| April | 7,599 | 13.1 | 4,378 | 13.2 | 3,221 | 13.0 | |
| May | 3,501 | 6.0 | 2,003 | 6.0 | 1,498 | 6.0 | |
| Tested sample from inpatient setting | 49,621 | 85.5 | 28,544 | 85.9 | 21,077 | 85.1 | 0.007 |
| Specimen positive for influenza | 11,444 | 19.7 | 6,177 | 18.6 | 5,267 | 21.3 | < 0.001 |
| Vaccinated against influenza in current season | 30,916 | 53.3 | 24,592 | 74.0 | 6,324 | 25.5 | < 0.001 |
NA: not applicable; SD: standard deviation.
a Includes acute ischaemic stroke, arrhythmias, congestive heart failure, ischaemic heart disease, and transient ischaemic attack.
Table 3. Descriptive characteristics of community-dwelling adults aged ≥ 70 years for the 2010/11 to 2015/16 influenza seasons, stratified by influenza vaccination history over five previous seasons, Ontario, Canada (n = 49,294).
| Characteristic | Vaccination history over five previous seasons | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 49,294) |
4–5 vaccinations (n = 24,664) |
1–3 vaccinations (n = 15,933) |
0 vaccinations (n = 8,697) |
p value | |||||
| Number | % | Number | % | Number | % | Number | % | ||
| Influenza season | |||||||||
| 2010/11 | 5,295 | 10.7 | 2,656 | 10.8 | 1,700 | 10.7 | 939 | 10.8 | 0.05 |
| 2011/12 | 3,046 | 6.2 | 1,589 | 6.4 | 932 | 5.8 | 525 | 6.0 | |
| 2012/13 | 9,216 | 18.7 | 4,563 | 18.5 | 3,023 | 19.0 | 1,630 | 18.7 | |
| 2013/14 | 9,328 | 18.9 | 4,609 | 18.7 | 3,011 | 18.9 | 1,708 | 19.6 | |
| 2014/15 | 14,192 | 28.8 | 7,178 | 29.1 | 4,618 | 29.0 | 2,396 | 27.5 | |
| 2015/16 | 8,217 | 16.7 | 4,069 | 16.5 | 2,649 | 16.6 | 1,499 | 17.2 | |
| Age (years), mean ± SD | 81.5 ± 7.1 | NA | 82.1 ± 6.9 | NA | 81.3 ± 7.2 | NA | 80.3 ± 7.1 | NA | < 0.001 |
| Age group in years | |||||||||
| 70–75 | 11,952 | 24.2 | 5,058 | 20.5 | 4,191 | 26.3 | 2,703 | 31.1 | < 0.001 |
| 76–85 | 22,290 | 45.2 | 11,553 | 46.8 | 6,963 | 43.7 | 3,774 | 43.4 | |
| ≥ 86 | 15,052 | 30.5 | 8,053 | 32.7 | 4,779 | 30.0 | 2,220 | 25.5 | |
| Male sex | 23,256 | 47.2 | 11,936 | 48.4 | 7,386 | 46.4 | 3,934 | 45.2 | < 0.001 |
| Neighbourhood income quintile | |||||||||
| 1 (lowest) | 10,872 | 22.1 | 5,102 | 20.7 | 3,652 | 22.9 | 2,118 | 24.4 | < 0.001 |
| 2 | 10,485 | 21.3 | 5,349 | 21.7 | 3,316 | 20.8 | 1,820 | 20.9 | |
| 3 | 9,356 | 19.0 | 4,690 | 19.0 | 3,068 | 19.3 | 1,598 | 18.4 | |
| 4 | 8,829 | 17.9 | 4,458 | 18.1 | 2,824 | 17.7 | 1,547 | 17.8 | |
| 5 (highest) | 9,470 | 19.2 | 4,934 | 20.0 | 2,994 | 18.8 | 1,542 | 17.7 | |
| Missing | 282 | 0.6 | 131 | 0.5 | 79 | 0.5 | 72 | 0.8 | |
| Medical conditions | |||||||||
| Cardiovascular diseasea | 32,830 | 66.6 | 16,841 | 68.3 | 10,610 | 66.6 | 5,379 | 61.8 | < 0.001 |
| Chronic obstructive pulmonary disease | 25,351 | 51.4 | 13,214 | 53.6 | 8,329 | 52.3 | 3,808 | 43.8 | < 0.001 |
| Diabetes | 21,154 | 42.9 | 10,910 | 44.2 | 6,850 | 43.0 | 3,394 | 39.0 | < 0.001 |
| Cancer | 14,559 | 29.5 | 7,563 | 30.7 | 4,682 | 29.4 | 2,314 | 26.6 | < 0.001 |
| Asthma | 13,725 | 27.8 | 7,561 | 30.7 | 4,381 | 27.5 | 1,783 | 20.5 | < 0.001 |
| Anaemia | 12,090 | 24.5 | 6,375 | 25.8 | 3,899 | 24.5 | 1,816 | 20.9 | < 0.001 |
| Chronic kidney disease | 11,272 | 22.9 | 5,706 | 23.1 | 3,804 | 23.9 | 1,762 | 20.3 | < 0.001 |
| Dementia/frailty | 10,845 | 22.0 | 5,345 | 21.7 | 3,877 | 24.3 | 1,623 | 18.7 | < 0.001 |
| Immunocompromised | 6,463 | 13.1 | 3,433 | 13.9 | 2,060 | 12.9 | 970 | 11.2 | < 0.001 |
| Any of the above medical conditions | 47,310 | 96.0 | 23,877 | 96.8 | 15,335 | 96.2 | 8,098 | 93.1 | < 0.001 |
| Received homecare services, past 1y | 25,184 | 51.1 | 12,567 | 51.0 | 8,456 | 53.1 | 4,161 | 47.8 | < 0.001 |
| Hospitalisations, past 3y, mean ± SD | 1.6 ± 2.1 | NA | 1.5 ± 2.1 | NA | 1.7 ± 2.3 | NA | 1.4 ± 2.1 | NA | < 0.001 |
| Outpatient visits, past 1y, mean ± SD | 14.0 ± 10.7 | NA | 15.5 ± 10.7 | NA | 13.3 ± 10.6 | NA | 11.0 ± 9.9 | NA | < 0.001 |
| Prescription medications, past 1y, mean ± SD | 16.6 ± 9.1 | NA | 17.5 ± 8.8 | NA | 16.8 ± 9.2 | NA | 13.7 ± 9.0 | NA | < 0.001 |
| Month of influenza testing | |||||||||
| November | 1,178 | 2.4 | 590 | 2.4 | 380 | 2.4 | 208 | 2.4 | 0.74 |
| December | 8,127 | 16.5 | 4,111 | 16.7 | 2,592 | 16.3 | 1,424 | 16.4 | |
| January | 12,920 | 26.2 | 6,376 | 25.9 | 4,250 | 26.7 | 2,294 | 26.4 | |
| February | 8,741 | 17.7 | 4,346 | 17.6 | 2,813 | 17.7 | 1,582 | 18.2 | |
| March | 9,009 | 18.3 | 4,533 | 18.4 | 2,939 | 18.4 | 1,537 | 17.7 | |
| April | 6,398 | 13.0 | 3,237 | 13.1 | 2,024 | 12.7 | 1,137 | 13.1 | |
| May | 2,921 | 5.9 | 1,471 | 6.0 | 935 | 5.9 | 515 | 5.9 | |
| Tested sample from inpatient setting | 42,652 | 86.5 | 21,487 | 87.1 | 13,767 | 86.4 | 7,398 | 85.1 | < 0.001 |
| Specimen positive for influenza | 9,877 | 20.0 | 4,743 | 19.2 | 3,117 | 19.6 | 2,017 | 23.2 | < 0.001 |
| Vaccinated against influenza in current season | 27,043 | 54.9 | 18,946 | 76.8 | 7,325 | 46.0 | 772 | 8.9 | < 0.001 |
NA: not applicable; SD: standard deviation.
a Includes acute ischaemic stroke, arrhythmias, congestive heart failure, ischaemic heart disease, and transient ischaemic attack.
Table 4. Descriptive characteristics of community-dwelling adults aged ≥ 75 years for the 2010/11 to 2015/16 influenza seasons, stratified by influenza vaccination history over 10 previous seasons, Ontario, Canada (n = 38,766).
| Characteristic | Vaccination history over 10 previous seasons | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 38,766) |
9–10 vaccinations (n = 13,036) |
7–8 vaccinations (n = 9,008) |
4–6 vaccinations (n = 7,416) |
1–3 vaccinations (n = 5,147) |
0 vaccinations (n = 4,159) |
p value | |||||||
| Number | % | Number | % | Number | % | Number | % | Number | % | Number | % | ||
| Influenza season | |||||||||||||
| 2010/11 | 4,144 | 10.7 | 1,268 | 9.7 | 1,024 | 11.4 | 852 | 11.5 | 539 | 10.5 | 461 | 11.1 | 0.02 |
| 2011/12 | 2,371 | 6.1 | 835 | 6.4 | 552 | 6.1 | 442 | 6.0 | 297 | 5.8 | 245 | 5.9 | |
| 2012/13 | 7,294 | 18.8 | 2,430 | 18.6 | 1,702 | 18.9 | 1,407 | 19.0 | 948 | 18.4 | 807 | 19.4 | |
| 2013/14 | 7,254 | 18.7 | 2,470 | 18.9 | 1,663 | 18.5 | 1,363 | 18.4 | 974 | 18.9 | 784 | 18.9 | |
| 2014/15 | 11,416 | 29.4 | 3,953 | 30.3 | 2,595 | 28.8 | 2,133 | 28.8 | 1,552 | 30.2 | 1,183 | 28.4 | |
| 2015/16 | 6,287 | 16.2 | 2,080 | 16.0 | 1,472 | 16.3 | 1,219 | 16.4 | 837 | 16.3 | 679 | 16.3 | |
| Age (years), mean ± SD | 83.9 ± 5.8 | NA | 84.4 ± 5.7 | NA | 84.3 ± 5.8 | NA | 83.6 ± 5.8 | NA | 83.2 ± 5.8 | NA | 83.1 ± 5.9 | NA | < 0.001 |
| Age group in years | |||||||||||||
| 75 | 2,011 | 5.2 | 509 | 3.9 | 410 | 4.6 | 442 | 6.0 | 343 | 6.7 | 307 | 7.4 | < 0.001 |
| 76–85 | 21,900 | 56.5 | 7,149 | 54.8 | 4,943 | 54.9 | 4,273 | 57.6 | 3,056 | 59.4 | 2,479 | 59.6 | |
| ≥ 86 | 14,855 | 38.3 | 5,378 | 41.3 | 3,655 | 40.6 | 2,701 | 36.4 | 1,748 | 34.0 | 1,373 | 33.0 | |
| Male sex | 17,936 | 46.3 | 6,291 | 48.3 | 4,060 | 45.1 | 3,408 | 46.0 | 2,358 | 45.8 | 1,819 | 43.7 | < 0.001 |
| Neighbourhood income quintile | |||||||||||||
| 1 (lowest) | 8,338 | 21.5 | 2,592 | 19.9 | 1,883 | 20.9 | 1,664 | 22.4 | 1,194 | 23.2 | 1,005 | 24.2 | < 0.001 |
| 2 | 8,264 | 21.3 | 2,846 | 21.8 | 1,876 | 20.8 | 1,588 | 21.4 | 1,090 | 21.2 | 864 | 20.8 | |
| 3 | 7,328 | 18.9 | 2,509 | 19.2 | 1,695 | 18.8 | 1,410 | 19.0 | 1,007 | 19.6 | 707 | 17.0 | |
| 4 | 6,988 | 18.0 | 2,350 | 18.0 | 1,671 | 18.6 | 1,321 | 17.8 | 889 | 17.3 | 757 | 18.2 | |
| 5 (highest) | 7,618 | 19.7 | 2,661 | 20.4 | 1,841 | 20.4 | 1,396 | 18.8 | 942 | 18.3 | 778 | 18.7 | |
| Missing | 230 | 0.6 | 78 | 0.6 | 42 | 0.5 | 37 | 0.5 | 25 | 0.5 | 48 | 1.2 | |
| Medical conditions | |||||||||||||
| Cardiovascular diseasea | 26,959 | 69.5 | 9,175 | 70.4 | 6,453 | 71.6 | 5,230 | 70.5 | 3,415 | 66.3 | 2,686 | 64.6 | < 0.001 |
| Chronic obstructive pulmonary disease | 19,818 | 51.1 | 6,819 | 52.3 | 4,809 | 53.4 | 3,935 | 53.1 | 2,537 | 49.3 | 1,718 | 41.3 | < 0.001 |
| Diabetes | 16,241 | 41.9 | 5,634 | 43.2 | 3,889 | 43.2 | 3,115 | 42.0 | 2,096 | 40.7 | 1,507 | 36.2 | < 0.001 |
| Cancer | 11,339 | 29.2 | 4,011 | 30.8 | 2,655 | 29.5 | 2,185 | 29.5 | 1,409 | 27.4 | 1,079 | 25.9 | < 0.001 |
| Asthma | 10,623 | 27.4 | 3,896 | 29.9 | 2,671 | 29.7 | 2,042 | 27.5 | 1,229 | 23.9 | 785 | 18.9 | < 0.001 |
| Anaemia | 9,559 | 24.7 | 3,428 | 26.3 | 2,309 | 25.6 | 1,764 | 23.8 | 1,181 | 22.9 | 877 | 21.1 | < 0.001 |
| Chronic kidney disease | 9,040 | 23.3 | 2,997 | 23.0 | 2,241 | 24.9 | 1,833 | 24.7 | 1,145 | 22.2 | 824 | 19.8 | < 0.001 |
| Dementia/frailty | 9,795 | 25.3 | 3,129 | 24.0 | 2,494 | 27.7 | 2,022 | 27.3 | 1,298 | 25.2 | 852 | 20.5 | < 0.001 |
| Immunocompromised | 4,514 | 11.6 | 1,551 | 11.9 | 1,124 | 12.5 | 937 | 12.6 | 529 | 10.3 | 373 | 9.0 | < 0.001 |
| Any of the above medical conditions | 37,380 | 96.4 | 12,645 | 97.0 | 8,743 | 97.1 | 7,182 | 96.8 | 4,927 | 95.7 | 3,883 | 93.4 | < 0.001 |
| Received homecare services, past 1y | 21,006 | 54.2 | 6,947 | 53.3 | 5,087 | 56.5 | 4,148 | 55.9 | 2,734 | 53.1 | 2,090 | 50.3 | < 0.001 |
| Hospitalisations, past 3y, mean ± SD | 1.5 ± 2.1 | NA | 1.5 ± 1.9 | NA | 1.7 ± 2.2 | NA | 1.7 ± 2.2 | NA | 1.6 ± 2.1 | NA | 1.3 ± 1.8 | NA | < 0.001 |
| Outpatient visits, past 1y, mean ± SD | 13.7 ± 10.3 | NA | 15.3 ± 10.3 | NA | 14.2 ± 10.4 | NA | 13.1 ± 10.3 | NA | 12.0 ± 10.0 | NA | 10.5 ± 9.3 | NA | < 0.001 |
| Prescription medications, past 1y, mean ± SD | 16.5 ± 8.8 | NA | 17.2 ± 8.5 | NA | 17.4 ± 8.8 | NA | 16.8 ± 9.0 | NA | 15.4 ± 8.8 | NA | 13.1 ± 8.8 | NA | < 0.001 |
| Month of influenza testing | |||||||||||||
| November | 908 | 2.3 | 286 | 2.2 | 232 | 2.6 | 161 | 2.2 | 146 | 2.8 | 83 | 2.0 | 0.27 |
| December | 6,426 | 16.6 | 2,226 | 17.1 | 1,479 | 16.4 | 1,217 | 16.4 | 834 | 16.2 | 670 | 16.1 | |
| January | 10,301 | 26.6 | 3,412 | 26.2 | 2,352 | 26.1 | 2,041 | 27.5 | 1,364 | 26.5 | 1,132 | 27.2 | |
| February | 6,853 | 17.7 | 2,263 | 17.4 | 1,620 | 18.0 | 1,300 | 17.5 | 924 | 18.0 | 746 | 17.9 | |
| March | 7,036 | 18.1 | 2,352 | 18.0 | 1,672 | 18.6 | 1,346 | 18.1 | 930 | 18.1 | 736 | 17.7 | |
| April | 4,976 | 12.8 | 1,694 | 13.0 | 1,152 | 12.8 | 930 | 12.5 | 654 | 12.7 | 546 | 13.1 | |
| May | 2,266 | 5.8 | 803 | 6.2 | 501 | 5.6 | 421 | 5.7 | 295 | 5.7 | 246 | 5.9 | |
| Tested sample from inpatient setting | 33,904 | 87.5 | 11,474 | 88.0 | 7,875 | 87.4 | 6,511 | 87.8 | 4,449 | 86.4 | 3,595 | 86.4 | 0.01 |
| Specimen positive for influenza | 8,043 | 20.7 | 2,653 | 20.4 | 1,783 | 19.8 | 1,502 | 20.3 | 1,095 | 21.3 | 1,010 | 24.3 | < 0.001 |
| Vaccinated against influenza in current season | 21,645 | 55.8 | 10,297 | 79.0 | 5,847 | 64.9 | 3,768 | 50.8 | 1,450 | 28.2 | 283 | 6.8 | < 0.001 |
NA: not applicable; SD: standard deviation.
a Includes acute ischaemic stroke, arrhythmias, congestive heart failure, ischemic heart disease, and transient ischaemic attack.
Figure 1.
Forest plots of (A) current season vaccine effectiveness estimates against any influenza for community-dwelling adults aged > 65 years, taking into account vaccination histories for one, five, and 10 previous seasons and stratifying according to number of vaccinations received and (B) also correcting for misclassification of current season vaccination status, Ontario, Canada
CI: confidence interval; prev.: previous; VE: vaccine effectiveness.
a The model adjusted for age, sex, census area-level neighbourhood income quintile, number of hospitalisations in the past 3 years, number of outpatient visits in the past year, receipt of home care services in the past year, number of prescription medications in the past year, comorbidities that increase the risk of influenza complications (anaemia, cancer, cardiovascular disease, dementia, diabetes, frailty, immunodeficiency due to underlying disease and/or therapy, as well as renal disease and respiratory disease), calendar time, and influenza season.
We observed similar trends against A(H3N2) (Supplementary Figure S1) but not against A(H1N1)pdm09 (Supplementary Figure S2) or influenza B (Supplementary Figure S3).
When correcting for misclassification of current season vaccination status, we found similar patterns as the primary analysis, but with VE estimates that were higher in magnitude ( Figure 1b ). Repeating the analyses restricted to patients aged ≥ 75 years in the current season, the patterns were similar to our primary analysis, but the VE estimates were slightly lower (Supplementary Figure S4a). After correcting for misclassification of current season vaccination status within this restricted cohort, VE estimates were higher but the overall trends were consistent (Supplementary Figure S4b). Results were similar when manually reclassifying vaccination status in past seasons based on current season misclassification (Supplementary Figure S5).
Using the conventional approach of comparing to a common reference group, VE did not differ substantially for patients vaccinated in both prior and current seasons (25%; 95%CI: 22 to 29%) and those vaccinated in the current season only (29%; 95%CI: 23 to 34%) (interaction test p = 0.31), but was lower for those vaccinated in the prior season only (18%; 95%CI: 13 to 23%) (p < 0.001) (Supplementary Figure S6). When accounting for 5-year vaccination history, significant protection against influenza was observed among patients with any previous vaccination, with or without current season vaccination. Notably, for similar levels of vaccination in previous seasons, receipt of current season vaccination was associated with higher VE estimates than being unvaccinated in the current season. VE decreased for current vaccine recipients as the number of previous vaccinations received increased (i.e. 36% vs 31% vs 26%) (trend test p = 0.007). In contrast, for those not vaccinated in the current season, residual protection increased as the number of previous vaccinations received increased (i.e. 13% vs 17%) (p < 0.001). Similar patterns were observed when considering 10-year vaccination history, with VE decreasing for current vaccine recipients with increasing numbers of previous vaccinations (from 33% to 22%) (p < 0.001), while the opposite trend in residual protection was observed for those without current season vaccination (from 9% to 16%) (p < 0.001).
Discussion
In this study of older adults, we estimated VE against laboratory-confirmed influenza healthcare use to be 21% (95%CI: 18 to 24%) during the 2010/11 to 2015/16 influenza seasons, which increased to 38% (95%CI: 35 to 42%) after correcting for misclassification of vaccination status. When we examined the impact of repeated vaccination during previous influenza seasons on VE for the current season, we observed a declining trend in VE as the number of previous vaccinations increased. Nevertheless, influenza vaccination during the current season was associated with some protection against influenza infection irrespective of the number of vaccinations over the previous 10 seasons, except for individuals vaccinated 9–10 times before we corrected for misclassification of vaccination status. After correcting for misclassification of vaccination status in the current season, influenza vaccination was associated with some protection even for those vaccinated 9–10 times during the previous 10 seasons. Reassuringly, the overall observed trends in VE were consistent when correcting for this misclassification, both for the current season only and when manually reclassifying vaccination status during past seasons based on current season misclassification. Similar patterns as any influenza were observed against A(H3N2) but not A(H1N1)pdm09 or influenza B, but interpretation of these results is challenging due to lower case counts for the latter analyses leading to less precision. The observed patterns for any influenza were likely driven by A(H3N2) since that subtype comprised 67% of specimens during the influenza seasons included in this study, if one assumes the subtype distribution for unsubtyped specimens is the same as for subtyped specimens. We noted that for patients who were not vaccinated in the current season, residual protection appeared to increase with increasing numbers of vaccines received during previous seasons. We also demonstrated that being vaccinated in the current season resulted in consistently greater protection, compared with not being vaccinated in the current season, regardless of the number of previous vaccinations.
While reduced VE from repeated vaccination has been reported previously [13,25], this has not been consistently found [11,12]. The antigenic distance hypothesis is one potential explanation for reduced VE; if the vaccine strains for the current season and prior season are similar but the current season’s vaccine strain is distinct from the current epidemic strain, negative interference leading to reduced VE for the current season may result [7]. However, this hypothesis does not consider the effects of multiple previous vaccine or virus exposures [25]. Thompson et al. [26] examined up to 4 years of previous vaccination history among healthcare workers and observed a greater blunting of serologic response to the A(H3N2) vaccine strain with more doses of previous vaccines received. In addition, two studies have examined vaccination history for up to five previous seasons, with one study observing reduced VE with repeated vaccination [17] while the other did not [18]. However, similar to our results, both studies showed that vaccination in the current season provided some protection against influenza regardless of the number of previous vaccinations. No study has ever examined the impact of repeated vaccination over 10 previous seasons. The residual protection from being vaccinated in previous seasons observed in our study has also been seen elsewhere; this phenomenon may result from cross-reactivity of immune responses elicited by previous vaccinations with current-season virus antigens [17]. It is possible that due to this potential residual protection, the incremental benefit of current season vaccination may be difficult to observe for those who have received many previous vaccinations.
It remains unclear whether true vaccine interference is occurring from repeated vaccination or whether the differences between studies are an artefact of residual confounding [17]. Individuals may be more inclined to be vaccinated for the first time if they were infected by influenza in the prior season. Vaccine responses may be enhanced with recent prior infection [27], such that those who were vaccinated repeatedly may appear to have lower VE. However, measuring immunity arising from previous infection is challenging [11]. In addition, while pooling of multiple seasons can increase statistical power, it can mask important variation at the individual season level [14]. Thus, a large knowledge gap persists regarding the immunologic mechanisms for potential vaccine interference. Future studies that longitudinally ascertain both influenza vaccination and influenza infection status over multiple seasons would be helpful to better understand the impact of repeated vaccination on current season VE as well as residual protection from previous vaccination.
This study has several limitations. First, the specimens were not collected through systematic screening and enrollment but rather as part of routine clinical care. However, we have validated the use of these specimens for estimating VE [19]. Second, while test-negative studies typically use symptom onset date as the index date, we were limited to using specimen collection date. This may have led to underestimation of VE. Third, the VE estimate against unsubtyped influenza A was outside of the range between those against A(H1N1)pdm09 and A(H3N2), raising the possibility of potential bias. Although only 49% of individuals positive for influenza A had their specimens subtyped, they are fairly representative of all individuals positive for influenza A (Supplementary Table S1). We speculate that the lower VE observed for unsubtyped specimens may be due to a greater proportion being collected during the 2014/15 season (a season with known poor match) and during later months of influenza season (with potentially lower VE due to intra-season waning of immunity). Fourth, receipt of influenza vaccines outside of physician offices and pharmacies leads to misclassification of vaccination status when relying on health administrative data to ascertain vaccination status. However, healthcare-seeking behaviour has been found to be similar between test-positive and test-negative individuals [28], so any misclassification would likely be non-differential and underestimate VE, as demonstrated in our sensitivity analyses. The low sensitivity value for the influenza vaccination billing claims used in these sensitivity analyses may have resulted from bias given the self-reported nature of the reference standard available for validation of the claims data, or because pharmacist billing claims data were not yet available for inclusion in the validation study [22]. Fifth, the macro programme used for our sensitivity analysis could not include interaction terms with the main exposure to determine whether subgroup similarities/differences in VE were maintained after misclassification was corrected, and could only correct for misclassification of current season vaccination status and not misclassification in past seasons. However, our sensitivity analyses involving reclassification of past vaccination based on misclassification of current season vaccination status found very similar trends as when accounting only for current season misclassification. Sixth, we used the same values of sensitivity and specificity of the vaccination billing claims for all strata of past vaccination history because we did not have stratum-specific parameters. The observed trend might not remain if the magnitude of the bias correction varies by past vaccination history. Seventh, our use of meta-regression to assess for trends in VE estimates between vaccination history strata does not capture season-to-season heterogeneity in terms of circulating viruses, vaccine match, and host-virus immunological interactions. Eighth, we did not have information on participants’ influenza infections in the previous seasons, as most infections do not result in laboratory testing. Ninth, we did not have information on vaccination history for > 10 previous influenza seasons; the impact of repeated vaccination over longer periods of time remain unknown. Finally, as an observational study, the possibility of residual confounding remains.
Conclusions
In summary, we observed modest VE for community-dwelling older adults in Ontario, Canada during the 2010/11 to 2015/16 influenza seasons. We observed declining VE associated with repeated vaccination, however current season vaccination likely provides some protection against influenza regardless of the number of vaccines received over the previous 10 influenza seasons. Moreover, among those not vaccinated in the current season, increasing residual protection was observed with increasing numbers of previous vaccines received. Therefore, until effective universal influenza vaccines are available and eliminate the need for annual influenza vaccination, our findings support current recommendations for annual vaccination among older adults given their higher risk for influenza-related morbidity and mortality.
Acknowledgements
This work was supported by the Canadian Immunization Research Network (CIRN) through a grant from the Public Health Agency of Canada and the Canadian Institutes of Health Research (CNF 151944). This study was also supported by Public Health Ontario (PHO) and ICES, which are funded by annual grants from the Ontario Ministry of Health and Long-Term Care (MOHLTC). JCK is supported by a Clinician Scientist Award from the University of Toronto Department of Family and Community Medicine. We thank IMS Brogan Inc. for use of their Drug Information Database.
The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI) and by Cancer Care Ontario (CCO). However, the analyses, conclusions, opinions, and statement expressed herein are those of the authors, and not necessarily those of CIHI or CCO. No endorsement by ICES, PHO, MOHLTC, CIHI, or CCO is intended or should be inferred.
Supplementary Data
Conflict of interest: AJM has received research funds from GSK and Sanofi Pasteur. MS has received research grants from Janssen Canada for respiratory virus clinical trials. JBG has received research grants from GSK and Hoffmann-LaRoche for antiviral resistance studies, and from Pfizer Inc. to conduct microbiological surveillance of Streptococcus pneumoniae. All other authors report no conflicts.
Authors’ contributions: JCK conceived and oversaw the study. JKHJ and HC extracted the data and conducted statistical analyses. AC, JBG, TK, KK, AJM, JDM, DCR, SER, AS, MS, and GZ provided respiratory virus laboratory data. SAB, MAC, NSC, LCR, and KLS provided methodological input. JKHJ and JCK drafted the manuscript. All authors interpreted the results, critically revised the manuscript, and approved the final version for publication.
References
- 1. Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices-United States, 2018-19 Influenza Season. MMWR Recomm Rep. 2018;67(3):1-20. 10.15585/mmwr.rr6703a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942-51. 10.1016/S1473-3099(16)00129-8 [DOI] [PubMed] [Google Scholar]
- 3. Rondy M, El Omeiri N, Thompson MG, Levêque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: A systematic review and meta-analysis of test-negative design case-control studies. J Infect. 2017;75(5):381-94. 10.1016/j.jinf.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Darvishian M, Bijlsma MJ, Hak E, van den Heuvel ER. Effectiveness of seasonal influenza vaccine in community-dwelling elderly people: a meta-analysis of test-negative design case-control studies. Lancet Infect Dis. 2014;14(12):1228-39. 10.1016/S1473-3099(14)70960-0 [DOI] [PubMed] [Google Scholar]
- 5. Hoskins TW, Davies JR, Smith AJ, Miller CL, Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ’s Hospital. Lancet. 1979;313(8106):33-5. 10.1016/S0140-6736(79)90468-9 [DOI] [PubMed] [Google Scholar]
- 6. Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine. 1997;15(10):1114-22. 10.1016/S0264-410X(97)00003-0 [DOI] [PubMed] [Google Scholar]
- 7. Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci USA. 1999;96(24):14001-6. 10.1073/pnas.96.24.14001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC, et al. Influenza Complications Alert Network (FluCAN) Investigators Repeated Vaccination Does Not Appear to Impact Upon Influenza Vaccine Effectiveness Against Hospitalization With Confirmed Influenza. Clin Infect Dis. 2017;64(11):1564-72. 10.1093/cid/cix209 [DOI] [PubMed] [Google Scholar]
- 9. Young B, Sadarangani S, Jiang L, Wilder-Smith A, Chen MI. Duration of Influenza Vaccine Effectiveness: A Systematic Review, Meta-analysis, and Meta-regression of Test-Negative Design Case-Control Studies. J Infect Dis. 2018;217(5):731-41. 10.1093/infdis/jix632 [DOI] [PubMed] [Google Scholar]
- 10. Russell K, Chung JR, Monto AS, Martin ET, Belongia EA, McLean HQ, et al. Influenza vaccine effectiveness in older adults compared with younger adults over five seasons. Vaccine. 2018;36(10):1272-8. 10.1016/j.vaccine.2018.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valenciano M, Kissling E, Larrauri A, Nunes B, Pitigoi D, O’Donnell J, et al. Exploring the effect of previous inactivated influenza vaccination on seasonal influenza vaccine effectiveness against medically attended influenza: Results of the European I-MOVE multicentre test-negative case-control study, 2011/2012-2016/2017. Influenza Other Respir Viruses. 2018;12(5):567-81. 10.1111/irv.12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Domínguez A, Soldevila N, Toledo D, Godoy P, Espejo E, Fernandez MA, et al. The Working Group Of The Project Pi/ The effectiveness of influenza vaccination in preventing hospitalisations of elderly individuals in two influenza seasons: a multicentre case-control study, Spain, 2013/14 and 2014/15. Euro Surveill. 2017;22(34):1-8. 10.2807/1560-7917.ES.2017.22.34.30602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ohmit SE, Thompson MG, Petrie JG, Thaker SN, Jackson ML, Belongia EA, et al. Influenza vaccine effectiveness in the 2011-2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58(3):319-27. 10.1093/cid/cit736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines. 2017;16(7):723-36. 10.1080/14760584.2017.1334554 [DOI] [PubMed] [Google Scholar]
- 15. Bartoszko JJ, McNamara IF, Aras OAZ, Hylton DA, Zhang YB, Malhotra D, et al. Does consecutive influenza vaccination reduce protection against influenza: A systematic review and meta-analysis. Vaccine. 2018;36(24):3434-44. 10.1016/j.vaccine.2018.04.049 [DOI] [PubMed] [Google Scholar]
- 16. Ramsay LC, Buchan SA, Stirling RG, Cowling BJ, Feng S, Kwong JC, et al. The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis. BMC Med. 2019;17(1):9. 10.1186/s12916-018-1239-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McLean HQ, Thompson MG, Sundaram ME, Meece JK, McClure DL, Friedrich TC, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis. 2014;59(10):1375-85. 10.1093/cid/ciu680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Örtqvist Å, Brytting M, Leval A, Hergens MP. Impact of repeated influenza vaccinations in persons over 65 years of age: A large population-based cohort study of severe influenza over six consecutive seasons, 2011/12-2016/17. Vaccine. 2018;36(37):5556-64. 10.1016/j.vaccine.2018.07.052 [DOI] [PubMed] [Google Scholar]
- 19. Kwong JC, Buchan SA, Chung H, Campitelli MA, Schwartz KL, Crowcroft NS, et al. Can routinely collected laboratory and health administrative data be used to assess influenza vaccine effectiveness? Assessing the validity of the Flu and Other Respiratory Viruses Research (FOREVER) Cohort. Vaccine. 2019;37(31):4392-400. 10.1016/j.vaccine.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 20. Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31(17):2165-8. 10.1016/j.vaccine.2013.02.053 [DOI] [PubMed] [Google Scholar]
- 21. Fox MP, Lash TL, Greenland S. A method to automate probabilistic sensitivity analyses of misclassified binary variables. Int J Epidemiol. 2005;34(6):1370-6. 10.1093/ije/dyi184 [DOI] [PubMed] [Google Scholar]
- 22. Schwartz KL, Jembere N, Campitelli MA, Buchan SA, Chung H, Kwong JC. Using physician billing claims from the Ontario Health Insurance Plan to determine individual influenza vaccination status: an updated validation study. CMAJ Open. 2016;4(3):E463-70. 10.9778/cmajo.20160009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. March 2011. (Version 5.1.0). [Accessed 4 Nov 2019]. Available from: https://handbook-5-1.cochrane.org/chapter_9/9_6_4_meta_regression.htm
- 24.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression methods in biostatistics: Linear, logistic, survival, and repeated measures models. 2nd ed. New York: Springer; 2012. [Google Scholar]
- 25. Skowronski DM, Chambers C, De Serres G, Sabaiduc S, Winter AL, Dickinson JA, et al. Serial vaccination and the antigenic distance hypothesis: Effects on influenza vaccine effectiveness during A(H3N2) epidemics in Canada, 2010-2011 to 2014-2015. J Infect Dis. 2017;215(7):1059-99. 10.1093/infdis/jix074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson MG, Naleway A, Fry AM, Ball S, Spencer SM, Reynolds S, et al. Effects of Repeated Annual Inactivated Influenza Vaccination among Healthcare Personnel on Serum Hemagglutinin Inhibition Antibody Response to A/Perth/16/2009 (H3N2)-like virus during 2010-11. Vaccine. 2016;34(7):981-8. 10.1016/j.vaccine.2015.10.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davies JR, Grilli EA. Natural or vaccine-induced antibody as a predictor of immunity in the face of natural challenge with influenza viruses. Epidemiol Infect. 1989;102(2):325-33. 10.1017/S0950268800030004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Belongia EA, Kieke BA, Donahue JG, Greenlee RT, Balish A, Foust A, et al. Marshfield Influenza Study Group Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004-2005 season to the 2006-2007 season. J Infect Dis. 2009;199(2):159-67. 10.1086/595861 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.