Abstract
Multiple sclerosis (MS) is an autoimmune and demyelinating disease of the central nervous system characterised by incoordination, sensory loss, weakness, changes in bladder capacity and bowel function, fatigue and cognitive impairment, creating a significant socioeconomic burden. The pathogenesis of MS involves both genetic susceptibility and exposure to distinct environmental risk factors. The gene x environment interaction is regulated by epigenetic mechanisms. Epigenetics refers to a complex system that modifies gene expression without altering the DNA sequence. The most studied epigenetic mechanism is DNA methylation. This epigenetic mark participates in distinct MS pathophysiological processes, including blood–brain barrier breakdown, inflammatory response, demyelination, remyelination failure and neurodegeneration. In this study, we also accurately summarised a list of environmental factors involved in the MS pathogenesis and its clinical course. A literature search was conducted using MEDLINE through PubMED and Scopus. In conclusion, an exhaustive study of DNA methylation might contribute towards new pharmacological interventions in MS by use of epigenetic drugs.
Keywords: Multiple sclerosis, DNA methylation, Environmental risk factors, Inflammation, Neurodegeneration
Background
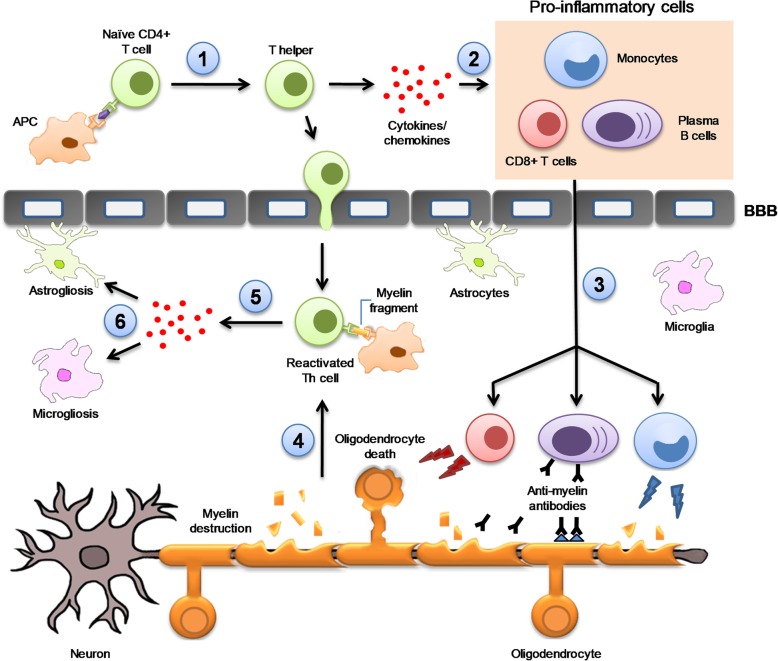
Multiple sclerosis (MS) is an autoimmune, inflammatory, demyelinating and neurodegenerative disease of the central nervous system (CNS) [1]. As a result of myelin sheath destruction, the electric impulse between neurons is inefficient, and thus the initial symptoms appear [2]. Although symptoms differ from each MS patient, the most common ones include incoordination, sensory loss, weakness, changes in bladder capacity and bowel function, fatigue, and cognitive impairment [3]. Therefore, MS has serious negative effects on the health-, social, and work-related issues of patients and their families, creating a significant socioeconomic burden [4]. The aetiology of MS is still unknown and requires a close interaction between genetic susceptibility and exposure to environmental agents. Synergistic effect of these risk factors would be responsible for triggering autoimmunity in MS patients. For this reason, the underlying mechanisms involved in the MS pathogenesis can differ among patients. Common mechanisms are observed in the pathophysiology of disease and listed next. Autoreactive CD4+ T cells are activated in the periphery [1] by antigen-presenting cells (APC), that present via the class II major histocompatibility complex (MHC) receptor an amino acid similar to myelin peptides synthesised in the CNS. This interaction activates the differentiation of the CD4+ T naïve cells into CD4+ T helper cells [5]. Upon activation, the Th1 subtype produces interferon gamma (IFN-γ) [6], a cytokine responsible for recruiting CD8+ T cells, B cells and monocytes in the periphery [7]. Then, these proinflammatory cells migrate to the blood–brain barrier (BBB) throughout the bloodstream, where they can adhere to the BBB endothelium [8]. In a healthy brain, immune cells are circulating freely in the meninges orchestrating immune surveillance of the CNS [9]. In MS, the BBB displays an aberrant expression and organisation of the endothelial tight junctions [10] that favours massive lymphocyte trafficking into the brain [11]. Infiltrated CD4+ T cells in the CNS are reactivated upon interaction with the resident APCs [12]. Afterwards, the reactivated CD4+ T cells release a variety of proinflammatory cytokines and chemokines [13], resulting in astrogliosis [14] and microgliosis [15]. This process is exacerbated when infiltrated cytotoxic CD8+ T cells attack oligodendrocytes, causing their destruction and neuronal death [16]. In parallel, plasma B cells produce antibodies against CNS self-antigens, contributing to myelin sheath damage [17]. Plasma B cells in coordination with monocytes increase the local inflammatory response by reactivating the autoreactive CD4+ T cells [18] (Fig. 1). T cell–mediated axonal injury contributes to trophic/metabolic support deficiency from oligodendrocytes as well as a lack of energy by releasing soluble inflammatory molecules [19]. The pathophysiology of MS suggests a complex interaction between the genetic and environmental risk factors [20] regulated by epigenetic mechanisms. Epigenetics can provide a stable heritable base for understanding the underlying mechanisms involved in MS [21].
Fig. 1.
The underlying pathophysiological mechanism of MS. In the first instance, autoreactive CD4+ T cells are activated in the periphery by antigen presenting cells (APC) that present, in conjunction with class II MHC molecules, similar antigens to those synthesised by the CNS. (1) This interaction activates the differentiation of CD4+ T naïve cells into CD4+ T helper cells (Th). (2) Upon activation, Th produces interferon-gamma (IFN-γ), a cytokine responsible for recruiting CD8+ T cells, B cells and monocytes in the periphery. (3) These proinflammatory cells migrate to the blood–brain barrier (BBB) and pass into the CNS. Inside the brain, plasma B cells produce auto-antibodies against CNS self-antigens contributing to myelin sheath damage. This process is aggravated when infiltrated cytotoxic CD8+ T cells attack oligodendrocytes causing their destruction and neuronal death. Monocytes, on the other hand, increase local inflammatory response by releasing proinflammatory cytokines and contributing to demyelination through myelin phagocytosis. (4) In parallel, infiltrated CD4+ T cells are reactivated upon interaction with myelin fragments presented by resident APCs which favours (5) proinflammatory cytokines and chemokines release, (6) astrogliosis and microgliosis
Genetic, epigenetic and environmental factors
The robust susceptibility loci that confer risk for MS are the human leukocyte antigen (HLA) system, which is located in the short arm of chromosome 6 [22]. However, only 27% of MS heritability can be explained by the genetic variants of the HLA system [23], which supports a prominent contribution of the environment to the MS pathogenesis. Indeed, Epstein Barr virus (EBV) infection, tobacco smoking, vitamin D deficiency, diet style and sun light exposure are critically involved in MS susceptibility [24, 25]. The individual genetic background in combination with the environmental risk factors increase the probability of developing MS. Epigenetic modifications do not alter the sequence of DNA, and they comprise distinct mechanisms such as DNA methylation (DNAme), histone modifications and micro-RNA [26]. Given the scope of this review, we focus our efforts on describing the contribution of DNAme in MS.
DNA methylation
DNAme is the most current epigenetic hallmark in human somatic cells [27]. The methylation of DNA occurs when a methyl group is transferred to the fifth carbon of a cytosine (5mC) through the action of DNA methyltransferases (DNMT). This process occurs mainly in CG dinucleotides, which are commonly found in the regulatory and promoter regions [28]. The addition of methyl groups in the promoter region contributes to gene silencing [28]. In mammals, DNMTs are mainly represented by DNMT1, DNMT3a and DNMT3b. DNMT1 acts during the cell cycle to maintain the DNAme pattern [29] and participates in the DNA repair system [30]. By contrast, DNMT3a and DNMT3b catalyse the de novo addition of a methyl group into a naked cytosine [31]. This fact occurs in cooperation with either the specific transcription factors or the binding transcription factors, which methylate all the CpG sites uncovered [32].
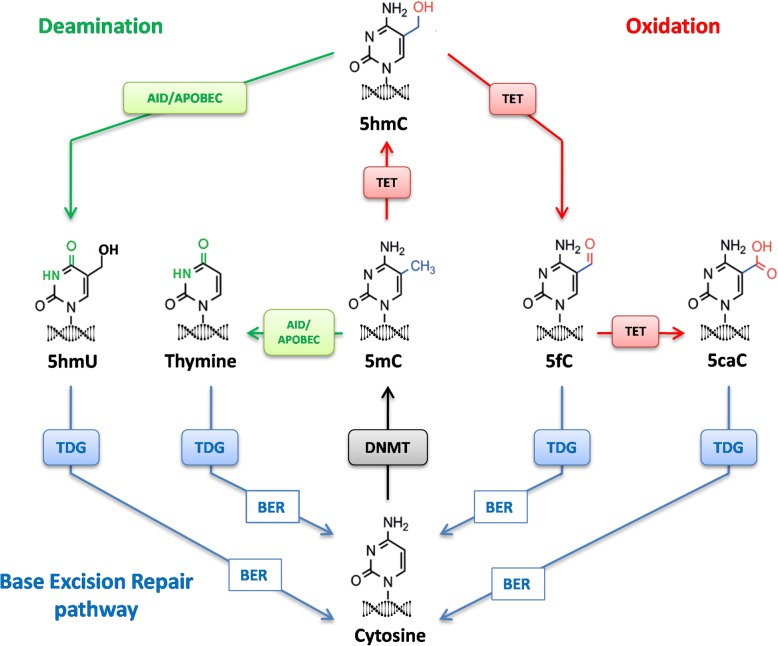
DNAme is a dynamic process that usually requires the removal of the methyl group (demethylation) to cope with environmental stimuli [33]. This process can be achieved in a passive or active manner. Passive DNA demethylation occurs when DNMT1 activity is misregulated and cannot maintain the integrity of the DNAme pattern during the DNA replication, leading to the incorporation of unmethylated cytosine into the genome [34]. Active DNA demethylation is achieved when a sequence of enzymatic reactions of oxidation and/or deamination modifies 5-methylcytosine (5mC) to obtain a naked cytosine. In the oxidation pathway, 5mC is oxidised by the ten–eleven translocation (TET) enzymes to 5-hydroxymethylcytosine (5hmC) [35], which can be further oxidised to 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) [36]. In the deamination pathway, AID or APOBEC deaminates 5hmC to 5-hydroxymethyluracil (5hmU) or 5mC to thymine [37]. Eventually, all these modified bases (5hmU, Thymine, 5fC, 5caC) can be recognised by thymine DNA glycosylase (TDG) [38] and converted to naked cytosine through the base excision repair pathway (Fig. 2).
Fig. 2.
DNA methylation metabolism. The addition of a methyl group to a naked cytosine is catalysed by DNMT (black arrow). 5-methylcytosine (5mC) is oxidised by TET enzymes to 5-hydroxymethylcytosine (5hmC) which can be further oxidised to 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) as indicated red arrows. In the deamination pathway (green arrows), AID or APOBEC can deaminate 5hmC to 5-hydroxymethyluracil (5hmU) or 5mC to thymine. Eventually, all these modified bases (5hmU, Thymine, 5fC, 5caC) are recognised by TDG and converted to naked cytosine through the base excision repair (BER) pathway (blue arrows)
Contribution of DNA methylation in patients with MS
Although the precise role of the DNAme in MS remains to be fully elucidated, several studies have reported differentially methylated regions in both the immune cells and brain tissue collected from MS patients (Table 1). Therefore, we summarise the current published research on DNAme as follows: BBB breakdown, inflammation, demyelination, remyelination failure and neurodegeneration.
Table 1.
DNA methylation changes in MS
| References | Comparison | Sample target | Method | Differentially methylated genes |
|---|---|---|---|---|
| [39] | MS vs CTR | CD8+ T cells | Illumina 450K array | ERG, FTL, DCAF4, NCAPH2, CDKN1C, ZNF462, CBX7, MIR492, HPS1, SASH1, MYL3, KCNG1, DYDC2, MEGF10, SP5, LMO3, SLC12A7, MORN1, IGF2BP1, PLCB3, ABCC4, CREG2, CDC42BPB, UGT1A10, TMEM125, ARHGAP22, DACH1, OR8B12, TMEM8C, BAI1, EIF2S1, CRTAC1, DHX36, C19orf41, DLGAP2, TNXB, PRDM8, HEATR2, WHSC2, CAMTA1, ALK, KCNQ2, SCTR, RHEB, LOC202181, RRP9, KRT75, DGKE, PLD5, ZC3H14. |
| [40] | RRMS vs CTR | CD4+ T cells | Illumina 450K array | MICA, MICB, HLA-DRB, MORN1, LCLAT1, PDCD1, MUC4, AHRR, ARSB, PCBD2, TGFBI, PCDHB13, PCDHB15, KIF25, CSGALNACT1, ADARB2, LDHAL6A, CORO1B, USP35, FUT4, ERC1, TCRA, PACS2, IL32, KCTD11, C17orf108, ARHGAP27, NPLOC4, SBNO2, GNG7, C21orf56, RIBC2. |
| [41] |
Myelinated vs demyelinated MS brains |
Hippocampus | Illumina 450K array | MLLT4, PPIF, SCRT2, SNRNP40, ISLR2, MEF2A, PMEPA1, ABCA4, ADAMTS12, AHRR, BEST3, CASP7, CCL4L2, CPXM2, FBXW8, HLA-B, LOC145845, MEIS1, MGMT, MYO7A, NXN, PKP2, PQLC1, PSD3, SCN4B, SDK2, SMYD3, TGFBI, TMEM165, PON1, HDLBP, MKKS, TRIM26, TRPS1, KRTAP27-1, MGP, AJAP1, C1orf106, C2orf62, DSE, EIF2C2, GATA5, HLA-B, IGSF9B, INSC, KIAA1026, KIF25, LOC100292680, NFASC, RASA3, SDK1, SHISA2, SOLH, SORBS2, TAGLN3, TBX5, TM9SF1, TOP1MT, ZSCAN1, AKNA, EBPL, FLJ42709, HERC6, OR52M1, SFRP1, C22orf43, LOC285830, NAPEPLD, NHLH2, PLCH1, SERPINA9, SLFN13, TMEM132B, TTLL3, WDR81. |
| [42] | SPMS vs CTR | PMBCs | Microarray dataset and RT-PCR | DNMT3A, GADD45A, GADD45B, MBD4, APOBEC3D, APOBEC4, GADD45G, TET1, TDG, APOBEC3C, APOBEC2, MBD2, MBD3, APOBEC3A, DNMT3B, APOBEC1, TET2, TET3. |
| [43] | RRMS vs PPMS vs CTR | PMBCs | Illumina 450K array | RRMS vs CTR: ASB2, ATP11A, CACNA2D3, CERS5, ESRRG, FRMD4A, GNAS, HOXC4-HOXC6, IFITM5, ILDR1, KCNK15, KLHL35, LEFTY2, PLEKHA2, RPH3AL, WRAP73, ZFYVE28.PPMS vs CTR: ATG16L2, CES1, CSGALNACT2, CYB5D1, LSMD1, FAM110A, GDF7, HKR1, HLA-F, HOXB13, IGSF9B, ILDR1, LDB2, MTPN, LUZP6, NTN1, OPCML, OR2L13, RBM46, TBX1, TCP10L, TMEM44, VIPR2, WRAP73. RRMS vs SPMS: ABCC5, AKAP12, CARS, CBFA2T3, CCDC67, FAM110A, FRMD4A, GIMAP5, HIVEP3, ICAM5, KCNQ1, KLF4, LEFTY2, OLFM3, PTH1R, RASA3, RNF39, RPH3AL, TRAF3, USP35, XKR5. |
| [44] | Smoker vs non-smoker MS | PMBCs | Bisulphite Illumina Methylation 450k Beadchip | SRM, GNG12, GFI1, ANXA4, NFE2L2, ABLIM2, AHRR, SMIM3, CDKN1A, TPST1, CNTNAP2, SNTG1, MTSS1, PTK2, ZC3H3, ZMIZ1, PTGDR2, PRSS23, GRIK4, ETV6, RARG, LOC348021, CCDC88C, ITPK1, ANPEP, RARA, SMIM6, RECQL5, F2RL3, LINC00111, ACOT9. |
| [45] | MS vs CTR | NAWM |
Direct BS-sequencing |
PAD2 |
| [46] | RRMS vs CTR | cfDNA (whole blood) | BS-PCR sequencing assay | MBP3, WM1. |
| [47] | MS vs CTR | NAWM | Bisulphite Illumina Methylation 450k Beadchip | ALDOA, ATP1A2, BCAR1, BRK1, CDK5, CORO1A, CSF3, DLC1, DTNBP1, FGD2, FMNL1, MLST8, MYBPC3, MYH6, MYH7, MYO1F, OBSCN, PDGFA, PRKCZ, SHC1, SIPA1L1, SSH3, TPM3, ADA, AGAP1, ALDOA, ARHGEF16, ATP1A1, ATP1A2, ATP1A4, ATP5H, BIN1, DAB2IP, DLC1, FGD2, LDHC, MACROD1, MLST8, MYBPC3, MYH6, MYH7, NME4, NT5C, PLXNB1, PTPRN2, RASA3, SEPT9, SIPA1L1, TBCD, TK1, ACSBG1, ACSL1, ACTR8, ADA, AGAP1, AGPAT1, AGRP, AKAP8, ALDH3A1, ALDOA, AMH, ANGPT2, APBB1IP, APEX, ARHGEF16, ATF6B, ATP11A, ATP1A1, ATP1A2, ATP1A4, ATP6V0E1, ATRIP, BBS2, BCAR1, BCL2L2, BIN1, BIRC5, BPI, BRD4, BRK1, C4B, CACNA1D, CASKIN1, CBX4, CCL17, CCL22, CD37, CD59, CDH1, CDK5, CHST3, CHURC1, CLASP1, CLIC5, CORO1A, CREB5, CRY2, CSF3, CSNK1E, CX3CL1, CXXC5, CYP21A2, DAB2IP, DAND5, DCPS, DHRS3, DLC1, DLL1, DOK4, DOT1L, DSCAML1, DTNBP1, DYRK1B, E2F6, E4F1, EDN2, EFS, ENTPD2, ERCC3, F7, FAM109A, FGD2, FGFR3, FMNL1, GBX1, GDF10, GPR114, GPR56, GTF2H1, GYLTL1B, HDAC11, HEG1, HEXIM1, HEXIM2, HIGD1A, HIST3H3, HLA-DMA, IL17RB, IL25, IL34, INO80E, INPP5J, INTS1, IRAK2, ITPKB, JARID2, LIMD1, LMF1, LPCAT1, MAB21L2, MADD, MAML3, MAP3K14, MAPK3, MBP, MCF2L, MED24, MEIS2, MLLT10, MLST8, MT1A, MT1E, MT1F, MT1G, MT1M, MT2A, MT4, MTCH1, MTSS1L, MUSK, MYBPC3, MYH6, MYH7, MYO1F, NARFL, NCOR2, NDRG1, NLRP3, NOTCH4, NR1H3, NUP210, OBSCN, OTX2, PABPN1, PAG1, PBX2, PCSK6, PDGFA, PEG10, PHF21A, PIK3R1, PLEKHG3, PLLP, PLXNB1, POLD4, POLR2C, POU2F1, PPARA, PPIL2, PPP1R13B, PPP4C, PRAM1, PRDM16, PRKCH, PRKCZ, PTGDS, PTPRN2, RAD9A, RAI1, RASA3, RBP1, RFX5, RIN2, RNF187, RPA1, RRM2, RXRA, SACS, SEMA4C, SETD1A, SHC1, SHISA5, SIPA1L1, SLC17A7, SLC22A17, SLC39A13, SLC7A8, SMAD6, SOX1, SOX8, SPI1, SPOCK2, SREBF1, SSH3, SSTR5, SUN1, TACC3, TBCD, TBX6, TEAD2, TEF, TEP1, THRA, TLN2, TNRC6C, TPM3, TRAF2, TSNARE1, UBE2L3, USP19, VAC14, WHSC1, WISP1, WISP2, WNK2, ZBTB47, ZFP1, ZIC1, ZNF135, ZNF256, ZNF329, ZNF362, ZNF414, ZNF418, ZNF488, ZNF606, ZNF664, ZNF687, ADAMDEC1, AIF1, AIRE, B2M, BPI, C1QA, C1QB, C1QC, C4BPA, C4BPB, CCR6, CD19, CD37, CD4, CD7, CD81, CFD, DLG1, FCER2, HAMP, HLA-DMA, HLA-DMB, HLA-DOA, HLA-DOB, HLA-DQA2, HLA-DQB2, HLA-F, IRF6, IRF8, IRF9, JAK1, JAK3, KYNU, LAG3, LAT, LBP, LCP2, LGMN, LST1, LTA, LTB, MBL2, MICB, NCR3, OSM, PSMB8, PTPN22, RARA, RNF31, SECTM1, SLAMF7, STXBP2, TAP1, TAP2, TAPBP, TNF, TNIP2, B2M, C1QA, C1QB, C1QC, C4BPA, C4BPB, DLG1, FCER2, HLA-DMA, LAG3, LTA, MBL2, NCR3, SLAMF7, TAP1, TAP2, TNF, B2M, C1QA, C1QB, C1QC, C4BPA, C4BPB, DLG1, FCER2, HLA-DMA, LAG3, LAT, LTA, MBL2, NCR3, SLAMF7, STXBP2, TAP1, TAP2, TNF, B2M, FCER2, HAMP, LAG3, MBL2, NCR3, SLAMF7, STXBP2, TAP1, TAP2, BHLHE23, CTSZ, DLG1, DLL1, DLX1, DLX2, EDARADD, EPHB4, FOXL2, GLI1, GNAS, HOXC11, HOXC13, HOXC4, HOXC8, HOXC9, HOXD10, HOXD11, HOXD13, HOXD3, HOXD4, HOXD8, HOXD9, MSX1, PHLDA2, PPP1R13L, PTCD2, RARA, RUNX3, SOX1, SOX8, TBX3, TEAD2, TGM1, TH, TNF, TWIST1, WNT2, ZIC1. |
| [48] | RRMS and CTR |
CD4+ T cells CD8+ T cells Whole blood |
Illumina 450K array | CD4+T cells: DCX, RDH13, DNHD1, TEKT5, TXNL1, MAGI2, TTC30B, APC2, TMEM48, ANGPTL2, RALGPS1, USP29, C20orf151, DLL1 6, DACH2, INPP5A, LOC727677, SEMA5B, SUGT1L1, HOXB2, OR10J5, RBMS1, C20orf151, AEN. CD8+T cells: APC2, HOXA2, HRNBP3, HEXDC, NTRK3, DCX, TRIL, ARHGEF17, ESPNP, LHX5, TEKT5, LRRC43, CYP27C1, TMEM48, HHATL, AMMECR1, C19orf45, SRRM3, PSD3, PTPRN2, LOC654342, ARHGEF17, DNHD1, KIF1C, INCA1, VSIG1. Whole blood: DACH2, LAMA2, TTLL8, GALNT9, POU3F4, NLRP12, PLS3, ANKRD1, CLSTN2, MAGEB4, APC2, PCDHA7, TMEM27, DNHD1, LGI1, PTCHD2, MMD2, HHATL, TMEM48, NXPH1, TDRD9, CDX1, YTHDC2, RGPD1, PLGLB2. |
| [49] | RRMS vs PPMS vs SPMS vs CTR | Buffy coat | BS-sequencing | SHP-1 |
| [50] | MS vs CTR |
Whole blood PBMCs NAWM |
Illumina 450K array | IL2RA |
| [51] | MS treatment-naïve vs 1 year IFN-b vs CTR | PMBCs | BS-PCR sequencing assay | LINE-1 |
| [52] | Discordant twins (RRMS vs CTR) | CD4+ T cells | RRBS | TMEM1, PEX14. |
| [53] |
RRMS(e)vs RRMS(r) vs CTR |
Serum | BS-PCR sequencing assay | MOG |
| [54] | RRMS vs CTR | cfDNA (serum) | BS-PCR sequencing assay | LINE-1 |
| [55] | RRMS vs CTR | CD3+ T cells | BS-PCR sequencing assay | VDR |
| [56] |
RRMS(e) vs RRMS(r) vs CTR |
cfDNA (plasma) |
MethDet-56 microarray based assay |
RRMS(r) vs CTR: CDH1, CDKN2A, CDKN2B, FAS, ICAM1, MCJ, MDGI, MUC2, MYF3, PAX5, PGK1, RB1, SOCS1, SYK, TP73. RRMS(e) vs CTR:BRCA1, CCND2, DAPK, FAS, FHIT, MCT1, MDGI, MCJ, CDKN2A, TP73, PGK1, PR-PROX.RRMS(r) vs RRMS(e): CDH1, CDKN2B, HIC1, PR-PROX, SYK. |
| [57] |
RRMS(e) vs RRMS(r) vs CTR |
Whole Blood | Methylation-Specific Multiple Ligation Probe Amplification PCR | CDKN2A, SOCS1, RUNX3, NEUROG1. |
| [58] |
Discordant twins (MS vs CTR) |
PMBCs CD4+ T cells |
Bisulphite Illumina Methylation 450k Beadchip | TMEM232, SEMA3C, YWHAGI, ZBTB16, MRI1. |
| [59] |
RRMS and SPMS vs CTR |
PMBCs | BS-PCR sequencing assay | PAD2 |
| [60] |
RRMS and SPMS vs CTR |
PMBCs | EpiTyper assay | DNMT1, TET2 |
| [61] |
RRMS vs SPMS vs CTR |
CD4+ T cells | Illumina 450K array | VMP1, MIR21 |
MS multiple sclerosis, CTR control, RRMS relapsing–remitting multiple sclerosis, PPMS primary progressive multiple sclerosis, SPMS secondary progressive multiple sclerosis, RRMS(e) RRMS in exacerbation, RRMS(r) RRMS in remission, cfDNA circulating-free DNA, PBMCs peripheral blood mononuclear cells, BS bisulphite, RRBS reduced representation bisulphite sequencing, NAWM normal appearing white matter
BBB breakdown
Infiltration of the autoreactive proinflammatory cells across the BBB into the brain is one of the pathological features of MS [62]. The BBB is a selective semi-permeable endothelium that separates the CNS from the circulating blood. This barrier is composed of a monolayer of endothelial cells tightly bound mainly by cadherins [63] and intercellular adhesion molecule (ICAM) proteins [64]. Cadherins are calcium-dependent adhesion molecules importantly involved in cell–cell adhesion [63]. The disruption of cell–cell interaction mediated by cadherins leads to BBB permeability [63]. A hypermethylated pattern of E-cadherin (CDH1) may increase the BBB permeability in relapsing–remitting MS (RRMS) patients favouring lymphocyte infiltration into the brain, and lastly, disease progression [47, 56]. The other adhesion molecules expressing on the BBB endothelium are the ICAM family. In particular, ICAM-1 is essential for leukocyte crawling prior to diapedesis from the bloodstream to the CNS [65] and plays a remarkable role in T cell proliferation [66]. Liggett et al. (2010) reported a hypermethylation pattern for ICAM1 in cell-free plasma DNA derived from RRMS patients in response to clinical remission, indicating an impairment of the T cell extravasation into the brain as a consequence of immune response mitigation [56]. These findings are in accordance with the results reported in knockout mice for Icam1 subjected to the experimental autoimmune/allergic encephalomyelitis (EAE) model [66].
Inflammation
The first inflammatory event in MS is conducted when APC through the class II MHC complex presents a specific antigen to naïve CD4+ T cells, which favour T cell differentiation and the recruitment of proinflammatory cells into the CNS [5]. MHC, also known as human leukocyte antigen (HLA), is responsible for presenting non-self-antigens to the T cell receptors and natural killer receptors (NKRs) [67] facilitating the inflammatory response. Leukocytes use the HLA complex to distinguish self-proteins from exogenous components [68]. In MS, certain HLA genes showed an aberrant methylation pattern contributing to MS aetiology [47]. For example, the hypomethylation of MHC class I polypeptide-related sequence B (MICB) has been reported in normal appearing white matter (NAWM) [47] and CD4+ T cells in MS patients [40]. In MS, a ligand codified by MICB activates the NK and CD8+ T cell destruction [69]. Similarly, the HLA-F variant is actively expressed in the inflammatory reaction [67] as a result of its promoter demethylation [43, 47]. Aside from the HLA complex, changes in DNAme are found in other inflammatory pathways reported in MS. Specifically, global CG island hypermethylation of the Src homology region 2 domain-containing phosphatase-1 and the suppressor of cytokine signalling 1 might aggravate the course of MS through the overactivation of the immune-mediated response [49, 56, 57]. Adhesion molecules such as ICAM5 are markedly present in the cerebral and hippocampal neurons [70]. In MS, the extracellular domain of ICAM5 is cleaved and released into the cerebrospinal fluid (CSF) and blood, where it modulates the synthesis of proinflammatory cytokines (TNF-α, IL-1β), stimulates the expression of anti-inflammatory cytokine IL-10 and represses T cell activation [71]. As a result of disease progression, primary progressive MS (PPMS) patients with non-recoverable demyelination and neurodegeneration showed higher methylation levels for ICAM5 than RRMS patients [43]. This result suggests that an overactivation of the inflammatory response in MS may be attributable to the aberrant methylation pattern of certain anti-inflammatory genes.
Demyelination
In MS, demyelination occurs when the myelin sheath of neurons is damaged by the action of the immune system [72]. The attack of immune surveillance is mainly directed against the myelin basic protein (MBP) [73], a protein that stabilises and maintains the correct structure of the myelin sheath around the axon [74]. An extensive hippocampal demyelination simultaneously coincides with the lower number of methyl groups to the DNMT promoters with an increase in their mRNA levels and a decrease in their TET enzymes [41]. Under normal conditions, approximately 20% of the total MBP is citrullinated (MBP-Cit). Citrullination is a post-translational modification catalysed by peptidyl arginine deiminase 2 (PAD2) [75]. The addition of citrullin groups leads to the loss of myelin compaction [76], and particularly, the percentage of MBP-Cit increases drastically [77] along with the promoter demethylation and overexpression of PAD2 as a result of the clinical course [45, 59]. The processing of MBP self-antigens and their presentation by APCs to T cells occurs during the negative selection of autoreactive T cells in the thymus [78]. An increase in the legumain (LGMN) activity, an enzyme involved in the self-antigen processing, prevents the development of immune tolerance against MBP [79]. Interestingly, the demethylation of the LGMN promoter could be responsible for favouring autoimmunity in MS patients [47, 74].
Remyelination failure
Following the myelin destruction, the recruitment of oligodendrocyte progenitor cells (OPCs) is necessary to rescue the demyelinated axons [80]. However, in MS patients, this process is not completely achieved [81], contributing to progressive neurodegeneration. The origin of this failure is not fully understood, but some hypotheses have been postulated in this regard [81]. Briefly, remyelination may be incomplete because of the inadequate recruitment of OPCs into the demyelinated lesion, an impairment of the OPC differentiation into myelinating oligodendrocytes, or the dysfunctions in oligodendrocytes when they attempt to wrap axons [81, 82]. In adults, OPC migration and recruitment require several growth factors including the platelet-derived growth factor (PDGF) and the fibroblast growth factor (FGF) [83–85]. Both growth factors are significantly methylated in the NAWM of MS patients [47]. In this regard, the addition of methyl groups to the DNA may be accompanied by the lower expression of PDGF and FGF during disease progression, thus gaining mechanistic insight into the oligodendrocyte dysfunction. Wnt signalling pathway is involved in the differentiation of precursor oligodendocytes into mature myelinating oligodendocytes, affecting remyelination of axons [86]. Fancy et al. (2009) found that MS demyelinating lesions display an activation of the Wnt signalling impairing the remyelination process due to lack of proliferation of premyelinating oligodendrocytes [86]. Interestingly, some of the negative regulators of the Wnt pathway (histone deacetylase inhibitors, or Shisha genes) [87, 88] are hypermethylated in the brain [47, 41]. This hypermethylation would facilitate Wnt pathway activation declining efficiency of endogenous remyelination. This hypothesis is reinforced by Chen et al. (2011). They reported that mice lacking HDAC1/2 displayed severe myelin deficiency [89]. The hypermethylation of neurofascin (NFAS) has been related to the improper myelin wrapping of paranodal segments and might respond to the pathophysiological mechanisms underlying MS [90, 91]. Self-antibodies against NFAS have been detected in MS patients [92]. In the hippocampus while neurodegeneration is ongoing, Chomyk et al. (2017) reported a decrease in the WD repeat domain 81 and AT-hook transcription factor methylation status, which could lead to a lower mRNA expression and neuronal injury [41].
Neurodegeneration
Progressive axonal loss contributes to brain atrophy and neurological disability [93]. Kulakova et al. (2016) examined the DNAme of the peripheral blood mononuclear cells (PBMCs) collected from secondary progressive (SPMS) and RRMS patients, providing a time course DNAme pattern [43]. They found a cluster of 21 differentially methylated genes. Gene ontology analysis revealed that four of these genes are hypermethylated while neurodegeneration is ongoing and involved in biological processes, such as cell proliferation (PTH1R, CBFA2T3, KLF4) and nuclear factor κ-light-chain-enhancer of activated B cell (NF-kβ) pathway (KLF4, TRAF3). NF-kβ is a pleiotropic regulator of neuroinflammation, neuronal protection and neurotoxicity [94] involved in distinct pathological events mediated by the glia once the earliest signs of MS are present [95]. During the course of neurodegeneration, an imbalance between the expression of enzymes participating in the addition and removal of methyl groups to the DNA has been reported in MS patients. Indeed, Fagone et al. (2016) found an upregulation of methyl CpG-binding protein 2 and 4 and a downregulation of enzymes such as TET3 and TDG in SPMS patients [92].
Environmental and lifestyle risk factors
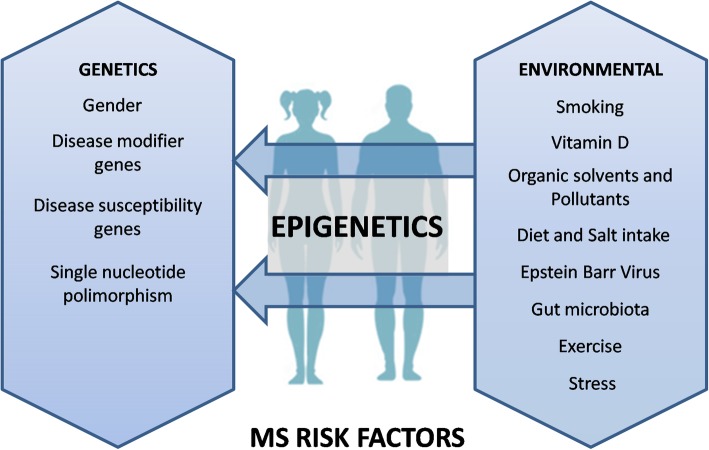
The influence of the environment on MS pathophysiology has been largely studied in twins discordant for the disease [52]. This divergence has been attributed to the effect of environmental risk factors through epigenetic modifications [96]. Thus, Handel et al. (2010) reported an additive deleterious effect of smoking, ultraviolet (UV) exposure and HLA alleles on MS prevalence [20]. Scientific evidence shows that environmental factors, such as smoking, Epstein Barr infection, vitamin D level, organic solvent and chemical pollutant exposure, diet style, gut microbiota, exercise and stress, are involved in the development and/or course of MS (Fig. 3).
Fig. 3.
Risk factors of multiple sclerosis. MS pathogenesis is influenced by both genetic and environmental factors. Among the genetic factors, gender, disease-modifier genes, disease susceptibility genes and single nucleotide polymorphisms are remarkably important in prevalence and MS pathogenesis. In contrast, environmental factors such as smoking, vitamin D deficiency, organic solvents and pollutants exposure, diet style, Epstein Barr infection, dysbiosis of the gut microbiota, lack of exercise and stress are critically associated with MS susceptibility and progression
Smoking
Smoking confers the risk for developing MS, and it is associated with disease onset and progression [97]. Degelman and Herman (2017) found a significant association between smoking frequency and conversion from RRMS to SPMS forms [98]. Nearly 98 chemical compounds of tobacco, including nicotine, cyanide and nitric oxide, are hazardous [99]. For example, nicotine increases the permeability of the BBB [100] in the earlier stages of MS [101], cyanide contributes to CNS demyelination [102] and nitric oxide promotes neurodegeneration [103]. Tobacco contains dioxins that activate the aryl-hydrocarbon receptor pathway [104], which modulates neuroinflammation [105] and Th17 and Treg activities [106] becoming a key player in the MS aetiology and disease progression. A well-designed study performed by Zeilinger et al. (2013) reported the differences in the DNAme of current, former and never smokers [107]. They found that the aryl-hydrocarbon receptor repressor (AHRR) was highly demethylated among the current smokers, leading to the inhibition of AhR signalling pathway and thus enhancing neuroinflammatory and neurodegeneration events [105, 106]. AhR can act as protective or deleterious pathway depending on the cell type where is expressed. In EAE model, AhR activation has a protective role in dendritic cells (DC), astrocytes and foxp3+ T reg cells, while it has a pro-inflammatory effect in Th17 cells [108]. AhR deletion is known to exacerbate EAE disease [109], altering myelin-associated proteins and increasing the production of proinflammatory cytokines [110]. In MS patients, low levels of AhR have been measured in serum, along with a reduced AhR activity in demyelinating lesions during disease progression [111]. Interestingly, Laquinimod, a phase III drug for MS treatment that activated AhR pathway, was shown to reduce brain atrophy in MS patients by counteracting the neuroinflammatory reaction [112].
Epstein Barr virus
The latent form of EBV is present in almost 90% of the world population within memory B cells [113]; more than 99% of MS patients are seropositive for EBV [24]. Infection with EBV increases the risk of developing MS by ~ 3.6-fold [114], and its effect can be exacerbated when interacts with other risk factor, such as HLA risk variants, rocketing the odd ratio for MS up to ~ 15-fold [115]. We can speculate that a cross-reaction may occur between the myelin self-antigens and certain viral proteins of the EBV in MS [116]. This hypothesis is underpinned by the identification of two EBV peptides (EBNA-1 and BRRF2) in the CSF of MS patients [117]. Indeed, the CD8+ T cells derived from MS patients displayed reactivity against some latent EBV proteins [117]. The reactivation of latent EBV in memory B cells may occur through specific epigenetic modifications. For example, the use of 5-azacytidine, a DNMT inhibitor, can switch the EBV latent form to a reactivated form [118].
Vitamin D
Vitamin D is synthesised upon exposure to UV [119] or can be obtained from diet [120]. Vitamin D deficiency is considered a risk factor for MS even before birth, altering the structure of embryonic tissues as a result of low maternal vitamin D [121]. Indeed, the prevalence of MS is lower near the equator, where UV radiation is at its maximum, than at higher and lower latitudes [122]. Low levels of vitamin D have been associated with a higher frequency of relapses [123] and disability [121].
Current evidence points out that the active form of vitamin D acts as an inducer of the promoter demethylation of multiple genes [124]. In accordance with these findings, Rawson et al. (2012) reported that a high intake of vitamin D is associated with lower methylation levels of certain genes involved in the Wnt signalling pathway [125], which could favour neurogenesis and neuronal plasticity [126]. In T cells from RRMS whole peripheral blood, the vitamin V receptor (VDR) showed a promoter demethylation pattern associated with an overexpression of VDR mRNA [55]. Nevertheless, the underlying mechanism behind vitamin D and its effect on demethylation remains unknown and requires further investigation.
Organic solvents and pesticides
Organic solvents are hydrocarbon compounds commonly used worldwide. The prolonged exposure to these compounds has severe effects on health and may be clinically important in autoimmune diseases [127]. Organic solvents are highly hydrophilic and lipophilic molecules able to pass through the BBB into the brain, resulting in distinct myelin pathologies [128]. Exposure to organic solvents induces changes in the DNAme of the immune system [129] and certain genes involved in cell survival [130]. Hexachlorobenzene, a pesticide widely used until 1965, modulates the expression of E-cadherin through the activation of the integrin-linked kinase signalling [131]. E-cadherin plays an important role in BBB integrity, and its promoter region is highly methylated in MS patients [49], facilitating the infiltration of immune cell into the brain. To evaluate the precise contribution of this pesticide in the context of MS, we should determine the exposure time frame and if it occurred before or after the diagnosis of disease. Remarkably, the precise effect of organic solvents on DNAme in MS warrants further investigation. However, exposure to organic solvents and the presence of HLA risk alleles present a fourfold increased risk for MS in comparison with exposure to organic solvents alone, and the additive effect of organic solvents, HLA risk alleles and smoking increase the risk 20-fold [132].
Diet
Recent evidence points out that food intake is important in the pathogenesis of MS and other autoimmune diseases. Seasonal variations in food intake in pregnant mothers may interfere with foetal development and confer the risk of MS in the later life of the offspring (for a review, see [133]). The intake of some nutrients, such as long-chain fatty acids and salt, is known to act as pro-inflammatory molecules, whereas other nutrients, such as short-chain fatty acids and flavonoids, possess anti-inflammatory properties [134]. A type of flavonoid called quercetin represses the capacity of monocytes to cross the BBB [135] and reduces the synthesis of proinflammatory cytokines produced by monocytes in MS [136]. Limited data are available on the intake of nutrients and their effect on DNAme. However, certain cofactors obtained from diet are required to maintain DNAme homeostasis. For example, vitamin B, folate, methionine, choline and zinc are essential to maintain the levels of Dnmt1 [137] and methyl-donor S-adenosylmethionine [137]. By contrast, vitamin C can act as a cofactor for TET enzymes [138]. In particular, vitamin B12 is necessary for the proper function of the CNS through the conversion of homocysteine to methionine. Methionine regulates the expression of DNA methyltransferase 3A (DNMT3A) [139] and participates in the transcription of pro-inflammatory genes and the formation of myelin sheath [140]. Interestingly, vitamin B12 and folate deficiency is reported in RRMS concomitantly with elevated levels of homocysteine. A misbalance in DNAme metabolism has been reported in MS patients [139, 141]. Indeed, some authors found that the levels of methyl group donors were lower in plasma [139] and post-mortem grey matter [141] in MS.
The elevated consumption of salt has many noxious effects, including the production of reactive oxygen species [142], deregulation of regulatory T cells [143] and macrophage [144], disruption of BBB permeability [145] and higher probability of new brain lesions in RRMS patients [146]. After the ingestion of a diet containing high levels of salt, patients with an autoimmune disease showed demethylation and elevated levels of hydroxymethylation in CD4+ T cells as a result of TET2 overexpression [25]. Consequently, the high consumption of salt may be a risk factor for MS. Note that the use of standardised diets or alternative therapies cannot substitute conventional MS treatments, but the intake of healthy food may ameliorate the inflammatory and physical status of MS patients. Therefore, it can be hypothesised that some components of diet may interfere with the DNAme metabolism even before childbirth or during lifetime contributing to both disease aetiology and progression.
Gut microbiota
Gut microbiota is a complex ecosystem of microorganisms that establish a symbiotic relationship with the host by favouring the vitamin production and fermentation of some components of diet [147]. However, the dysbiosis of microbiota increases the risk of developing autoimmune diseases [148, 149]. DC from germ-free mice subjected to the EAE model were less reactive to stimulated proinflammatory T cells than conventionally colonised germ mice [149]. Berer et al. (2011) found that gut microbiota in cooperation with myelin auto-antigens is necessary to stimulate the immune response [148], consistent with [149]. Dysbiosis has been associated with an inflammatory phenotype in MS patients [150, 151]. Resident microbiota can alter the epigenetic signature of the host through the production of specific metabolites (Table 2). Pregnant women showed a different DNAme profile according to their predominant microbiota in the gut [166]. Therefore, dysbiosis of microbiota can be involved in disease onset by an overactivation of T cells [148, 149] or exacerbating inflammatory events in patients diagnosed with MS [150, 151]. However, the contribution of microbiota in disease aetiology and progression warrants further investigation.
Table 2.
List of metabolites released by microbiota
| Metabolite | Effect on DNA methylation |
|---|---|
| p-Cresol | It induces the expression of DNA methyltransferases 1, 3a, and 3b and it is associated with CpG hypermethylation of Klotho gene [152], a regulator of vitamin D metabolism [153]. |
| Hydrogen sulphide (H2S) | Involved in the neutralisation of ROS. It increases DNA methylation [154]. |
|
Riboflavin (vitamin B2) Pyridoxine (vitamin B6) Cobalamin (vitamin B12) |
Cofactor involved in DNA methylation metabolism [155, 156]. |
| Folate (vitamin B9) | It acts as a methyl donor involved in DNA methylation metabolism [155, 156]. |
| It reduces the activity of DNA methyltransferase [157]. | |
| Choline | It acts as a methyl donor that can be recruited by human gut microbiota, reducing its availability [158]. |
| Involved in DNA methylation and gene expression in murine colitis model, an inflammatory disease [159]. | |
| Betaine | It acts as a methyl donor involved in DNA methylation reactions [156, 160]. |
| Associated with changes in DNA methyltransferases and coupled with changes in DNA methylation [161]. | |
| Ammonium (NH4) | Inverse correlation between faecal NH3 and LINE-1 gene methylation [162]. |
| α-ketoglutarate | Involved in (de)methylation as a co-factor of histone demethylases and TET family [163, 164]. |
| L-ascorbic acid (vitamin C) | It exerts a strong influence on active DNA demethylation. It enhances TET-mediated generation of 5-hydroxymethylation [165]. |
ROS reactive oxygen species, NH3 ammonia, LINE-1 long interspersed element-1, TET ten–eleven translocation
Adapted from Mischke et al. [147]
Physical activity
Physical exercise has been demonstrated to produce changes in the leukocyte DNAme pattern [167] and thus changes in gene expression [168]. In the context of neurodegenerative diseases, the influence of physical exercise has a direct effect on the brain-derived neurotrophic factor (BDNF) [169, 170]. In a healthy brain, BDNF is mainly expressed in neurons [171]. However, following a demyelinating insult, this gene is transcriptionally active in astrocytes [172], regulatory T cells, B cells and monocytes [172, 173], favouring brain plasticity [174] and myelin formation [175]. Recently, Briken et al. (2016) found elevated protein levels of serum BDNF after 30 min of exercise in progressive MS patients [170], and this result was probably attributable to the demethylation of its promoter region [169]. Similarly, 2 weeks of physical exercise promotes the overexpression of TET1 and the demethylation of the vascular endothelial growth factor A (VEGF-A) [176]. VEGF-A may potentiate neurogenesis and neuroprotection in the EAE model as postulated by [177]. The overexpression of apoptosis-associated, speck-like protein containing a C-terminal caspase recruitment domain gene (ASC) activates inflammatory signalling and may exacerbate MS progression [178]. In addition, Nakajima et al. (2010) found that following 6 months of moderate exercise, the mRNA levels of ASC were lower because its promoter region was hypermethylated [179]. Therefore, the current data support the notion that moderate exercise can reduce pro-inflammatory cytokines and improve the clinical MS course. However, some studies failed to validate this evidence [180–182]. The findings reported here point out that moderate exercise can ameliorate some symptoms but cannot stop the progression of MS.
Stress
Stressful life events have a negative effect on MS by increasing the risk of clinical exacerbation and disease progression [183]. However, the contribution of stress in the pathophysiology of the disease remains under discussion. A well-designed study by Liu et al. (2009) reported a strong association between stress and MS aetiology [184]. In accordance with these findings, Babenko et al. (2015) demonstrated that prenatal stress causes a demethylation of the nuclear subfamily 3 group C member 1 glucocorticoid receptor (NR3C1) [185] and changes in the nervous, immune and musculoskeletal systems [186]. Interestingly, the differences in NR3C1 gene expression have been reported in MS patients [187], suggesting that early life stressors can present susceptibility to developing MS in adulthood.
DNA methylation in animal models of MS
None of the current experimental animal models can reproduce the complexity and heterogeneity of MS. In particular, both disease onset and clinical course in animals differ considerably from those in humans [188]. However, in vivo experimental models are widely used to understand certain aspects of the disease. In general, we consider three models to study MS pathophysiology: (a) EAE, (b) Theiler’s murine encephalomyelitis virus (TMEV) and (c) use of toxins such as cuprizone (CPZ) or lysolecithin. However, not all of them have been addressed to study the changes in DNAme. For example, no current study has been conducted on DNAme in the TMEV model.
EAE model
EAE is a well-established model of autoimmunity induced by the subcutaneous injection of self-antigens derived from myelin proteins, such as the myelin oligodendrocyte glycoprotein (MOG) [189] and the proteolipid protein (PLP) [190]. Catanzaro et al. (2016) characterised the DNAme profile in the striatum of EAE mice showing a global DNA hypomethylation of interneurons positive for neuronal nitric oxide synthase [191]. Interestingly, they found a demethylation of Ras-related protein-1 (Dexras-1) in parallel with elevated levels of iron inside the cells and thus neurotoxicity and neuronal death. Furthermore, they reported that the hypomethylation of Dexras-1 was reverted when mice were subjected to an enriched environment in their home cage, emphasising an epigenetic-mediated effect [191]. Recently, Noori-Zadeh et al. (2017) found that the promoter region for forkhead box P3 was hypermethylated in T cells collected from EAE mice [192], thus indicating a dysfunction of regulatory T lineage and the lack of auto-immune tolerance [193].
Toxin-induced demyelination
Demyelination can be induced by copper-chelating agents (e.g. CPZ) or lysolecithin [194]. In the CPZ model, young adult mice fed with this neurotoxicant showed a significant loss of mature oligodendrocytes, astrocytosis, microgliosis and demyelination, followed by spontaneous remyelination [195]. The CPZ model was used by Olsen et al. (2019) to identify novel biomarkers related to the demyelination course [53]. Specifically, they isolated circulating-free DNA from mice blood at the end of the CPZ treatment, and identified a specific methylation pattern associated with oligondendrocyte apoptosis. Conversely, the use of lysolecithin in mice induced demyelination accompanied by a high expression of DNMT1 in the OPCs at the early stages of remyelination. By contrast, DNMT3A is highly expressed in oligodendrocytes at the later stages when remyelination is achieved. The study revealed a global hypermethylation in the oligodendrocyte lineage during remyelination, demonstrating that DNMT1 plays a crucial role in the proliferation and differentiation of OPCs into mature oligodendrocytes, while DNMT3A has a dominant role in the remyelination phase [196].
Conclusions
MS is an inflammatory autoimmune disease of the CNS caused by a complex interaction between genetic and environmental factors [20]. Emerging evidence indicates that DNAme actively participates in gene x environment interactions [33]. As previously mentioned, several studies showed an aberrant DNAme profile in relapsing–remitting forms and in progressive MS forms. Remarkably, most of the studies reported in this work were based on the bisulphite technique (Table 1). However, this approximation does not discriminate between 5-methylcytosine and 5-hydroxymethylcytosine and thus may contribute to a misinterpretation of the data. To avoid this bias, we recommend an alternative method for studying DNAme, such as the methylated-DNA immunoprecipitation and the TET-assisted bisulphate sequencing. As far as we know, genetic factors can explain approximately 30% of worldwide MS prevalence [23], and the remaining 70% may correspond to the influence of environmental risk factors. As described in this study, UV radiation, cigarette smoking and infection with the Epstein Barr virus are clinically relevant for MS, although other environmental factors, such as diet style, microbiota profile, exposure to organic solvents and pollutants, exercise and long-term stress, have a clear effect in MS. All of the aforementioned risk factors can modify the DNAme pattern in humans, but further studies are required to expand our knowledge of the molecular basis of the disease and elucidate the underlying mechanisms behind MS pathophysiology. Furthermore, DNA methylation is currently the best surrogate marker for epigenetic change in disease, because methylation alterations track with disease state. DNA methylation markers can also indicate success or failure of drug treatment, are stable in isolated DNA, and can be measured by a variety of quantitative and qualitative methods. We postulate that epigenetic DNAme marks described in the context of the disease can potentially be used in a specific, substantial, and credible way in clinical interventions. It is conceivable that, in the near future, we will be able to design drugs modifying DNAme metabolism to stop the progression of MS.
Acknowledgements
Not applicable.
Abbreviations
- 5caC
5-Carboxylcytosine
- 5fC
5-Formylcytosine
- 5hmC
5-Hydroxymethylcytosine
- 5hmU
5-Hydroxymethyluracil
- 5mC
5-Methylcytosine
- AHRR
Aryl-hydrocarbon receptor repressor
- APC
Antigen presenting cells
- ASC
Apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain
- BBB
Blood–brain barrier
- BDNF
Brain-derived neurotrophic factor
- BER
Base excision repair
- BS
Bisulphite
- CDH1
E-cadherin
- cfDNA
Circulating-free DNA
- Cit
Citrullination
- CNS
Central nervous system
- CPZ
Cuprizone
- CSF
Cerebrospinal fluid
- CTR
Control
- CYP1A1
Cytochrome P450 family 1 subfamily A member 1
- DC
Dendritic cell
- Dexras-1
Ras-related protein-1
- DNAme
DNA methylation
- DNMT
DNA methyltransferases
- EAE
Experimental autoimmune/allergic encephalomyelitis
- EBV
Epstein Barr virus
- FGF
Fibroblast growth factor
- FOXP3
Forkhead box P3
- H2S
Hydrogen sulphide
- HCB
Hexachlorobenzene
- HDACs
Histone deacetylase inhibitors
- HLA
Human leukocyte antigen
- ICAM
Intercellular adhesion molecules
- IFN-γ
Interferon-gamma
- ILK
Integrin-linked kinase
- LCFA
Long-chain fatty acids
- LGMN
Legumain
- LINE-1
Long interspersed element-1
- MBD
Methyl CpG-binding protein
- MBP
Myelin basic protein
- MeDIP
Methylated-DNA immunoprecipitation
- MHC
Major histocompatibility complex
- MICB
MHC class I polypeptide-related sequence B
- MOG
Myelin oligodendrocyte glycoprotein
- MS
Multiple sclerosis
- NaCl
Salt
- NAWM
Normal appearing white matter
- NFAS
Neurofascin
- NF-kβ
Nuclear factor k-light-chain-enhancer of activated B cells
- NH3
Ammonia
- NH4
Ammonium
- NKRs
Natural killer receptors
- nNOS
Neuronal nitric oxide synthase
- NR3C1
Nuclear subfamily 3 group C member 1 glucocorticoid receptor
- OPCs
Oligodendrocyte progenitor cells
- PAD2
Peptidil arginine deiminase 2
- PBMCs
Peripheral blood mononuclear cells
- PDGF
Platelet-derived growth factor
- PLP
Proteolipid protein
- PPMS
Primary progressive multiple sclerosis
- ROS
Reactive oxygen species
- RRBS
Reduced representation bisulphite sequencing
- RRMS(r)
RRMS in remission
- RRMS
Relapsing–remitting multiple sclerosis
- RRMS(e)
RRMS in exacerbation
- SAM
Methyl-donor S-adenosylmethionine
- SCFA
Short-chain fatty acids
- SHP-1
Src homology region 2 domain-containing phosphatase-1
- SOCS1
Suppressor of cytokine signalling 1
- SPMS
Secondary progressive multiple sclerosis
- TAB-seq
TET-assisted bisulphate sequencing
- TCRs
T cell receptors
- TDG
Thymine DNA glycosylase
- TET
Ten–eleven translocation
- Th
T helper
- TMEV
Theiler’s murine encephalomyelitis virus
- VDR
Vitamin D receptor
- VEGF-A
Vascular endothelial growth factor A
- Vit D
Vitamin D
Authors’ contributions
NC and JTR researched the literature and drafted the manuscript. JTR critically reviewed and edited the work. Both authors read and approved the final manuscript.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This review was funded by the Deutsche Forschungsgemeinschaft to Dr. Jordi Tomas Roig (ref. TO 977/1-1) and the University of Girona to Mrs Naiara Celarain Sanz (ref. IFUdG2017).
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Naiara Celarain, Email: ncelarain@idibgi.org.
Jordi Tomas-Roig, Email: jtomas@idibgi.org.
References
- 1.Ghasemi N, Razavi S, Nikzad E. Multiple sclerosis: pathogenesis, symptoms, diagnoses and cell-based therapy citation. Cell J. 2017;19:1–10. doi: 10.22074/cellj.2016.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Stefano N, Narayanan S, Francis GS, Arnaoutelis R, Tartaglia MC, Antel JP, et al. Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Arch Neurol. 2001;58:65–70. doi: 10.1001/archneur.58.1.65. [DOI] [PubMed] [Google Scholar]
- 3.Compston A, Coles A. Seminar multiple sclerosis. 2008. [Google Scholar]
- 4.Jennum P, Wanscher B, Frederiksen J, Kjellberg J. The socioeconomic consequences of multiple sclerosis: a controlled national study. Eur Neuropsychopharmacol. 2012;22:36–43. doi: 10.1016/j.euroneuro.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi R, Laroni A, Weiner HL. Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2010;221:7–14. doi: 10.1016/j.jneuroim.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sattler Arne, Wagner Ulf, Rossol Manuela, Sieper Joachim, Wu Peihua, Krause Andreas, Schmidt Wolfgang A., Radmer Sebastian, Kohler Siegfried, Romagnani Chiara, Thiel Andreas. Cytokine-induced human IFN-γ–secreting effector-memory Th cells in chronic autoimmune inflammation. Blood. 2009;113(9):1948–1956. doi: 10.1182/blood-2008-02-139147. [DOI] [PubMed] [Google Scholar]
- 7.Young HA, Hardy KJ. Role of interferon-γ in immune cell regulation. J Leukoc Biol. 1995;58:373–381. doi: 10.1002/jlb.58.4.373. [DOI] [PubMed] [Google Scholar]
- 8.Takeshita Y, Ransohoff RM. Inflammatory cell trafficking across the blood-brain barrier: chemokine regulation and in vitro models. Immunol Rev. 2012;248:228–239. doi: 10.1111/j.1600-065X.2012.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louveau A, Harris TH, Kipnis J. Revisiting the concept of CNS immune privilege. Trends Immunol. 2016;36:569–577. doi: 10.1016/j.it.2015.08.006.Revisiting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leech S, Kirk J, Plumb J, McQuaid S. Persistent endothelial abnormalities and blood-brain barrier leak in primary and secondary progressive multiple sclerosis. Neuropathol Appl Neurobiol. 2007;33:86–98. doi: 10.1111/j.1365-2990.2006.00781.x. [DOI] [PubMed] [Google Scholar]
- 11.Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011;585:3770–3780. doi: 10.1016/j.febslet.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y, Goods BA, Raddassi K, Nepom GT, Kwok WW, Love JC, et al. Distinct inflammatory profiles of myelin-reactive t cells from patients with multiple sclerosis HHS Public Access. Sci Transl Med. 2015;7:287–274. doi: 10.1126/scitranslmed.aaa8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chitnis T. The role of CD4 T cells in the pathogenesis of multiple sclerosis. Int Rev Neurobiol. 2007;79:43–72. doi: 10.1016/S0074-7742(07)79003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Correale J, Farez MF, Cardona AE. The role of astrocytes in multiple sclerosis progression. Front Neurol. 2015;6:180. doi: 10.3389/fneur.2015.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najafi S, Mirshafiey A. The effect of activated microglia in progression of multiple sclerosis. Int Trends Immun. 2015;3:96–104. [Google Scholar]
- 16.Denic A, Wootla B, Rodriguez M. CD8 + T cells in multiple sclerosis. Expert Opin Ther Targets. 2013;17:1053–1066. doi: 10.1517/14728222.2013.815726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krumbholz M, Derfuss T, Hohlfeld R, Meinl E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat Rev Neurol. 2012;8:613–623. doi: 10.1038/nrneurol.2012.203. [DOI] [PubMed] [Google Scholar]
- 18.Jelcic I, Al Nimer F, Wang J, Lentsch V, Planas R, Jelcic I, et al. Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell. 2018;175:85–100. doi: 10.1016/j.cell.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stassart RM, Möbius W, Nave KA, Edgar JM. The axon-myelin unit in development and degenerative disease. Front Neurosci. 2018;12:467. [DOI] [PMC free article] [PubMed]
- 20.Handel A. E., Handunnetthi L., Giovannoni G., Ebers G. C., Ramagopalan S. V. Genetic and environmental factors and the distribution of multiple sclerosis in Europe. European Journal of Neurology. 2010;17(9):1210–1214. doi: 10.1111/j.1468-1331.2010.03003.x. [DOI] [PubMed] [Google Scholar]
- 21.Meaney MJ. Epigenetics and the biological definition of gene X environment interactions. Child Dev. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 22.Gourraud PA, Harbo HF, Hauser SL, Baranzini SE. The genetics of multiple sclerosis: an up-to-date review. Immunol Rev. 2012;248:87–103. doi: 10.1111/j.1600-065X.2012.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lill CM. Recent advances and future challenges in the genetics of multiple sclerosis. Front Neurol. 2014;5:1–5. doi: 10.3389/fneur.2014.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 25.Wu H, Huang X, Qiu H, Zhao M, Liao W, Yuan S, et al. High salt promotes autoimmunity by TET2-induced DNA demethylation and driving the differentiation of Tfh cells. Sci Rep. 2016;6:1–14. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allis CD, Jenuwein T. The molecular hallmarks of epigenetic control. Nat Rev Genet. 2016;17:487–500. doi: 10.1038/nrg.2016.59. [DOI] [PubMed] [Google Scholar]
- 27.Ehrlich M, Gama-Sosa MA, Huang LH, Midgett RM, Kuo KC, Mccune RA, et al. Amount and distribution of 5-methylcytosine in human DNA from different types of tissues or cells. Nucleic Acids Res. 1982;10:2709–2721. doi: 10.1093/nar/10.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber M, Hellmann I, Stadler MB, Ramos L, Pääbo S, Rebhan M, et al. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet. 2007;39:457–466. doi: 10.1038/ng1990. [DOI] [PubMed] [Google Scholar]
- 29.Svedružić ŽM. Dnmt1: Structure and function. Prog Mol Biol Transl Sci. 2011;101:221–254. doi: 10.1016/B978-0-12-387685-0.00006-8. [DOI] [PubMed] [Google Scholar]
- 30.Mortusewicz O, Schermelleh L, Walter J, Cardoso MC, Leonhardt H. Recruitment of DNA methyltransferase I to DNA repair sites. Proc Natl Acad Sci. 2005;102:8905–8909. doi: 10.1073/pnas.0501034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Emburgh BO, Robertson KD. Modulation of Dnmt3b function in vitro by interactions with Dnmt3L, Dnmt3a and Dnmt3b splice variants. Nucleic Acids Res. 2011;39:4984–5002. doi: 10.1093/nar/gkr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lienert F, Wirbelauer C, Som I, Dean A, Mohn F, Schübeler D. Identification of genetic elements that autonomously determine DNA methylation states. Nat Genet. 2011;43:1091–1097. doi: 10.1038/ng.946. [DOI] [PubMed] [Google Scholar]
- 33.Flores KB, Wolschin F, Amdam GV. The role of methylation of DNA in environmental adaptation. Integr Comp Biol. 2013;53:359–372. doi: 10.1093/icb/ict019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kagiwada S, Kurimoto K, Hirota T, Yamaji M, Saitou M. Replication-coupled passive DNA demethylation for the erasure of genome imprints in mice. EMBO J. 2013;32:340–353. doi: 10.1038/emboj.2012.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito S, Dalessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, et al. Tet proteins can convert 5-Methylcytosine to 5-Formylcytosine and 5-Carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell. 2011;145:423–434. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortellino S, Xu J, Sannai M, Moore R, Caretti E, Cigliano A, et al. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell. 2011;146:67–79. doi: 10.1016/j.cell.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maltby VE, Graves MC, Lea RA, Benton MC, Sanders KA, Tajouri L, et al. Genome-wide DNA methylation profiling of CD8+ T cells shows a distinct epigenetic signature to CD4+ T cells in multiple sclerosis patients. Clin Epigenetics. 2015;7:1–6. 10.1186/s13148-015-0152-7 [DOI] [PMC free article] [PubMed]
- 40.Graves MC, Benton M, Lea RA, Boyle M, Tajouri L, Macartney-Coxson D, Scott RJ, Lechner-Scott J. Methylation differences at the HLA-DRB1 locus in CD4+ T-Cells are associated with multiple sclerosis. Multiple Sclerosis Journal. 2013;20(8):1033–1041. doi: 10.1177/1352458513516529. [DOI] [PubMed] [Google Scholar]
- 41.Chomyk AM, Volsko C, Tripathi A, Deckard SA, Trapp BD, Fox RJ, et al. DNA methylation in demyelinated multiple sclerosis hippocampus. Sci Rep. 2017;7:1–10. 10.1038/s41598-017-08623-5. [DOI] [PMC free article] [PubMed]
- 42.Fagone Paolo, Mangano Katia, Di Marco Roberto, Touil-Boukoffa Chafia, Chikovan Tinatin, Signorelli Santo, Lombardo Giuseppe A.G., Patti Francesco, Mammana Santa, Nicoletti Ferdinando. Expression of DNA methylation genes in secondary progressive multiple sclerosis. Journal of Neuroimmunology. 2016;290:66–69. doi: 10.1016/j.jneuroim.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Kulakova OG, Kabilov MR, Danilova LV, Popova EV, Baturina OA, Tsareva EY, et al. Whole-genome DNA methylation analysis of peripheral blood mononuclear cells in multiple sclerosis patients with different disease courses. Acta Naturae. 2016;8:103–110. doi: 10.32607/20758251-2016-8-3-103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marabita F, Almgren M, Sjöholm LK, Kular L, Liu Y, James T, et al. Smoking induces DNA methylation changes in multiple sclerosis patients with exposure-response relationship. Sci Rep. 2017;7:1–15. [DOI] [PMC free article] [PubMed]
- 45.Mastronardi FG, Noor A, Wood DD, Paton T, Moscarello MA. Peptidyl argininedeiminase 2 CpG island in multiple sclerosis white matter is hypomethylated. J Neurosci Res. 2007;85:2006–2016. doi: 10.1002/jnr.21329. [DOI] [PubMed] [Google Scholar]
- 46.Lehmann-Werman Roni, Neiman Daniel, Zemmour Hai, Moss Joshua, Magenheim Judith, Vaknin-Dembinsky Adi, Rubertsson Sten, Nellgård Bengt, Blennow Kaj, Zetterberg Henrik, Spalding Kirsty, Haller Michael J., Wasserfall Clive H., Schatz Desmond A., Greenbaum Carla J., Dorrell Craig, Grompe Markus, Zick Aviad, Hubert Ayala, Maoz Myriam, Fendrich Volker, Bartsch Detlef K., Golan Talia, Ben Sasson Shmuel A., Zamir Gideon, Razin Aharon, Cedar Howard, Shapiro A. M. James, Glaser Benjamin, Shemer Ruth, Dor Yuval. Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proceedings of the National Academy of Sciences. 2016;113(13):E1826–E1834. doi: 10.1073/pnas.1519286113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huynh JL, Garg P, Thin TH, Yoo S, Dutta R, Trapp BD, et al. Epigenome-wide differences in pathology-free regions of multiple sclerosis-affected brains. Nat Neurosci. 2014;17:121–130. doi: 10.1038/nn.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bos Steffan D., Page Christian M., Andreassen Bettina K., Elboudwarej Emon, Gustavsen Marte W., Briggs Farren, Quach Hong, Leikfoss Ingvild S., Bjølgerud Anja, Berge Tone, Harbo Hanne F., Barcellos Lisa F. Genome-Wide DNA Methylation Profiles Indicate CD8+ T Cell Hypermethylation in Multiple Sclerosis. PLOS ONE. 2015;10(3):e0117403. doi: 10.1371/journal.pone.0117403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumagai C, Kalman B, Middleton FA, Vyshkina T, Massa PT. Increased promoter methylation of the immune regulatory gene SHP-1 in leukocytes of multiple sclerosis subjects. J Neuroimmunol. 2012;246:51–57. doi: 10.1016/j.jneuroim.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Field J, Fox A, Jordan M A, Baxter A G, Spelman T, Gresle M, Butzkueven H, Kilpatrick T J, Rubio J P. Interleukin-2 receptor-α proximal promoter hypomethylation is associated with multiple sclerosis. Genes & Immunity. 2017;18(2):59–66. doi: 10.1038/gene.2016.50. [DOI] [PubMed] [Google Scholar]
- 51.Pinto-Medel MJ, Oliver-Martos B, Urbaneja-Romero P, Hurtado-Guerrero I, Ortega-Pinazo J, Serrano-Castro P, et al. Global methylation correlates with clinical status in multiple sclerosis patients in the first year of IFNbeta treatment. Sci Rep. 2017;7:1–9. [DOI] [PMC free article] [PubMed]
- 52.Baranzini SE, Mudge J, Van Velkinburgh JC, Khankhanian P, Khrebtukova I, Miller NA, et al. Genome, epigenome and RNA sequences of monozygotic twins discordant for multiple sclerosis. Nature. 2010;464:1351–6. 10.1038/nature08990. [DOI] [PMC free article] [PubMed]
- 53.Olsen John A., Kenna Lauren A., Tipon Regine C., Spelios Michael G., Stecker Mark M., Akirav Eitan M. A Minimally-invasive Blood-derived Biomarker of Oligodendrocyte Cell-loss in Multiple Sclerosis. EBioMedicine. 2016;10:227–235. doi: 10.1016/j.ebiom.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunaeva Marina, Derksen Merel, Pruijn Ger J. M. LINE-1 Hypermethylation in Serum Cell-Free DNA of Relapsing Remitting Multiple Sclerosis Patients. Molecular Neurobiology. 2017;55(6):4681–4688. doi: 10.1007/s12035-017-0679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ayuso Teresa, Aznar Patricia, Soriano Luis, Olaskoaga Ander, Roldán Miren, Otano María, Ajuria Iratxe, Soriano Gerardo, Lacruz Francisco, Mendioroz Maite. Vitamin D receptor gene is epigenetically altered and transcriptionally up-regulated in multiple sclerosis. PLOS ONE. 2017;12(3):e0174726. doi: 10.1371/journal.pone.0174726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liggett T, Melnikov A, Tilwalli S, Yi Q, Chen H, Replogle C, et al. Methylation patterns of cell-free plasma DNA in relapsing-remitting multiple sclerosis. J Neurol Sci. 2010;290:16–21. doi: 10.1016/j.jns.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sokratous M, Dardiotis E, Bellou E, Tsouris Z, Michalopoulou A, Dardioti M, et al. CpG island methylation patterns in relapsing-remitting multiple sclerosis. J Mol Neurosci. 2018;64:478–484. doi: 10.1007/s12031-018-1046-x. [DOI] [PubMed] [Google Scholar]
- 58.Souren NY, Gerdes LA, Lutsik P, Gasparoni G, Beltran E, Salhab A, et al. DNA methylation signatures of a large cohort monozygotic twins clinically discordant for multiple sclerosis; 2018. 10.1101/381822. [DOI] [PMC free article] [PubMed]
- 59.Calabrese R, Zampieri M, Mechelli R, Annibali V, Guastafierro T, Ciccarone F, et al. Methylation-dependent PAD2 upregulation in multiple sclerosis peripheral blood. Mult Scler J. 2012;18:299–304. doi: 10.1177/1352458511421055. [DOI] [PubMed] [Google Scholar]
- 60.Calabrese R., Valentini E., Ciccarone F., Guastafierro T., Bacalini M.G., Ricigliano V.A.G., Zampieri M., Annibali V., Mechelli R., Franceschi C., Salvetti M., Caiafa P. TET2 gene expression and 5-hydroxymethylcytosine level in multiple sclerosis peripheral blood cells. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2014;1842(7):1130–1136. doi: 10.1016/j.bbadis.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 61.Ruhrmann Sabrina, Ewing Ewoud, Piket Eliane, Kular Lara, Cetrulo Lorenzi Julio Cesar, Fernandes Sunjay Jude, Morikawa Hiromasa, Aeinehband Shahin, Sayols-Baixeras Sergi, Aslibekyan Stella, Absher Devin M, Arnett Donna K, Tegner Jesper, Gomez-Cabrero David, Piehl Fredrik, Jagodic Maja. Hypermethylation of MIR21 in CD4+ T cells from patients with relapsing-remitting multiple sclerosis associates with lower miRNA-21 levels and concomitant up-regulation of its target genes. Multiple Sclerosis Journal. 2017;24(10):1288–1300. doi: 10.1177/1352458517721356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hemmer B, Cepok S, Zhou D, Sommer N. Multiple sclerosis -- a coordinated immune attack across the blood brain barrier 2. Curr Neurovasc Res. 2004;1:141–150. doi: 10.2174/1567202043480152. [DOI] [PubMed] [Google Scholar]
- 63.On NH, Kiptoo P, Siahaan TJ, Miller DW. Modulation of blood-brain barrier permeability in mice using synthetic E-cadherin peptide. Mol Pharm. 2014;11:974–981. doi: 10.1021/mp400624v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Montesinos ML. Cell adhesion molecules: implications in neurological diseases. 2014. [PubMed] [Google Scholar]
- 65.Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Review: Leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 66.Bullard DC, Hu X, Schoeb TR, Collins RG, Beaudet AL, Barnum SR. Intercellular adhesion molecule-1 expression is required on multiple cell types for the development of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:851–857. doi: 10.4049/jimmunol.178.2.851. [DOI] [PubMed] [Google Scholar]
- 67.Dulberger CL, McMurtrey CP, Hölzemer A, Neu KE, Liu V, Steinbach AM, et al. Human leukocyte antigen F presents peptides and regulates immunity through interactions with NK cell receptors. Immunity. 2017;46:1018–1029. doi: 10.1016/j.immuni.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frohn Christoph, Fricke Lutz, Puchta Jan‐Christoph, Kirchner Holger. The effect of HLA‐C matching on acute renal transplant rejection. Nephrology Dialysis Transplantation. 2001;16(2):355–360. doi: 10.1093/ndt/16.2.355. [DOI] [PubMed] [Google Scholar]
- 69.Meresse B, Curran SA, Ciszewski C, Orbelyan G, Setty M, Bhagat G, et al. Reprogramming of CTLs into natural killer–like cells in celiac disease. J Exp Med. 2006;203:1343–1355. doi: 10.1084/jem.20060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshihara Y, Oka S, Nemoto Y, Watanabe Y, Nagata S, Kagamiyama H, et al. An ICAM-related neuronal glycoprotein, telencephalin, with brain segment-specific expression. Neuron. 1994;12:541–553. doi: 10.1016/0896-6273(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 71.Paetau S, Rolova T, Ning L, Gahmberg CG. Neuronal ICAM-5 inhibits microglia adhesion and phagocytosis and promotes an anti-inflammatory response in LPS stimulated microglia. Front Mol Neurosci. 2017;10:1–12. doi: 10.3389/fnmol.2017.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lubetzki C, Stankoff B. Demyelination in multiple sclerosis. Handb Clin Neurol. 2014;122:89–99. http://sci-hub.tw/10.1016/B978-0-444-52001-2.00004-2. [DOI] [PMC free article] [PubMed]
- 73.Karin N. Reversal of experimental autoimmune encephalomyelitis by a soluble peptide variant of a myelin basic protein epitope: T cell receptor antagonism and reduction of interferon gamma and tumor necrosis factor alpha production. J Exp Med. 2004;180:2227–2237. doi: 10.1084/jem.180.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krogsgaard M, Wucherpfennig KW, Cannella B, Hansen BE, Svejgaard A, Pyrdol J, et al. Visualization of myelin basic protein (Mbp) T cell epitopes in multiple sclerosis lesions using a monoclonal antibody specific for the human histocompatibility leukocyte antigen (Hla)-Dr2–Mbp 85–99 complex. J Exp Med. 2000;191:1395–1412. doi: 10.1084/jem.191.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Falcão AM, Meijer M, Scaglione A, Rinwa P, Agirre E, Liang J, et al. PADI2-mediated citrullination is required for efficient oligodendrocyte differentiation and myelination. bioRxiv. 2018. 10.1101/425348. [DOI] [PMC free article] [PubMed]
- 76.Pritzker LB, Joshi S, Harauz G, Moscarello MA. Deimination of myelin basic protein. 2. Effect of methylation of MBP on its deimination by peptidylarginine deiminase. Biochemistry. 2000;39:5382–5388. doi: 10.1021/bi9925571. [DOI] [PubMed] [Google Scholar]
- 77.Wood DD, Bilbao JM, O’Connors P, Moscarello MA. Acute multiple sclerosis (Marburg type) is associated with developmentally immature myelin basic protein. Ann Neurol. 1996;40:18–24. doi: 10.1002/ana.410400106. [DOI] [PubMed] [Google Scholar]
- 78.Watts C, Matthews SP, Mazzeo D, Manoury B, Moss CX. Asparaginyl endopeptidase: case history of a class II MHC compartment protease. Immunol Rev. 2005;207:218–228. doi: 10.1111/j.0105-2896.2005.00312.x. [DOI] [PubMed] [Google Scholar]
- 79.Manoury B, Mazzeo D, Fugger L, Viner N, Ponsford M, Streeter H, et al. Destructive processing by asparagine endopeptidase limits presentation of a dominant T cell epitope in MBP. Nat Immunol. 2002;3:169–174. doi: 10.1038/ni754. [DOI] [PubMed] [Google Scholar]
- 80.Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Franklin RJM. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- 82.Kuhlmann T, Miron V, Cuo Q, Wegner C, Antel J, Brück W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- 83.Redwine JM, Armstrong RC. In vivo proliferation of oligodendrocyte progenitors expressing PDGFαR during early remyelination. J Neurobiol. 1998;37:413–428. doi: 10.1002/(SICI)1097-4695(19981115)37:3<413::AID-NEU7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 84.Hinks GL, Franklin RJM. Distinctive patterns of PDGF-A, FGF-2, IGF-I, and TGF-β1 gene expression during remyelination of experimentally-induced spinal cord demyelination. Mol Cell Neurosci. 1999;14:153–168. doi: 10.1006/mcne.1999.0771. [DOI] [PubMed] [Google Scholar]
- 85.Messersmith DJ, Murtie JC, Le TQ, Frost EE, Armstrong RC. Fibroblast growth factor 2 (FGF2) and FGF receptor expression in an experimental demyelinating disease with extensive remyelination. J Neurosci Res. 2000;62:241–256. doi: 10.1002/1097-4547(20001015)62:2<241::AID-JNR9>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fancy SPJ, Baranzini SE, Zhao C, Yuk D-I, Irvine K-A, Kaing S, et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamaguchi M. Histone deacetylase 1 regulates retinal neurogenesis in zebrafish by suppressing Wnt and Notch signaling pathways. Development. 2005;132:3027–3043. doi: 10.1242/dev.01881. [DOI] [PubMed] [Google Scholar]
- 88.Yamamoto A, Nagano T, Takehara S, Hibi M, Aizawa S. Shisa promotes head formation through the inhibition of receptor protein maturation for the caudalizing factors, Wnt and FGF. Cell. 2005;120:223–235. doi: 10.1016/j.cell.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 89.Chen Y, Wang H, Yoon SO, Xu X, Hottiger MO, Svaren J, et al. HDAC-mediated deacetylation of NF-κB is critical for Schwann cell myelination. Nat Neurosci. 2011;14:437–441. doi: 10.1038/nn.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, Martin MS, et al. Axon-glia interactions and the domain organization of myelinated axons requires Neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/S0896-6273(01)00294-X. [DOI] [PubMed] [Google Scholar]
- 91.Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, et al. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 92.Mathey EK, Derfuss T, Storch MK, Williams KR, Hales K, Woolley DR, et al. Neurofascin as a novel target for autoantibody-mediated axonal injury. J Exp Med. 2007;204:2363–2372. doi: 10.1084/jem.20071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Linington C, Engelhardt B, Kapocs G, Lassman H. Induction of persistently demyelinated lesions in the rat following the repeated adoptive transfer of encephalitogenic T cells and demyelinating antibody. J Neuroimmunol. 1992;40:219–224. doi: 10.1016/0165-5728(92)90136-9. [DOI] [PubMed] [Google Scholar]
- 94.Kaltschmidt B., Kaltschmidt C. NF- B in the Nervous System. Cold Spring Harbor Perspectives in Biology. 2009;1(3):a001271–a001271. doi: 10.1101/cshperspect.a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akama KT, Albanese C, Pestell RG, Van Eldik L. Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism. Proc Natl Acad Sci U S A. 1998;95:5795–800. [DOI] [PMC free article] [PubMed]
- 96.Castillo-Fernandez JE, Spector TD, Bell JT. Epigenetics of discordant monozygotic twins: Implications for disease. Genome Med. 2014;6:1–16. doi: 10.1186/s13073-014-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Healy BC, Ali EN, Guttmann CRG, Chitnis T, Glanz BI, Buckle G, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66:858–864. doi: 10.1001/archneurol.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Degelman ML, Herman KM. Smoking and multiple sclerosis: a systematic review and meta-analysis using the Bradford Hill criteria for causation. Mult Scler Relat Disord. 2017;17:207–216. doi: 10.1016/j.msard.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 99.Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health. 2011;8:613–628. doi: 10.3390/ijerph8020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, et al. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027:48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 101.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Multiple Sclerosis. 2003;9:540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 102.Funata N, Song SY, Okeda R, Funata M, Higashino F. A study of experimental cyanide encephalopathy in the acute phase -physiological and neuropathological correlation. Acta Neuropathol. 1984;64:99–107. doi: 10.1007/BF00695572. [DOI] [PubMed] [Google Scholar]
- 103.Wang L, Hagemann TL, Kalwa H, Michel T, Messing A, Feany MB. Nitric oxide mediates glial-induced neurodegeneration in Alexander disease. Nat Commun. 2015;6:8966. [DOI] [PMC free article] [PubMed]
- 104.Kitamura M, Kasai A. Cigarette smoke as a trigger for the dioxin receptor-mediated signaling pathway. Cancer Lett. 2007;252:184–194. doi: 10.1016/j.canlet.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 105.Quintana FJ. Regulation of central nervous system autoimmunity by the aryl hydrocarbon receptor. Semin Immunopathol. 2013;35:627–635. doi: 10.1007/s00281-013-0397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quintana Francisco J., Basso Alexandre S., Iglesias Antonio H., Korn Thomas, Farez Mauricio F., Bettelli Estelle, Caccamo Mario, Oukka Mohamed, Weiner Howard L. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 107.Zeilinger Sonja, Kühnel Brigitte, Klopp Norman, Baurecht Hansjörg, Kleinschmidt Anja, Gieger Christian, Weidinger Stephan, Lattka Eva, Adamski Jerzy, Peters Annette, Strauch Konstantin, Waldenberger Melanie, Illig Thomas. Tobacco Smoking Leads to Extensive Genome-Wide Changes in DNA Methylation. PLoS ONE. 2013;8(5):e63812. doi: 10.1371/journal.pone.0063812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neavin Drew, Liu Duan, Ray Balmiki, Weinshilboum Richard. The Role of the Aryl Hydrocarbon Receptor (AHR) in Immune and Inflammatory Diseases. International Journal of Molecular Sciences. 2018;19(12):3851. doi: 10.3390/ijms19123851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Duarte JH, Di Meglio P, Hirota K, Ahlfors H, Stockinger B. Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo. PLoS One. 2013;8:e79819. doi: 10.1371/journal.pone.0079819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Juricek L, Carcaud J, Pelhaitre A, Riday TT, Chevallier A, Lanzini J, et al. AhR-deficiency as a cause of demyelinating disease and inflammation. Sci Rep. 2017;7:9794. doi: 10.1038/s41598-017-09621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rothhammer V, Borucki DM, Garcia Sanchez MI, Mazzola MA, Hemond CC, Regev K, et al. Dynamic regulation of serum aryl hydrocarbon receptor agonists in MS. Neurol Neuroimmunol Neuroinflamm. 2017;4:e359. doi: 10.1212/NXI.0000000000000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kaye J, Piryatinsky V, Birnberg T, Hingaly T, Raymond E, Kashi R, et al. Laquinimod arrests experimental autoimmune encephalomyelitis by activating the aryl hydrocarbon receptor. Proc Natl Acad Sci. 2016;113:e6145–e6152. doi: 10.1073/pnas.1607843113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hatton OL, Harris-Arnold A, Schaffert S, Krams SM, Martinez OM. The interplay between Epstein-Barr virus and B lymphocytes: implications for infection, immunity, and disease. Immunol Res. 2014;58:268–276. doi: 10.1007/s12026-014-8496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ascherio A, Munger KL, Lennette ET, Spiegelman D, Hernán MA, Olek MJ, et al. Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study. J Am Med Assoc. 2001;286:3083–3088. doi: 10.1001/jama.286.24.3083. [DOI] [PubMed] [Google Scholar]
- 115.Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. 2017;13:25–36. doi: 10.1038/nrneurol.2016.187. [DOI] [PubMed] [Google Scholar]
- 116.Niller HH, Wolf H, Minarovits J. Regulation and dysregulation of Epstein - Barr virus latency: implications for the development of autoimmune diseases. Autoimmunity. 2008;41:298–328. doi: 10.1080/08916930802024772. [DOI] [PubMed] [Google Scholar]
- 117.Cepok Sabine, Zhou Dun, Srivastava Rajneesh, Nessler Stefan, Stei Susanne, Büssow Konrad, Sommer Norbert, Hemmer Bernhard. Identification of Epstein-Barr virus proteins as putative targets of the immune response in multiple sclerosis. Journal of Clinical Investigation. 2005;115(5):1352–1360. doi: 10.1172/JCI200523661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Robertson KD, Hayward SD, Ling PD, Samid D, Robertson KD, Hayward SD, et al. Transcriptional activation of the Epstein-Barr virus latency C promoter after 5-azacytidine treatment: evidence that demethylation at a single CpG site is crucial. Mol Cell Biol. 1995;15:6150–6159. doi: 10.1128/MCB.15.11.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Engelsen Ola. The Relationship between Ultraviolet Radiation Exposure and Vitamin D Status. Nutrients. 2010;2(5):482–495. doi: 10.3390/nu2050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Holick Michael F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. The American Journal of Clinical Nutrition. 2004;80(6):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 121.Smolders Joost, Damoiseaux Jan, Menheere Paul, Hupperts Raymond. Vitamin D as an immune modulator in multiple sclerosis, a review. Journal of Neuroimmunology. 2008;194(1-2):7–17. doi: 10.1016/j.jneuroim.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 122.Sloka Scott, Silva Claudia, Pryse-Phillips William, Patten Scott, Metz Luanne, Yong V. Wee. A Quantitative Analysis of Suspected Environmental Causes of MS. Canadian Journal of Neurological Sciences / Journal Canadien des Sciences Neurologiques. 2011;38(1):98–105. doi: 10.1017/S0317167100011124. [DOI] [PubMed] [Google Scholar]
- 123.Simpson S, Taylor B, Blizzard L, Ponsonby AL, Pittas F, Tremlett H, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68:193–203. [DOI] [PubMed]
- 124.Fetahu IS, Höbaus J, Kállay E. Vitamin D and the epigenome. Front Physiol. 2014;5:1–13. [DOI] [PMC free article] [PubMed]
- 125.Rawson James B., Sun Zhouyu, Dicks Elizabeth, Daftary Darshana, Parfrey Patrick S., Green Roger C., Gallinger Steven, McLaughlin John R., Wang Peizhong P., Knight Julia A., Bapat Bharati. Vitamin D Intake Is Negatively Associated with Promoter Methylation of the Wnt Antagonist GeneDKK1in a Large Group of Colorectal Cancer Patients. Nutrition and Cancer. 2012;64(7):919–928. doi: 10.1080/01635581.2012.711418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Inestrosa Nibaldo C., Varela-Nallar Lorena. Wnt signalling in neuronal differentiation and development. Cell and Tissue Research. 2014;359(1):215–223. doi: 10.1007/s00441-014-1996-4. [DOI] [PubMed] [Google Scholar]
- 127.Barragán-Martínez Carolina, Speck-Hernández Cesar A., Montoya-Ortiz Gladis, Mantilla Rubén D., Anaya Juan-Manuel, Rojas-Villarraga Adriana. Organic Solvents as Risk Factor for Autoimmune Diseases: A Systematic Review and Meta-Analysis. PLoS ONE. 2012;7(12):e51506. doi: 10.1371/journal.pone.0051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Al-Hajri Z, Del Bigio MR. Brain damage in a large cohort of solvent abusers. Acta Neuropathol. 2010;119:435–445. doi: 10.1007/s00401-010-0653-6. [DOI] [PubMed] [Google Scholar]
- 129.Godderis L, De Raedt K, Tabish AM, Poels K, Maertens N, De Ruyck K, et al. Epigenetic changes in lymphocytes of solvent-exposed individuals. Epigenomics. 2012;4:269–277. doi: 10.2217/epi.12.23. [DOI] [PubMed] [Google Scholar]
- 130.Hong Ji Young, Yu So Yeon, Kim Seol Young, Ahn Jeong Jin, Kim Youngjoo, Kim Gi Won, Son Sang Wook, Park Jong-Tae, Hwang Seung Yong. Association analysis of toluene exposure time with high-throughput mRNA expressions and methylation patterns using in vivo samples. Environmental Research. 2016;146:59–64. doi: 10.1016/j.envres.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 131.Wu C, Keightley SY, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, et al. Integrin-linked protein kinase regulates fibronectin matrix assembly, E- cadherin expression, and tumorigenicity. J Biol Chem. 1998;273:528–536. doi: 10.1074/jbc.273.1.528. [DOI] [PubMed] [Google Scholar]
- 132.Hedström AK, Hössjer O, Katsoulis M, Kockum I, Olsson T, Alfredsson L. Organic solvents and MS susceptibility. Neurology. 2018;91:e455–e462. doi: 10.1212/WNL.0000000000005906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bagur M José, Murcia M Antonia, Jiménez-Monreal Antonia M, Tur Josep A, Bibiloni M Mar, Alonso Gonzalo L, Martínez-Tomé Magdalena. Influence of Diet in Multiple Sclerosis: A Systematic Review. Advances in Nutrition: An International Review Journal. 2017;8(3):463–472. doi: 10.3945/an.116.014191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang WW, Lu L, Bao TH, Zhang HM, Yuan J, Miao W, et al. Scutellarin alleviates behavioral deficits in a mouse model of multiple sclerosis, possibly through protecting neural stem cells. J Mol Neurosci. 2016;58:210–220. doi: 10.1007/s12031-015-0660-0. [DOI] [PubMed] [Google Scholar]
- 135.Hendriks JJA, Alblas J, van der Pol SMA, van Tol EAF, Dijkstra CD, de Vries HE. Flavonoids influence monocytic GTPase activity and are protective in experimental allergic encephalitis. J Exp Med. 2004;200:1667–1672. doi: 10.1084/jem.20040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sternberg Z, Chadha K, Lieberman A, Hojnacki D, Drake A, Zamboni P, et al. Quercetin and interferon-β modulate immune response(s) in peripheral blood mononuclear cells isolated from multiple sclerosis patients. J Neuroimmunol. 2008;205:142–147. doi: 10.1016/j.jneuroim.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 137.Ross Sharon A., Poirier Lionel. Proceedings of the Trans-HHS Workshop: Diet, DNA Methylation Processes and Health. The Journal of Nutrition. 2002;132(8):2329S–2332S. doi: 10.1093/jn/132.8.2329S. [DOI] [PubMed] [Google Scholar]
- 138.Dickson KM, Gustafson CB, Young JI, Züchner S, Wang G. Ascorbate-induced generation of 5-hydroxymethylcytosine is unaffected by varying levels of iron and 2-oxoglutarate. Biochem Biophys Res Commun. 2013;439:522–527. doi: 10.1016/j.bbrc.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Singhal NK, Freeman E, Arning E, Wasek B, Clements R, Sheppard C, et al. Dysregulation of methionine metabolism in multiple sclerosis. Neurochem Int. 2018;112:1–4. doi: 10.1016/j.neuint.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 140.Strickland Faith M., Hewagama Anura, Wu Ailing, Sawalha Amr H., Delaney Colin, Hoeltzel Mark F., Yung Raymond, Johnson Kent, Mickelson Barbara, Richardson Bruce C. Diet Influences Expression of Autoimmune-Associated Genes and Disease Severity by Epigenetic Mechanisms in a Transgenic Mouse Model of Lupus. Arthritis & Rheumatism. 2013;65(7):1872–1881. doi: 10.1002/art.37967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Singhal NK, Li S, Arning E, Alkhayer K, Clements R, Sarcyk Z, et al. Changes in methionine metabolism and histone H3 trimethylation are linked to mitochondrial defects in multiple sclerosis. J Neurosci. 2015;35:15170–15186. doi: 10.1523/JNEUROSCI.4349-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou X, Zhang L, Ji WJ, Yuan F, Guo ZZ, Pang B, et al. Variation in dietary salt intake induces coordinated dynamics of monocyte subsets and monocyteplatelet aggregates in humans: implications in end organ inflammation. PLoS One. 2013;8;e60332. [DOI] [PMC free article] [PubMed]
- 143.Hernandez AL, Kleinewietfeld M, David A, Hernandez AL, Kitz A, Wu C, et al. Sodium chloride inhibits the suppressive function of FOXP3 + regulatory T cells. J Clin Invest. 2015;125:4212–4222. doi: 10.1172/JCI81151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hucke S, Eschborn M, Liebmann M, Herold M, Freise N, Engbers A, et al. Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J Autoimmun. 2016;67:90–101. doi: 10.1016/j.jaut.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 145.Zhang T, Fang S, Wan C, Kong Q, Wang G, Wang S, et al. Excess salt exacerbates blood-brain barrier disruption via a p38/MAPK/SGK1-dependent pathway in permanent cerebral ischemia. Sci Rep. 2015;5. 10.1038/srep16548. [DOI] [PMC free article] [PubMed]
- 146.Farez MF, Fiol MP, Gaitán MI, Quintana FJ, Correale J. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:26–31. doi: 10.1136/jnnp-2014-307928. [DOI] [PubMed] [Google Scholar]
- 147.Mischke M, Plösch T. The gut microbiota and their metabolites: potential implications for the host epigenome. Adv Exp Med Biol. 2016;902:33–44. doi: 10.1007/978-3-319-31248-4_3. [DOI] [PubMed] [Google Scholar]
- 148.Berer K, Mues M, Koutrolos M, AlRasbi Z, Boziki M, Johner C, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 149.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci. 2011;108(Supplement_1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Soldan MMP, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6:1–10. doi: 10.1038/srep28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci. 2017;114:10719–10724. doi: 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sun Chiao-Yin, Chang Shih-Chung, Wu Mai-Szu. Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney International. 2012;81(7):640–650. doi: 10.1038/ki.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Torres P.-Ureña, Prié D., Molina-Blétry V., Beck L., Silve C., Friedlander G. Klotho: An antiaging protein involved in mineral and vitamin D metabolism. Kidney International. 2007;71(8):730–737. doi: 10.1038/sj.ki.5002163. [DOI] [PubMed] [Google Scholar]
- 154.Afanas’ev Igor. New Nucleophilic Mechanisms of Ros-Dependent Epigenetic Modifications: Comparison of Aging and Cancer. Aging and Disease. 2014;5(1):52–62. doi: 10.14336/AD.2014.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Anderson Olivia S., Sant Karilyn E., Dolinoy Dana C. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. The Journal of Nutritional Biochemistry. 2012;23(8):853–859. doi: 10.1016/j.jnutbio.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kalhan SC. One-carbon metabolism, fetal growth and long-term consequences. In: Nestle Nutrition Institute Workshop Series; 2013. [DOI] [PMC free article] [PubMed]
- 157.Ly A., Lee H., Chen J., Sie K. K. Y., Renlund R., Medline A., Sohn K.-J., Croxford R., Thompson L. U., Kim Y.-I. Effect of Maternal and Postweaning Folic Acid Supplementation on Mammary Tumor Risk in the Offspring. Cancer Research. 2010;71(3):988–997. doi: 10.1158/0008-5472.CAN-10-2379. [DOI] [PubMed] [Google Scholar]
- 158.Dumas M.-E., Barton R. H., Toye A., Cloarec O., Blancher C., Rothwell A., Fearnside J., Tatoud R., Blanc V., Lindon J. C., Mitchell S. C., Holmes E., McCarthy M. I., Scott J., Gauguier D., Nicholson J. K. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proceedings of the National Academy of Sciences. 2006;103(33):12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schaible Tiffany D., Harris R. Alan, Dowd Scot E., Smith C. Wayne, Kellermayer Richard. Maternal methyl-donor supplementation induces prolonged murine offspring colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Human Molecular Genetics. 2011;20(9):1687–1696. doi: 10.1093/hmg/ddr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Canani RB, Costanzo MD, Leone L. The epigenetic effects of butyrate: potential therapeutic implications for clinical practice. Clin Epigenetics. 2012;4:1–7. [DOI] [PMC free article] [PubMed]
- 161.Kanai Y., Hirohashi S. Alterations of DNA methylation associated with abnormalities of DNA methyltransferases in human cancers during transition from a precancerous to a malignant state. Carcinogenesis. 2007;28(12):2434–2442. doi: 10.1093/carcin/bgm206. [DOI] [PubMed] [Google Scholar]
- 162.Worthley Daniel L., Whitehall Vicki L. J., Le Leu Richard K., Irahara Natsumi, Buttenshaw Ronald L., Mallitt Kylie-Ann, Greco Sonia A., Ramsnes Ingunn, Winter Jean, Hu Ying, Ogino Shuji, Young Graeme P., Leggett Barbara A. DNA Methylation in the Rectal Mucosa Is Associated with Crypt Proliferation and Fecal Short-Chain Fatty Acids. Digestive Diseases and Sciences. 2010;56(2):387–396. doi: 10.1007/s10620-010-1312-4. [DOI] [PubMed] [Google Scholar]
- 163.Hou Haifeng, Yu Hongtao. Structural insights into histone lysine demethylation. Current Opinion in Structural Biology. 2010;20(6):739–748. doi: 10.1016/j.sbi.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang L, Chang J, Varghese D, Dellinger M, Kumar S, Best AM, et al. A small molecule modulates Jumonji histone demethylase activity and selectively inhibits cancer growth. Nat Commun. 2013;4:2035. [DOI] [PMC free article] [PubMed]
- 165.Minor Emily A., Court Brenda L., Young Juan I., Wang Gaofeng. Ascorbate Induces Ten-Eleven Translocation (Tet) Methylcytosine Dioxygenase-mediated Generation of 5-Hydroxymethylcytosine. Journal of Biological Chemistry. 2013;288(19):13669–13674. doi: 10.1074/jbc.C113.464800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kumar H, Lund R, Laiho A, Lundelin K, Ley RE, Isolauri E, et al. Gut microbiota as an epigenetic regulator: pilot study based on whole-genome methylation analysis. MBio. 2014;5:e02113–14. [DOI] [PMC free article] [PubMed]
- 167.Denham J, O’Brien BJ, Marques FZ, Charchar FJ. Changes in the leukocyte methylome and its effect on cardiovascular-related genes after exercise. J Appl Physiol. 2015;118:475–488. doi: 10.1152/japplphysiol.00878.2014. [DOI] [PubMed] [Google Scholar]
- 168.Radom-Aizik S, Zaldivar F, Leu SY, Adams GR, Oliver S, Cooper DM. Effects of exercise on microRNA expression in young males peripheral blood mononuclear cells. Clin Transl Sci. 2012;5:32–38. doi: 10.1111/j.1752-8062.2011.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci. 2011;33:383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Briken S, Rosenkranz SC, Keminer O, Patra S, Ketels G, Heesen C, et al. Effects of exercise on Irisin, BDNF and IL-6 serum levels in patients with progressive multiple sclerosis. J Neuroimmunol. 2016;299:53–58. doi: 10.1016/j.jneuroim.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 171.Lewin Gary R., Barde Yves-Alain. Physiology of the Neurotrophins. Annual Review of Neuroscience. 1996;19(1):289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 172.Stadelmann C, Kerschensteiner M, Misgeld T, Brück W, Hohlfeld R, Lassmann H. BDNF and gp145trkB in multiple sclerosis brain lesions: neuroprotective interactions between immune and neuronal cells? Brain. 2002;125:75–85. doi: 10.1093/brain/awf015. [DOI] [PubMed] [Google Scholar]
- 173.Kerschensteiner Martin, Gallmeier Eike, Behrens Lüder, Leal Vivian Vargas, Misgeld Thomas, Klinkert Wolfgang E.F., Kolbeck Roland, Hoppe Edmund, Oropeza-Wekerle Rosa-Laura, Bartke Ilse, Stadelmann Christine, Lassmann Hans, Wekerle Hartmut, Hohlfeld Reinhard. Activated Human T Cells, B Cells, and Monocytes Produce Brain-derived Neurotrophic Factor In Vitro and in Inflammatory Brain Lesions: A Neuroprotective Role of Inflammation? The Journal of Experimental Medicine. 1999;189(5):865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 175.Xiao Junhua, Wong Agnes W., Willingham Melanie M., van den Buuse Maarten, Kilpatrick Trevor J., Murray Simon S. Brain-Derived Neurotrophic Factor Promotes Central Nervous System Myelination via a Direct Effect upon Oligodendrocytes. Neurosignals. 2010;18(3):186–202. doi: 10.1159/000323170. [DOI] [PubMed] [Google Scholar]
- 176.Sølvsten Christina A. E., de Paoli Frank, Christensen Jane H., Nielsen Anders L. Voluntary Physical Exercise Induces Expression and Epigenetic Remodeling of VegfA in the Rat Hippocampus. Molecular Neurobiology. 2016;55(1):567–582. doi: 10.1007/s12035-016-0344-y. [DOI] [PubMed] [Google Scholar]
- 177.Lin Wensheng. Neuroprotective effects of vascular endothelial growth factor A in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neural Regeneration Research. 2017;12(1):70. doi: 10.4103/1673-5374.198982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Keane RW, Dietrich WD, de Rivero Vaccari JP. Inflammasome proteins as biomarkers of multiple sclerosis. Front Neurol. 2018;9:135. [DOI] [PMC free article] [PubMed]
- 179.Nakajima K., Takeoka M., Mori M., Hashimoto S., Sakurai A., Nose H., Higuchi K., Itano N., Shiohara M., Oh T., Taniguchi S. Exercise Effects on Methylation ofASCGene. International Journal of Sports Medicine. 2010;31(09):671–675. doi: 10.1055/s-0029-1246140. [DOI] [PubMed] [Google Scholar]
- 180.Slattery Martha L., Curtin Karen, Sweeney Carol, Levin Theodore R., Potter John, Wolff Roger K., Albertsen Hans, Samowitz Wade S. Diet and lifestyle factor associations with CpG island methylator phenotype and BRAF mutations in colon cancer. International Journal of Cancer. 2006;120(3):656–663. doi: 10.1002/ijc.22342. [DOI] [PubMed] [Google Scholar]
- 181.Zhang Fang Fang, Cardarelli Roberto, Carroll Joan, Zhang Shun, Fulda Kimberly G., Gonzalez Karina, Vishwanatha Jamboor K., Morabia Alfredo, Santella Regina M. Physical activity and global genomic DNA methylation in a cancer-free population. Epigenetics. 2011;6(3):293–299. doi: 10.4161/epi.6.3.14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.White Alexandra J., Sandler Dale P., Bolick Sophia C.E., Xu Zongli, Taylor Jack A., DeRoo Lisa A. Recreational and household physical activity at different time points and DNA global methylation. European Journal of Cancer. 2013;49(9):2199–2206. doi: 10.1016/j.ejca.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mohr David C, Hart Stacey L, Julian Laura, Cox Darcy, Pelletier Daniel. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731. doi: 10.1136/bmj.38041.724421.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu X.J., Ye H.X., Li W.P., Dai R., Chen D., Jin M. Relationship between Psychosocial Factors and Onset of Multiple Sclerosis. European Neurology. 2009;62(3):130–136. doi: 10.1159/000226428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Babenko Olena, Kovalchuk Igor, Metz Gerlinde A.S. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neuroscience & Biobehavioral Reviews. 2015;48:70–91. doi: 10.1016/j.neubiorev.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 186.Garabedian Michael J., Harris Charles A., Jeanneteau Freddy. Glucocorticoid receptor action in metabolic and neuronal function. F1000Research. 2017;6:1208. doi: 10.12688/f1000research.11375.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Matysiak M, Makosa B, Walczak A, Selmaj K. Patients with multiple sclerosis resisted to glucocorticoid therapy: abnormal expression of heat-shock protein 90 in glucocorticoid receptor complex. Multiple Sclerosis Journal. 2008;14(7):919–926. doi: 10.1177/1352458508090666. [DOI] [PubMed] [Google Scholar]
- 188.Constantinescu Cris S, Farooqi Nasr, O'Brien Kate, Gran Bruno. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) British Journal of Pharmacology. 2011;164(4):1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Barthelmes J, Tafferner N, Kurz J, de Bruin N, Parnham MJ, Geisslinger G, et al. Induction of experimental autoimmune encephalomyelitis in mice and evaluation of the disease-dependent distribution of immune cells in various tissues. J Vis Exp. 2016:1–10. 10.3791/53933. [DOI] [PMC free article] [PubMed]
- 190.Schmitz Katja, Bruin Natasja, Bishay Philipp, Männich Julia, Häussler Annett, Altmann Christine, Ferreirós Nerea, Lötsch Jörn, Ultsch Alfred, Parnham Michael J, Geisslinger Gerd, Tegeder Irmgard. R‐flurbiprofen attenuates experimental autoimmune encephalomyelitis in mice. EMBO Molecular Medicine. 2014;6(11):1398–1422. doi: 10.15252/emmm.201404168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Catanzaro G., Pucci M., Viscomi M.T., Lanuti M., Feole M., Angeletti S., Grasselli G., Mandolesi G., Bari M., Centonze D., D’Addario C., Maccarrone M. Epigenetic modifications of Dexras 1 along the nNOS pathway in an animal model of multiple sclerosis. Journal of Neuroimmunology. 2016;294:32–40. doi: 10.1016/j.jneuroim.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 192.Noori-Zadeh Ali, Mesbah-Namin Seyed Alireza, Saboor-Yaraghi Ali Akbar. Epigenetic and gene expression alterations of FOXP3 in the T cells of EAE mouse model of multiple sclerosis. Journal of the Neurological Sciences. 2017;375:203–208. doi: 10.1016/j.jns.2017.01.060. [DOI] [PubMed] [Google Scholar]
- 193.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. J Immunol. 2017;198:986–92. [PubMed]
- 194.Blakemore WF, Franklin RJM. Remyelination in experimental models of toxin-induced demyelination. Curr Top Microbiol Immunol. 2008;318:193–212. [DOI] [PubMed]
- 195.Matsushima Glenn K., Morell Pierre. The Neurotoxicant, Cuprizone, as a Model to Study Demyelination and Remyelination in the Central Nervous System. Brain Pathology. 2006;11(1):107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Moyon Sarah, Ma Dan, Huynh Jimmy L., Coutts David J.C., Zhao Chao, Casaccia Patrizia, Franklin Robin J.M. Efficient Remyelination Requires DNA Methylation. eneuro. 2017;4(2):ENEURO.0336-16.2017. doi: 10.1523/ENEURO.0336-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Minor EA, Court BL, Young JI, Wang G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J Biol Chem. 2013;288:13669–13674. doi: 10.1074/jbc.C113.464800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the manuscript.