Version Changes
Revised. Amendments from Version 2
One reviewer suggested that we make a clarification regarding the study selection. We have modified the corresponding lines to better reflect our methodology. We then modified the limitations section accordingly.
Abstract
Background: We conducted a systematic review to study the association between diabetes and malaria as well as malaria severity.
Methods: The search was conducted in Embase, Global Health, MEDLINE, Scopus and Web of Science. Titles and abstracts were screened, full-text studied and information extracted for qualitative synthesis. Risk of bias was assessed with ROBINS-I criteria. The exposure was diabetes and the outcome malaria or malaria severity.
Results: Of 1992 results, three studies were included (n=7,226). Two studies found strong associations: people with diabetes had higher odds of malaria (adjusted odds ratio (aOR): 1.46 (95% CI: 1.06-2.03)) and severe malaria (aOR: 2.98 (95% CI: 1.25-7.09)). One study did not find conclusive evidence: aOR for severe malaria was 0.95 (95% CI: 0.71-1.28). Risk of bias was high in all the studies.
Conclusions: Although the available evidence on the association between diabetes and malaria is limited, the results may suggest there is a non-trivial positive relationship between these conditions.
Keywords: malaria, diabetes, multi-morbidity, co-morbidity, syndemics, Peru
Introduction
In a global context where non-communicable diseases lead the ranking of mortality and disease burden, malaria is a tropical disease still accountable for thousands of years of life lost and years lived with disability disproportionally affecting low- and middle-income countries (LMICs) 1, 2. The current multi-morbidity and syndemics paradigm calls to study diseases as clusters rather than isolate entities 3, 4, and new links between diseases could signal innovative prevention paths, e.g., adequate control of socio-demographic determinants for both conditions. Moreover, this knowledge could also provide relevant evidence on treatment and management, e.g., medications and their interactions for subjects with both illnesses. Therefore, looking at unknown associations between non-communicable diseases and malaria seems relevant, particularly for diseases for which incidence has swiftly risen in the last decades such as diabetes 5– 7. Even though there is evidence about the increasing co-morbidity between diabetes and infectious diseases 7, 8, the concept that diabetes could be a “risk factor” for malaria is relatively new 7, 9. Although relevant, available evidence is still sparse, including letters, brief reviews and animal models 10– 12. To the best of our knowledge, no systematic review has yet summarised the evidence on the association between diabetes and malaria. In so doing, the magnitude of this association could be explored, and research gaps identified. Consequently, we aimed to conduct a systematic review of observational studies addressing the association between diabetes and malaria as well as the association between diabetes and malaria severity.
Methods
Study design
This is a systematic review of the literature following the PRISMA guidelines 13 Extended data (p. 2); the checklist is available on Figshare 14. This work was registered at PROPSERO (ID CRD42018105771).
Search strategy
The search was conducted in Ovid, including Embase, Global Health and MEDLINE, as well as in Scopus and Web of Science; for specific terms used in these search engines please refer to Extended data (pp. 4–7). The search was conducted from inception to July 31 st 2018, and no language restrictions were set. However, the search in Ovid was restricted to human subjects; the search in Scopus was restricted to articles and medicine as subject area; and the search in Web of Science was restricted to articles.
Selection criteria
We sought studies that included humans subjects, had a comparator group (e.g., healthy individuals or subjects without diabetes), and the outcome was malaria diagnosis or severity. In detail, studies were selected if they included human subjects regardless of where they had been enrolled, i.e., these could have been population-based/community studies or hospital-based samples; no age restrictions were set. The study followed an observational design, e.g., cross-sectional, case-control or cohort study. Case reports and non-comparative studies were excluded. The exposure variable was diabetes defined as either a laboratory test (e.g., fasting plasma glucose ≥126 mg/dL), self-reported diagnosis or currently receiving medication for diabetes; these variables could have been actively collected or retrieved from medical records. The exposure of interest could have been any kind of diabetes (e.g., type 1 or type 2). The outcome was either malaria diagnosis or malaria severity regardless of the species as defined in each original report; for example, cases could should have had laboratory confirmation (e.g., blood smears, rapid diagnostic tests or polymerase chain reactions (PCR)) or based on clinical or discharge records.
Data collection
Using the Rayyan online tool 15, two reviewers (RMC-L and CA-F) independently screened titles and abstracts retrieved from the search strategy (agreement 0.99 and Kappa 0.99, Extended data (p. 8)). The full text of the studies that both reviewers agreed met selection criteria were sought; in addition, studies on which both reviewers had a discrepant opinion were also retrieved in full text. These full texts were studied by two reviewers (RMC-L and CA-F) independently to select those for final inclusion. The authors agreed on items that needed to be extracted from each study and RMC-L sought these pieces of information; all the authors reviewed the extraction process.
Risk of bias was assessed using the ROBINS-I criteria 16; nevertheless, the “Bias due to deviations from intended interventions” criterion was not considered because it did not apply to the studies herein included. RMC-L conducted the risk of bias assessment and all the authors reviewed the results.
The results are presented as a qualitative synthesis. No meta-analysis was planned because very few studies were expected, with high variability among them. Therefore, summary estimates (e.g., odds ratio) are summarized and presented as they were reported by each original study.
Results
Search strategy
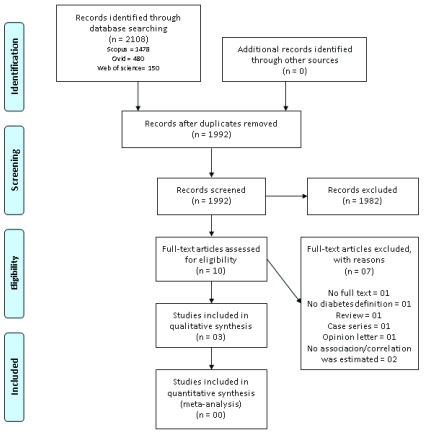
The search strategy retrieved 2108 results, 1992 were screened and 10 selected for in-depth scrutiny; finally, three studies were included for qualitative synthesis. Figure 1 depicts the number of studies at each stage of the screening process.
Figure 1. Flow chart of the study selection process.
Study characteristics
Table 1 shows the overall characteristics of the selected studies. Of the three studies, two were conducted in high-income countries: USA 17 and Sweden 18; whereas one was in Africa (Ghana) 19. All of them were published in the last 8 years and followed a retrospective design. There was heterogeneity in diabetes definition, including blood tests (e.g., fasting glucose) and history based on clinical records.
Table 1. Characteristic of the studies included in the systematic review and reported measures of association.
| Study | Country | Design | Setting | Study
population |
Sample size | % diabetes/
% malaria |
Exposure | Outcome | Main result |
|---|---|---|---|---|---|---|---|---|---|
| Malaria Susceptibility | |||||||||
| Danquah I,
et al.
19
(2010) |
Ghana | Case-control
study |
Health
centre |
Men and women
aged ≥18 years |
1,466 | 46% / by PCR
14.1% had Plasmodium spp. infection (91.8% P. falciparum) |
Type 2
diabetes: treatment or fasting plasma glucose ≥7 mmol/L |
Malaria: PCR was
used to identify Plasmodium infection and species |
Type 2 diabetes was
associated with higher odds of Plasmodium falciparum infection: aOR = 1.46 (95% CI: 1.06-2.03) |
| Malaria Severity | |||||||||
| Wyss K,
et al.
18
(2017) |
Sweden | Retrospective
observational study |
National
surveillance data (Public Health Agency) |
All adults
(≥18 years) with Plasmodium falciparum microbiologically confirmed |
937 individuals
with P. falciparum, of which 9.8% were severe |
In severe cases
9.8% had diabetes |
Diabetes:
as per the ICD-10 codes registered, including type 1, type 2 and unspecified |
Severe malaria:
2012 WHO criteria (modified by the authors) |
Regardless of
hyperparasitemia, diabetes was associated with higher odds of severe malaria: aOR = 2.98 (95% CI: 1.25-7.09) |
| Khuu D,
et al.
17
(2018) |
USA | Retrospective
observational study |
Based on
hospital records from State Inpatient Database |
Men and women
whose discharge records had malaria (ICD-9 codes) as the primary or secondary diagnoses |
4,823 severe
malaria hospitalizations |
Among severe
malaria inpatients, 10.4% type 2 diabetes |
Based on
the hospital records; no further details |
Severe malaria:
CDC criteria (modified by the authors). Malaria complications: where the discharge record noted malaria plus one or more complications (e.g., neurological symptoms) |
Type 2 diabetes was not
associated with severe malaria or malaria plus complications: aOR = 0.95 (95% CI: 0.71- 1.28) [severe malaria]; aOR = 1.06 (95% CI: 0.60-1.88) [malaria with ARDS]; aOR = 0.93 (95% CI: 0.55-1.57) [cerebral malaria]; aOR = 1.16 (95% CI: 0.75-1.80) [malaria with severe anaemia]; aOR = 1.20 (95% CI: 0.82-1.74) [malaria with renal failure]; aOR = 0.83 (95% CI: 0.36-1.92) [malaria with jaundice] |
Diabetes and malaria susceptibility
One of the three selected reports showed a strong positive association between diabetes and malaria susceptibility. People with type 2 diabetes had higher odds of malaria: adjusted odds ratios (aOR) = 1.46; 95% CI: 1.06-2.03 ( Table 1) 19.
Diabetes and malaria severity
Of the three selected reports, one provided strong evidence that people with diabetes (including type 1 and 2) had higher odds of severe malaria, aOR = 2.98; 95% CI: 1.25-7.09 18. One study did not find a strong association, reporting that type 2 diabetes and severe malaria had an aOR = 0.95; 95% CI: 0.71-1.28 ( Table 1) 17.
Risk of bias
The risk of bias assessment showed that the three retrieved studies had critical risk of bias. They all had low bias in classification of the intervention, and they presented poor information to assess bias due to missing data. Conversely, the report by Wyss and colleagues was deemed as low risk of bias in the participant selection domain 18. Table 2 summarizes all criteria for risk of bias assessment, and specific items within each criterion are shown in Extended data (pp. 9–11) 14.
Table 2. Risk of bias assessment.
| Variable | Study | ||
|---|---|---|---|
| Danquah I, et al. 19 | Wyss K, et al. 18 | Khuu D, et al. 17 | |
| Bias due to confounding | Serious risk of bias | Serious risk of bias | Serious risk of bias |
| Bias in selection of participants into the study | Critical risk of bias | Low risk of bias | Critical risk of bias |
| Bias in classification of interventions | Low risk of bias | Low risk of bias | Low risk of bias |
| Bias due to missing data | No information | No information | No information |
| Bias in measurement of outcomes | Moderate risk of bias | Moderate risk of bias | Moderate risk of bias |
| Bias in selection of the reported result | Moderate risk of bias | Moderate risk of bias | Moderate risk of bias |
| Judgement | Critical risk of bias | Critical risk of bias | Critical risk of bias |
Discussion
Summary of evidence
There were three reports addressing the association between diabetes and malaria, one studied malaria diagnosis 19 whereas two malaria severity or complications 17, 18. There was a strong positive association between diabetes and malaria diagnosis 19, and only one study found compelling evidence of an association between diabetes and malaria severity 18. Across these three studies, overall risk of bias was high.
Limitations
The study designs followed by the reviewed studies were case-control 19 and retrospective analyses 17, 18. Although these methodologies provide relevant evidence and have several strengths, regarding the topic at hand the evidence they offer still needs further verification with stronger observational approaches. These could include prospective cohorts or thoroughly conceived analysis following a casual-inference methodology. On the other hand, we acknowledge that the reviewed studies ascertained both the exposure and outcome following valid methodologies, which in turn suggests that addressing the association of interest is feasible and supports our call for further studies in this field.
Differences in malaria severity definition could limit our conclusions too. One report ascertained malaria severity with laboratory tests 18, and the other extracted the information from hospital discharge records 17. The second report was included in the review because hospital records are most likely to be based on laboratory tests, even though this was not disclosed in the publication. The different methodologies these studies followed could explain why the former report showed a strong association whereas the latter failed to show compelling evidence, even though they both had a similar proportion of people with diabetes among severe malaria cases 17, 18.
This review has some strengths and limitations. First, we searched five international search engines, which allowed us to cover thousands of published materials. Therefore, our results show what there is available at a global scale and thus signal a dearth of strong evidence on the association between diabetes and malaria. Second, there are several tools to assess risk of bias. The one we followed, ROBINS-I, is a domain-based approach which seems to be a better approach in comparison to tools without domains; nevertheless, it has also been suggested that ROBINS-I labels more studies as of high risk of bias 20. Regardless of the assessed domains and other properties of the ROBINS-I, the study design (e.g., case-control) followed by the reviewed reports already suggest high risk of bias. This is not a critique on the available evidence, but rather a call to build on the available literature and conduct more comprehensive research. Third, we defined malaria and malaria severity as in the original publications. Differences in malaria definitions could explain some results as discussed above 17, 18. Nonetheless, two of the three selected reports confirmed their cases with laboratory methods while one used discharge records. The positive and strong association these two publications found 18, 19, supports the potential association between diabetes and malaria, while also signalling the dearth of evidence on this subject.
Implications for research
This systematic review found three studies following a case-control 19 and retrospective analysis approach 17, 18. Although they did a great effort to ascertain the exposure and outcome with precision, e.g., using biomarkers for diabetes or PCR for malaria, they lacked generalizability because their study populations were based on (small) patients samples instead of general population samples. The selected reports also followed the most suitable research design given their locations; for example, a case-control study was feasible for Danquah and colleagues in Ghana where malaria is prevalent 19, though it would be challenging for other researchers in USA and Sweden where retrospective analysis based on clinical records is much efficient 17, 18. All these studies have provided relevant evidence and have sparked interest in this novel yet relevant study field: diabetes and malaria.
A relevant research approach, as suggested by Broz et al., would be how to improve diabetes control in people with malaria 21. Because malaria would imply haemolysis, this could impair the accuracy of HbA1c to inform about diabetes control; potential solutions could include daily glucose monitor or fructosamine 22. We believe that a population-based cohort study that has assessed diabetes would be a key asset in further elucidating the (true) association between diabetes and malaria. We are confident that these studies are available somewhere, with interest in LMICs, and that soon they will produce evidence in order to identify whether addressing diabetes could reduce malaria burden.
Implications for public health and clinical practice
At this this time we believe it is premature to draw any strong conclusions for clinical practice or public health. Nevertheless, we could suggest strengthening malaria prevention strategies for people with diabetes, particularly in highly endemic areas or among travellers to these settings. In accordance to international guidelines suggesting prophylactic treatment for travels to malaria highly-endemic areas 23, 24, these recommendations could be stronger for people with diabetes.
Diabetes and malaria: the epidemiological context
Global estimates inform that Africa is the region with the largest number of Malaria cases, where Nigeria, followed by the Democratic Republic of Congo, accounts for most of these 25. Although there has been a decrease since 1990, West and Central Africa exhibit the highest malaria death rates, with Mali, Burkina Faso and Niger leading the ranking 26. On the other hand, epidemiological evidence shows that from 1980 to 2014, diabetes prevalence has doubled in most sub-Saharan nations 5, 6. Regarding the above highlighted countries, diabetes prevalence in Nigeria and the Democratic Republic of Congo has increased by 2- and 2.5-fold, respectively; these estimates for Mali, Burkina Faso and Niger were 2.6-, 2.8- and 2.3-fold 5, 6. These country-level estimates could have several potential implications. First, countries with high malaria prevalence have experienced a dramatic increase in diabetes prevalence. Perhaps, this could imply that the rising diabetes burden makes it difficult to lessen malaria burden despite large efforts on this matter. Second, Burkina Faso, one of the countries with high malaria death rates have also had an utterly increase in diabetes prevalence. This could suggest that the increasing diabetes burden may be leading to more severe cases with fatal outcomes. Third, as diabetes prevalence keeps rising in malaria endemic areas, larger populations could be at high risk of malaria. This could lead to call to strengthen diabetes prevention and early diagnosis programs, not only to stop non-communicable diseases but also because of a potential positive impact on malaria control. These ecological arguments, along with the evidence summarized in this review, suggest of a possible co-morbidity (if not synergism) between diabetes and malaria that deserves further and comprehensive scrutiny.
Diabetes and malaria: pathways
The aim of this review was to synthetize the evidence about diabetes as an associated factor with higher malaria prevalence, incidence or severity. Even though we could not assess diabetes as a ‘risk factor’ in the strict definition of the term because of the lack of prospective studies, we summarized preliminary evidence that suggest diabetes could be associated with malaria and malaria severity. In addition to this finding, biological pathways have been proposed to explain this association.
Olivier and colleagues summarized key aspects of immune response in malaria, highlighting features of early infection including that phagocytes such as monocytes, macrophages and neutrophils lead the immunological response 27; this role of monocytes and macrophages during erythrocyte infection has been studied before, particularly in response to Plasmodium hemozoin and Glycosylphosphatidylinositol 28– 31. However, macrophage phagocytic activity seems to be reduced in diabetics 32– 35, and this is paramount in the association between diabetes and tuberculosis 36. This impaired immunity, particularly regarding those cells with responsibilities against malaria, could explain the association between diabetes and malaria 11. These arguments, i.e. impaired immunity, support solid hypotheses to explain higher malaria susceptibility and malaria severity among people with diabetes.
Other possible pathways between diabetes and malaria include hyperinsulinemia, which has been suggested to affect mosquitos in a way that they have a reduced immune response against Plasmodium falciparum 37. This implies there would be more mosquitos to spread malaria. Although we are unaware of robust evidence to support this potential explanation, Raghunath hypothesised that diabetics who take metformin would attract more mosquitos because this medication increases the concentration of lactic acid in their sweat, a compound that attracts mosquitoes 10; however, biguanides appear to positively interact with anti-malaria drugs 38. These additional arguments to support the association between diabetes and malaria render further importance to the study of this co-morbidity; not only because this would enrich science, but because it could also provide innovative prevention or treatment strategies.
Conclusions
Despite conducting a comprehensive systematic review, the results showed there is paucity in the evidence about the association between diabetes and malaria as well as between diabetes and malaria severity. Of the three reviewed studies, one found a strong association between diabetes and malaria diagnosis, and one between diabetes and malaria severity; one study did not find strong evidence of association between diabetes and malaria severity. The three studies had high risk of bias, with one following a case-control design while the other two conducted a retrospective analysis. This limited evidence precludes drawing a strong conclusion on the associations of interest. Although there is evidence signalling biologically plausible pathways between diabetes and malaria as well as malaria severity. It is still premature to make clinical or public health recommendations to address the (possible) synergism between diabetes and malaria. On the other hand, this review signals the dearth of evidence on this association, both in terms of quantity and quality of available research; this calls to further study these associations and others between communicable and non-communicable diseases particularly in places with high or raising burden of both.
Data availability
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
Extended data
Figshare: IS DIABETES ASSOCIATED WITH MALARIA AND MALARIA SEVERITY? A SYSTEMATIC REVIEW OF OBSERVATIONAL STUDIES - SUPPLEMENTARY MATERIAL. https://doi.org/10.6084/m9.figshare.9789740.v2 14.
Reporting guidelines
Figshare: PRISMA checklist for ‘Is diabetes associated with malaria and malaria severity? A systematic review of observational studies’. https://doi.org/10.6084/m9.figshare.9789740.v1 14.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Funding Statement
Strategic Award, Wellcome Trust-Imperial College Centre for Global Health Research (100693) and Imperial College London Wellcome Trust Institutional Strategic Support Fund [Global Health Clinical Research Training Fellowship] (294834 ISSF ICL). Rodrigo M Carrillo-Larco is supported by a Wellcome Trust International Training Fellowship (214185).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 3; peer review: 2 approved]
References
- 1. GBD 2016 Causes of Death Collaborators: Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators: Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Medical Research Council-UK: Multimorbidity: a priority for global health research. Reference Source [Google Scholar]
- 4. Mendenhall E: Syndemics: a new path for global health research. Lancet. 2017;389(10072):889–891. 10.1016/S0140-6736(17)30602-5 [DOI] [PubMed] [Google Scholar]
- 5. NCD Risk Factor Collaboration (NCD-RisC) – Africa Working Group: Trends in obesity and diabetes across Africa from 1980 to 2014: an analysis of pooled population-based studies. Int J Epidemiol. 2017;46(5):1421–1432. 10.1093/ije/dyx078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. NCD Risk Factor Collaboration (NCD-RisC): Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–1530. 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Crevel R, van de Vijver S, Moore DAJ: The global diabetes epidemic: what does it mean for infectious diseases in tropical countries? Lancet Diabetes Endocrinol. 2017;5(6):457–468. 10.1016/S2213-8587(16)30081-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Critchley JA, Restrepo BI, Ronacher K, et al. : Defining a Research Agenda to Address the Converging Epidemics of Tuberculosis and Diabetes: Part 1: Epidemiology and Clinical Management. Chest. 2017;152(1):165–173. 10.1016/j.chest.2017.04.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oni T, Unwin N: Why the communicable/non-communicable disease dichotomy is problematic for public health control strategies: implications of multimorbidity for health systems in an era of health transition. Int Health. 2015;7(6):390–399. 10.1093/inthealth/ihv040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raghunath P: Impact of type 2 diabetes mellitus on the incidence of malaria. J Infect Public Health. 2017;10(3):357–358. 10.1016/j.jiph.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 11. Kalra S, Khandelwal D, Singla R, et al. : Malaria and diabetes. J Pak Med Assoc. 2017;67(5):810–813. [PubMed] [Google Scholar]
- 12. Pakpour N, Cheung KW, Luckhart S: Enhanced transmission of malaria parasites to mosquitoes in a murine model of type 2 diabetes. Malar J. 2016;15:231. 10.1186/s12936-016-1277-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liberati A, Altman DG, Tetzlaff J, et al. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carrillo Larco R: IS DIABETES ASSOCIATED WITH MALARIA AND MALARIA SEVERITY? A SYSTEMATIC REVIEW OF OBSERVATIONAL STUDIES - SUPPLEMENTARY MATERIAL. figshare.Online resource.2019. [DOI] [PMC free article] [PubMed]
- 15. Ouzzani M, Hammady H, Fedorowicz Z, et al. : Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterne JA, Hernán MA, Reeves BC, et al. : ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khuu D, Eberhard ML, Bristow BN, et al. : Risk factors for severe malaria among hospitalized patients in the United States, 2000–2014. Infect Dis Health. 2018;23(2):93–106. 10.1016/j.idh.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 18. Wyss K, Wangdahl A, Vesterlund M, et al. : Obesity and Diabetes as Risk Factors for Severe Plasmodium falciparum Malaria: Results From a Swedish Nationwide Study. Clin Infect Dis. 2017;65(6):949–958. 10.1093/cid/cix437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Danquah I, Bedu-Addo G, Mockenhaupt FP: Type 2 diabetes mellitus and increased risk for malaria infection. Emerg Infect Dis. 2010;16(10):1601–1604. 10.3201/eid1610.100399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Losilla JM, Oliveras I, Marin-Garcia JA, et al. : Three risk of bias tools lead to opposite conclusions in observational research synthesis. J Clin Epidemiol. 2018;101:61–72. 10.1016/j.jclinepi.2018.05.021 [DOI] [PubMed] [Google Scholar]
- 21. Broz J, Hoskovcova L, Brunerova L: Comment on Sanjay Kalra et al (J Pak Med Assoc. 2017; 5: 810-813) - Diabetes mellitus, malaria and HbA1c levels. J Pak Med Assoc. 2017;67(11):1786. [PubMed] [Google Scholar]
- 22. Kalra S, Khandelwal D, Singla R, et al. : Response to comment on Sanjay Kalra et al (J Pak Med Assoc. 2017; 5: 810-813) - Malaria, red blood cells turnover and glycated hemoglobin: Pitfalls and challenges. J Pak Med Assoc. 2017;67(11):1786. [PubMed] [Google Scholar]
- 23. Public Health England: Guidelines for malaria prevention in travellers from the UK: 2017.2017. Reference Source [Google Scholar]
- 24. CDC Centers for Disease Control and Prevention: Malaria and Travellers for U.S. Residents. Reference Source [Google Scholar]
- 25. World Health Organiztion: World malaria report 2017. Geneva: WHO;2017; [Accessed on 16 Sept, 2018]. Reference Source [Google Scholar]
- 26. Gething PW, Casey DC, Weiss DJ, et al. : Mapping Plasmodium falciparum Mortality in Africa between 1990 and 2015. N Engl J Med. 2016;375(25):2435–2445. 10.1056/NEJMoa1606701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olivier M, Van Den Ham K, Shio MT, et al. : Malarial pigment hemozoin and the innate inflammatory response. Front Immunol. 2014;5(5):25. 10.3389/fimmu.2014.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lindner SE, Miller JL, Kappe SH: Malaria parasite pre-erythrocytic infection: preparation meets opportunity. Cell Microbiol. 2012;14(3):316–324. 10.1111/j.1462-5822.2011.01734.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chua CL, Brown G, Hamilton JA, et al. : Monocytes and macrophages in malaria: protection or pathology? Trends Parasitol. 2013;29(1):26–34. 10.1016/j.pt.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 30. Punsawad C: Effect of malaria components on blood mononuclear cells involved in immune response. Asian Pac J Trop Biomed. 2013;3(9):751–756. 10.1016/S2221-1691(13)60151-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aldridge JR, Vogel IA: Macrophage biology and their activation by protozoan-derived glycosylphosphatidylinositol anchors and hemozoin. J Parasitol. 2014;100(6):737–742. 10.1645/14-646.1 [DOI] [PubMed] [Google Scholar]
- 32. Lecube A, Pachón G, Petriz J, et al. : Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One. 2011;6(8):e23366. 10.1371/journal.pone.0023366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Geerlings SE, Hoepelman AI: Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26(3–4):259–265. 10.1111/j.1574-695X.1999.tb01397.x [DOI] [PubMed] [Google Scholar]
- 34. Pavlou S, Lindsay J, Ingram R, et al. : Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol. 2018;19(1):24. 10.1186/s12865-018-0261-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Knapp S: Diabetes and infection: is there a link?--A mini-review. Gerontology. 2013;59(2):99–104. 10.1159/000345107 [DOI] [PubMed] [Google Scholar]
- 36. Hodgson K, Morris J, Bridson T, et al. : Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2015;144(2):171–185. 10.1111/imm.12394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pakpour N, Corby-Harris V, Green GP, et al. : Ingested human insulin inhibits the mosquito NF-κB-dependent immune response to Plasmodium falciparum. Infect Immun. 2012;80(6):2141–2149. 10.1128/IAI.00024-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones K, Ward SA: Biguanide-atovaquone synergy against Plasmodium falciparum in vitro. Antimicrob Agents Chemother. 2002;46(8):2700–3. 10.1128/aac.46.8.2700-2703.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]