Abstract
Introduction:
Numerous biomarkers have been investigated for the diagnosis and follow-up of patients with bladder cancer, but none has achieved desirable acceptability. In the search of biomarkers, minichromosome maintenance protein 2 (MCM2), a cell cycle regulatory protein, was investigated and the preliminary results were promising. Hence, we conducted a study to investigate the role of immunocytochemical (ICC) detection of MCM2 in voided urinary samples of patients with bladder cancer in an Indian population.
Materials and Methods:
A prospective comparative observational study was performed. One hundred and fifty patients with a mass lesion in the bladder and 100 controls were enrolled in this prospective study from June 2017 to–December 2018. Fifty-milliliter of voided urine sample was collected and processed for ICC staining of MCM2.
Results:
Fifty, 100, and 200 positive MCM2 cells as a cutoff value has shown a sensitivity of 87.33% (80.93%–92.20%), 84.67% (77.89%–90.02%), and 80.67% (73.43%–86.65%), respectively. The specificity of 50, 100, and 200 positive MCM2 cells was 97% (91.48%–99.38%), 99% (94.55%–99.97%), and 100% (96.38%–100.0%), respectively.
Conclusion:
ICC detection of MCM2 in voided urinary samples has good sensitivity and specificity for the detection of bladder cancer. Hence, it can be used as a potential marker for the detection of bladder cancer.
INTRODUCTION
Bladder neoplasm is a standout amongst the most widely recognized cancers.[1] It adds 3% to the cancer-related mortality.[2] Its mortality and morbidity can be diminished by early detection and prompt management.[3]
Hematuria is the most common manifestation that leads to the evaluation and detection of bladder neoplasms. Cystoscopy, upper tract imaging, and cytology are the established modalities for the evaluation of hematuria.[4] Amongst them, cystoscopy and urine cytology are the widely employed modalities for the diagnosis, follow-up, and surveillance of bladder cancer. Cystoscopy is most sensitive but operator dependent and is an invasive procedure for the patients. Urinary cytology is tumor grade dependent and has overall low sensitivity.[5,6]
To overcome the aforementioned lacunae, numerous biomarkers have been assessed. At present, the quantitative BTA TRAK, qualitative BTA stat, quantitative immunoassay NMP22, qualitative test NMP22 (bladder check), UroVysion (fluorescence in situ hybridization test), and ImmunoCyt test are approved by the US Food Drug Administration (FDA).[7] However, requisite sensitivity and specificity are not achieved by the available urinary biomarkers.[8]
Another potential biomarker is minichromosome maintenance protein (MCM). MCM as a biomarker has been evaluated for epithelial cancer since 1900.[9,10,11,12] The MCM proteins are important nuclear regulators of cell cycle and are highly specific for cellular proliferation. MCM protein is expressed in proliferating cells and cells with potential of proliferation.[13] In normal cells, MCM proteins are degraded in quiescent phase, but in malignancy and premalignancy, MCM proteins do not disappear and get accumulated in the nucleus of proliferating cells.[10] This attribute of the MCM protein leads to its expression and may serve as a breakthrough in the detection of malignancy.
There are six types of MCM protein (MCM2–7). Korkolopoulou et al. concluded that MCM2 and MCM5 proteins are useful and reliable proliferation and prognostic markers in patients with muscle-invasive bladder carcinomas.[14] Diagnostic role of the detection of MCM2 in urine of bladder cancer was also found to be promising.[15,16]
Despite the early promising results of MCM2 (good sensitivity and specificity), a limited number of studies are available in literature, and as per our knowledge, no study has been performed in Indian subjects. We conducted this study to assess the role of immunocytochemical detection of MCM2 in voided urine of bladder cancer in an Indian population.
MATERIALS AND METHODS
The study was approved by our institutional ethics committee and written informed consent was obtained before enrollment in the study. The study was conducted between June 2017 and–December 2018. Patients who were diagnosed with a bladder mass and were admitted for transurethral resection of tumor were included and labeled as the study group (SG). Patients with urinary tract infection (UTI), concomitant upper tract tumor, and any recent (within 1 week) urethral instrumentation were excluded. The reason of exclusion was avoiding contamination of the result from upper tract malignancy, undesired contamination of basal urothelial cells in collected samples due to instrumentation, and infection. 100 subjects with renal stone disease, ureteric stone disease, and pelviureteric junction obstruction, who had no known urothelial malignancy, active UTI, or history of gross hematuria, were included as controls and labeled as CG. All CG patients underwent cystoscopic examination during their respective surgery, and bladder malignancy was further ruled out.
Comprehensive history was recorded and 50-ml freshly voided samples (irrespective of the presence of hematuria) were collected. All collected samples were processed within 1 h. Liquid-based cytology (LBC) slides were prepared with BD sure Path automated processor. Prepared unstained LBC slides were dipped for 2 min in methanol and relocated into overnight freeze acetone for 1 h, followed by peroxidase blocking which was performed for 10 min and washed with buffer, and then ready-to-use rabbit monoclonal minichromosome maintenance protein (Pathn Situ biotechnologies Pvt. Ltd., EP40) was incorporated and stored for 40 min at room condition. Envision-labeled polymer was incorporated for 30 min after washing. Commercially provided ready-to-use 3,3′-Diaminobenzidine chromogen was then applied over the slide and stored for 10 min. Counterstaining with hematoxylin was performed after washing and kept for 5 min at ambient temperature. Slides were viewed and reported by a pathologist in the department of pathology. MCM2-positive cells showed nuclear staining, whereas negative cells did not take nuclear stain of MCM2 antibody stain [Figure 1a-c].
Figure 1.
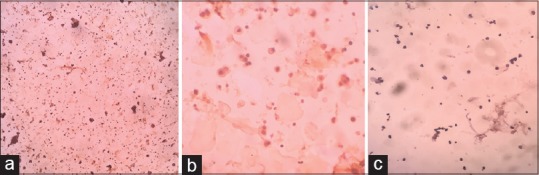
(a) ICC-minichromosome maintenance protein 2-positive exfoliated urothelial cell (specimen: Voided urine) low-magnification view (×10 view). (b) ICC-minichromosome maintenance protein 2-positive exfoliated cell (specimen: Voided urine) high-magnification view (×40 view). (c) ICC-minichromosome maintenance protein 2-negative exfoliated urothelial cell (Specimen: Voided urine) high-magnification view (×40 view)
An average number of MCM-positive cells per high-power field (×40 view) were estimated after counting of ten high-power fields per slide. An equivalent number of positive cells were further estimated with multiplication factors (nine cells/high-power field are equal to 5000 equivalent cells; round-off multiplication factor 550[17]). If ten high-power fields did not show positive cells, then further part of slide was assessed, and a mean positive value per high-power field (and equivalents positive cells) was calculated. For adequacy of slide, 5000 cells were considered a minimum number of equivalent cells. Three cutoff values were taken as under:
50 cells positive for MCM2 (50+ cells)
100 cells positive for MCM2 (100+ cells)
200 cells positive for MCM2 (200+ cells).
A higher number of positive MCM2 cells above the cutoff value were considered positive for MCM2. Conventional cytology of urinary samples was also performed by Papanicolaou's method, and reporting was done according to the Paris system. For better clinical significance, suspicious and atypical cytology was considered negative in the current study. Cystoscopy and transurethral resection of tumor of SG were performed usually 3–7 days after collection of urinary samples. Tumor staging and grading were recorded after transurethral resection of bladder tumors.
After obtaining data, the analysis was performed with SPSS 23 (Statistics for Windows, IBM Corp. 2015, Armonk, NY, USA). Pearson's Chi-square test and McNemar-Bowker test were used. Sensitivity and specificity were calculated, and their confidence interval was calculated by Clopper–Pearson method.
RESULTS
During the study period, we enrolled 150 patients of known bladder SOL as SG after exclusion of 39 patients (as per exclusion criteria) and 100 patients were enrolled as CG. CG included 49 renal stone disease patients, 18 pelviureteric junction obstruction patients, and 33 ureteric stone patients. Most of the patients of bladder SOL were older in age (more than 50 years), were male, and had history of smoking [Table 1]. Mostly, they presented with hematuria, and histopathological examination revealed nonmuscle-invasive tumor and low-grade tumor [Table 1].
Table 1.
Clinical features, stage and grading of tumor of study group
| Variables | Frequency | Percentage |
|---|---|---|
| Age (years) | 150 {mean age 59.74±21.5} | |
| <41 years | 7 | 4.7 |
| 41-50 | 17 | 11.3 |
| 51-60 | 57 | 38.0 |
| 61-70 | 51 | 34.0 |
| More than 71 years | 18 | 12.0 |
| Sex | ||
| Male | 126 | 84 |
| Female | 24 | 16 |
| Family history | ||
| Present | 17 | 11.3 |
| Absent | 133 | 88.7 |
| Smoker | ||
| Smoker | 119 (112 male & 7 female) | 79.3 |
| Non -smoker | 31 (14 male & 17 female) | 20.7 |
| Mode of presentation | ||
| Hematuria | 141 | 94 |
| Others | 9 | 6 |
| Incidental | 2 | |
| Dysuria | 5 | |
| LUTS | 2 | |
| Tumor stage | ||
| Benign | 2 | 1.3 |
| PUNLMP | 2 | 1.3 |
| Ta | 49 | 32.7 |
| T1 | 82 | 54.7 |
| T2 | 14 | 9.3 |
| CIS | 1 | 0.7 |
| Grade of tumor | ||
| Benign | 2 | 1.3 |
| PUNLMP | 2 | 1.3 |
| Low grade | 87 | 58 |
| High Grade | 59 | 39.3 |
LUTS: lower urinary tract symptom, CIS: carcinoma in situ, PUNLMP: papillary urothelial neoplasia of low malignant potential
When a cutoff of 50 MCM2-positive cells was taken, then 87.33% (n = 131) of bladder SOL (SG) were positive for malignancy, as compared to 3% (n = 3) of CG. But, when the cutoff value was increased to 200 positive cells, 80.67% (n = 121) of the SG was positive without any false positives [Table 2]. For all the cutoff values, the positivity of SG was significantly more than the CG (P < 0.0001).
Table 2.
ICC -MCM2 positivity
| MCM2 cut off value | Study Group (positive) | Control Group (Positive) | P* |
|---|---|---|---|
| 50+cells | 131 (87.33%) | 3 (3%) | <0.000001 |
| 100+cells | 127 (84.67%) | 1 (1%) | |
| 200+cells | 121 (80.67%) | 0 (0%) |
MCM2: Mini Chromosome maintenance protein 2, *Pearson’s Chi-square test and Mc Nemar -Bowker
The sensitivity for detection of ICC-MCM2-positive exfoliated urothelial cells in the voided urine samples was >80% for all the cutoff values, whereas the specificity was greater than 95% and increased to 100% when the cutoff value was 200 MCM2-positive cells [Table 3]. Urinary ICC-MCM2 detection remained unaffected by depth and grade of tumor (P > 0.05) [Table 4]. Cancer detection by ICC-MCM2 was statistically better than urinary cytology in the SG (P < 0.005) [Table 5].
Table 3.
Sensitivity and specificity of ICC-MCM2 positivity
| Cut off value (MCM2) | Sensitivity (confidence interval) % | Specificity (confidence interval) % |
|---|---|---|
| 50+cells | 87.33 (80.93-92.20) | 97 (91.48-99.38) |
| 100+cells | 84.67 (77.89-90.02) | 99 (94.55-99.97) |
| 200+cells | 80.67 (73.43-86.65) | 100 (96.38-100.0) |
*Confidence interval was calculated by Clopper-Pearson method
Table 4.
Relationship of MCM2 with Subgroup of bladder tumor
| Variables | MCM2 cut off value (50+cells) | MCM2 cut off value (100+cells) | MCM2 cut off value (200+cells) |
|---|---|---|---|
| Non-muscle invasive* | 118 (88.05%) | 114 (85.07%) | 108 (80.59%) |
| Muscle invasive | 13 (92.85%) | 13 (92.85%) | 13 (92.85%) |
| P (Pearson Chi square test) | 0.592 | 0.427 | 0.258 |
| High grade Tumor | 77 (86.51%) | 75 (84.26%) | 73 (82.02%) |
| Low grade tumor | 54 (91.52%) | 52 (88.13%) | 48 (81.35%) |
| P (Pearson Chi square test) | 0.349 | 0.509 | 0.918 |
*Non-muscle invasive bladder tumor includes PUNLMP, Ta, T1 and CIS tumor
Table 5.
Relationship of MCM2 and cytology
| MCM2 cut off value | Study group (positive) | Cytology (malignant cells) | P (Pearson-Chi square test) |
|---|---|---|---|
| 50+cells | 131 (87.33%) | 38 (25.3%) | <0.005 |
| 100+cells | 127 (84.67%) | 38 (25.3%) | |
| 200+cells | 121 (80.67%) | 38 (25.3%) |
MCM2: Mini Chromosome maintenance protein 2
DISCUSSION
Biomarkers are emerging tools for the detection and follow-up of various neoplasms. Different biomarkers have been used and approved by the FDA for bladder cancer, but none of them have achieved desirable sensitivity. Therefore, the pursuit of a new biomarker is essential.
One of the biomarkers is MCM. It is a nuclear regulatory protein, which is expressed during the division of cells. It is expressed in malignant and premalignant cells and basal layer of urothelial layers (which are normally not exfoliated in the urine).[13] This gives us an opportunity to detect MCM in urine of bladder cancer patients. MCM protein complex constitutes six subtypes (MCM2–7).[18]
After the late 1990, MCM has been studied in different epithelial carcinomas such as cervical, laryngeal, esophagus, and anal carcinomas.[9,10,11,12,19] Initially, immunostaining of MCM5 subtype of MCM protein complex was examined in histology of the bladder cancer by Stoeber et al.[20] Proportionated immunostained cells with MCM5 were correlated with grade of bladder transitional cell carcinoma, which amounted to 45%, 70%, and 78% for well-differentiated (G1), moderately differentiated (G2), and poorly differentiated (G3) tumors, respectively.
After antecedent positive results, Stoeber et al. had used the detection of minichromosome maintenance 5 protein in urine sediments to diagnose genitourinary tract carcinoma.[21] They concluded that the elevated levels of MCM5 in urine sediments was highly predictive of bladder cancer. Similarly, MCM2 was studied by Burger et al.[22] and Krüger et al.[23] in the histological specimens of bladder cancer and found it to be of good prognostic significance.
Diagnostic role of MCM2 in urine sediment cells was investigated by Gordon et al.[16] MCM2 levels were determined in LBC urine samples from 56 patients who had a diagnosis of bladder cancer as determined by cystoscopy which showed a sensitivity of. 93%.
Saeb-Parsy et al. had also conducted a study to investigate the diagnostic accuracy of immunocytochemistry (ICC) of MCM2 in bladder cancer. Four hundred and ninety-seven patients (patients with gross hematuria or follow-up case of bladder cancer on cystoscopic surviellance) were enrolled in their study. Adequate urinary samples were obtained and a cutoff value 50+ MCM2-positive cells in the specimens of patients with gross hematuria and 200+ cells in patients on cystoscopic surveillance based on a minimum total cell number of 5000 was set. Sensitivity, specificity, and negative predictive value were 81.3%, 76.0%, and 92.7% in gross hematuria and 63.2%, 89.9%, and 89.9% in cystoscopic follow-up patients, respectively.[15]
In the present study, we had set three cutoff values of MCM2-positive exfoliated cells (50, 100, and 200 MCM2-positive cells). The above values were considered positive for MCM2. At a cutoff value of 50 MCM2-positive cells, 87.33% in the SG were true positive, but the false positive rate was 3% whereas at a cut off of 200 positive MCM2 cells there were no false positives with >80% true positive rate in the SG [Table 2].
The present study revealed high sensitivity and specificity of immunocytochemical (ICC) detection of MCM2. The sensitivity of the present study is similar to the previous work performed by Gordon et al.[16] and Saeb-Parsy et al.[15] However, specificity is higher as compared to the study by Saeb-Parsy et al.
The positive detection of urinary MCM2 was not affected by depth and grade of the bladder tumor [Table 4]. Malignant cell detection with cytology was also compared with urinary ICC-MCM2 in the present study. MCM2 positivity was statistically superior in all threshold values [Table 5].
CONCLUSION
We conclude that urinary immunocytochemical detection of MCM2 in exfoliated cells show a promising role in patients with bladder tumor. It can be used as a potential biomarker for the detection of bladder cancer as it had very high sensitivity and specificity. However, to generalize this statement, further multi-institutional, the inclusion of large number of patients, follow-up patients, and patients recieving bacillus Calmette–Guerin is required.
Acknowledgments
The authors express their indebtedness to Dr. Ankit Vaishnav for his constant support during the study period.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of Interest: There are no conflicts of interest.
REFERENCES
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Messing EM, Madeb R, Young T, Gilchrist KW, Bram L, Greenberg EB, et al. Long-term outcome of hematuria home screening for bladder cancer in men. Cancer. 2006;107:2173–9. doi: 10.1002/cncr.22224. [DOI] [PubMed] [Google Scholar]
- 4.Grossfeld GD, Wolf JS, Jr, Litwan MS, Hricak H, Shuler CL, Agerter DC, et al. Asymptomatic microscopic hematuria in adults: Summary of the AUA best practice policy recommendations. Am Fam Physician. 2001;63:1145–54. [PubMed] [Google Scholar]
- 5.Raitanen MP, Aine R, Rintala E, Kallio J, Rajala P, Juusela H, et al. Differences between local and review urinary cytology in diagnosis of bladder cancer. An interobserver multicenter analysis. Eur Urol. 2002;41:284–9. doi: 10.1016/s0302-2838(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 6.van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: A systematic review. Eur Urol. 2005;47:736–48. doi: 10.1016/j.eururo.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Herman MP, Svatek RS, Lotan Y, Karakiewizc PI, Shariat SF. Urine-based biomarkers for the early detection and surveillance of non-muscle invasive bladder cancer. Minerva Urol Nefrol. 2008;60:217–35. [PubMed] [Google Scholar]
- 8.Vriesema JL, Poucki MH, Kiemeney LA, Witjes JA. Patient opinion of urinary tests versus flexible urethrocystoscopy in follow-up examination for superficial bladder cancer: A utility analysis. Urology. 2000;56:793–7. doi: 10.1016/s0090-4295(00)00777-9. [DOI] [PubMed] [Google Scholar]
- 9.Williams GH, Romanowski P, Morris L, Madine M, Mills AD, Stoeber K, et al. Improved cervical smear assessment using antibodies against proteins that regulate DNA replication. Proc Natl Acad Sci U S A. 1998;95:14932–7. doi: 10.1073/pnas.95.25.14932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman A, Morris LS, Mills AD, Stoeber K, Laskey RA, Williams GH, et al. Minichromosome maintenance proteins as biological markers of dysplasia and malignancy. Clin Cancer Res. 1999;5:2121–32. [PubMed] [Google Scholar]
- 11.Davies RJ, Freeman A, Morris LS, Bingham S, Dilworth S, Scott I, et al. Analysis of minichromosome maintenance proteins as a novel method for detection of colorectal cancer in stool. Lancet. 2002;359:1917–9. doi: 10.1016/S0140-6736(02)08739-1. [DOI] [PubMed] [Google Scholar]
- 12.Sirieix PS, O’Donovan M, Brown J, Save V, Coleman N, Fitzgerald RC. Surface expression of minichromosome maintenance proteins provides a novel method for detecting patients at risk for developing adenocarcinoma in Barrett's esophagus. Clin Cancer Res. 2003;9:2560–6. [PubMed] [Google Scholar]
- 13.Stoeber K, Tlsty TD, Happerfield L, Thomas GA, Romanov S, Bobrow L, et al. DNA replication licensing and human cell proliferation. J Cell Sci. 2001;114:2027–41. doi: 10.1242/jcs.114.11.2027. [DOI] [PubMed] [Google Scholar]
- 14.Korkolopoulou P, Givalos N, Saetta A, Goudopoulou A, Gakiopoulou H, Thymara I, et al. Minichromosome maintenance proteins 2 and 5 expression in muscle-invasive urothelial cancer: A multivariate survival study including proliferation markers and cell cycle regulators. Hum Pathol. 2005;36:899–907. doi: 10.1016/j.humpath.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Saeb-Parsy K, Wilson A, Scarpini C, Corcoran M, Chilcott S, McKean M, et al. Diagnosis of bladder cancer by immunocytochemical detection of minichromosome maintenance protein-2 in cells retrieved from urine. Br J Cancer. 2012;107:1384–91. doi: 10.1038/bjc.2012.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon L, Stewart K, McKean M. Minichromosome maintenance protein-2 as a diagnostic marker in bladder cancer. Biomed Sci. 2010:32–3. [Google Scholar]
- 17.Sørbye SW, Pedersen MK, Ekeberg B, Williams ME, Sauer T, Chen Y. Can an inadequate cervical cytology sample in ThinPrep be converted to a satisfactory sample by processing it with a SurePath preparation? Cytojournal. 2017;14:20. doi: 10.4103/cytojournal.cytojournal_34_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carpentieri F, De Felice M, De Falco M, Rossi M, Pisani FM. Physical and functional interaction between the mini-chromosome maintenance-like DNA helicase and the single-stranded DNA binding protein from the crenarchaeon Sulfolobus solfataricus. J Biol Chem. 2002;277:12118–27. doi: 10.1074/jbc.M200091200. [DOI] [PubMed] [Google Scholar]
- 19.Chatrath P, Scott IS, Morris LS, Davies RJ, Rushbrook SM, Bird K, et al. Aberrant expression of minichromosome maintenance protein-2 and Ki67 in laryngeal squamous epithelial lesions. Br J Cancer. 2003;89:1048–54. doi: 10.1038/sj.bjc.6601234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoeber K, Halsall I, Freeman A, Swinn R, Doble A, Morris L, et al. Immunoassay for urothelial cancers that detects DNA replication protein Mcm5 in urine. Lancet. 1999;354:1524–5. doi: 10.1016/S0140-6736(99)04265-8. [DOI] [PubMed] [Google Scholar]
- 21.Stoeber K, Swinn R, Prevost AT, de Clive-Lowe P, Halsall I, Dilworth SM, et al. Diagnosis of genito-urinary tract cancer by detection of minichromosome maintenance 5 protein in urine sediments. J Natl Cancer Inst. 2002;94:1071–9. doi: 10.1093/jnci/94.14.1071. [DOI] [PubMed] [Google Scholar]
- 22.Burger M, Denzinger S, Hartmann A, Wieland WF, Stoehr R, Obermann EC. Mcm2 predicts recurrence hazard in stage Ta/T1 bladder cancer more accurately than CK20, Ki67 and histological grade. Br J Cancer. 2007;96:1711–5. doi: 10.1038/sj.bjc.6603784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krüger S, Thorns C, Stöcker W, Müller-Kunert E, Böhle A, Feller AC. Prognostic value of MCM2 immunoreactivity in stage T1 transitional cell carcinoma of the bladder. Eur Urol. 2003;43:138–45. doi: 10.1016/s0302-2838(02)00580-8. [DOI] [PubMed] [Google Scholar]


