Abstract
Background:
Platelet products play a fundamental role in the process of healing. The new generation of platelet-rich fibrin (PRF), namely advanced PRF (A-PRF), has different biological and mechanical properties compared to those of leukocyte-PRF (L-PRF). This study aimed to compare the effects of L-PRF and A-PRF on the viability and migration of human gingival fibroblasts (HGFs).
Materials and Methods:
In this in vitro study, the effects of A-PRF and L-PRF on the viability and migration of HGFs after 24 and 48 h were evaluated using the methyl thiazolyl tetrazolium assay. The viability of the negative control culture medium was considered to be 100%. The mean optical density of the test groups was divided by that of the negative control group and reported as percentage. One-way ANOVA was applied to assess the effects of time and type of PRF on the viability and migration of HGFs. Pairwise comparisons were made using the Tukey's test.
Results:
At 24 h, cell viability in the L-PRF group was significantly higher than that in the A-PRF group (P < 0.05). However, no significant difference was noted between the two groups at 48 h. At 24 h, L-PRF caused significantly higher cell migration compared to the negative control group, whereas at 48 h, both A-PRF and L-PRF significantly increased cell migration compared to the control group.
Conclusion:
Within the limitations of this study, L-PRF and A-PRF had significant effects on the viability and migration of HGFs. Further studies on these platelet concentrates are warranted.
Key words: Cell migration, cell viability, fibroblast, platelet-rich fibrin
INTRODUCTION
Fibroblasts are the main cells of the gingival connective tissue and play a fundamental role in the integrity, longevity, and healing of the gingival connective tissue.[1] Healing of an injured tissue requires the presence of cells with regenerative capacity at the site. Fibroblasts should be able to synthesize the matrix while preserving their attachment to the extracellular matrix. This is particularly important in preserving the morphology and function of the cells as well as tissue integrity.[2] Wound healing is a complex process, which includes four phases of hemostasis, inflammation, proliferation, and remodeling.[3] The proliferation phase is particularly important due to the metabolic activity of the cells that cause tissue healing.[4]
Periodontal wound healing is a unique process due to the mutual interactions of the hard and soft tissues in the periodontium.[5] The final morphology and function of tissue are influenced by a number of factors that affect different phases of healing. The final tissue can be a nonfunctional fibrous tissue (scar tissue), relatively functional repaired tissue, or a completely functional tissue.[6]
Growth factors play a critical role in the process of healing.[7] Platelets contain different growth factors that guide the regenerating cells toward the wound site. The use of platelet-rich plasma is one method to procure a concentrated volume of growth factors for use at the wound site.[8] However, platelet-rich plasma releases the growth factors only for a short period of time and has poor mechanical properties. Thus, further research has been focused on platelet-rich fibrin (PRF), which is the second generation of platelet concentrates. No anticoagulant or activator is used for the preparation of this product.[9,10] Slow and sustained release of growth factors (minimum of 7 days) is among the characteristics of PRF.[8]
Recently, a modified form of PRF, namely advanced PRF (A-PRF), was introduced to the market.[11] Considering its lower speed of centrifugation, A-PRF contains higher amounts of platelets and growth factors and possesses better mechanical properties. To the best of authors' knowledge, rare study has compared the effects of leukocyte–PRF (L-PRF) and A-PRF on cell behavior. Thus, this study aimed to compare the effects of L-PRF and A-PRF on the viability and proliferation of human gingival fibroblasts (HGFs) in vitro.
MATERIALS AND METHODS
Blood collection
Blood samples were collected from two eligible candidates after obtaining their written informed consent. The volunteers were systemically healthy, had normal platelet count and blood factors, and had no intake of aspirin or other medications within the past 2 weeks before the onset of the study. This study was approved by the ethics committee (ethical code: IR.SBMU. RIDS. REC.1396.501).
Preparation of leukocyte–platelet-rich fibrin
A total of 27 mm of venous blood was collected in three 9-mm dry glass collecting tubes (Blood tubes®, Process, Nice, France) with no anticoagulant. According to the Choukroun's standard protocol, the tubes were immediately centrifuged at 2700 rpm (around 700 g) for 12 min. A fibrin blood clot was formed at the center of the tube between the blood cell sediments at the bottom and the overlaying serum at the top (containing plasma-poor platelet). The PRF clot was separated from the blood cells by scissors under a sterile hood. PRF box (Process, Nice, France) was used for the standardization of samples and their homogenization.
Preparation of advanced platelet-rich fibrin
A total of 10 mm of whole blood was centrifuged at 1500 rpm (100 g) for 14 min with no anticoagulant. The absence of anticoagulant in the blood sample allowed the formation of a fibrin clot at the center of the glass test tube between the red blood cell sediment at the bottom and acellular plasma at the top.
Cell culture and treatment
The HGF cell line was obtained from the National Cell Bank of Iran (NCBI, Pasteur Institute of Iran, Tehran). The fourth passage cells were seeded into 6-well plates (SPL Life Science, Korea) with 50,000 cell density in each dish containing Dulbecco's modified Eagle's medium (DMEM) (Gibco, Paisley, UK), plus 10% fetal bovine serum (FBS) (Gibco, Paisley, UK). After 24 h, the complete medium was replaced with Lowe serum medium (DMEM + 1% FBS) to stop cell proliferation. After 24 h, the cells were divided into 12 groups as follows:
Group 1: four culture media containing L-PRF, Group 2: four culture media containing A-PRF, Group 3 (negative control): two culture media containing 1% FBS, and Group 4 (positive control): two culture media containing 10% FBS.
To prevent accumulation and scraping of cells due to direct contact with PRF, the cell culture inserts (SPL Life Science, Korea) with 0.4 μm pore size were placed on the well plates. Next, PRF was transferred into the inserts such that the culture medium completely covered the PRF membranes.
Evaluation of proliferation and viability of the cells
After 24 and 48 h of culture, the cell cultures were rinsed with sterile phosphate-buffered saline (PBS) twice, and the proliferation and viability of the cells were evaluated using the methyl thiazolyl tetrazolium (MTT) assay. In brief, the MTT solution with a final concentration of 0.5 mg/mL was added to each culture dish at 24 and 48 h. The dishes were incubated at 37°C. After 4 h of incubation, the medium containing MTT was extracted and dimethyl sulfoxide was added to each dish, which resulted in the formation of insoluble crystals. Next, 100 μL of the solution was added to 96-well plates of enzyme-linked immunosorbent assay reader. The optical density was read at 570–620 nm wavelength.
Assessment of cell migration
In vitro scratch test or wound healing test was used for assessment of cell migration. The cells were seeded into 6-well plates with 50,000 cell density. Using a sterile sampler, 100 vertical scratches were created in each well (time zero). Each well was rinsed with complete culture medium twice to remove the cells scraped off from the bottom of the wells and were stuck in the scratches. Each well was then treated with PRF using cell culture inserts. The control group did not receive any treatment. The insert along with the PRF membrane was removed from the wells after 24 and 48 h of exposure, and the wells were rinsed with cold PBS, fixed with ice-cold 100% methanol, and stained with 20% ethanol containing 0.5% crystal violet. The stained cells were inspected under an inverted microscope at ×4 magnification, and digital images were obtained. The rate of cell migration was determined using Image J software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Each test was repeated in triplicate, and the results were analyzed using SPSS version 25 (SPSS Inc., IL, USA) through one-way ANOVA. Pairwise comparisons were carried out using the Tukey's test. The level of significance was set at P < 0.005.
RESULTS
As shown in Figure 1, at 24 h, a significant increase in cell proliferation was noted in A-PRF (152 ± 4%), L-PRF (187.5 ± 4%), and positive control groups (154.7 ± 5%) compared to the negative control group (100% viability). Furthermore, L-PRF caused a significant increase in cell proliferation compared to the positive control group (P < 0.05), but the difference between the A-PRF and positive control groups was not significant. At 48 h, cell proliferation in the A-PRF (140 ± 4%), L-PRF (132.3 ± 2%), and positive control groups (165.3 ± 3%) significantly increased compared to the negative control (P < 0.05). Furthermore, the A-PRF and L-PRF groups showed a significant increase in cell proliferation compared to the positive control. On the other hand, a significant difference was noted in cell viability and proliferation at 24 and 48 h in the L-PRF and A-PRF groups, respectively, such that cell viability at 24 h was significantly higher than that at 48 h (P < 0.05).
Figure 1.
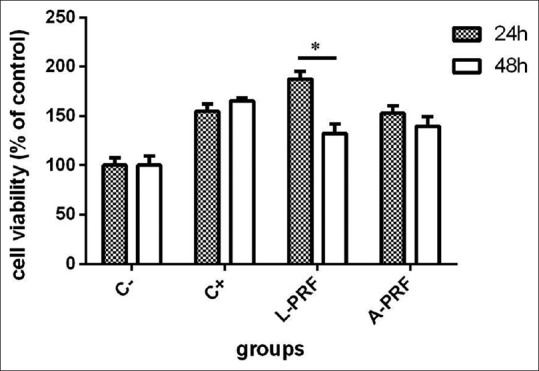
Viability of human gingival fibroblasts in different groups at 24 and 48 h (*P < 0.05). C- – negetive control, C+ – positive control, L-PRF – Leucocyte platelet rich fibrin, A-PRF – Advanced platelet rich fibrin
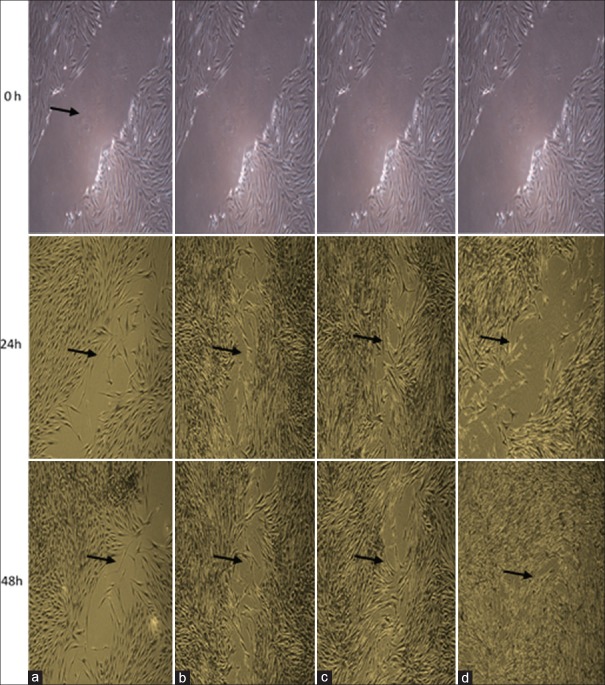
As shown in Figures 2 and 3, the migration of HGFs at 24 h significantly increased in the L-PRF group compared to the negative control (P < 0.05). However, at 48 h, a significant increase in migration and improvement of wound healing was noted in both the L-PRF (P < 0.05) and A-PRF (P < 0.01) groups compared to the negative control. Comparison of 24- and 48-h time points revealed a significant increase in cell migration in only A-PRF group (P < 0.001). No significant difference was noted between the two time points in other groups.
Figure 2.
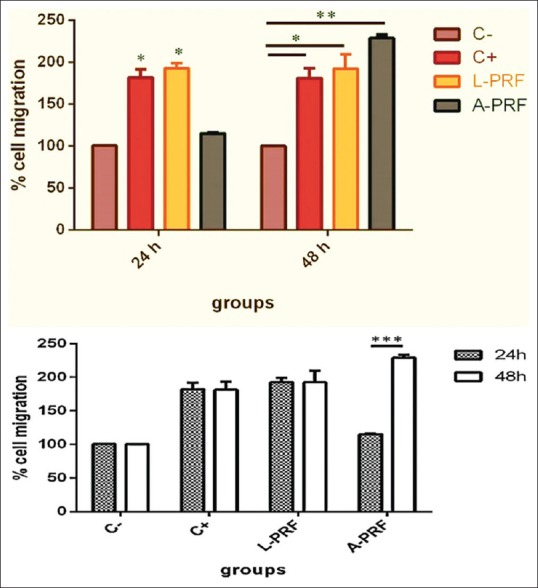
Migration of human gingival fibroblasts in different groups at 24 and 48 h (*P < 0.05, **P < 0.01, ***P < 0.001). C- – negetive control, C+ – positive control L-PRF – Leucocyte platelet rich fibrin, A-PRF – Advanced platelet rich fibrin
Figure 3.
Cell migration at 24 and 48 h after treatment (a) negative control; (b) positive control; (c) leukocyte–platelet-rich fibrin; (d) advanced platelet-rich fibrin
Comparison of the groups revealed no significant difference in cell migration between the positive control and L-PRF groups at 24 h (P > 0.05). However, cell migration in the aforementioned two groups had significant differences with that in the negative control and A-PRF groups (P < 0.05). At this time point, the difference in cell migration between the negative control and A-PRF group was not significant (P > 0.05).
At 48 h, cell migration in L-PRF, A-PRF, and positive control groups had significant differences with that in the negative control group. The highest difference was noted between A-PRF and negative control groups (P < 0.01). The difference in cell migration of L-PRF and positive control groups with that of negative control was also significant (P < 0.05). Although maximum cell migration at 48 h was noted in the A-PRF group, this value had no significant difference with that in the L-PRF and positive control groups (P > 0.05).
DISCUSSION
According to the current findings, cell viability and proliferation at 24 h after treatment were higher than those at 48 h. At 24 h, cell viability and proliferation in the L-PRF group were significantly higher than those in the A-PRF and positive control groups. In a study by Vahabi et al., exposure of cultured cells to PRF membrane had significant effects on their proliferation at 24 h compared to the negative control group. Nonetheless, at 48 and 72 h, PRF showed an inverse effect and caused 38% and 60% reduction in viability and proliferation of HGFs, respectively, compared to the negative control group, which was in agreement with our findings.[12] They also used the MTT assay similar to our study to assess the viability and proliferation of HGFs. Dohan Ehrenfest et al. evaluated the effects of PRF on primary culture of gingival fibroblasts using the MTT assay and showed that PRF induced significant and continuous proliferation of different types of cells, which was different from our findings.[13] Evidence shows that a platelet concentrate with a concentration 2.5 times the normal value in blood is ideal for cell proliferation.[14]
Fundamental changes in platelet concentrates such as PRF are not possible, and growth factors are released slowly within 7 days.[8] Furthermore, it should be noted that PRF includes all platelets derived from blood products, and the blood clot volume before concentration accounts for 40% of the volume of the final product. Accordingly, in such concentrates, the product has 2.5 times the platelet count of the blood. However, this topic is in need of further investigation.
According to the current results, the effect of L-PRF on the viability of HGFs at 24 h was greater than that of A-PRF; but, the viability of cells after 48 h of exposure was the same for both products. Evidence shows that platelet concentrates contain a number of factors with different effects.[15] Variations in the amounts of these factors in different platelet concentrates can lead to different outcomes in terms of cell viability. Furthermore, some studies only assessed the effects of supernatants of platelet concentrates, and the effects of the whole product were disregarded.[15] PRF is a concentrated fibrin with a specific matrix structure and special cell content, and its exudate is not the only part derived from it.
In the present study, we tried our best to standardize the process of production of L-PRF and A-PRF. The preparation of these concentrates was relatively easy and affordable and did not require any special equipment. L-PRF is a novel advancement in the field of platelet concentrates because it is a fibrin membrane containing platelets and trapped leukocytes. Such strong membranes have excellent properties and can be firmly sutured in suitable anatomical sites during open surgery. However, the physical and biological properties of L-PRF have been less commonly investigated. One important criterion in the preparation of L-PRF membrane is the time lapse between blood collection and centrifugation. The success of L-PRF technique, in general, depends on fast blood collection, its immediate transfer for centrifugation (within approximately 1 min), and 21°C centrifugation temperature. If blood collection takes too long and adequate, homogeneity is not achieved or if the centrifugation temperature is 21°C to 30°C, a good-quality L-PRF with a suitable cell content, efficient matrix structure, and adequate growth factor release profile is not achieved.[16]
Evidence shows that L-PRF has the ability to induce guided tissue regeneration through endogenous cell homing and is, therefore, an important biomaterial in regenerative medicine.[17] A-PRF has higher amounts of platelets and growth factors and superior mechanical properties compared to PRF due to lower speed of centrifugation. It also has higher white blood cell content, and due to low speed of centrifugation, this fibrin clot is softer than the original PRF.[11]
Fujioka-Kobayashi et al. demonstrated that A-PRF concentrates caused higher proliferation and migration of HGFs compared to L-PRF.[18] In the present study, cell migration in the A-PRF group was lower than that in the L-PRF group after 24 h of exposure and higher than that in the L-PRF group after 48 h of exposure, which was relatively in line with the current findings.
In previous studies, blood was collected from several candidates; thus, between-group comparisons were problematic. Evidence shows that significant differences exist between individuals with similar platelet concentrations in terms of concentration of growth factors.[19,20,21] Interindividual differences are partly responsible for the difference in the reported results of previous studies regarding the effects of platelet concentrates. In the present study, all blood samples were collected from two healthy, nonsmoker candidates to eliminate the confounding effect of interindividual differences on the results.
In vitro studies are beneficial for the assessment of the biological effects of PRF on periodontal cells. However, in vitro results have limitations in terms of generalization to the clinical setting. Further studies are required to compare different platelet formulations to determine different therapeutic scenarios in the clinical setting.
CONCLUSION
Within the limitations of this study, L-PRF and A-PRF had significant effects on the viability of HGFs. Further studies on these platelet concentrates are warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
This article is taken from a thesis submitted in partial fulfillment of the requirements for the degree of dentistry in International Branch Shahid Beheshti University of Medical Sciences, Tehran, Iran.
REFERENCES
- 1.Cate AR, Deporter DA. The degradative role of the fibroblast in the remodelling and turnover of collagen in soft connective tissue. Anat Rec. 1975;182:1–3. doi: 10.1002/ar.1091820102. [DOI] [PubMed] [Google Scholar]
- 2.Götz W, Gerber T, Michel B, Lossdörfer S, Henkel KO, Heinemann F. Immunohistochemical characterization of nanocrystalline hydroxyapatite silica gel (NanoBone(r)) osteogenesis: A study on biopsies from human jaws. Clin Oral Implants Res. 2008;19:1016–26. doi: 10.1111/j.1600-0501.2008.01569.x. [DOI] [PubMed] [Google Scholar]
- 3.Diegelmann RF, Evans MC. Wound healing: An overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 4.Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64:217–24. [PubMed] [Google Scholar]
- 5.Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256–60. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 6.Arnoczky S. We Improve Mother Nature's Recipe. Las Vegas: Arthroscopy Association of North America Specialty Day; 2009. Biologic adjuncts to connective tissue healing; pp. 101–7. [Google Scholar]
- 7.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 8.Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun's platelet-rich fibrin (PRF): A gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27:63–9. doi: 10.1080/08977190802636713. [DOI] [PubMed] [Google Scholar]
- 9.Bowers GM, Chadroff B, Carnevale R, Mellonig J, Corio R, Emerson J, et al. Histologic evaluation of new attachment apparatus formation in humans. Part I. J Periodontol. 1989;60:664–74. doi: 10.1902/jop.1989.60.12.664. [DOI] [PubMed] [Google Scholar]
- 10.Cortellini P, Bowers GM. Periodontal regeneration of intrabony defects: An evidence-based treatment approach. Int J Periodontics Restorative Dent. 1995;15:128–45. [PubMed] [Google Scholar]
- 11.Choukroun J. Advanced PRF and i-PRF: Platelet concentrate or blood concentrate? J Periodont Med Clin Pract. 2014;1:3. [Google Scholar]
- 12.Vahabi S, Vaziri S, Torshabi M, Rezaei Esfahrood Z. Effects of Plasma Rich in Growth Factors and Platelet-Rich Fibrin on Proliferation and Viability of Human Gingival Fibroblasts. J Dent (Tehran) 2015;12:504–12. [PMC free article] [PubMed] [Google Scholar]
- 13.Dohan Ehrenfest DM, Diss A, Odin G, Doglioli P, Hippolyte MP, Charrier JB. In vitro effects of Choukroun's PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:341–52. doi: 10.1016/j.tripleo.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17:212–9. doi: 10.1111/j.1600-0501.2005.01203.x. [DOI] [PubMed] [Google Scholar]
- 15.Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, et al. Angiogenesis is regulated by a novel mechanism: Pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–33. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crisci A, Lombardi D, Serra E, Lombardi G, Cardillo F, Crisci M. Standardized protocol proposed for clinical use of L-PRF and the use of L-PRF Wound Box®. J Unexplored Med Data. 2017;2:78. [Google Scholar]
- 17.Di Liddo R, Bertalot T, Borean A, Pirola I, Argentoni A, Schrenk S, et al. Leucocyte and platelet-rich Fibrin: A carrier of autologous multipotent cells for regenerative medicine. J Cell Mol Med. 2018;22:1840–54. doi: 10.1111/jcmm.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. Optimized platelet-rich fibrin with the low-speed concept: Growth factor release, biocompatibility, and cellular response. J Periodontol. 2017;88:112–21. doi: 10.1902/jop.2016.160443. [DOI] [PubMed] [Google Scholar]
- 19.Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30:97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 20.Lacoste E, Martineau I, Gagnon G. Platelet concentrates: Effects of calcium and thrombin on endothelial cell proliferation and growth factor release. J Periodontol. 2003;74:1498–507. doi: 10.1902/jop.2003.74.10.1498. [DOI] [PubMed] [Google Scholar]
- 21.Martineau I, Lacoste E, Gagnon G. Effects of calcium and thrombin on growth factor release from platelet concentrates: Kinetics and regulation of endothelial cell proliferation. Biomaterials. 2004;25:4489–502. doi: 10.1016/j.biomaterials.2003.11.013. [DOI] [PubMed] [Google Scholar]